Abstract
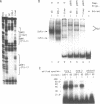
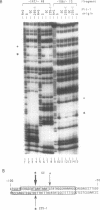
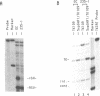
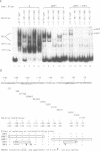
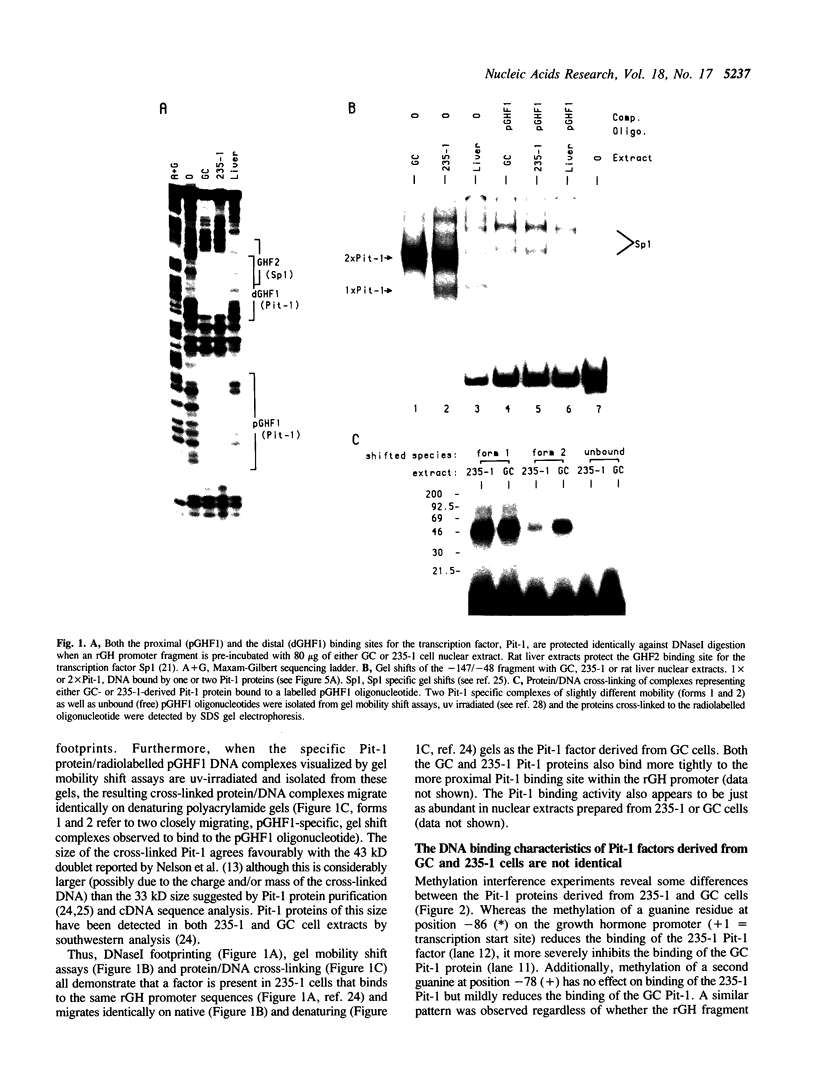
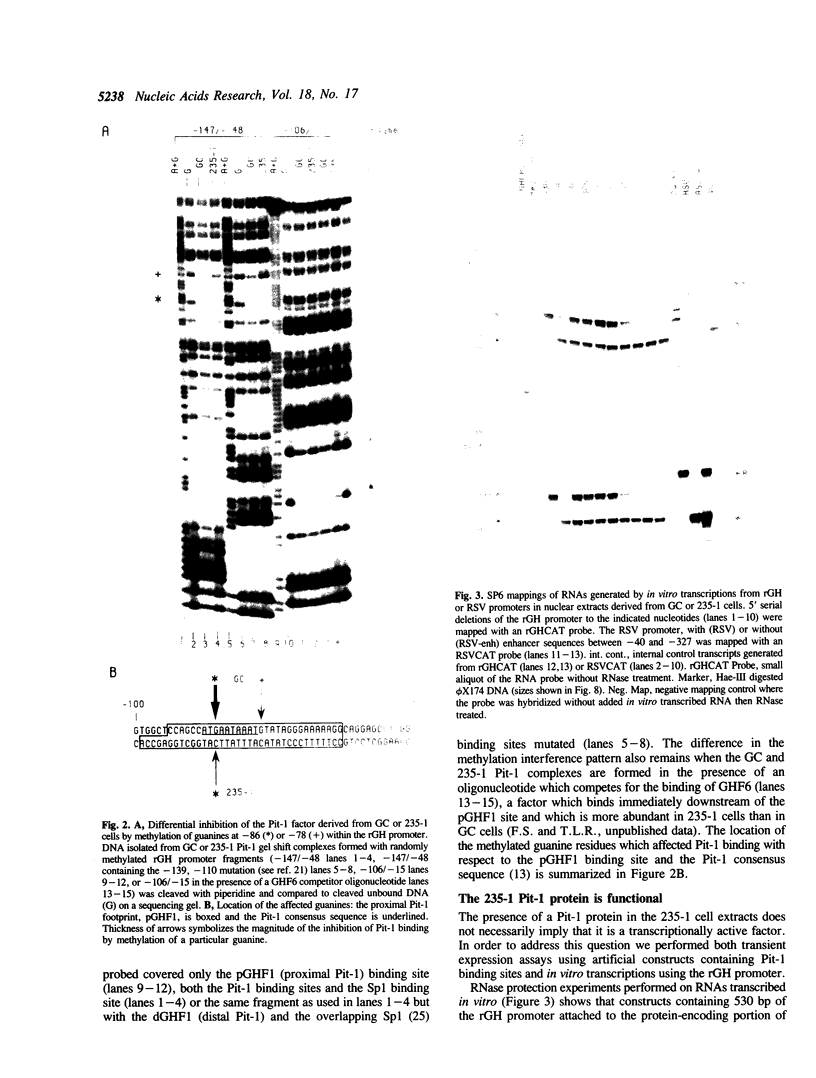
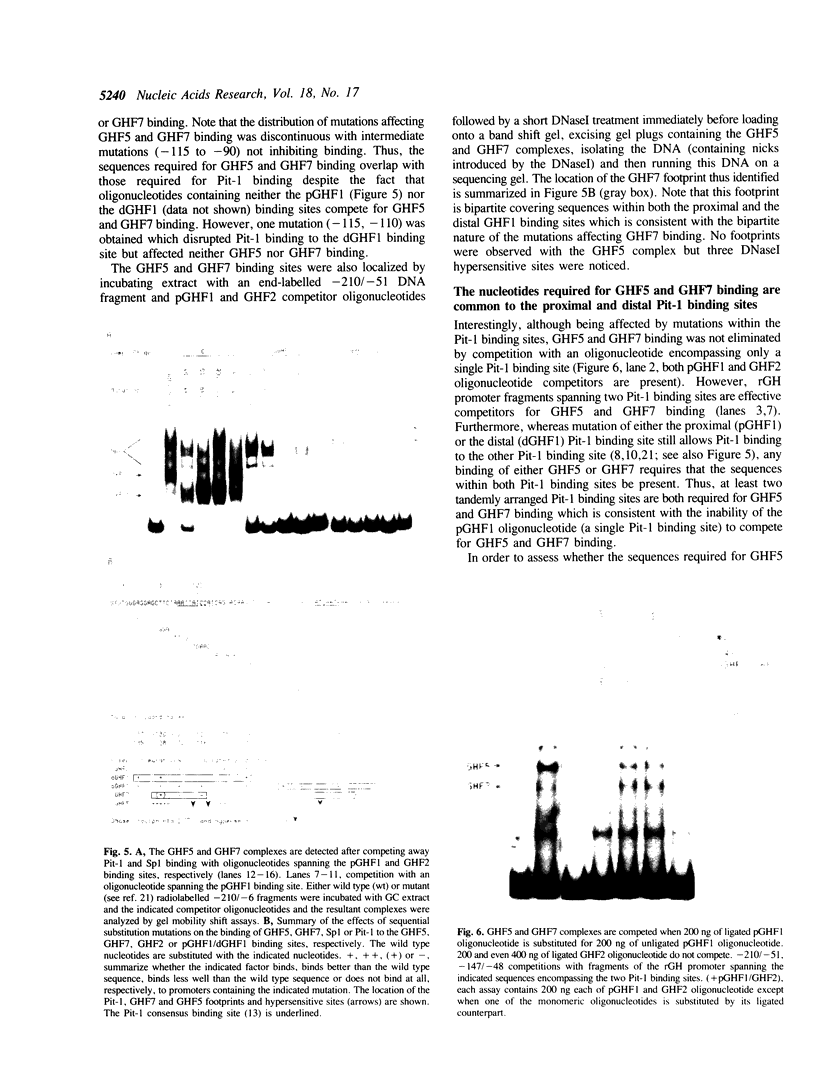
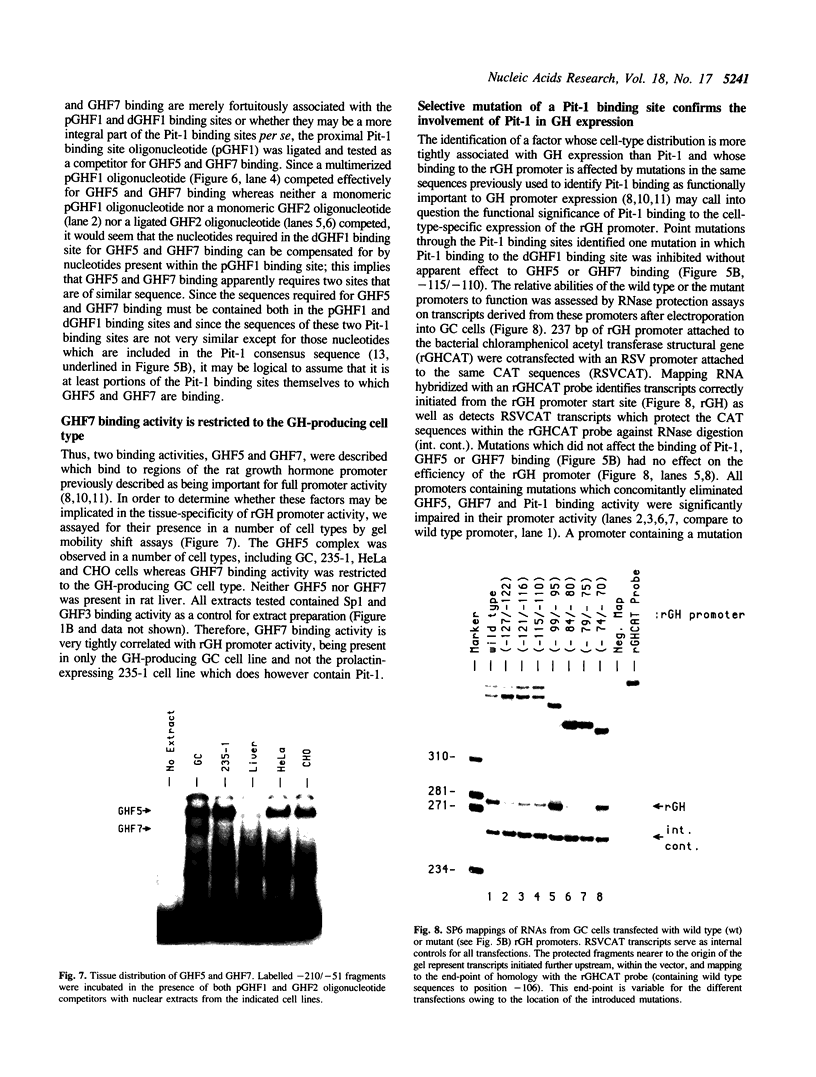
Nuclear extracts prepared from growth hormone-secreting (GC) and prolactin-secreting (235-1) rat anterior pituitary cell lines were compared for their ability to bind to the DNA sequences conferring tissue-specificity to the expression of the rat growth hormone (rGH) gene promoter. Cell-specific differences in the interaction of Pit-1, a tissue-specific member of the POU-domain transcription factor family, with the pGHF1 binding site were detected by methylation interference experiments; otherwise the Pit-1 proteins present in GC cell and 235-1 cell extracts were similar. Two other protein/DNA complexes, GHF5 and GHF7, were detected by gel mobility shift assays and the binding of both complexes to the rGH promoter depended upon DNA sequences contained within the two binding sites for Pit-1. In contrast to Pit-1 which can bind to either of the two sites independently, a single Pit-1 binding site was insufficient for GHF5 and GHF7 binding; i.e. both Pit-1 binding sites within the rGH promoter were required. Whereas GHF5 was present in nuclear extracts of GC cells and a variety of cells not producing growth hormone, GHF7 binding activity was detected only in the GC cell line (and not in the 235-1 cell line). GHF7 binding activity was therefore more closely correlated with growth hormone gene transcription than was Pit-1. rGH promoters containing mutations which inhibited GHF5, GHF7 and Pit-1 binding were expressed less efficiently than the