Abstract
Study Objectives:
Approximately 8-10% of the general population suffers from chronic insomnia, whereas another 20-30% of the population has insomnia symptoms at any given time (i.e., poor sleep). However, few longitudinal studies have examined risk factors of the natural history of poor sleep, and none have examined the role of polysomnographic (PSG) variables.
Design:
Representative longitudinal study.
Setting:
Sleep laboratory.
Participants:
From a random, general population sample of 1,741 individuals of the adult Penn State Cohort, 1,395 were followed up after 7.5 yr.
Measurements:
Full medical evaluation and 1-night PSG at baseline and telephone interview at follow-up.
Results:
The rate of incident poor sleep was 18.4%. Physical (e.g., obesity, sleep apnea, and ulcer) and mental (e.g., depression) health conditions and behavioral factors (e.g., smoking and alcohol consumption) increased the odds of incident poor sleep as compared to normal sleep. The rates of persistent, remitted, and poor sleepers who developed chronic insomnia were 39%, 44%, and 17%, respectively. Risk factors for persistent poor sleep were physical health conditions combined with psychologic distress. Shorter objective sleep duration and a family history of sleep problems were risk factors for poor sleep evolving into chronic insomnia.
Conclusions:
Poor sleep appears to be primarily a symptom of physical and mental health conditions, whereas the persistence of poor sleep is associated with psychologic distress. Importantly, sleep apnea appears to be associated with incident poor sleep but not with chronic insomnia. Finally, this study suggests that objective short sleep duration in poor sleepers is a biologic marker of genetic predisposition to chronic insomnia.
Citation:
Fernandez-Mendoza J; Vgontzas AN; Bixler EO; Singareddy R; Shaffer ML; Calhoun SL; Karataraki M; Vela-Bueno A; Liao D. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. SLEEP 2012;35(5):689-697.
Keywords: Incidence, persistence, poor sleep, chronic insomnia, polysomnography
INTRODUCTION
The prevalence of insomnia varies widely among epidemiologic studies ranging from 8-40%. A possible explanation for this wide variability is the different criteria used to define insomnia. For example, approximately 8-10% of the general population suffers from chronic insomnia,1–3 whereas another 20-30% of the population has insomnia symptoms, i.e., difficulties initiating sleep, difficulties maintaining sleep, early morning awakening, and/or nonrestorative sleep, at any given time.1–3 When current Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria are used, the prevalence of insomnia is approximately 20%,4,5 a figure that decreases to 9% when further criteria is used to define insomnia as severe.4
In our most recent studies, we have used the term poor sleep to refer to the presence in an individual of 1 or more insomnia symptoms.6–12 This term is very similar to those used in other studies, such as insomnia symptoms, symptoms of insomnia, or sleep difficulties.2,3,12,13 Despite the high prevalence of poor sleep or insomnia symptoms in the general population,2,3 its effect on physical and mental health is not clear. We have recently reported that poor sleep with objective short sleep duration is a risk factor for hypertension.6 Moreover, poor sleep is an independent risk factor for the development of chronic insomnia,7 which is associated with significant medical morbidity6,8–10 and mortality.11 Together, these findings suggest that poor sleep is a very prevalent condition whose medical effect has been underestimated.
Very little is known about the incidence and persistence of insomnia symptoms or poor sleep, with 2 studies suggesting rates of approximately 30% and 46%, respectively.12,13 Even less is known about the risk factors involved in the natural history of poor sleep in the general population. Furthermore, no study to date has explored the role of polysomnographic (PSG) variables, such as sleep apnea or sleep duration, in the natural history of poor sleep. The 3 objectives of this study were to examine the risk factors involved in the incidence of poor sleep, the persistence of poor sleep, and the evolution of poor sleep into chronic insomnia in a large general random sample (the Penn State Cohort Study) using both subjective and objective (i.e., PSG) data and a longitudinal design.
