Abstract
Motility generated by 9+2 organelles, variably called cilia or flagella, evolved before divergence from the last common ancestor of extant eukaryotes. In order to better understand how motility in these organelles is regulated, evolutionary steps that led to the present 9+2 morphology are considered. In addition, recent advances in our knowledge of flagellar assembly, together with heightened appreciation of the widespread role of cilia in sensory processes, suggest that these organelles may have served multiple roles in early eukaryotic cells. In addition to their function as undulating motility organelles, we speculate that protocilia were the primary determinants of cell polarity and directed motility in early eukaryotes, and that they provided the first defined membrane domain for localization of receptors that allowed cells to respond tactically to environmental cues. Initially, motility associated with these protocilia may have been gliding motility rather than the more complex bend propagation. Once these protocilia became functional motile organelles for beating, we believe that addition of an asymmetric central apparatus, capable of transducing signals to dynein motors and altering beat parameters, provided refined directional control in response to tactic signals. The rest of this paper presents a more detailed description of hypothesized steps in this evolution, and examples to support these hypotheses.
The origins of 9+2 organelles
Phylogenetic analysis of eukaryotic organisms based on morphological characteristics failed to identify connections among unicellular eukaryotes. In recent years, more extensive molecular phylogenetic studies have shown that such simple organisms are not necessarily closer to the root of the eukaryotic tree. Thus fungi, once thought to have branched before the divergence of plants and animals, are now considered to group with Animalia and Choanozoa in the Bikont clade. The other major branch of eukaryotes, Unikonts, includes several distantly related groups, including algae and plants, Chromalveolates (e.g. ciliates, dinoflagellates), Rhizaria (e.g., radiolarians, foraminiferans), and Excavates (e.g., euglenids, diplomonads). Branching earlier, probably within Unikonts rather than Bikonts, are Ameobozoa, including such 9+2 amoeboflagellates as the Mastigamoebidae. Because organisms with 9+2 organelles are found in all eukaryotic phyla, these organelles must have been present in the last common ancestor of all extant eukaryotes, probably as a single posterior flagellum (Cavalier-Smith, 2002; Cavalier-Smith and Chao, 2003). All present day cilia and flagella, motile or not, clearly evolved from the 9+2 versions. The rarely encountered motile 14+0, 12+0, 9+0, 6+0, or 3+0 axonemes, and the widespread non-motile metazoan 9+0 axonemes, are all derived through loss from ancestral 9+2 organelles.
Two common features of the root eukaryote, mitochondria and a 9+2 flagellum, clearly provided that organism with a tremendous competitive advantage and led to rapid radiation into the ancestors of the major groups present today. Acquisition of a mitochondrion may have occurred before or after the evolution of a flagellum, but the combination was clearly advantageous enough to have out competed early organisms having only one or the other. While the endosymbiotic origin of mitochondria is easy to understand, evolution of 9+2 flagella is less obvious.
Dyneins and kinesins must surely have existed as microtubule motors for mitosis and other cytoplasmic movements before 9+2 organelles evolved (Cavalier-Smith, 1978; Cavalier-Smith, 1982). Initially, polarized assembly of microtubules from nucleus-associated organizing centers (centrosomes) provided a scaffold for mitosis during cell division and for directed vesicle motility, secretion, and membrane assembly during interphase. As the interphase array would emanate from a single centrosome, it would define an internal cell polarity radiating in one direction from the nucleus (Fig. 1A). Interphase microtubule interactions with the cell cortex, perhaps in concert with the actin cytoskeleton, could distend the membrane and create a polarized morphology. As a polarized extension with associated motors, this simple axoneme might have been important for gliding motility. Interaction of motors with transmembrane proteins, together with a clutch mechanism to engage and disengage the motors and a system for returning motors to the tip of the axoneme, could have evolved with little modification of pre-existing systems (Fig. 1B). Today, flagellar-dependent gliding is only well-documented as a form of motility in a few Bikont organisms such as Chlamydomonas (Bloodgood, 1981) and Peranema (Tamm, 1967; Saito et al., 2003) but ciliary surface motility has also been observed in Unikont metazoa, including sea urchin embryo cilia (Bloodgood, 1980) and mammalian primary cilia (Bowser and Leonard, 1992), and the underlying motility, intraflagellar transport (IFT), is widespread and essential for the assembly and maintenance of both motile and non-motile cilia and flagella (Pazour and Rosenbaum, 2002). Reassessment of the distribution of flagellar gliding motility, especially among the numerous soil amoeboflagellates, may show that it is more widespread than we know at present.
