Abstract
METCAM, an integral membrane cell adhesion molecule (CAM) in the Ig-like gene superfamily, is capable of performing typical functions of CAMs, such as mediating cell-cell and cell-extracellular interactions, crosstalk with intracellular signaling pathways, and modulating social behaviors of cells. METCAM is expressed in about nine normal cells/tissues. Aberrant expression of METCAM has been associated with the progression of several epithelial tumors. Further in vitro and in vivo studies show that METCAM plays a dual role in the progression of different tumors. It can promote the malignant progression of several tumors. On the other hand, it can suppress the malignant progression of other tumors. We suggest that the role of METCAM in the progression of different cancer types may be modulated by different intrinsic factors present in different cancer cells and also in different stromal microenvironment. Many possible mechanisms mediated by this CAM during early tumor development and metastasis are suggested.
1. Introduction
Human METCAM (huMETCAM), a CAM in the immunoglobulin-like gene superfamily, is an integral membrane glycoprotein. Alternative names for METCAM are MUC18 [1], CD146 [2], MCAM [3], MelCAM [4], A32 [5], and S-endo 1 [6]. To avoid confusion with mucins and to reflect its biological functions, we have renamed MUC18 as METCAM (metastasis CAM), which means an immunoglobulin-like CAM that affects or regulates metastasis, [7]. The huMETCAM has 646 aminoacids that include a N-terminal extracellular domain of 558 aminoacids, which has 28 aminoacids characteristics of a signal peptide sequence at its N-terminus, a transmembrane domain of 24 aminoacids (amino acid number 559–583), and a cytoplasmic domain of 64 aminoacids at the C-terminus. HuMETCAM has eight putative N-glycosylation sites (Asn-X-Ser/Thr), of which six are conserved, and are heavily glycosylated and sialylated resulting in an apparent molecular weight of 113,000–150,000. The extracellular domain of the protein comprises five immunoglobulin-like domains (V-V-C2-C2-C2) [1, 7] and an X domain [7]. The cytoplasmic tail contains peptide sequences that will potentially be phosphorylated by protein kinase A (PKA), protein kinase C (PKC), and casein kinase 2 (CK 2) [1, 7, 8]. My lab has also cloned and sequenced the mouse METCAM (moMETCAM) cDNA, which contains 648 aminoacids with a 76.2% identity with huMETCAM, suggesting that moMETCAM is likely to have biochemical properties and biological functions similar to the human counterpart [9]. The structure of the huMETCAM protein is depicted in Figure 1, suggesting that METCAM, similar to most CAMs, plays an active role in mediating cell-cell and cell-extracellular interactions, crosstalk with many intracellular signaling pathways, and modulating the social behaviors of cells [7].
Figure 1.
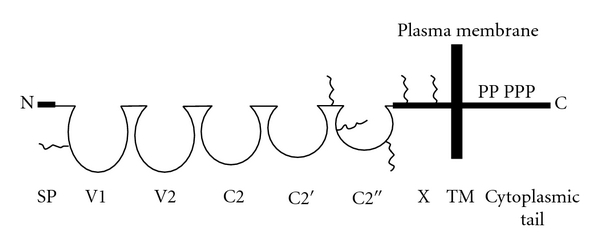
HuMETCAM protein structure. SP stands for signal peptide sequence: V1, V2, C2, C2′, and C2′′ for five Ig-like domains (each held by a disulfide bond) and X for one domain (without any disulfide bond) in the extracellular region, and TM for transmembrane domain. P stands for five potential phosphorylation sites (one for PKA, three for PKC, and one for CK2) in the cytoplasmic tail. The six conserved N-glycosylation sites are shown as wiggled lines in the extracellular domains of V1, the region between C2′ and C2′′, C2′′, and X.
HuMETCAM is expressed in a limited number of normal tissues, such as hair follicular cells, smooth muscle cells, endothelial cells, cerebellum, normal mammary epithelial cells, basal cells of the lung, activated T cells, intermediate trophoblast [10], and normal nasopharyngeal epithelial cells [11]. The protein is overly expressed in most (67%) malignant melanoma cells [1], and in most (more than 80%) premalignant prostate epithelial cells (PIN), high-grade prostatic carcinoma cells, and metastatic lesions [12, 13]. HuMETCAM is also expressed in other cancers, such as gestational trophoblastic tumors, leiomyosarcoma, angiosarcoma, haemangioma, Kaposi's sarcoma, schwannoma, some lung squamous and small cell carcinomas, some breast cancer, some neuroblastoma [10], and also nasopharyngeal carcinoma [11] and ovarian cancer [14].
It is now well documented that in addition to tissue-specific signatures in different cancer types, cancers from different tissues also express some common genes [15–17]. One group of them is cell adhesion molecules (CAMs). CAMs do not merely act as a molecular glue to hold together homotypic cells in a specific tissue or to facilitate interactions of heterotypic cells; CAMs also actively govern the social behaviors of cells by affecting the adhesion status of cells and modulating cell signaling [18]. They control cell motility and invasiveness by mediating the remodeling of cytoskeleton [18]. They also actively mediate the cell-to-cell and cell-to-extracellular matrix interactions to allow cells to constantly respond to physiological fluctuations and to alter/remodel the surrounding microenvironment for survival [19]. They do so by crosstalk with cellular surface growth factor receptors, which interact with growth factors that may be secreted from stromal cells or released from circulation and embedded in the extracellular matrix [18, 19]. Thus, an altered expression of CAMs affects the motility and invasiveness of many tumor cells in vitro and metastasis in vivo [18, 19]. CAMs also play an important role in the favorable soil that provides a proper microenvironment at a suitable period to awaken the dormant metastatic tumor cells to enter into an aggressive growth phase. Actually, the metastatic potential of a tumor cell, as documented in many carcinomas, is the consequence of a complex participation of many over- and under-expressed CAMs [18, 19]. Based on the above information, aberrant expression of huMETCAM may also affect the motility and invasiveness of many tumor cells in vitro and metastasis in vivo. It is logical to hypothesize that HuMETCAM/MUC18 should play an important role in promoting the malignant progression of many cancer types [7, 18]. However, recently we observed an unexpected opposite function of METCAM/MUC18 in the malignant progression of a mouse melanoma subline and ovarian cancer cells, in which it functioned as a tumor and metastasis suppressor (Wu, unpublished results). In this paper, we will review its dual roles in the tumorigenesis and metastasis in different cancer types.
2. METCAM and Tumorigenesis
METCAM-induced tumorigenesis has been studied in melanoma, prostate cancer, breast cancer, and ovarian cancer. Overexpression of METCAM may have no effect, a negative effect, or a positive effect on tumorigenesis, dependent upon the cell lines used, as shown in the following.
2.1. METCAM and Melanoma Tumorigenesis
Overexpression of METCAM had a slight tumor suppression effect on tumorigenesis of human melanoma cells in xenograft mice [20], as shown in Figure 2, but it had no effect on tumorigenesis of two sublines, number 3 and number 10, of the mouse melanoma cell line K1735 in syngeneic mice [21]. Figure 3 shows only the effect of moMETCAM on the tumorigenesis of K1735-3.
Figure 2.
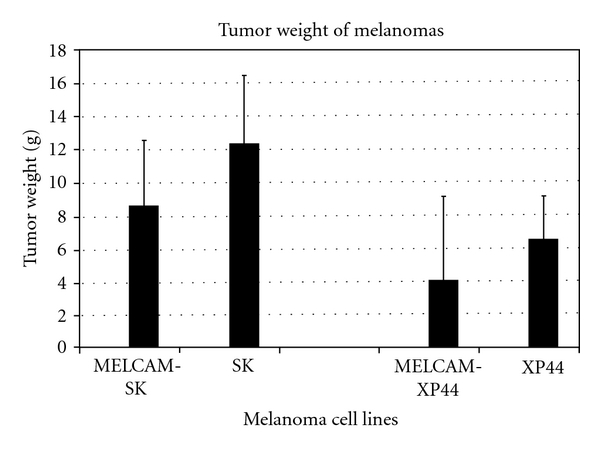
Effect of overexpression of huMETCAM on tumor formation of two human melanoma cell lines, SK and XP-44 [20]. MELCAM-SK and MELCAM-XP-44 were two clones of human melanoma cell lines, SK and XP-44, respectively, which were transfected with huMETCAM and expressed a high level of huMETCAM. Statistical analysis was not possible because detailed data was not provided.
Figure 3.