METHODS
Population
The data presented here were collected as part of a population-based study of sleep disorders, which used a 2-phase protocol to recruit participants from various age groups.2,14,15 In the first phase of the study, a sample of adult men and women (age 20 yr or older) was randomly selected from local telephone households in 2 counties of central Pennsylvania (Dauphin and Lebanon) using the Mitofsky-Waksberg 2-stage random digit dialing procedure.16 A within-household selection procedure described by Kish17 was used to select the specific man or woman to be interviewed. Telephone interviews were conducted with 4,364 age-eligible men and 12,219 age-eligible women residing in the sample households, for a total sample of 16,583 with response rates of 73.5% and 74.1%, respectively. The questionnaire used in this interview included basic demographic and sleep information. In the second phase of this study, a subsample of 741 men and 1,000 women, selected randomly from those individuals previously interviewed by telephone, were studied in our sleep laboratory. The response rates for this phase were 67.8% and 65.8% for men and women, respectively. After providing a complete description of the study to the individuals, written informed consent was obtained. We contrasted those individuals who were recorded in the laboratory with those who were selected but not recorded in terms of age, body mass index, and prevalence of sleep disorders. There were no significant differences between these 2 groups on any of these variables.
Of the 1,741 individuals who completed the comprehensive sleep evaluation, 1,395 were followed up after an average duration of 7.5 yr (mean duration of 4.5 yr for women and 10.5 yr for men) by 1 of the investigators (S.L.C.) via telephone interview. The response rate of the follow-up study was 79.7%. However, if one considers that 215 individuals died between baseline and follow-up, then the response rate of those alive was 90.9%. In the Penn State Cohort Study, men were recruited first and women 5 yr later. This explains the 5-yr difference in the follow-up period between men and women. After complete description of the follow-up study to the individuals, verbal informed consent was obtained. The entire study procedure was approved by the University's Institutional Review Board.
Key Measurements
Each participant selected for laboratory evaluation completed a comprehensive sleep history and physical examination. All participants were evaluated for 1 night in the sleep laboratory in sound-attenuated, light- and temperature-controlled rooms. During this evaluation, each participant was continuously monitored for 8 h (fixed-time period) using 16-channel PSG including electroencephalogram, electrooculogram, and electromyogram. Bedtimes were adjusted to conform to participants' usual bedtimes, and participants were recorded between 22:00-23:00 and 06:00-07:00. The sleep recordings were subsequently scored independently, according to Rechtschaffen and Kales criteria.18 The percentage of sleep time is total sleep time divided by recorded time in bed and multiplied by 100. Additional information obtained during the PSG included that assessing sleep apnea. Respiration was monitored throughout the night by use of thermocouples at the nose and mouth and thoracic strain gauges. All-night recordings of hemoglobin oxygen saturation (SpO2) were obtained with an oximeter attached to the finger. Apnea was considered present if a breath cessation exceeded 10 sec and each apnea was categorized in terms of obstructive (chest wall movement present) or central (chest wall movement absent). In addition, hypopnea was considered present when a reduction in air flow of approximately 50% was indicated at the nose or mouth and was associated with a reduction of 4% arterial blood oxygen saturation.14,15 For the purpose of this study, the presence of sleep apnea was established on 3 levels of severity. The absence of sleep apnea was defined as an apnea/hypopnea index (AHI) < 5, mild sleep apnea was defined as an AHI 5-14.9, and moderate to severe sleep apnea was defined as an AHI ≥ 15. Body mass index was based on measured height (cm) and weight (kg) during the participants' sleep laboratory visit, and data are presented in terms of mean and percentage within each category.