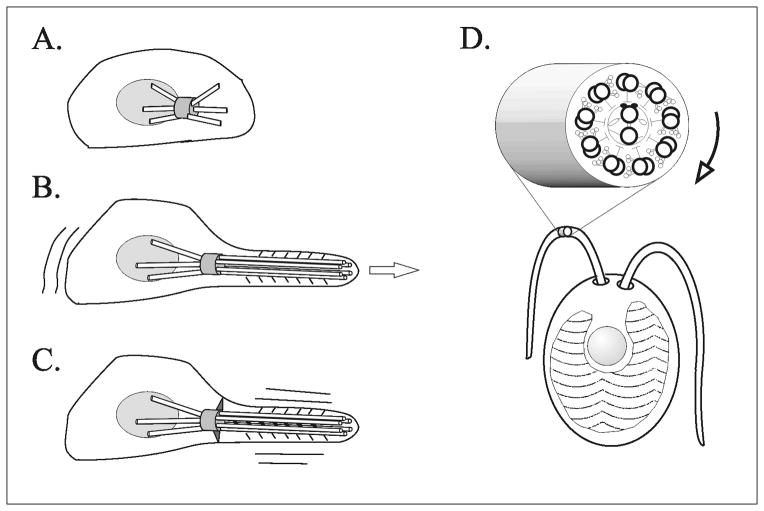
Figure 1.
Diagrams of proposed stages in evolution of 9+2 cilia and flagella. (A) A centrosome nucleates microtubules bidirectionally, toward and away from the nucleus. (B) Lengthening microtubules that radiate away from the nucleus generate a polarized cell. Addition of membrane-associated microtubule motors allows gliding motility. (C) Sliding motors between parallel microtubules leads to vibrating/undulating motility, and necessitates stabilization through additional links between the centrosome and the membrane, defining two membrane domains (cell body and flagellum). (D) Formation of doublets and addition of the radial spoke-central and pair apparatus allow complex regulation of undulating motility, including receptor-dependent tactic responses. Rotation of a twisted central pair promotes effective interaction of specific central pair projections with radial spokes to control dyneins. (A–C) modified from Cavalier-Smith (1982), (D) modified from Mitchell (2003b).
The ability to move in a specific direction becomes more advantageous if the direction benefits the organism. By coupling the regulation of directed motility to receptors that are sensitive to environmental cues, cells could develop tactic responses. Although taxis does not require localization of receptors to polarized domains like the axoneme membrane, such localization might have advantages in response time and directional sensitivity. Initially, the simple bundle of microtubules of this protocilium axoneme would not require extensive anchoring in the cytoplasm or clear delineation of cytoplasmic membrane from ciliary membrane, so the formation of an undulating axoneme may have preceded receptor localization. Undulating motility exerts transverse forces and requires stronger axoneme anchoring in the cytoplasm and to the cell membrane, and it is membrane anchors in particular that separate the flagellar membrane from the rest of the cell surface (Fig. 1C). Today it is clear that 9+2 organelles do have isolated membrane domains and that many receptors are specifically localized to the flagellar/ciliary membrane. These include chemosensory and touch receptors in single-celled organisms as well as the sensory receptors widespread in non-motile 9+0 cilia and ciliary derivatives of multicellular organisms. Among the latter are sensory neurons in C. elegans (Perkins et al., 1986) and insects (Dubruille et al., 2002), retinal rod and cone cells, olfactory cells, and taste buds in mammals, and a growing number of tissue-specific receptors localized to primary cilia found on most differentiated cell types in adult mammals (Pazour and Witman, 2003). That such receptors are evolutionarily ancient can be deduced from the presence of GABA, insulin, FGF and PDGF receptors, related to those of mammals (Unikont branch), on ciliary membranes of Tetrahymena and Paramecium (Bikont lineage) (Leick et al., 2001; Christensen et al., 2003; Ramoino et al., 2003). For two recent analyses of genes common to all organisms with cilia and flagella, including genes needed for motility, assembly, and receptor functions, and genes implicated in human diseases, see Li et al. (Li et al., 2004) and Avidor-Reiss et al. (Avidor-Reiss et al., 2004).
