Abstract
Study Objectives:
Neuronal histamine shows diurnal rhythms in rodents and plays a major role in the maintenance of vigilance. No data are available on its diurnal fluctuation in humans, either in health or in neurodegenerative disorders such as Parkinson disease (PD), Alzheimer disease (AD), or Huntington disease (HD), all of which are characterized by sleep-wake disturbances.
Design:
Quantitative in situ hybridization was used to study the mRNA expression of histidine decarboxylase (HDC), the key enzyme of histamine production in the tuberomammillary nucleus (TMN) in postmortem human hypothalamic tissue, obtained from 33 controls and 31 patients with a neurodegenerative disease—PD (n = 15), AD (n = 9), and HD (n = 8)—and covering the full 24-h cycle with respect to clock time of death.
Results:
HDC-mRNA levels in controls were found to be significantly higher during the daytime than at night (e.g., 08:01-20:00 versus 20:01-08:00, P = 0.004). This day-night fluctuation was markedly different in patients with neurodegenerative diseases.
Conclusion:
The diurnal fluctuation of HDC-mRNA expression in human TMN supports a role for neuronal histamine in regulating day-night rhythms. Future studies should investigate histamine rhythm abnormalities in neurodegenerative disorders.
Citation:
Shan L; Hofman MA; van Wamelen DJ; Van Someren EJW; Bao AM; Swaab DF. Diurnal fluctuation in histidine decarboxylase expression, the rate limiting enzyme for histamine production, and its disorder in neurodegenerative diseases. SLEEP 2012;35(5):713-715.
Keywords: Diurnal fluctuations, histamine, tuberomammillary nucleus, histidine decarboxylase, neurodegenerative disorders
INTRODUCTION
Neuronal histamine is produced from L-histidine by L-histidine decarboxylase (HDC), the key enzyme for histamine production, in the tuberomammillary nucleus (TMN) of the posterior hypothalamus. Knockout or pharmacological manipulation of HDC significantly affects histamine production. From the TMN, histaminergic projections run to a large number of brain areas, such as the preoptic and anterior hypothalamus, brainstem, pineal gland, and cortex.1 Many, if not all, of these structures are involved in the generation of the sleep-wake cycle or show functional day-night fluctuations.1 In many species—rodents and cats, for instance—neuronal histamine displays a diurnal rhythm with high levels during the waking period and low levels during sleep.1 In humans, however, no data are available concerning diurnal fluctuations in neuronal histamine production. Sleep-wake perturbations are frequently observed in patients suffering from neurodegenerative disorders. In Parkinson disease (PD) patients, sleeping disorders are a common and early feature and even seem to contribute to disease progression. In Alzheimer disease (AD) and Huntington disease (HD) patients, too, circadian and sleep disturbances are a common feature.2,3 Therefore, we aimed at analyzing the changes in day-night rhythm of HDC-mRNA expression in the human TMN both in control subjects and in patients suffering from a neurodegenerative disorder.
METHODS
Paraffin-embedded hypothalamic material from 33 control subjects and 31 patients suffering from a neurodegenerative disorder (NDD) was obtained from the Netherlands Brain Bank (NBB). Permission for a brain autopsy and for the use of brain material and clinical data for research purposes was obtained by the NBB from the patients or their next of kin. The control subjects had no primary neurological or psychiatric disorders and no neuropathological changes, while the AD changes were Braak stage 0-2. The group of neurodegenerative disorder patients consisted of 31 patients (9 AD, 9 PD, 6 preclinical PD, and 8 HD patients) matched well with controls for age, sex, postmortem interval (PMI), month of death, clock time of death (CTD), fixation time (FT), and cerebrospinal fluid (CSF)-pH (a measure of agonal state). For clinicopathological information, see Supplementary Table 1 and Supplementary Table 2. It should be noted that in both hospitals and nursing homes, lights were switched on at 07:00-08:00 and off at 22:00-23:00.
The HDC-mRNA levels in the TMN were measured by quantitative in situ hybridization (ISH), as described extensively before.4 Neurodegenerative patients and their matched controls were always tested in the same ISH session. The various ISH batches always included all the material of ≥ 3 overlapping subjects in order to normalize the data among the various batches. The coefficient of variation was 9%.
The differences between day and night (8:01-20:00 versus 20:01-8:00) were statistically evaluated by one-way ANOVA. The effects of AD-Braak stage, PMI, and FT were tested by a 2-factor analysis of variance (ANCOVA); correlations were tested by the Pearson correlation. P value < 0.05 was considered to be significant (2-tailed). All values are expressed as mean ± standard error of the mean (SEM). In addition, a two-harmonic 24-hr cosine function (constrained nonlinear regression, Matlab R2011b, Mathworks, Inc., Natick, MA, USA) was fitted to the HDC-mRNA data to evaluate the phase and amplitude of the rhythms in patients and controls.