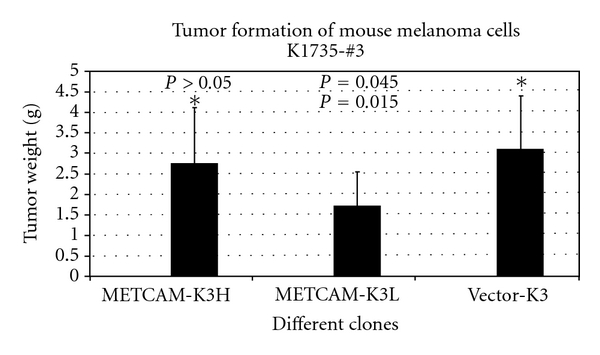
Effect of overexpression of moMETCAM on tumor formation of a mouse melanoma cell line K1735 subline number 3 [21]. METCAM-K3H and METCAM-K3L were two K3 clones transfected with moMETCAM cDNA; expressed a high and a low level of moMETCAM, respectively. Vector-K3, as a negative control, was one clone transfected with an empty vector and did not express any moMETCAM. Asterisk was the reference for the P value calculation. The P values should be compared with the reference (asterisk) on the same row.
Only one group showed that overexpression of METCAM increased tumorigenesis of a human melanoma cell line in xenograft mice [3]; however, the results were questionable because only the tumorigenicity of one mouse injected with METCAM-expressing clone and one mouse with control cells was determined, and thus no standard deviations were indicated and no statistical analysis was done, as shown in Figure 4.
Figure 4.
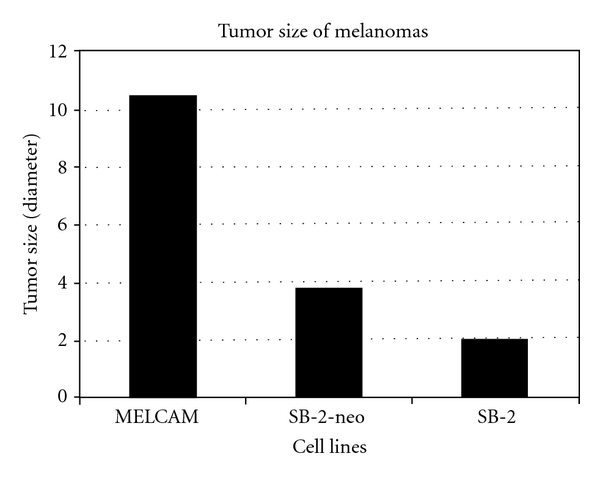
Effect of overexpression of huMETCAM on tumor formation of a human melanoma cell line SB-2 [3]. SB-2 is a human melanoma cell line, which did not express any huMETCAM. SB-2-neo is the SB-2 cells transected with the empty vector, as a negative control. MELCAM is a clone of the SB-2 cells which were transfected with huMETCAM cDNA and expressed a high level of huMETCAM. Since tumor formation was only shown in one nude mouse for each clone, statistical analysis was not possible.
The most compelling evidence for its tumor suppressor effect is in the subline number 9 of the mouse melanoma cell line K1735 (K9) in syngeneic C3H mice. Overexpression of moMETCAM in the K9 cells decreased subcutaneous tumorigenesis in immunocompetent syngeneic C3H mice [22, 23], as shown in Figure 5.
Figure 5.
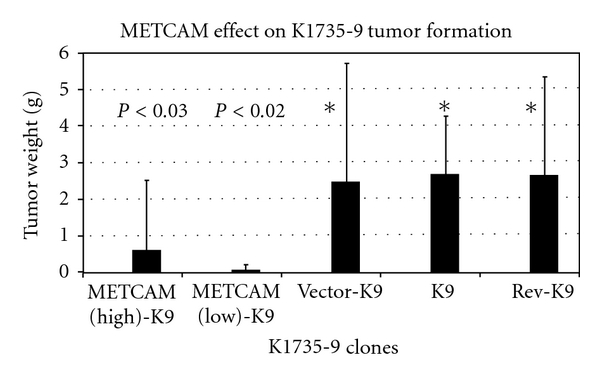
Effect of overexpression of moMETCAM on tumor formation of a mouse melanoma K1735 subline number 9 (K9) in immune competent syngeneic C3H mice. METCAM-K9H and METCAM-K9L were two transfected clones, which expressed a high and a low level of moMETCAM, respectively. Vector-K9 was one clone transfected with the empty vector, as a negative control. K9 was parental K1735 subline number 9 cells, also as a negative control. Rev-K9, in which the moMETCAM cDNA was inserted into the expression vector in antisense orientation, is also as a control clone. Vector-K9, K9, and Rev-K9 did not express any moMETCAM.
2.2. METCAM and Breast Cancer Tumorigenesis
Shih et al. showed that METCAM was not expressed in MCF-7 cell line [24], and they showed that the overexpression of huMETCAM in MCF-7 cells suppressed tumor formation of the cells in SCID mice, as shown in Figure 6, suggesting that METCAM is a possible tumor suppressor in breast cancer [24].
Figure 6.
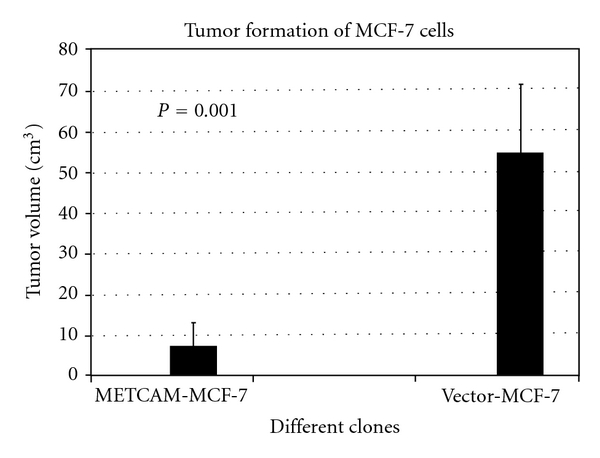
Effect of overexpression of huMETCAM on tumor formation of a human breast cancer cell line MCF-7 [24]. METCAM-MCF-7 was a clone, which expressed a high level of huMETCAM/MUC18 after transfection with the cDNA. Vector MCF-7 was a clone, which did not express any huMETCAM after transfection with an empty vector. Cells were injected subcutaneously into female SCID mice [24].
We have confirmed from their Western blot and immunohistochemistry results that METCAM is not expressed in MCF-7 cells (0%), very weakly expressed in SK-BR-3 cells (5%), and weakly expressed, though slightly higher levels than the above two cells lines, in the human mammary cancer cell lines, MDA-MB-231 (a low metastatic cell line) (16%), and MDA-MB-468 (a high metastatic cell line) (22%), as shown in Figure 7.
Figure 7.
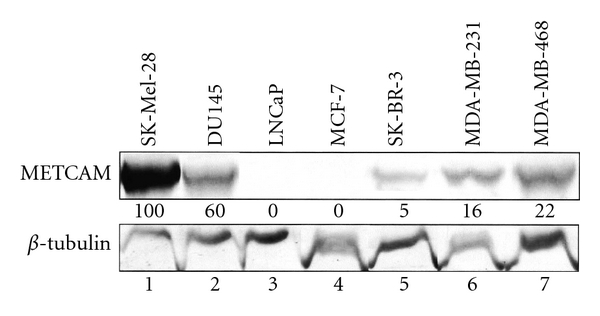
Expression of huMETCAM in four human breast cancer cell lines, MCF-7, SK-BR-3, MDA-MB-231, and MDA-MB-468. SK-Mel-28, a human melanoma cell line, which expressed a very high level of huMETCAM, was used as a positive control (100%). Two human prostate cancer cell lines, DU145 and LNCaP, which expressed different levels of huMETCAM (60% and 0%, resp.) were used as positive and negative controls.
Recently gene expression profiles of breast cancer cell lines have indicated that the gene expression profiles of MCF-7 and SK-BR3 are more closely related to the luminal subtype of the breast cancers, whereas those of MDA-MB-231 and MDA-MB-468 are more closely related to the basal-like subtype [25, 26]. It appeared that METCAM is not or weakly expressed in cell lines established from luminal subtypes, but it is moderately expressed in cell lines established from basal-like subtypes, MDA-MB-231 and MDA-MB-468. Recently Ouhtit et al. [27] found that overexpression of METCAM inhibited the in vitro invasiveness of MDA-MB-231 cells, supporting the notion of Shih et al. On the contrary, Garcia et al. [28] and Zabouo et al. [29] supported the opposite role of METCAM in the progression of human breast cancer cells in that it plays a role of tumor promoter. However, all three groups did not substantiate their claim with studies in animal models. To resolve this controversy, we recently reinvestigated the role of METCAM in the tumorigenesis of human breast cancer cells in animal models and found that overexpression of METCAM promoted the tumorigenesis of four human breast cancer cell lines, MCF7, SK-BR-3, MDA-MB-231, and MDA-MB-468 [30, 31]. Tumorigenesis of MCF-7 in female SCID mice [30] is shown in Figure 8, and that of SK-BR-3 in female nude mice [31] in Figure 9.