An ability to vibrate or bend its axoneme provided our early eukaryote with two advantages. First, such movement can create currents that draw nutrients toward the organism. Second, if the organism is suspended in liquid, undulation is a way to swim. Both uses of axonemes are widespread throughout protozoan and metazoan phyla. However, undulation of a bundle of parallel microtubules does not require any specific microtubule geometry, as motile organelles have evolved with spirals of singlet microtubules (Baccetti et al., 1982) as well as with doublets arranged in 3+0, 6+0, 9+0, 12+0, 14+0, or even hundreds (Baccetti, 1986; Omoto et al., 1999) rather than 9+2 arrays. What then led to the dominance of the 9+2 array? The relative order of establishment of 9-fold symmetry and doublet microtubule structure cannot be readily deduced, but we favor doublet formation as the earlier adaptation. Doublet microtubules presumably have superior mechanical properties for an undulatory organelle that must withstand continuous cycles of deformation and the exertion of transverse forces against the surrounding medium. They also provide anchorage for unidirectional orientation of motors, an essential property of motility based on parallel (rather than antiparallel) bundles of microtubules. This advantage is independent of the number or arrangement of doublet microtubules in the protocilium.
General constraints on axoneme diameter must include the ability to anchor such an organelle and should be related to the size of the microtubule organizing center that initially evolved to support the mitotic apparatus. Generation of useful propulsive force with existing motors and without substantial changes in microtubule bending resistance would also constrain the useful minimum diameter of a beating axoneme, although effective hydrodynamic diameter has since been modified upward in many phyla by the addition of mastigonemes to flagellar membranes. Let us assume that axonemes of 3 to 20 doublet microtubules evolved in a variety of configurations in an early eukaryotic radiation, and that the 9-doublet cylinder was fixed by development of an evolutionarily superior motility mechanism. We suggest that selective advantage came from superior regulation of motility to provide rapid tactic responses, and that this regulation was provided by an asymmetric central apparatus.
Regulation of motility by the central pair
Motility changes in response to external stimuli can take one of two forms. The stimulus can alter the frequency of random re-orientation, as occurs in bacteria, so that motility in a favorable direction is rewarded, or the stimulus can directly regulate motility so that the organism turns in a defined direction relative to the stimulus (toward the stimulus for positive taxis, away for negative taxis). Such tactic turning requires localized receptors that can function as an antenna, and a motility apparatus that can be directionally controlled (Foster and Smyth, 1980). A simple stop-reorient-start type of regulation does not require a sophisticated regulation of motility. In flagella, directional control requires coordinated changes in waveform and beat frequency and therefore a more sophisticated regulatory apparatus. The central pair-radial spoke system fulfills that role in 9+2 cilia and flagella, as demonstrated most elegantly in Chlamydomonas (Fig. 1D). This single-celled biflagellate alga responds to phototactic stimuli by altering the relative waveform, stroke velocity, and beat frequency of its two flagella (Witman, 1993). Available evidence indicates that phototaxis involves a signal transduction pathway from the central apparatus, through radial spokes, to a doublet-associated regulatory complex, which then modifies the pattern of dynein activity and hence of bend formation and propagation through changes in dynein-associated protein kinases and phosphatases (Porter and Sale, 2000; Smith and Yang, 2004).