As part of this protocol we also assessed for the presence of all sleep disorders, based on a standardized questionnaire completed by the participants on the evening of their sleep laboratory visit. This questionnaire consists of 53 questions (7 demographic, 20 sleep related, and 26 general health questions). In addition, women responded to 8 questions related to menstrual history, menopause, and hormone therapy. Sleep related questions were qualified in terms of severity on a scale of 0-3 (0 = none, 1 = mild, 2 = moderate, 3 = severe) and duration. Health conditions were also qualified in terms of severity, type of treatment (on a scale of 0-7), and duration. The presence of sleep difficulty was established on 3 levels of severity. First, chronic insomnia was defined as a complaint of insomnia (i.e., a positive response to the question “Do you feel you have insomnia?”) with a duration of ≥ 1 yr. Second, poor sleep was defined as a moderate to severe complaint (based on a mild to severe scale) of difficulty falling asleep (“Do you have difficulty falling asleep?”), difficulty staying asleep (“Do you have difficulty staying asleep?”), early final awakening (“Do you wake up in the morning earlier than desired?”), or unrefreshing sleep (“Do you still feel groggy and unrefreshed after morning awakening?”). Third, normal sleep was defined as the absence of either of these 2 categories. To create 3 mutually exclusive categories, none in the poor sleep group reported having chronic insomnia and none in the normal sleeping group reported either chronic insomnia or poor sleep.
Additional information obtained from the standardized questionnaire included assessing physical and mental health, substance use, and family history of sleep problems. We ascertained whether the respondent was currently being treated for physical (e.g., hypertension, diabetes, thyroidism) and/or mental (e.g., depression, alcohol use, drug use) health conditions. A composite variable for each general category of physical or mental health conditions was calculated by indicating a positive response when at least 1 health condition within each category was present, as we have described in other studies.2,9,10 We also ascertained respondents' daily consumption of caffeine (number of cups/day), tobacco (number of cigarettes/day), and alcohol (number of drinks/day). We defined a positive family history of sleep problems as a moderate to severe answer (based on a mild to severe scale) to the question “Does anyone in your family (blood relatives) have sleep problems?” From the 1,741 individuals, a total of 1,300 completed a valid Minnesota Multiphasic Personality Inventory-2 (MMPI-2) at baseline. The MMPI-2 was administered following the standardized rules and scored accordingly.19
Follow-up measures taken through telephone interview included the standardized questionnaire that participants completed at baseline during their sleep laboratory visit. Sleep related questions were also used to establish the presence of sleep difficulty at follow-up based on 3 levels of severity as defined previously (i.e., normal sleep, poor sleep, and chronic insomnia).
Sample
Of the 1,395 participants who were followed up, 149 had chronic insomnia, 403 had poor sleep, and 843 had normal sleep at baseline. Because the focus of the current study is the natural history of poor sleep, those individuals with chronic insomnia at baseline were excluded from further analysis; these individuals were part of a study on risk factors for persistent chronic insomnia.20 All participants selected for the current study were further classified according to their baseline and follow-up status into 4 groups: normal sleep (individuals with normal sleep both at baseline and at follow-up; n = 590), incident poor sleep (individuals with normal sleep at baseline and with poor sleep at follow-up; n = 188), persistent poor sleep (individuals with poor sleep both at baseline and at follow-up; n = 155), and remitted poor sleep (individuals with poor sleep at baseline and with normal sleep at follow-up; n = 180). Two additional groups were obtained for secondary analysis of the natural history of poor sleep: poor sleepers who developed chronic insomnia (individuals with poor sleep at baseline and with chronic insomnia at follow-up; n = 68) and normal sleepers who developed chronic insomnia (individuals with normal sleep at baseline and with chronic insomnia at follow-up; n = 65). Figure 1 shows the participant flow in the study.
Figure 1.
Participants' flow in the study.
Statistical Analyses
The design of this study included oversampling of those at higher risk for sleep disordered breathing and women with markedly higher levels of body mass index to increase the precision of the risk estimates. Because of this sampling strategy, numeric sampling weights were developed for the analysis so that the estimates could be inferred to the general population. A comprehensive presentation of this sampling strategy has been described,2,–11,14,15 including the use of the NHANES III laboratory data as the standard to adjust both the men and women in terms of sociodemographics to be representative of the national population. Therefore, we adjusted for the sampling weight in all of our analyses, including those estimating incidence and persistence rates.