Figure 8.
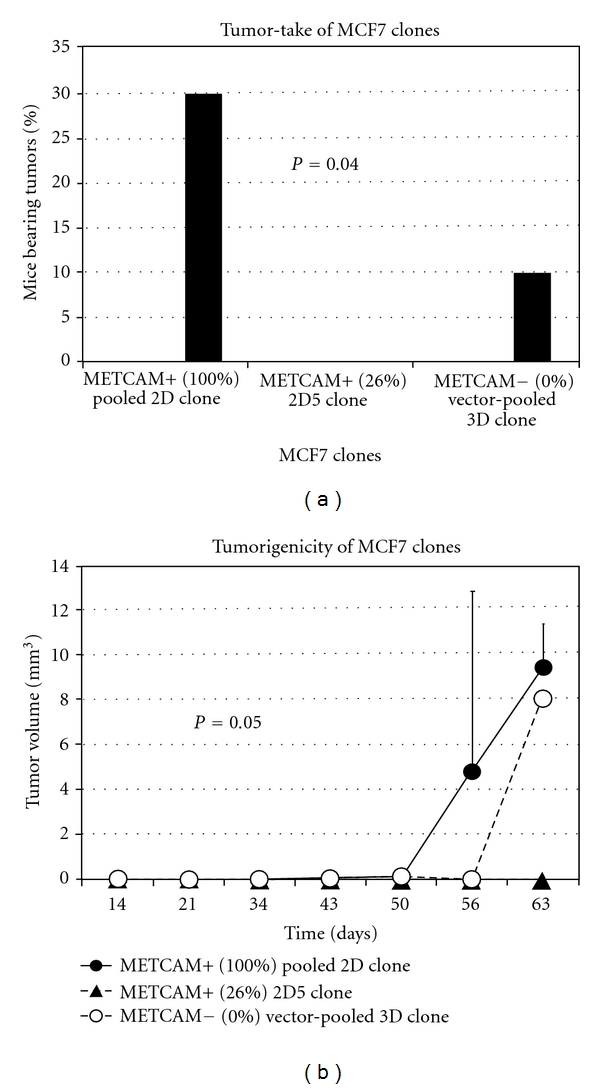
Effect of overexpression of huMETCAM on tumor-take (a) and tumorigenicity (b) of a human breast cancer cell line MCF7 in female SCID mice. METCAM+ clone 2D pooled was a pooled clone, which expressed 100% of huMETCAM, and METCAM+ clone 2D5, which expressed 26% of huMETCAM, after transfection with the huMETCAM cDNA. Vector clone 3D pooled was a pooled clone, which did not express any huMETCAM, after transfection with an empty vector. Cells were injected subcutaneously into female SCID mice [30].
Figure 9.
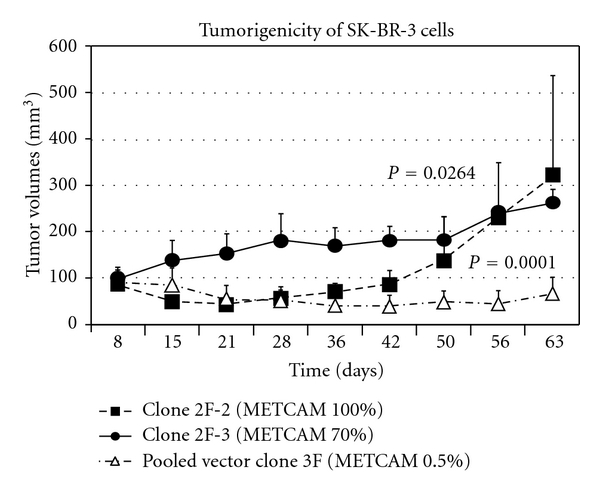
Effect of overexpression of huMETCAM on tumor formation of a human breast cancer cell line SK-BR-3 in female nude mice [31]. Clone 2F-2 and clone 2F-3 expressed 100% and 70% of METCAM, respectively. Pooled vector clone 3F expressed only about 0.5% of METCAM, as a vector control.
Thus, the tumor suppression role of METCAM in tumorigenesis of human breast cancer cells is not supported by the above evidence. On the contrary, it suggests the alternative notion that METCAM increased tumorigenesis and perhaps also the metastasis of human breast cancer cells.
2.3. METCAM and Ovarian Cancer Tumorigenesis
Recently, both our group and another group found that METCAM was upregulated in human ovarian cancer specimens, suggesting that METCAM may be a marker for the poor prognosis of ovarian cancer patients [14, 32], and that METCAM may play a positive role in the development of ovarian cancer [14, 32]. However, preliminary animal tests (injection of BG-1 cells in nonorthotopic, subcutaneous sites of female nude mice) show that overexpression of METCAM did not have any significant effect on the tumor formation of a human ovarian cancer cell line, BG-1 (data not shown). To rule out the possibility that this effect might be an artifact because the tests were carried out in the nonorthotopic, subcutaneous sites, which did not provide a proper microenvironment for tumorigenesis, we carried out further tests of the effect of overexpression of METCAM on tumorigenesis of BG-1 cells by injecting the clones in an orthotopic site, the intraperitoneal cavity of female SCID mice. We found that tumorigenesis of BG-1 clones was also very poor, suggesting that estrogen supplement by subcutaneous implantation may enhance the tumorigenesis of BG-1 cells in both immunodeficient mice. Nevertheless, the test of the effect of overexpression of METCAM on tumorigenesis of BG-1 cells in orthotopic sites had a somewhat suppressive effect, as shown in Figure 10 [33].
Figure 10.
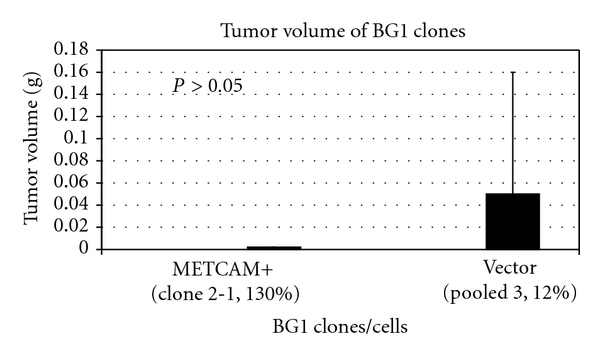
Effect of overexpression of huMETCAM on tumorigenicity of a human ovarian cancer cell line BG-1 in an orthotopic site (the intraperitoneal cavity) in female SCID mice. Clone 2-1 expressed 130% of METCAM. The vector control, pooled clone 3, expressed 12% of METCAM.
We also carried out animal studies by using another human ovarian cancer cell line, SK-OV-3 and found that overexpression of METCAM suppressed tumorigenesis of SK-OV-3 cells at both nonorthotopic, subcutaneous sites, as well as an orthotopic site (the intraperitoneal cavity) [34], as shown in Figure 11.
Figure 11.
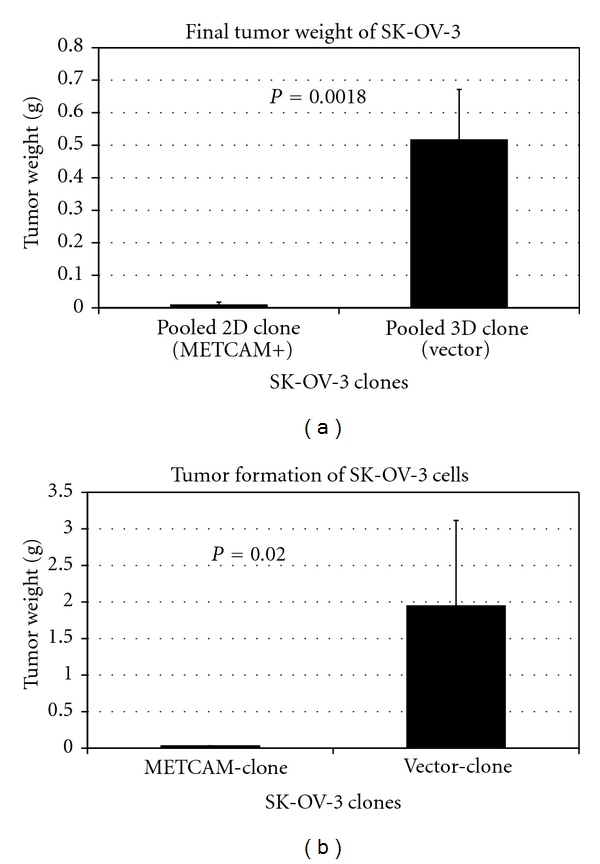
Effect of overexpression of huMETCAM on the final tumor weight at S.C. sites (a) and orthotopic sites (the intraperitoneal cavity) (b) of a human ovarian cancer cell line SK-OV-3. Both Pooled 2D clone and METCAM-clone expressed 100% of METCAM. Both pooled 3D clone (vector) and vector clone expressed 0.5% of METCAM.