RESULTS
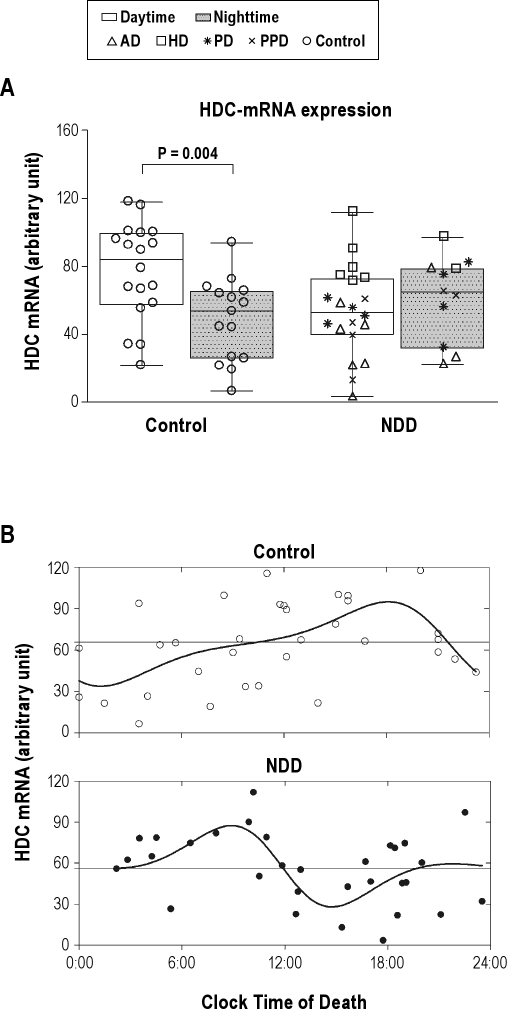
In controls, HDC-mRNA expression was significantly higher for those with a clock time of death during daytime (77.10 ± 6.67 arbitrary units [a.u.], n = 18, 08:01-20:00) than for those with a clock time of death at night (48.36 ± 6.24 a.u., n = 15, 20:01-08:00; P = 0.004) (Figure 1A). In marked contrast, NDD patients showed a nonsignificant lower daytime (53.3 ± 5.9 a.u., n = 20) than nighttime (61.4 ± 7.5 a.u., n = 11; P = 0.410) HDC-mRNA expression.
Figure 1.
(A) Box plots show the median, 25th-75th percentiles and the total range of radioactivity in arbitrary units. The total amount of radioactivity of histidine decarboxylase (HDC)-mRNA expression is given for control subjects between daytime (08:01-20:00; n = 18) and nighttime (20:01-08:00; n = 15) on the left side, and for neurodegenerative diseases group (NDD, daytime n = 20, nighttime n = 11) on the right side. Note that there is a significant difference (P = 0.004) between daytime and nighttime in control subjects, but not in NDD (P = 0.410). (B) Raw data of HDC-mRNA expression plotted along the clock time of death. The nonlinear periodic functions describe the circadian cycles. The model in controls (open dots) reach an estimated maximum at the end of the afternoon (Tmax = 18:09) and a minimum shortly after midnight (Tmin = 01:09). The model in NDD group (block dots) reaches an estimated maximum in the morning (Tmax = 08:56) and a minimum in the afternoon (Tmin = 14:43).The horizontal lines indicate the 24-h mean of HDC-mRNA expression in controls and NDD group, respectively.
The daytime and nighttime control subgroups (Supplementary Table 1) did not differ significantly regarding possible confounders—age, sex, PMI, FT, or CSF-pH (P ≥ 0.17). The day-night difference in TMN HDC-mRNA levels was independent of age, sex, or AD-Braak stages, both in controls (P ≥ 0.33) and in the NDD group (P ≥ 0.07). None of the individual NDD groups, i.e., preclinical PD, PD, AD, and HD, showed day-night differences (P ≥ 0.10, Figure 1A), although it should be noted that the sample sizes were small.
The goodness of fit of the two-harmonic cosine curve indicated about equally significant 24-h rhythms in HDC-mRNA expression for the control (R2 = 0.28, P < 0.05) and NDD (R2 = 0.27, P < 0.05) group (Figure 1B). The estimated circadian amplitudes were, respectively, 30.55 a.u. (47% of the 24-h mean for the control group), and 24.37 a.u. (43% of the 24-h mean for the NDD group). A marked difference occurred in the acrophase and bathyphase of HDC-mRNA expression. Whereas the control group showed maximal expression in the early evening (Tmax = 18:09) and minimal expression shortly after midnight (Tmin = 01:09), the NDD group showed maximal expression in the morning (Tmax = 08:56) and minimal expression in the afternoon (Tmin = 14:43), suggesting a phase advance of about 9 to 10 hours.
DISCUSSION
The current study demonstrates for the first time a significant diurnal fluctuation in the expression of the total amount of HDC-mRNA in the human TMN. In addition, it suggests a disturbed diurnal fluctuation of neural histamine production in neurodegenerative diseases.