Mean (standard deviation, SD) and proportions of the demographic characteristics were calculated for the entire population, as well as stratified according to poor sleep status. The normal sleep group was used as the reference for incident poor sleep, whereas the remitted poor sleep group was used as the reference for persistent poor sleep. Analysis of variance (ANOVA) was used to examine mean differences on quantitative variables between the study groups. Chi-square tests were used to examine differences between the study groups on qualitative variables. Multivariable logistic regression models were used to assess the independent association of each risk factor with incident or persistent poor sleep. We calculated the odds ratios (OR) and the 95% confidence intervals (95% CIs) from the regression models to estimate the risk of incident or persistent poor sleep associated with each risk factor, simultaneously adjusting for covariates. Multivariate analysis of covariance (MANCOVA) was used to examine mean differences on MMPI-2 scores between the study groups, while controlling for potential confounding factors. Bonferroni corrections were applied to control for type I errors when performing post hoc multiple comparisons. All analyses were conducted with SPSS version 17.0 for Windows.
RESULTS
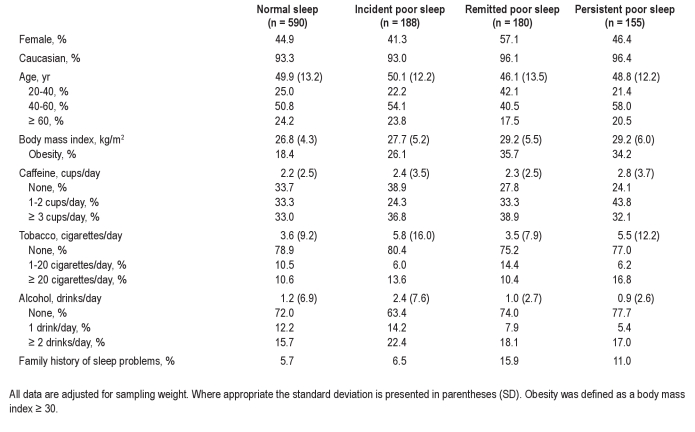
The rate of incident poor sleep was 18.4%. The rates of persistent poor sleepers, remitted poor sleepers, and poor sleepers who developed chronic insomnia were 39%, 44%, and 17%, respectively. The demographic, clinical, and behavioral characteristics of the study sample and stratified by poor sleep status are presented in Table 1.
Table 1.
Sociodemographic and behavioral characteristics at baseline
Incident Poor Sleep
Individuals with incident poor sleep were significantly more obese (P = 0.020), smoked more cigarettes (P = 0.015), and consumed more alcohol (P = 0.040) at baseline when compared with normal sleepers (Table 1). Having ulcer (P = 0.0001) and depression (P = 0.002) at baseline were significantly associated with incident poor sleep when compared with normal sleep (Table 2). Moderate to severe sleep apnea (AHI ≥ 15) at baseline was significantly associated with incident poor sleep (P = 0.039; Table 3). Moreover, the group with incident poor sleep showed significantly higher scores in all MMPI-2 scales, except 3-Hysteria, when compared with normal sleepers (Table 4).
Table 2.
Physical and mental health conditions at baseline
Table 3.
Polysomnographic characteristics at baseline
Table 4.