2.4. METCAM and Prostate Cancer Tumorigenesis
Overexpression of METCAM significantly increases the tumor-take and promote tumorigenicity and tumorigenesis of a human prostate cancer cell line, LNCaP, as shown in Figure 12 [35, 36].
Figure 12.
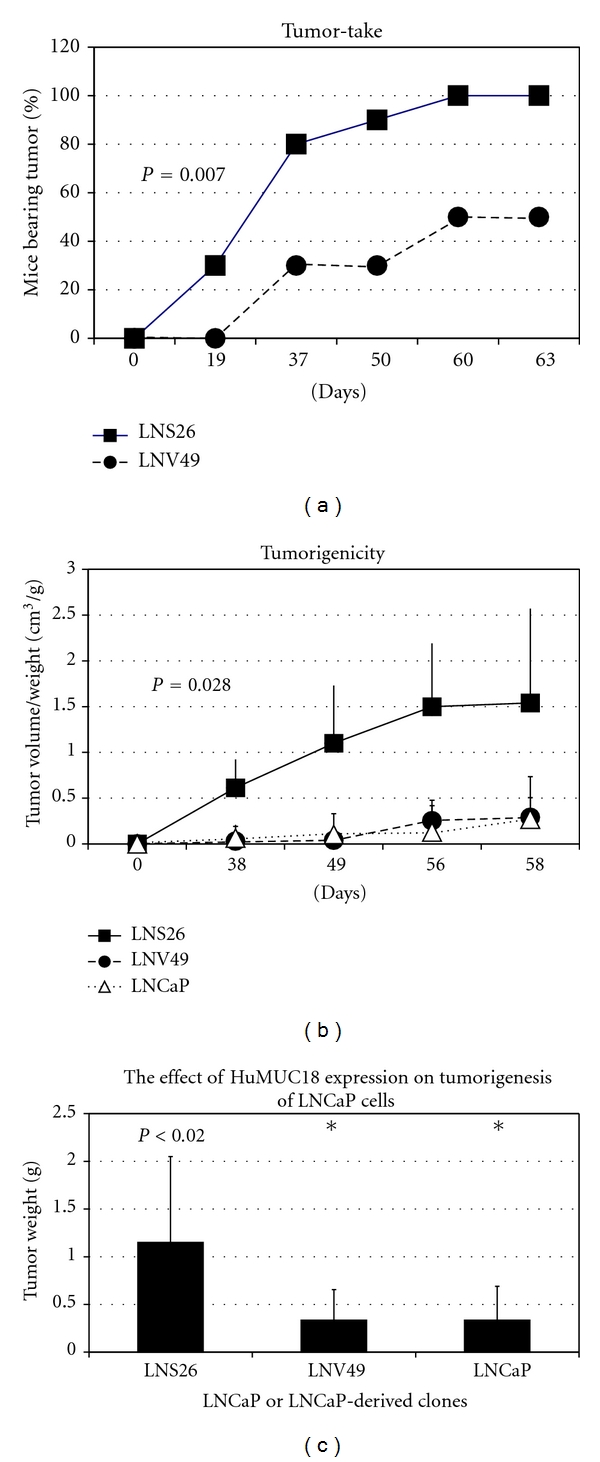
Enforced expression of huMETCAM in LNCaP cells resulted in an increased tumor-take (a), tumorigenicity (b), and final tumor weight (c) [35]. Clone LNS26 expressed METCAM. Both the vector control clone, LNV49, and the parental LNCaP cells did not express any METCAM.
2.5. METCAM Tumorigenesis of Other Cancer Cell Lines
We found that moMETCAM was expressed at a higher level in a mouse angiosarcoma clone, SVR, which was transfected with H-Ras, than in the immortalized normal endothelial cell line control, MS-1 (Figure 13). The higher level of moMETCAM expression appeared to correlate with the higher tumorigenicity of the SVR cell line [7, 37], suggesting a positive role for METCAM in promoting angiosarcoma [7].
Figure 13.
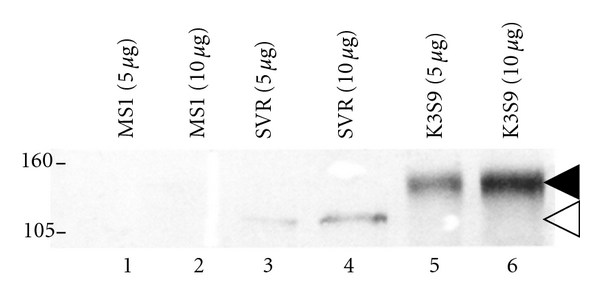
Expression of moMETCAM in mouse angiosarcoma cell lines [7]. MS1 is an immortalized mouse endothelial cell line, which expressed a barely detectable (low) level of moMETCAM and was nontumorigenic. SVR is a mouse angiosarcoma cell line, which had been transfected with H-Ras gene, also expressed moMETCAM, and was tumorigenic. K3S9, a clone derived from mouse melanoma K1735 subline number 3 which had been transfected with a moMETCAM cDNA gene, expressed a high level of moMETCAM and formed tumor efficiently in syngeneic mice (C3H). Western blot was carried out and detected by our chicken anti-moMETCAM antibody [9]. The smaller molecular weight of the moMETCAM (about 115 kDa) in angiosarcoma cell lines was probably due to less glycosylation than that in the mouse melanoma cell lines or in most human cancer cell lines (about 150 kDa).
There is a negative correlation of METCAM expression with the human nasopharyngeal carcinoma specimens, suggesting that METCAM may also play a tumor suppressor role in the tumorigenesis of nasopharyngeal carcinoma [11]. A tumor suppressor role of METCAM may also be implicated in haemangiomas, since METCAM expression was decreased during the progression of haemangiomas [38].
3. METCAM and Metastasis
METCAM-induced metastasis has been studied in melanoma, prostate cancer, osteosarcoma, and ovarian cancer lines. Overexpression of METCAM in melanoma cells mostly have a positive effect on the metastasis of human melanoma cell lines in immunodeficient mice (both athymic nude and SCID mice) [3, 20], mouse melanoma cell lines in syngeneic mice [7, 21], and a human prostate cancer cell line, LNCaP, in nude mice [7, 13, 36]. Overexpression of METCAM also has a positive effect on the metastasis of osteosarcoma cell lines [39]. Surprisingly, we have recently found that overexpression of METCAM has a negative effect on the metastasis of one subline, number 9, of mouse melanoma cell K1735 [22, 23] and ovarian cancer cell lines [30–32]. Details are described in the following.
3.1. METCAM and Melanoma Metastasis
HuMETCAM was originally found to be abundantly expressed on the cellular surface of most malignant human melanomas; since then, it has been postulated to play a role in the progression of human melanoma [1]. This notion is also supported by the positive correlation of moMETCAM expression with the metastatic ability of several mouse melanoma cells lines [9]. Definitive proof comes from the results that the stably ectopic expression of the huMETCAM cDNA gene in three nonmetastatic human cutaneous melanoma cell lines increases the metastatic abilities of these cell lines in immune-deficient mouse models [3, 20]. Furthermore, the stable, ectopic expression of moMETCAM cDNA in two low-metastatic mouse melanoma cell lines increases the metastatic abilities of these cell lines in immune-competent syngeneic mice [21]. However, METCAM enables melanoma cells to establish pulmonary metastasis only when the cells are injected into the tail vein (experimental metastasis assay) [3, 20, 21], thus bypassing the initial stages of metastasis. No metastasis was found when METCAM-expressing melanoma cells were injected subcutaneously (spontaneous metastasis assay) either in immune-deficient mouse models [3, 20] or in immune-competent syngeneic mouse models [21]. Taken together, METCAM definitely promotes the metastasis of melanoma cells, but at later stages [7]; thus overexpression of METCAM did not initiate the metastasis of melanoma cells. This result is consistent with the recent observation that fibroblast growth factor 2, but neither huMETCAM nor integrin actually initiates the malignant progression of subcutaneous melanocyte into melanoma [40].
In contrast to these results, overexpression of moMETCAM in one mouse melanoma cell line K1735 subline number 9 (K9) decreased pulmonary lung nodule formation when cells were injected into tail veins (experimental metastasis test) [22, 23], as shown in Figure 14.
Figure 14.