wild type promoter after transfection into GC cells. One promoter mutation to which the GHF7 complex but not the Pit-1 factor can bind was also transcription deficient demonstrating that Pit-1 binding, independent of GHF7 binding, was nevertheless important to the expression of the rat growth hormone promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. Drosophila development: making stripes inelegantly. Nature. 1989 Sep 28;341(6240):282–283. doi: 10.1038/341282a0. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Mathews L. S., Palmiter R. D., Brinster R. L. Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes Dev. 1988 Apr;2(4):453–461. doi: 10.1101/gad.2.4.453. [DOI] [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Bodner M., Karin M. A pituitary-specific trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cells. Cell. 1987 Jul 17;50(2):267–275. doi: 10.1016/0092-8674(87)90222-4. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Bodner M., Karin M. Purification of growth hormone-specific transcription factor GHF-1 containing homeobox. Science. 1989 Feb 10;243(4892):814–817. doi: 10.1126/science.2563596. [DOI] [PubMed] [Google Scholar]
- Catanzaro D. F., West B. L., Baxter J. D., Reudelhuber T. L. A pituitary-specific factor interacts with an upstream promotor element in the rat growth hormone gene. Mol Endocrinol. 1987 Jan;1(1):90–96. doi: 10.1210/mend-1-1-90. [DOI] [PubMed] [Google Scholar]
- Crenshaw E. B., 3rd, Kalla K., Simmons D. M., Swanson L. W., Rosenfeld M. G. Cell-specific expression of the prolactin gene in transgenic mice is controlled by synergistic interactions between promoter and enhancer elements. Genes Dev. 1989 Jul;3(7):959–972. doi: 10.1101/gad.3.7.959. [DOI] [PubMed] [Google Scholar]