MMPI-2 scores at baseline
A multivariable logistic regression model showed that ulcer (OR = 7.00; 95% CI = 3.31-14.8; P = 0.0001), depression (OR = 2.04; 95% CI = 1.17-3.57; P = 0.012), obesity (OR = 1.70; 95% CI = 1.13-2.55; P = 0.010), and smoking (OR = 1.02; 95% CI = 1.01-1.03; P = 0.024) significantly increased the odds of incident poor sleep compared with normal sleep, whereas sleep apnea (AHI ≥ 15) was only marginally associated with incident poor sleep (OR = 1.83; 95% CI = 0.94-3.54; P = 0.074) and alcohol consumption (OR = 1.01; 95% CI = 0.99-1.04; P = 0.159) did not remain as a significant predictor. The risk of incident poor sleep associated with physical health conditions, i.e., ulcer, obesity, and AHI ≥ 15 (OR = 2.32; 95% CI = 1.56-3.45; P < 0.001) and mental health conditions (OR = 2.19; 95% CI = 1.38-3.47; P = 0.001) was of similar magnitude. Smoking (OR = 1.02; 95% CI = 1.01-1.03; P = 0.031) was also a significant predictor of incident poor sleep in this secondary model.
Persistent Poor Sleep
Persistent poor sleep was significantly associated with middle age (P = 0.003), heart disorder (P = 0.002), ulcer (P = 0.012), and allergy/asthma (P = 0.040), and marginally associated with depression (P = 0.056), smoking (P = 0.059), and male sex (P = 0.099), when compared with remitted poor sleep. Furthermore, the group of participants with persistent poor sleep showed significantly higher scores in the 1-Hypochondriasis, 2-Depression, and 7-Psychasthenia clinical scales of the MMPI-2, when compared with remitted poor sleepers (Table 4).
In a multivariable logistic regression model, the risk factors that significantly increased the odds of persistent poor sleep were: heart disorder (OR = 3.43; 95% CI = 1.41-8.33; P = 0.007), ulcer (OR = 2.62; 95% CI = 1.07-6.45; P = 0.036), middle age (OR = 2.19; 95% CI = 1.11-4.33; P = 0.024), and allergy/asthma (OR = 1.99; 95% CI = 1.05-3.76; P = 0.035).
Development of Chronic Insomnia
As shown in Table 5, the risk factors associated with the development of chronic insomnia from poor sleep, as compared with normal sleep, in a multivariable logistic regression model were female sex, age younger than 40 yr, depression, smoking, fewer physical health conditions, and shorter objective sleep duration. Furthermore, a higher percentage of poor sleepers, as compared with normal sleepers who developed chronic insomnia, had a family history of sleep problems (35% versus 3%, respectively; P < 0.0001). Finally, poor sleepers who developed chronic insomnia showed higher scores in all MMPI-2 clinical scales except 9-Hypomania when compared with normal sleepers who also developed chronic insomnia (data not shown; e.g., mean number of MMPI-2 elevations, 2.0 ± 2.2 versus 0.8 ± 2.1).
Table 5.
Development of chronic insomnia from poor sleep versus normal sleep at baseline
DISCUSSION
The main findings of this population-based, longitudinal study are that incident poor sleep is associated with both physical and mental health conditions, psychologic distress is the link between physical problems and the persistence of poor sleep, and objective short sleep duration in poor sleepers is a biologic marker of predisposition to chronic insomnia.
Limited information exists on the risk factors associated with incident poor sleep. A previous general population study showed that the incidence of insomnia symptoms was associated primarily with baseline self-reported anxiety, depression, and pain.21 In the current study the incidence of poor sleep, i.e., moderate-to-severe insomnia symptoms, was associated with both physical (e.g., ulcer, obesity, and sleep apnea) and mental health conditions (e.g., depression and maladaptive personality traits) as well as behavioral (e.g., smoking and alcohol consumption) factors. An interesting finding of our study is that obesity was an independent risk factor for incident poor sleep. In addition, individuals in whom chronic insomnia developed, either from a premorbid status of normal sleep or poor sleep, were also significantly more obese at baseline when compared with normal sleepers. Taken together, our results suggest that metabolic aberrations associated with obesity may play a role in the development of poor sleep and chronic insomnia.