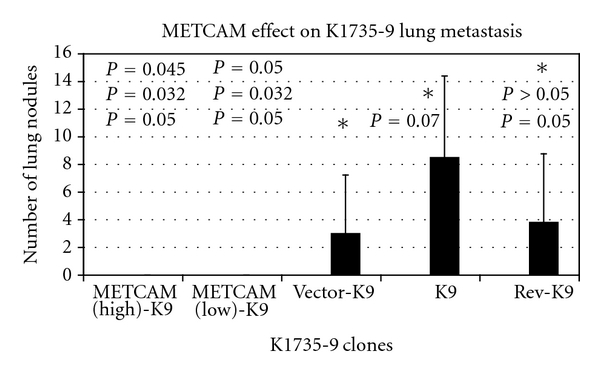
Enforced expression of moMETCAM suppressed lung nodule formation of mouse melanoma K9 cells in syngeneic C3H mice. Clones METCAM (high)-K9 and METCAM (low)-K9 clones expressed high and low levels of moMETCAM, respectively. Vector-K9, K9 parental cells, and Rev-K9, in which the moMETCAM cDNA was inserted into the expression vector in antisense orientation, were the control clones that did not express any moMETCAM.
3.2. METCAM and Prostate Cancer Metastasis
Overexpression of METCAM is not limited to melanoma as previously thought [7, 10]. Our group has pioneered the successful determination of huMETCAM expression in prostate cancer cells and tissues using our chicken polyclonal anti-huMETCAM and carried out extensive studies of huMETCAM-mediated prostate cancer metastasis [8]. We have used molecular biological and immunological methods to study the expression of huMETCAM in three established prostate cancer cell lines and human prostate cancer tissues, and in immunohistochemical studies of paraffin-embedded human prostate cancer tissue sections [7, 8, 12, 13]. From the results, we have suggested that huMETCAM may be a new diagnostic marker for the metastatic potential of human prostate cancer. This is further corroborated by results of a positive correlation of moMETCAM expression with the progression of mouse prostate adenocarcinoma in a transgenic mouse model, TRAMP [41]. From these results, we have also suggested that huMETCAM may be a key determinant in promoting tumorigenesis and metastasis of human prostate cancer cells [7]. To test this hypothesis, we determined the effect of ectopic expression of huMETCAM on the ability of human prostate LNCaP cells to form tumor in the prostate gland and to initiate metastasis in nude mice. We found that overexpression of METCAM had a positive effect on the metastasis of the human prostate cancer cell, LNCaP, when the cells were injected at the orthotopic site (the dorsolateral lobes of the nude mice) [36]. The metastatic lesions were found in multiple organs, such as seminal vesicles, ureter, kidney, and periaortic lymph nodes [36]. Different mice had metastatic lesions in one or two organs, but all of them had metastatic lesions in the lymph nodes. The parental LNCaP cells, which do not express any METCAM, can form tumors in the prostate, but these tumors did not manifest any metastasis. The metastatic lesions in the bones were not examined. But our recent preliminary results appear to show that overexpression of METCAM may be able to enhance establishment of the growth of a bone-homing C42B clone of LNCaP cells in nude mice. Further tests are in process [Wu et al., unpublished results].
Taken together, METCAM can actually initiate the metastasis of LNCaP cells, thus affecting the progression of prostate cancer cells at the early stage of metastasis [7, 36].
3.3. METCAM and Osteosarcoma Metastasis
Recently, one group has shown that METCAM is overly expressed in two of the six human osteosarcoma cell lines. Overexpression of METCAM increased the spontaneous lung metastasis of an osteosarcoma cell line KRIB. The metastasis can be blocked by a humanized antibody against METCAM, suggesting METCAM plays a positive role in the progression of osteosarcomas [39].
3.4. METCAM and Ovarian Cancer Metastasis
Recently we found that overexpression of METCAM/MUC18 suppressed metastasis and ascites formation of SK-OV-3 cells in the intraperitoneal cavity [34], as shown in Figure 15.
Figure 15.
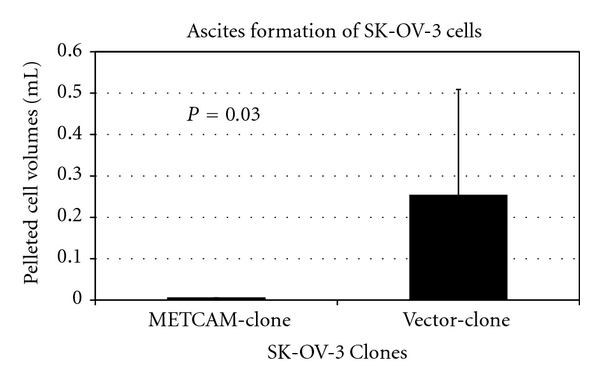
Enforced expression of huMETCAM in human ovarian SK-OV-3 cells suppressed solid tumor formation and ascites formation in the intraperitoneal cavity. METCAM-clone expressed 100% of METCAM. Vector-clone expressed 0.5% of METCAM.
3.5. METCAM and Metastasis of Other Cancer Cell Lines
Decreased expression of METCAM has been correlated with the progression of haemangioma, suggesting the possible negative effect of METCAM on progression of haemangioma [38]. Though METCAM was downregulated in nasopharyngeal carcinoma, interestingly it was upregulated again in metastatic lesions in nasopharyngeal patients, suggesting that METCAM may play a positive role in the malignant progression of nasopharyngeal carcinoma after a transient suppression of tumorigenesis [11].
Taken together, we suggest that the possible tumor and metastasis suppressor role of METCAM may not be limited to melanoma and ovarian cancers, and that this may be a new function of METCAM yet to be explored.
Summary 3.5 —
Table 1 summarizes the possible role of METCAM in the tumorigenesis and metastasis of various tumors/cancers.Taken together, huMETCAM is a tumor promoter for prostate and breast cancers, and a metastatic gene for most melanoma cell lines, prostate cancer, osteosarcoma, and perhaps, breast cancer and nasopharyngeal carcinoma. It is a tumor suppressor for a mouse melanoma subline and ovarian cancers, and perhaps, haemangioma and nasopharyngeal carcinoma; it is a metastasis suppressor for a mouse melanoma subline, ovarian cancer, and perhaps, haemangioma.
Table 1.
The role of METCAM in the tumorigenesis and metastasis of various cancer cells.
| Cancer cells | Tumorigenesis | Metastasis | References |
|---|---|---|---|
| Clinical prostate cancer and human prostate cancer cell lines | Increasing | Increasing (effect is in the early stage of initiation) | [7, 8, 12, 13, 35, 36] |
| Mouse prostate carcinomas in TRAMP mice | Increasing | Increasing | [41] |
| Clinical melanoma and human melanoma cell lines | No effect | Increasing (effect is in the late stages) | [3, 20] |
| Mouse melanoma cell line of K1735 subline number 3 and 10s | No effect or slight suppression | Increasing (effect is in the late stages) | [9, 21] |
| Mouse melanoma cell line K1735 subline number 9 | Suppression | Suppression | [22, 23] and Wu et al. unpublished results |
| Angiosarcoma | Increasing | Not determined | [7, 37] and Wu et al. unpublished results |
| Human ovarian cancer cell lines BG-1 and SK-OV-3 | Suppression | suppression | [33, 34] |
| Human osteosarcoma cell lines | Not determined | Increasing | [39] |
| Human breast cancer cell line MCF-7 | Suppression | Not determined | [24] |
| Human breast cancer cell lines MCF-7 and SK-BR-3 | Increasing | Not determined | [30, 31] |
| Haemangiomas | Suppression? | Not determined | [38] |
| Nasopharyngeal carcinoma | Suppression? | Not determined | [7, 11] |
4. Mechanisms of METCAM-Mediated Tumorignesis and Metastasis
How does METCAM mediate or regulate tumorigenesis and metastasis of cancer cells? By deducing knowledge learned from the tumorigenesis of other tumors [15–19, 42] and the huMETCAM-mediated progression of melanoma [43–45] and angiogenesis [2, 46–51], we may be able to find some common clues to begin understanding its mechanisms.
First, the transcriptional expression of METCAM gene may be regulated by PKA/CREB (cAMP-responsive element binding protein), AP-2α [44, 45], and other transcription factors, such as SP-1, c-Myb, N-Oct2, ETs, CArG, Egr-1, and transcription factors binding to insulin-response elements, as shown in Figure 16 [7].
Figure 16.
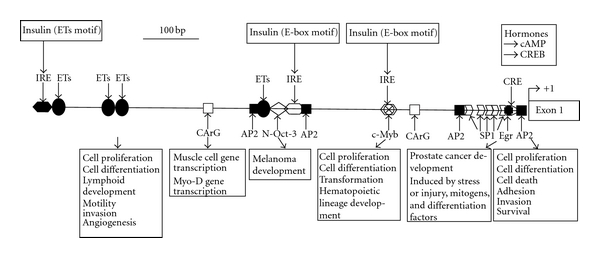
The promoter of the huMETCAM gene. The locations of various transcriptionally regulatory elements are shown in the 900 bp promoter region of the huMETCAM gene. The possible function of each element is also indicated. The promoter contains four AP-2 (activator protein-2, GCCNNNGGC), one CRE (cAMP response element, TGACGTCA), one Egr (early growth response element, CCCTG), five SP-1 (CCGCCC), two CArG (CC(A/t)6GG), three IRE (two insulin responsive elements with E-box motif, CANNTG, and one with Ets motif, ACGGAT), one c-Myb (coincided with one IRE with E-box motif, CACCTG), one N-Oct-3 (or brn-2, GCCTGAAT), and four Ets elements (GGAA).