- Crew M. D., Spindler S. R. Thyroid hormone regulation of the transfected rat growth hormone promoter. J Biol Chem. 1986 Apr 15;261(11):5018–5022. [PubMed] [Google Scholar]
- Flug F., Copp R. P., Casanova J., Horowitz Z. D., Janocko L., Plotnick M., Samuels H. H. cis-acting elements of the rat growth hormone gene which mediate basal and regulated expression by thyroid hormone. J Biol Chem. 1987 May 5;262(13):6373–6382. [PubMed] [Google Scholar]
- Forman B. M., Yang C. R., Stanley F., Casanova J., Samuels H. H. c-erbA protooncogenes mediate thyroid hormone-dependent and independent regulation of the rat growth hormone and prolactin genes. Mol Endocrinol. 1988 Oct;2(10):902–911. doi: 10.1210/mend-2-10-902. [DOI] [PubMed] [Google Scholar]
- Frawley L. S., Boockfor F. R., Hoeffler J. P. Identification by plaque assays of a pituitary cell type that secretes both growth hormone and prolactin. Endocrinology. 1985 Feb;116(2):734–737. doi: 10.1210/endo-116-2-734. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Homeo boxes in the study of development. Science. 1987 Jun 5;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Hodin R. A., Lazar M. A., Wintman B. I., Darling D. S., Koenig R. J., Larsen P. R., Moore D. D., Chin W. W. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989 Apr 7;244(4900):76–79. doi: 10.1126/science.2539642. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Schacter B. S., O'Farrell P. H. The level of expression of the rat growth hormone gene in liver tumor cells is at least eight orders of magnitude less than that in anterior pituitary cells. Mol Cell Biol. 1983 Aug;3(8):1460–1467. doi: 10.1128/mcb.3.8.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N. C. The effects of 5-azacytidine on the expression of the rat growth hormone gene. Methylation modulates but does not control growth hormone gene activity. J Biol Chem. 1984 Sep 25;259(18):11601–11606. [PubMed] [Google Scholar]