While there might have been more than one possible way to form an asymmetric central apparatus, one built on a minimal scaffold of two microtubules may have been the first to evolve and would require a minimal change to the protocilium, primarily the addition of a new assembly initiation site in the transition zone together with a structure (radial spoke) for transmitting signals from the central apparatus to doublet-associated dyneins. We imagine that radial spokes evolved from dynein regulation in sheets of doublets, where they interacted at their bases with a doublet-associated dynein regulatory complex and at their tips with microtubule-associated proteins on another row of doublets or on a cortical singlet microtubule cytoskeleton. It seems remarkable, given the likelihood that flagellar dyneins evolved from cytoplasmic dyneins, that no common regulatory mechanisms are known for these two dynein families. However, this may reflect our current lack of knowledge rather than an absence of conserved mechanisms. Recent molecular analysis of a protein in the Chlamydomonas dynein regulatory complex revealed a primary structure that does have similarities to cytoplasmic proteins, but a relationship to cytoplasmic dynein has not been established (Rupp and Porter, 2003).
Figuring out specific patterns of interaction between projections from the central pair microtubules and radial spokes that elicit specific changes in dynein activity remains a great puzzle. Recent results from our lab, including the analysis of normal central pair structure, characterization of central pair assembly defective mutants, and determination of central pair orientation during bend propagation, constrain models of how the CP regulates dynein. Here we attempt to synthesize a hypothesis of central pair regulation consistent with these results.
Previous thin section electron microscopy of Chlamydomonas and Tetrahymena axonemes, together with negative stain preparations of central pair complexes from Tetrahymena cilia (Chasey, 1969) and rat sperm flagella (Olson and Linck, 1977) revealed the asymmetric structure of central pair complexes and defined the CP microtubule with longer projections in cross section and 32 nm repeat periodicities as C1, whereas the other (C2) has shorter projections with only 16 nm repeats. By comparing transverse and longitudinal thin sections of wild type central pair complexes with central pair complexes from assembly mutants pf6 and cpc1, I determined the structural relationships and repeat periods for most of the C1 and C2-associated projections in Chlamydomonas. Surface views provided by stereo images of quick-frozen, deep etch preparations confirmed and expanded those conclusions and resulted in a fairly complete 3-d reconstruction of the central pair (Mitchell, 2003a). These studies provide a model of potential spoke interaction sites on the central pair, and in particular show discontinuities in the otherwise cylindrical surface of the CP along the microtubule surfaces that face toward adjacent spoke heads. Also emphasized is the overall asymmetry of the CP complex, which suggests unique spoke interactions at different radial positions around the CP cylinder. Cloning of the pf6 (Rupp et al., 2001) and cpc1 (Mitchell and Sale, 1999; Zhang and Mitchell, 2004) genes and identification of their gene products did not identify obvious candidates for proteins that interact with radial spokes, but a kinesin-like protein (Klp1) on the C2 microtubule (Bernstein et al., 1994) is an attractive candidate for a spoke binding protein. We have recently shown that flagella in Klp1 knockdown cells beat with dramatically reduced frequencies (Mitchell and Yokoyama, 2003).
EM studies show that the CP maintains a fixed orientation with respect to the cell body and outer doublets in some organisms, while in others the CP has a variable orientation. Phylogenetically, a fixed orientation appears to be a derived simplification in organelles that have a fixed bend plane, such as ctenophore comb plate cilia (Tamm and Tamm, 1981) and many metazoan spermatozoa (Sale, 1986). In extreme examples, the C1 and C2 microtubules are attached to doublets 8 and 3, respectively, by permanent links (either modified spokes, or accessory structures). At the opposite extreme are cilia and flagella of unicellular organisms that rely on rapid changes in waveform, beat frequency, and effective stroke orientation to respond to environmental cues. The CP in these organelles are twisted, so that they do not maintain a fixed orientation from base to tip within the surrounding 9 doublets. In addition, these twisted CP rotate during bend propagation (Omoto et al., 1999). We have recently shown that the Chlamydomonas CP is oriented parallel to the bend plane within each bend (Fig. 1D), and twists by 180° between successive principal and reverse bends, and that the C1 microtubule is always closest to doublets on the outer side of each bend (Mitchell, 2003b). This constant orientation of the CP relative to a bend in Chlamydomonas allows one set of CP projections to interact with radial spokes attached to doublets with active dyneins, while another set of CP projections interacts with radial spokes on doublets with inactive dyneins.