Another important finding of our study is that moderate to severe sleep apnea (AHI ≥ 15) is a risk factor of incident poor sleep. Since the 1970s, there have been reports suggesting that sleep apnea is associated with chronic insomnia,22–30 whereas other large studies have not shown a significant association between sleep apnea and chronic insomnia.2,–37 Our findings based on a general population longitudinal study appear to shed new light on this old controversy. Whereas in our previous cross-sectional and longitudinal studies there was a lack of association between sleep apnea with prevalent,2 incident,7 or persistent20 chronic insomnia, poor sleep, i.e., insomnia symptoms, can indeed be a consequence of sleep apnea. Thus, it appears that sleep apnea through breathing pauses and intermittent hypoxia can lead to sleep disruption, i.e., frequent brief awakenings, but this sleep disruption does not evolve into chronic insomnia, which is usually associated with prolonged periods of wake at the beginning or in the middle of the sleep period. This hypothesis is based on the fact that sleep loss in the form of sleep deprivation or sleep fragmentation in individuals without insomnia is associated with physiologic sleepiness, i.e., shortened sleep latencies in the multiple sleep latency test (MSLT),38 whereas in chronic insomnia sleep disruption, i.e., prolonged sleep latency and/or awakenings, is not associated with physiologic sleepiness, as revealed by increased sleep latencies in the MSLT.39 Thus, these findings are consistent with a model of chronic insomnia being a disorder of physiologic hyperarousal.40–43
The rate of persistent poor sleep in the current study was 39%, which is similar to that reported by Morin and colleagues,13 who followed individuals with insomnia symptoms for 3 yr. Other studies have reported persistence rates that ranged from 44-69%; however, they were limited by either focusing on elderly populations, men, or having a shorter follow-up period.21,44,45 Furthermore, our study showed for the first time that middle age and physical health (e.g., heart disorder, ulcer, and allergy/asthma), when associated with psychologic distress, are the primary risk factors of the persistence of poor sleep. This is supported by the fact that remitted poor sleepers did not show significant overall psychologic distress as compared with normal sleepers.
In approximately 17% of individuals, poor sleep evolved into chronic insomnia. This means that approximately 4% of the general population that perceive themselves as poor sleepers will develop chronic insomnia. Given that chronic insomnia is associated with significant morbidity6,–10 and mortality,11 identifying risk factors associated with this outcome is crucial to developing preventive strategies. In our study, young age, female sex, mental health conditions (including maladaptive personality traits), objective short sleep duration, and a family history of sleep problems were significant risk factors for poor sleep evolving into chronic insomnia. Focusing on these demographic groups (i.e., young women) or addressing modifiable factors (i.e., mental health and smoking) will be associated with higher success of preventive strategies.
A novel finding of our study is that objective short sleep duration is a risk factor predisposing to chronic insomnia. We have previously postulated that objective short sleep duration is an index of the biologic severity of chronic insomnia6,–11,40,41 and a predictor of its persistence over time.20 This study suggests that objective short sleep duration is also a marker of predisposition to this disorder, i.e., who among poor sleepers will develop chronic insomnia. We believe that, in chronic insomnia, objective short sleep duration is a marker of physiologic hyperarousal40–42 determined by biologic/genetic factors. In this regard, the strong family history of moderate to severe sleep complaints in poor sleepers who developed chronic insomnia (35%) compared with normal sleepers (3%) who also developed chronic insomnia lends support to this hypothesis.
The issue of whether insomnia is a disorder or a symptom of an underlying physical or mental health condition has long been debated but no definite conclusions have been reached. We have previously proposed that chronic insomnia with objective short sleep duration is a disorder associated with physiologic hyperarousal and significant morbidity and mortality. In contrast, chronic insomnia with objective normal sleep duration is not associated with physiologic hyperarousal or significant medical sequelae. This study shows that poor sleep is primarily a symptom of underlying physical or mental health conditions, whereas poor sleep with objective short sleep duration is in a continuum with chronic insomnia.