- Larsen P. R., Harney J. W., Moore D. D. Repression mediates cell-type-specific expression of the rat growth hormone gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8283–8287. doi: 10.1073/pnas.83.21.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverriere J. N., Muller M., Buisson N., Tougard C., Tixier-Vidal A., Martial J. A., Gourdji D. Differential implication of deoxyribonucleic acid methylation in rat prolactin and rat growth hormone gene expressions: a comparison between rat pituitary cell strains. Endocrinology. 1986 Jan;118(1):198–206. doi: 10.1210/endo-118-1-198. [DOI] [PubMed] [Google Scholar]
- Lefevre C., Imagawa M., Dana S., Grindlay J., Bodner M., Karin M. Tissue-specific expression of the human growth hormone gene is conferred in part by the binding of a specific trans-acting factor. EMBO J. 1987 Apr;6(4):971–981. doi: 10.1002/j.1460-2075.1987.tb04847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira S. A., Crenshaw E. B., 3rd, Glass C. K., Swanson L. W., Rosenfeld M. G. Identification of rat growth hormone genomic sequences targeting pituitary expression in transgenic mice. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4755–4759. doi: 10.1073/pnas.85.13.4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam H. J., Albert V. R., Ingraham H. A., Kapiloff M., Wilson L., Nelson C., Elsholtz H., Rosenfeld M. G. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989 Jul;3(7):946–958. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Nelson C., Crenshaw E. B., 3rd, Franco R., Lira S. A., Albert V. R., Evans R. M., Rosenfeld M. G. Discrete cis-active genomic sequences dictate the pituitary cell type-specific expression of rat prolactin and growth hormone genes. Nature. 1986 Aug 7;322(6079):557–562. doi: 10.1038/322557a0. [DOI] [PubMed] [Google Scholar]