Among these potential regulators, it is well documented that the AP-2α transcription factor plays a crucial tumor suppressor role in the progression of melanoma, prostate, and breast cancer [45]. It has been shown that PKA/CREB plays a positive role in the progression of melanoma, and perhaps also applicable to breast cancer and prostate cancer, by inhibiting the expression of AP-2αand increasing the expression of METCAM [45]. However, the expression level of AP-2αin other cancers has not been explored. The roles of other transcription regulators, tissue-specific enhancers and repressors, epigenetic control, and control at the level of chromatin remodeling of the gene have still yet to be investigated [7].
Second, since the cytoplasmic tail of METCAM contains consensus sequences potentially to be phosphorylated by PKA, PKC, and CK2, it may manifest its functions by crosstalk with various signaling pathways mediated by these protein kinases [7]. For example, METCAM expression in melanoma cells is reciprocally regulated by AKT, in which AKT up-regulates the level METCAM and overexpression of METCAM activates endogenous AKT, which in turn inhibits apoptosis and increases survival ability [43]. However, it is not clear if a similar mechanism is also used in breast, prostate, and other cancers. Also, the detailed mechanism of how AKT up-regulates the expression of METCAM has not been worked out. PKA, PKC, and CK2 may phosphorylate the cytoplasmic tail of METCAM, which then facilitates its interaction with FAK, thus promoting cytoskeleton remodeling. Alternatively, after phosphorylation of its cytoplasmic tail by these protein kinases, METCAM may interact with the downstream effectors of Ras, activating ERK and JNK, which in turn may transcriptionally activate the expression of AKT or other genes that promote the proliferation and angiogenesis of tumor cells. Though METCAM has not been shown to be a substrate of CK2, which has been shown to phosphorylate other CAMs, such as CD44, E-cadherin, L1-CAM, and vitronectin, it is also likely that CK2 may be able to phosphorylate METCAM [46] and link it to AKT to affect the proliferation, survival, and other tumorigenesis-related functions of tumor cells [47].
Third, after the engagement of METCAM with the ligand(s) or extracellular matrix, it may transmit the outside-in signals into tumor cells by activating FAK and the downstream-signaling components, promoting cytoskeleton remodeling and increasing tumor cell motility and invasiveness [2, 7].
Fourth, from what we know about the roles of other CAMs in the progression of other tumors [15–19, 42], it is logical to postulate that METCAM may affect cancer cell progression by crosstalk with signaling pathways that affect apoptosis, survival and proliferation, and angiogenesis of tumor cells [7, 18, 42]. Thus, METCAM may affect tumorigenesis and metastasis by altering the expression of various indexes in apoptosis, survival signaling, proliferation signaling, and angiogenesis. To support this notion, we have found that METCAM promotes the progression of prostate cancer cells by rendering the cells with increased proliferative ability by elevating levels of Ki67 and PCNA, with increased survival ability by elevating the level of phosphorylated AKT, and with increased angiogenic ability by elevating levels of VEGF, VEGFR2, and CD31 [35]; but it has no effect on the process of apoptosis. In contrast to this, METCAM promotes the progression of melanoma cells differently by preventing the apoptosis of melanoma cells [47] and reciprocally affecting the expression of a survival index, phospho-AKT [43]. Further systematic studies by using specific RNAis to knockdown the downstream effectors one-by-one in the METCAM-expressing clones may be necessary to further understand this aspect of mechanism.
Fifth, METCAM may mediate hematogenous spreading of melanoma cells, which had been implicated by its expression in endothelial cells, as well as in malignant melanoma cells [48], further shown to be present in the junctions of endothelial cells [49, 50] and essential for tumor angiogenesis in at least three tumor cell lines [51] and human prostate cancer LNCaP cells [52]. It is highly likely that METCAM expression may promote hematogenous spreading of prostate cancer cells, similar to melanoma cells [49]. Similar mechanisms may also be used for the METCAM-mediated hematogenous spreading of breast cancer and osteosarcoma cells. However, it is not known if METCAM also plays a role in the lymphatic spread of cancer cells. Recent results from one group showed that METCAM is one of the lymphatic metastasis-associated genes, which is upregulated in malignant mouse hepatocarcinoma [53]; suggesting that METCAM may also play a role in promoting lymphatic metastasis of cancer cells. However, the details of how METCAM mediates hematogenous or lymphatic spreading of cancer cells have still yet to be investigated. Labeling the cells with viable dyes and following the process in real time by using a newly developed nonintruding, but highly photo-penetrating imaging method of photoacoustic tomography (PAT) [54, 55] may be useful for monitoring each step in the METCAM-mediated progression. For the METCAM-mediated dynamic spreading of melanoma cells in vivo, the PAT imaging method coupled with using hairless syngeneic mouse animal models [56] should reveal more clearly the process in real time.
Sixth, METCAM has been shown to express in normal mesenchymal cells (smooth muscle, endothelium, and Schwann cells) in the tissue stroma and be a marker for the mesenchymal stem cells [57], METCAM may play an important role in regulating tumor dormancy or awakening, driving or preventing cancer cells to premetastatic niche, and formatting a microenvironment for favorable or unfavorable tumor growth in secondary sites.
Seventh, METCAM may affect the progression of cancer cells by interactions with the host immune system, which, however, has been shown to have a paradoxical role in tumor progression [58]. Recently, one group has shown that a subset of host B lymphocytes may control melanoma metastasis through METCAM-dependent interaction [59]. On the other hand, it is highly likely that the tumor suppression effect of METCAM expression in melanoma K1735-9 subline may be due to the interaction of METCAM-expressing cells with the host immune defense system in the immunocompetent syngeneic C3H brown mouse, since the intrinsic motility and invasiveness of mouse melanoma K1735-9 was increased by the METCAM expression [22, 23]. For example, the surface METCAM expressed in this particular melanoma cell line may have a homophilic interaction with the NK cells, which also express METCAM and enhance cytotoxic functions of NK cells [60]. This hypothesis should be testable by studying the METCAM-mediated metastasis of METCAM-expressing K1735-9 cells in various genetically altered mice with a knockout of CD4+ T cells, CD8+ T cells, or NK cells, or mice with a combined knockout of these immune cells.
Eighth, malignant progression of cancer cells has been shown to associate with an abnormal glycosylation, resulting in expression of altered carbohydrate determinants [61]. Thus, the glycosylated status of METCAM in different cancer types may be different from normal cells, thus manifesting positive or negative effect on the progression of different cancer types. This aspect of the METCAM-mediated cancer progression has not been well studied, but is especially intriguing since METCAM possesses six conserved N-glycosylation sites in the extracellular domain [7, 8].
We should always keep in mind that mechanisms of METCAM-mediated cancer progression may be slightly different in different cancer cells due to their different intrinsic properties, which provides different cofactors and/or different ligand(s) that either positively or negatively regulate the METCAM-mediated tumorigenesis and metastasis. To further understand the role of METCAM in these processes, it is essential to diligently identify the cofactors and the METCAM-cognate heterophilic ligand(s), which modulate the biological functions of METCAM. The endeavor in this direction appears to be promising from our preliminary attempts that we may have successfully found a possible candidate of METCAM's heterophilic ligand in METCAM-expressing human prostate cancer cells [7].
Mechanisms of METCAM-mediated negative role in the progression of some cancer cells have not been studied at all. Does METCAM in some cancers behave like E-cadherin, which always plays a negative role in the tumorigenesis and metastasis of most epithelial cancer cells [18]? But even E-cadherin may function differently in different cancer cells. For example, its expression is temporally different and correlates with different stages during the progression of ovarian cancer [62]: E-cadherin is not expressed in the ovarian surface epithelial cells, expressed in premalignant lesions and in well-differentiated tumors, and finally not expressed in late-stage invasive tumors [62]. Likewise, METCAM may express and function normally in the normal nasopharyngeal epithelium, transiently reduce its expression and lose its function during the development of nasopharyngeal carcinoma, resume its expression, and function in the invasion stage of the cancer. Alternatively, METCAM may behave differently from E-cadherin by being modulated by different cofactors or ligands, which are expressed at different stages of the cancer. The tumor suppressor role of METCAM in ovarian cancer cells may not be due to the altered intrinsic properties of the cancer cells, since the intrinsic motility and invasiveness of human ovarian cancer BG-1 and SK-OV-3 cells was not affected by the METCAM expression [34, 35]. Our preliminary results appear to suggest a special mechanism that a soluble form of METCAM, which is produced by MMPs in the METCAM-expressing cells, may mediate the suppressive effect in ovarian cancer cells, similar to the production of a soluble form of P-cadherin by the induced MMPs in breast cancer cells, which then dictates, instead of suppresses, the aggressive behavior of the breast cancer cells [63].