Our findings in the control group agree well with rat data of Mochizuki et al.,5 who showed that histamine release increased in the active period (20:00-08:00) as compared to the sleep period (08:00-20:00). The estimated acrophase of HDC-mRNA expression in controls at 18:09 in our study agrees very well with the recent finding of an acrophase for the histamine rhythm at 17:49 in a diurnal nonhuman primate.6
Our findings also showed a strongly deviating profile of the timing of HDC-mRNA expression in neurodegenerative disorders. It is tempting to suggest that the relatively high nocturnal and low diurnal expression could be involved in the restless nights and listless days that are so often seen in these patients. Sleep-wake rhythm disturbances are commonly seen in AD patients3 and are the most common reason for nursing home admission, taking precedence even over cognitive impairment as a reason for admission.7 The sleep disturbances in AD are most likely to have multiple causes, e.g., the function of the circadian pacemaker, the suprachiasmatic nucleus, and one of its main target areas, the pineal gland, are severely affected in AD even from the earliest stages onwards.7 The stage in the course of AD where the disorder of TMN fluctuations emerges still needs to be determined. In HD patients, circadian and sleep disorders are also prevalent and correlate with cognitive decline and frontal lobe dysfunction.2 In addition, an augmentation of neuronal histaminergic signaling in HD patients has been reported.8 Our current finding supports the view that a disturbed neuronal histaminergic signaling rhythm may contribute to a disturbed day-night pattern in HD patients. Because circadian abnormalities in AD and HD are already present in preclinical stages,7 the alteration of day-night patterns found in the present study is most probably an inherent part of the disease process. On the basis of the evidence of a strong accumulation of the characteristic neuropathological PD lesions, i.e., Lewy bodies and Lewy neurites, wakefulness-promoting cell groups in the TMN appear to be already affected in the preclinical stage of PD. It has therefore been hypothesized that the arousal system is damaged in PD. Together with previous evidence showing that excessive daytime sleepiness as occurs in, for instance, narcolepsy9,10 is accompanied by low levels of histamine in the CSF, and studies into the question whether alterations in histaminergic neurotransmission are indeed involved in sleep-wake disorders in neurodegenerative diseases would make interesting future projects.
Potential limitations of our study include the use of a variety of medications in patients. In addition, the subgroups of patients suffering from a particular neurodegenerative disease were small. Future studies are required to investigate the day-night fluctuations in the various neurodegenerative disorders in larger groups separately.
CONCLUSION
The total expression of HDC-mRNA in the human TMN exhibits a diurnal rhythm, which supports a role for neuronal histamine in regulating day-night patterns. The inverted profile in neurodegenerative diseases may be involved in the restless nights and listless days typical of this disorder.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the China Scholarship Council for State Scholarship Fund [grant number (2007) 3020] to [L. S.], and by the KNAW-CAS project 10CDPO37 of the China Exchange Programme to [A.-M. B.] and [D.F.S.]. We thank the Netherlands Brain Bank (director Dr I. Huitinga), for providing the brain material. The authors thank Mr. B. Fisser and Mr. T. Put for their technical assistance and Ms. W. Verweij for secretarial help.
REFERENCES
- 1.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 2.Aziz NA, Anguelova GV, Marinus J, Lammers GJ, Roos RA. Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington's disease. Parkinsonism Relat Disord. 2011;16:345–50. doi: 10.1016/j.parkreldis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–55. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 4.Liu CQ, Shan L, Balesar R, et al. A quantitative in situ hybridization protocol for formalin-fixed paraffin-embedded archival post-mortem human brain tissue. Methods. 2010;52:359–66. doi: 10.1016/j.ymeth.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki T, Yamatodani A, Okakura K, Horii A, Inagaki N, Wada H. Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol Behav. 1992;51:391–4. doi: 10.1016/0031-9384(92)90157-w. [DOI] [PubMed] [Google Scholar]
- 6.Zeitzer JM, Kodama T, Buckmaster CL, et al. Time-course of cerebrospinal fluid histamine in the wake-consolidated squirrel monkey. J Sleep Res. 2011 doi: 10.1111/j.1365-2869.2011.00957.x. in press, http://dx.doi.org/10.1111/j.1365-2869.2011.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer's disease. Sleep Med. 2007;8:623–36. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 8.van Wamelen DJ, Shan L, Aziz NA, et al. Functional increase of brain histaminergic signaling in Huntington's disease. Brain Pathol (Zurich, Switzerland) 2011;21:419–27. doi: 10.1111/j.1750-3639.2010.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanbayashi T, Kodama T, Kondo H, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep. 2009;32:181–7. doi: 10.1093/sleep/32.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino S, Sakurai E, Nevsimalova S, et al. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep. 2009;32:175–80. doi: 10.1093/sleep/32.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]