- Norman M. F., Lavin T. N., Baxter J. D., West B. L. The rat growth hormone gene contains multiple thyroid response elements. J Biol Chem. 1989 Jul 15;264(20):12063–12073. [PubMed] [Google Scholar]
- Pan W. T., Liu Q. R., Bancroft C. Identification of a growth hormone gene promoter repressor element and its cognate double- and single-stranded DNA-binding proteins. J Biol Chem. 1990 Apr 25;265(12):7022–7028. [PubMed] [Google Scholar]
- Reymond M. J., Nansel D. D., Burrows G. H., Neaves W. B., Porter J. C. A new clonal strain of rat pituitary tumour cells: a model for non-regulated secretion of prolactin. Acta Endocrinol (Copenh) 1984 Aug;106(4):459–470. doi: 10.1530/acta.0.1060459. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling E. Autoregulation--a common property of eukaryotic transcription factors? Trends Genet. 1989 May;5(5):131–133. doi: 10.1016/0168-9525(89)90049-8. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Theill L. E., Castrillo J. L., Wu D., Karin M. Dissection of functional domains of the pituitary-specific transcription factor GHF-1. Nature. 1989 Dec 21;342(6252):945–948. doi: 10.1038/342945a0. [DOI] [PubMed] [Google Scholar]
- West B. L., Catanzaro D. F., Mellon S. H., Cattini P. A., Baxter J. D., Reudelhuber T. L. Interaction of a tissue-specific factor with an essential rat growth hormone gene promoter element. Mol Cell Biol. 1987 Mar;7(3):1193–1197. doi: 10.1128/mcb.7.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. S., Samuels H. H. Cell- and sequence-specific binding of nuclear proteins to 5'-flanking DNA of the rat growth hormone gene. J Biol Chem. 1987 May 5;262(13):6313–6317. [PubMed] [Google Scholar]