5. Conclusions and Clinical Applications
METCAM may have a key positive function in the progression of angiosarcoma, breast cancer, osteosarcoma, prostate cancer, and most melanoma cell lines. On the other hand, it may also have a key function in suppressing the progression of a few melanoma cell lines, ovarian cancer, haemangioma, and other cancers. To further understand its mechanisms in these processes, it is crucial to define its functional domains, identify its cognate ligand(s) and cofactor regulators, and study its crosstalk with members of various signaling pathways [7]. These model systems may be useful for real-time observation of the dynamic process of cancer progression by using a nonintrusive and high photo-penetrating imaging system, such as the newly developed photoacoustic tomography (PAT), to further understanding the process in mouse models [54, 55]. The knowledge gained would also be useful for designing effective means to decrease, or even to block the metastatic potential of these cancers. Along these lines, a preclinical trial of using doxazosin, an α1-adrenergic antagonist that has been used to treat the BPH patients, has been shown to be able to suppress prostate cancer metastasis in the TRAMP mouse model [64]. Furthermore, preclinical trials using a fully humanized anti-METCAM antibody against melanoma growth and metastasis [65, 66] and using a mouse anti-METCAM monoclonal antibody against angiogenesis and tumor growth of hepatocarcinoma, leiomyosarcoma, and pancreatic cancer [51] have been successfully demonstrated. Alternatively, small soluble peptides derived from METCAM may also be useful for blocking the tumor formation and tumor angiogenesis [52, 67, 68]. The attachment of these reagents to nanoparticles may be another alternative for therapeutic use [69].
Acknowledgment
The author thanks Mr. Jonathan C.-Y. Wu for critical reading of the paper and proof reading of the English Language.
References
- 1.Lehmann JM, Riethmuller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(24):9891–9895. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anfosso F, Bardin N, Vivier E, Sabatier F, Sampol J, Dignat-George F. Outside-in signaling pathway linked to CD146 engagement in human endothelial cells. Journal of Biological Chemistry. 2001;276(2):1564–1569. doi: 10.1074/jbc.M007065200. [DOI] [PubMed] [Google Scholar]
- 3.Xie S, Luca M, Huang S, et al. Expression of MCAM/MUC18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Research. 1997;57(11):2295–2303. [PubMed] [Google Scholar]
- 4.Shih IM, Elder DE, Hsu MY, Herlyn M. Regulation of Mel-CAM/MUC18 expression on melanocytes of different stages of tumor progression by normal keratinocytes. American Journal of Pathology. 1994;145(4):837–845. [PMC free article] [PubMed] [Google Scholar]
- 5.Shih IM, Elder DE, Speicher D, Johnson JP, Herlyn M. Isolation and functional characterization of the A32 melanoma-associated antigen. Cancer Research. 1994;54(9):2514–2520. [PubMed] [Google Scholar]
- 6.Bardin N, George F, Mutin M, et al. S-Endo 1, a pan-endothelial monoclonal antibody recognizing a novel human endothelial antigen. Tissue Antigens. 1996;48(5):531–539. doi: 10.1111/j.1399-0039.1996.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu GJ. METCAM/MUC18 expression and cancer metastasis. Current Genomics. 2005;6(5):333–349. [Google Scholar]
- 8.Wu GJ, Wu MWH, Wang SW, et al. Isolation and characterization of the major form of human MUC18 cDNA gene and correlation of MUC18 over-expression in prostate cancer cell lines and tissues with malignant progression. Gene. 2001;279(1):17–31. doi: 10.1016/s0378-1119(01)00736-3. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Wu MWH, Wang SW, et al. Isolation and characterization of mouse MUC18 cDNA gene, and correlation of MUC18 expression in mouse melanoma cell lines with metastatic ability. Gene. 2001;265(1-2):133–145. doi: 10.1016/s0378-1119(01)00349-3. [DOI] [PubMed] [Google Scholar]
- 10.Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. Journal of Pathology. 1999;189(1):4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Lin JC, Chiang CF, Wang SW, et al. Decreased expression of METCAM/MUC18 correlates with the appearance of, but its increased expression with metastasis of nasopharyngealcarcinoma. In press. [Google Scholar]
- 12.Wu GJ, Varma VA, Wu MWH, et al. Expression of a human cell adhesion molecule, MUC18, in prostate cancer cell lines and tissues. Prostate. 2001;48(4):305–315. doi: 10.1002/pros.1111. [DOI] [PubMed] [Google Scholar]
- 13.Wu GJ. The role of MUC18 in prostate carcinoma. In: Hayat MA, editor. Immunohistochemistry and in Situ Hybridization of Human Carcinoma. Vol 1. Molecular Pathology, Lung Carcinoma, Breast Carcinoma, and Prostate Carcinoma. chapter 7. Elsevier Science/Academic Press; 2004. pp. 347–358. [Google Scholar]
- 14.Wu GJ, Son ES, Dickerson EB, et al. METCAM/MUC18 over-expression in human ovarian cancer tissues and metastatic lesions is associated with clinical progression. doi: 10.1016/j.tjog.2014.03.003. In press. [DOI] [PubMed] [Google Scholar]
- 15.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nature Medicine. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 16.Christofori G. New signals from the invasive front. Nature. 2006;441(7092):444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 17.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nature Reviews Cancer. 2004;4(2):118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 19.Chambers A, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 20.Schlagbauer-Wadl H, Jansen B, Muller M, et al. Influence of MUC18/MCAM/CD146 expression on human melanoma growth and metastasis in SCID mice. International Journal of Cancer. 1999;81(6):951–955. doi: 10.1002/(sici)1097-0215(19990611)81:6<951::aid-ijc18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Wu GJ, Fu P, Wang SW, Wu MWH. Enforced expression of MCAM/MUC18 increases in vitro motility and invasiveness and in vivo metastasis of two mouse melanoma K1735 sublines in a syngeneic mouse model. Molecular Cancer Research. 2008;6(11):1666–1677. doi: 10.1158/1541-7786.MCR-07-2200. [DOI] [PubMed] [Google Scholar]
- 22.Wu GJ, Peng Q, Wang SW, Yang H, Wu MWH. Effect of MUC18 expression on the in vitro invasiveness and in vivo tumorigenesis and metastasis of mouse melanoma cell lines in a syngeneic mouse model. In: Proceedings of the 92nd Annual Meeting of American Association for the Cancer Research, vol. 42, abstract no. 2776; 2001; p. 516. [Google Scholar]
- 23.Wu GJ, Wu MWH. Ectopic expression of MCAM/MUC18 increases in vivo motility and invasiveness, but decreases tumorigenesis and metastasis of a mouse melanoma K1735-9 subline in a syngeneic mouse model. doi: 10.1007/s10585-016-9812-z. In press. [DOI] [PubMed] [Google Scholar]
- 24.Shih IM, Hsu MY, Palazzo JP, Herlyn M. The cell-cell adhesion receptor Mel-CAM acts as a tumor suppressor in breast carcinoma. American Journal of Pathology. 1997;151(3):745–751. [PMC free article] [PubMed] [Google Scholar]
- 25.Charafe-Jauffret E, Ginestier C, Monville F, et al. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25(15):2273–2284. doi: 10.1038/sj.onc.1209254. [DOI] [PubMed] [Google Scholar]
- 26.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouhtit A, Gaur RL, Abd Elmageed ZY, et al. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochimica et Biophysica Acta. 2009;1795(2):130–136. doi: 10.1016/j.bbcan.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Garcia S, Dales JP, Charafe-Jauffret E, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Human Pathology. 2007;38(6):830–841. doi: 10.1016/j.humpath.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Zabouo G, Imbert AM, Jacquemier J, et al. CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Research. 2009;11(1, article no. R1) doi: 10.1186/bcr2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng G, Cai S, Wu GJ. Up-regulation of METCAM/MUC18 promotes motility, invasiveness and tumorigenesis of human breast cancer cells. BMC Cancer. 2011;11:p. 113. doi: 10.1186/1471-2407-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng G, Cai S, Liu Y, Wu GJ. METCAM/MUC18 augments promotes migration, invasion and tumorigenicity of human breast cancer SK-BR-3 cells. Gene. 2012;492:229–238. doi: 10.1016/j.gene.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Aldovini D, Demichelis F, Doglioni C, et al. M-cam expression as marker of poor prognosis in epithelial ovarian cancer. International Journal of Cancer. 2006;119(8):1920–1926. doi: 10.1002/ijc.22082. [DOI] [PubMed] [Google Scholar]