Some limitations should be taken into account when interpreting our results. The objective sleep in this study was based on 1 night of PSG, which may not be representative of the participants' typical or optimal objective sleep duration and may be affected by the so-called first night effect. In large epidemiologic studies the average objective sleep duration is approximately 6 hr, which is independent of whether sleep is recorded at home with PSG (Sleep Heart Health Study)46 or for 3 consecutive nights with actigraphy (CARDIA Study)47 or in the sleep laboratory with PSG (Penn State Cohort Study).6–11 Thus, the consistency among these 3 large epidemiologic studies in terms of objective sleep duration supports the validity of our findings. In the current study, we did not assess daytime consequences of insomnia; however, 3 lines of evidence support the validity of our definitions of poor sleep and chronic insomnia. First, all of those that identified themselves as having chronic insomnia reported 1 or more of the 4 nighttime symptoms used in the diagnosis of insomnia, i.e., difficulty falling asleep, difficulty staying asleep, early morning awakening, or unrefreshing sleep. Second, the prevalence estimates in the Penn State Cohort using these definitions2 are similar to those of other population-based studies where insomnia symptoms and chronic insomnia/insomnia syndrome are defined as 2 mutually exclusive groups.3 Third, in our previous studies, chronic insomnia was strongly associated with cardiometabolic morbidity6,8 and mortality,11 whereas the association of poor sleep was much smaller or nonsignificant. These findings give further support to the face, construct, and predictive validity of the definitions used. Another potential limitation is that we have only 1 follow-up time point that precludes a more accurate assessment of the waxing and waning typical of poor sleep and chronic insomnia over time.13 Finally, effort-related breathing arousals were not quantified during the sleep study,14,15 which did not allow examination of their relative contribution in the natural history of poor sleep.
In summary, our data suggest that poor sleep is primarily a symptom of diverse physical and mental health conditions, whereas the persistence of poor sleep is associated with psychologic distress. Furthermore, objective short sleep duration and a family history of sleep problems are predisposing factors for poor sleep evolving into chronic insomnia. Finally, poor sleep and chronic insomnia with normal sleep duration appear to be in a continuum with strong psychologic roots, whereas poor sleep and chronic insomnia with objective short sleep duration appear to be in a continuum with a strong biological background (e.g., genetics and physiologic hyperarousal).
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was funded in part by the National Institutes of Health grants RO1 51931, RO1 40916 (to Dr. Bixler), and RO1 64415 (to Dr. Vgontzas). The work was performed at the Sleep Research and Treatment Center at the Penn State University Milton Hershey Hospital, and the staff is especially commended for their efforts. The principal investigator, Dr. Vgontzas, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in central Pennsylvania. J Psychosom Res. 2002;53:589–92. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 3.Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res. 2000;9:35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 5.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–97. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. doi: 10.1016/j.sleep.2011.10.033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1–6. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al. Sleep misperception and chronic insomnia in the general population: the role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73:88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State Cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeBlanc M, Mérette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 14.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–48. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 15.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 16.Waksberg J. Sampling methods for random digit dialing. J Am Stat Assoc. 1978;73:40–6. [Google Scholar]
- 17.Kish L. Survey sampling. New York: John Wiley – Sons Inc; 1965. [Google Scholar]
- 18.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, MD: National Institutes of Health; 1968. [Google Scholar]