- 33.Wu GJ, Zeng G, Son EL. METCAM/MUC18 over-expression suppresses in vitro motility and invasiveness and in vivo progression of human ovarian cancer BG-1 cells. In press. [Google Scholar]
- 34.Zeng G, Wu GJ. METCAM/MUC18 over-expression suppresses in vitro motility and invasiveness and in vivo tumor formation of human ovarian cancer SKOV3 cells. In press. [Google Scholar]
- 35.Wu GJ, Wu MWH, Liu Y. Enforced expression of human METCAM/MUC18 increases the tumorigenesis of human prostate cancer cells in nude mice. Journal of Urology. 2011;185:1504–1512. doi: 10.1016/j.juro.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 36.Wu GJ, Peng Q, Fu P, et al. Ectopical expression of human MUC18 increases metastasis of human prostate cancer cells. Gene. 2004;327(2):201–213. doi: 10.1016/j.gene.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 37.LaMontagne KR, Jr., Moses MA, Wiederschain D, et al. Inhibition of MAP kinase kinase causes morphological reversion and dissociation between soft agar growth and in vivo tumorigenesis in angiosarcoma cells. American Journal of Pathology. 2000;157(6):1937–1945. doi: 10.1016/s0002-9440(10)64832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Yu Y, Bischoff J, Mulliken JB, Olsen BR. Differential expression of CD146 in tissues and endothelial cells derived from infantile haemangioma and normal human skin. Journal of Pathology. 2003;201(2):296–302. doi: 10.1002/path.1443. [DOI] [PubMed] [Google Scholar]
- 39.McGary EC, Heimberger A, Mills L, et al. A fully human antimelanoma cellular adhesion molecule/muc18 antibody inhibits spontaneous pulmonary metastasis of osteosarcoma cells in vivo . Clinical Cancer Research. 2003;9(17):6560–6566. [PubMed] [Google Scholar]
- 40.Meier F, Caroli U, Satyamoorthy K, et al. Fibroblast growth factor-2 but not Mel-CAM and/or β3 integrin promotes progression of melanocyte to melanoma. Experimental Dermatology. 2003;12(3):296–306. doi: 10.1034/j.1600-0625.2003.120310.x. [DOI] [PubMed] [Google Scholar]
- 41.Wu GJ, Chiang CF, Fu P, Huss W, Greenberg N, Wu MWH. Increased expression of MUC18 correlates with the metastatic progression of mouse prostate adenocarcinoma in the (TRAMP) model. Journal of Urology. 2005;173(5):1778–1783. doi: 10.1097/01.ju.0000154643.30048.2c. [DOI] [PubMed] [Google Scholar]
- 42.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 43.Li G, Kalabis J, Xu X, et al. Reciprocal regulation of MelCAM and AKT in human melanoma. Oncogene. 2003;22(44):6891–6899. doi: 10.1038/sj.onc.1206819. [DOI] [PubMed] [Google Scholar]
- 44.Melnikova VO, Balasubramanian K, Villares GJ, et al. Crosstalk between protease-activated receptor 1 and platelet-activating factor receptor regulates melanoma cell adhesion molecule (MCAM/MUC18) expression and melanoma metastasis. Journal of Biological Chemistry. 2009;284(42):28845–28855. doi: 10.1074/jbc.M109.042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melnikova VO, Dobroff AS, Zigler M, et al. CREB inhibits AP-2αexpression to regulate the malignant phenotype of melanoma. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012452. Article ID e12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB Journal. 2003;17(3):349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 47.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three AKTs. Genes and Development. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 48.Sers C, Riethmuller G, Johnson JP. MUC18, a melanoma-progression associated molecule, and its potential role in tumor vascularization and hematogenous spread. Cancer Research. 1994;54(21):5689–5694. [PubMed] [Google Scholar]
- 49.Bardin N, Anfosso F, Masse J, et al. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell adhesion. Blood. 2001;98:3677–3684. doi: 10.1182/blood.v98.13.3677. [DOI] [PubMed] [Google Scholar]
- 50.Kang Y, Wang F, Feng J, Yang D, Yang X, Yan X. Knockdown of CD146 reduces the migration and proliferation of human endothelial cells. Cell Research. 2006;16(3):313–318. doi: 10.1038/sj.cr.7310039. [DOI] [PubMed] [Google Scholar]
- 51.Yan X, Lin Y, Tang D, et al. A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood. 2003;102(1):184–191. doi: 10.1182/blood-2002-04-1004. [DOI] [PubMed] [Google Scholar]
- 52.Wu GJ, Son EL. Soluble METCAM/MUC18 blocks angiogenesis during tumor formation of human prostate cancer cells. In: Proceedings of the 97th Annual Meeting of American Association for the Cancer Research, vol. 47; 2006; p. 252. [Google Scholar]
- 53.Song B, Tang JW, Wang B, Cui XN, Zhou CH, Hou L. Screening for lymphatic metastasis-associated genes in mouse hepatocarcinoma cell lines Hca-F and Hca-P using gene chip. Chinese Journal of Cancer. 2005;24(7):774–780. [PubMed] [Google Scholar]
- 54.Zhang Q, Liu Z, Carney PR, et al. Non-invasive imaging of epileptic seizures in vivo using photoacoustic tomography. Physics in Medicine and Biology. 2008;53(7):1921–1931. doi: 10.1088/0031-9155/53/7/008. [DOI] [PubMed] [Google Scholar]
- 55.Wang LV. Prospects of photoacoustic tomography. Medical Physics. 2008;35(12):5758–5767. doi: 10.1118/1.3013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaffer BS, Grayson MH, Wortham JM, et al. Immune competency of a Hairless mouse strain for improved preclinical studies in genetically engineered mice. Molecular Cancer Therapeutics. 2010;9(8):2354–2364. doi: 10.1158/1535-7163.MCT-10-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorrentino A, Ferracin M, Castelli G, et al. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Experimental Hematology. 2008;36(8):1035–1046. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 58.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature Reviews Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 59.Staquicini F, Tandle A, Libutti SK, et al. A subset of host B lymphocytes controls melanoma metastasis through a melanoma cell adhesion molecule/MUC18-dependent interaction: evidence from mice and humans. Cancer Research. 2008;68(20):8419–8428. doi: 10.1158/0008-5472.CAN-08-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Despoix N, Walzer T, Jouve N, et al. Mouse CD146/MCAM is a marker of natural killer cell maturation. European Journal of Immunology. 2008;38(10):2855–2864. doi: 10.1002/eji.200838469. [DOI] [PubMed] [Google Scholar]
- 61.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Science. 2004;95(5):377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C, Cipollone J, Maines-Bandiera S, et al. The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation. 2008;76(2):193–205. doi: 10.1111/j.1432-0436.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 63.Ribeiro AS, Albergaria A, Sousa B, et al. Extracellular cleavage and shedding of P-cadherin: a mechanism underlying the invasive behaviour of breast cancer cells. Oncogene. 2010;29(3):392–402. doi: 10.1038/onc.2009.338. [DOI] [PubMed] [Google Scholar]
- 64.Chiang CF, Son EL, Wu GJ. Oral treatment of the TRAMP mice with doxazosin suppresses prostate tumor growth and metastasis. Prostate. 2005;64(4):408–418. doi: 10.1002/pros.20260. [DOI] [PubMed] [Google Scholar]
- 65.Mills L, Ellez C, Huang S, et al. Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Research. 2002;62(17):5106–5114. [PubMed] [Google Scholar]
- 66.Leslie MC, Zhao YJ, Lachman LB, Hwu P, Wu GJ, Bar-Eli M. Immunization against MUC18/MCAM, a novel antigen that drives melanoma invasion and metastasis . Gene Therapy. 2007;14(4):316–323. doi: 10.1038/sj.gt.3302864. [DOI] [PubMed] [Google Scholar]
- 67.Satyamoorthy K, Muyrers J, Meier F, Patel D, Herlyn M. Mel-CAM-specific genetic suppressor elements inhibit melanoma growth and invasion through loss of gap junctional communication. Oncogene. 2001;20(34):4676–4684. doi: 10.1038/sj.onc.1204616. [DOI] [PubMed] [Google Scholar]
- 68.Hafner C, Samwald U, Wagner S, et al. Selection of mimotopes of the cell surface adhesion molecule Mel-CAM from a random pVIII-28aa phage peptide library. Journal of Investigative Dermatology. 2002;119(4):865–869. doi: 10.1046/j.1523-1747.2002.00171.x. [DOI] [PubMed] [Google Scholar]
- 69.Nie S. Nanotechnology for personalized and predictive medicine. Nanomedicine. 2006;4(1):p. 305. [Google Scholar]