- 19.Butcher JN, Graham JR, Ben-Porath YS, Tellegen A, Dahkstrom WG. MMPI-2: Manual for administration, scoring and interpretation. Revised ed. Minneapolis: University of Minnesota Press, ; 2001. [Google Scholar]
- 20.Vgontzas AN, Fernandez-Mendoza J, Bixler EO, et al. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep, 2012;35:61–68. doi: 10.5665/sleep.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–80. [PubMed] [Google Scholar]
- 22.Guilleminault C, Eldridge FL, Dement WC. Insomnia with sleep apnea: a new syndrome. Science. 1973;181:856–8. doi: 10.1126/science.181.4102.856. [DOI] [PubMed] [Google Scholar]
- 23.Stone J, Morin CM, Hart RP, Remsberg S, Mercer J. Neuropsychological functioning in older insomniacs with or without obstructive sleep apnea. Psychol Aging. 1994;9:231–6. doi: 10.1037//0882-7974.9.2.231. [DOI] [PubMed] [Google Scholar]
- 24.Lichstein KL, Riedel BW, Lester KW, Aguillard RN. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67:405–10. doi: 10.1037//0022-006x.67.3.405. [DOI] [PubMed] [Google Scholar]
- 25.Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49:948–53. doi: 10.1016/s0006-3223(00)01087-8. [DOI] [PubMed] [Google Scholar]
- 26.Smith S, Sullivan K, Hopkins W, Douglas J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS) Sleep Med. 2004;5:449–56. doi: 10.1016/j.sleep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Krell SB, Kapur VK. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005;9:104–10. doi: 10.1007/s11325-005-0026-x. [DOI] [PubMed] [Google Scholar]
- 28.Gooneratne NS, Gehrman PR, Nkwuo JE, et al. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–8. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 29.Krakow B, Ulibarri VA, Romero EA. Patients with treatment-resistant insomnia taking nightly prescription medications for sleep: a retrospective assessment of diagnostic and treatment variables. Prim Care Companion J Clin Psychiatry. 2010;12 doi: 10.4088/PCC.09m00873bro. PCC.09m00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krakow B, Ulibarri VA, Romero E. Persistent insomnia in chronic hypnotic users presenting to a sleep medical center: a retrospective chart review of 137 consecutive patients. J Nerv Ment Dis. 2010;198:734–41. doi: 10.1097/NMD.0b013e3181f4aca1. [DOI] [PubMed] [Google Scholar]
- 31.Kales A, Bixler EO, Soldatos CR, Vela-Bueno A, Caldwell AB, Cadieux RJ. Biopsychobehavioral correlates of insomnia, part 1: role of sleep apnea and nocturnal myoclonus. Psychosomatics. 1982;23:589–600. doi: 10.1016/S0033-3182(82)73359-6. [DOI] [PubMed] [Google Scholar]
- 32.Edinger JD, Hoelscher TJ, Webb MD, Marsh GR, Radtke RA, Erwin CW. Polysomnographic assessment of DIMS: empirical evaluation of its diagnostic value. Sleep. 1989;12:315–22. [PubMed] [Google Scholar]
- 33.Vgontzas AN, Bixler EO, Kales A, Manfredi RL, Tyson K. Validity and clinical utility of sleep laboratory criteria for insomnia. Int J Neurosci. 1994;77:11–21. doi: 10.3109/00207459408986015. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF, 3rd, Hauri PJ, et al. Diagnostic concordance for DSM-IV sleep disorders: a report from the APA/NIMH DSM-IV field trial. Am J Psychiatry. 1994;151:1351–60. doi: 10.1176/ajp.151.9.1351. [DOI] [PubMed] [Google Scholar]
- 35.Hagen C, Patel A, McCall WV. Prevalence of insomnia symptoms in sleep laboratory patients with and without sleep apnea. Psychiatry Res. 2009;170:276–7. doi: 10.1016/j.psychres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34:859–67. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gooneratne NS, Bellamy SL, Pack F, et al. Case-control study of subjective and objective differences in sleep patterns in older adults with insomnia symptoms. J Sleep Res. 2011;20:434–44. doi: 10.1111/j.1365-2869.2010.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 39.Bonnet MH, Arand DL. Activity, arousal, and the MSLT in patients with insomnia. Sleep. 2000;23:205–12. [PubMed] [Google Scholar]
- 40.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 42.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and the stress system. Sleep Med Clin. 2007;2:279–91. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Quan SF, Katz R, Olson J, et al. Factors associated with incidence and persistence of symptoms of disturbed sleep in an elderly cohort: the Cardiovascular Health Study. Am J Med Sci. 2005;329:163–172. doi: 10.1097/00000441-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men: a 10-year prospective population based study. Sleep. 2001;24:425–430. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 46.Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- 47.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]