Although the beat envelope is nearly planar in Chlamydomonas, and the direction of principal bends does not change dramatically away from a constant plane, this is not true in other organisms. If CP orientation also follows bend orientation in these other organisms, as I propose, then the CP is always positioned to provide flexible motility control. Our recent results indicate that CP orientation passively conforms to bends as they form at the base of a Chlamydomonas flagellum, and then this bend-dependent orientation translocates as each bend propagates from base to tip. An analogy from engineering is a worm gear, in which worm (central pair) rotation is keyed to the perpendicular motion (bend propagation) of interdigitating cogs of a toothed gear (axonemal bends). The direction of a principal bend therefore cannot be determined by central pair orientation, which passively conforms to bends, but must be regulated at the outer doublet level, through initiation patterns at the flagellar base. Regulatory signals from the CP may then determine shape and beat frequency through direct modulation of dynein activity patterns within developing and propagating bends. This hypothesis is consistent with results from vibrational re-orientation of the beat plane of sea urchin axonemes (Shingyoji et al., 1991; Takahashi et al., 1991). If the new bend plane induced by vibration forces a new orientation of the central pair, then relaxation of the system after removal of the imposed vibration from these cells would require a gradual rotation of the central pair back to its resting position. Unfortunately, information on actual CP orientation was not obtained in those experiments. This hypothesis is also consistent with the pattern of doublet sliding observed in protease-treated Chlamydomonas axonemes, in which doublet sliding patterns maintain a constant relationship to central pair orientation (Wargo and Smith, 2003; Wargo et al., 2004), and with studies of dynein activity in disrupted Chlamydomonas (Smith, 2002) and sea urchin (Yoshimura and Shingyoji, 1999; Nakano et al., 2003) axonemes, which show calcium- and central pair-dependent activity modulation.
Of what predictive value are these speculations about the evolution of cilia and flagella? First, we hypothesize that development of flagellar surface motility is primitive and likely to be encountered broadly, even in ciliary derivatives than do not beat. Clearly IFT is an essential and universal motility for assembly of both motile and non-motile organelles, so the linkage of this machinery to extracellular movement may be equally widespread. Second, the sequestering of receptors on ciliary membranes is also primitive, and likely to be a major selective pressure for continued existence of non-motile primary cilia, as well as more highly modified cilia of sensory organs. As ciliary derivatives provide essential functions in sensory neurons of many organisms, only a small leap of the imagination is needed to suggest that the protocilium also formed the ancestral platform for all sensory processes, and that additional features of this organelle may be common in sensory transduction cascades. Third, orientation of the centrosome as an indicator of cell polarity and of the direction of migration for motile cells is also very primitive. If so, the importance of the protocilium as an early determinant of cell polarity and directed migration suggests that more links should be sought between mechanisms that determine cell polarity and mechanisms that orient centrosomes/centrioles along with the cytoplasmic microtubule array. Finally, regulation of cilia and flagella by the central pair must also have developed very early in eukaryotic evolution, prior to the radiation of all extant eukaryotic phyla. Although differences undoubtedly exist in the detailed regulation required by these organelles in different organisms and cell types, we should expect to find many universal traits in the way that central pair-radial spoke interactions regulate dynein activity, and may yet find common themes in the regulation of axonemal and cytoplasmic dynein motors.
References
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- Baccetti B. Evolutionary trends in sperm structure. Comp Biochem Physiol A. 1986;85:29–36. doi: 10.1016/0300-9629(86)90457-3. [DOI] [PubMed] [Google Scholar]
- Baccetti B, Burrini AG, Dallai R, Pallini V. A motile system of singlet microtubules in spermatozoa. Cell Motility. 1982;2:93–101. [Google Scholar]
- Bernstein M, Beech PL, Katz SG, Rosenbaum JL. A new kinesin-like protein (Klp1) localized to a single microtubule of the Chlamydomonas flagellum. J Cell Biol. 1994;125:1313–1326. doi: 10.1083/jcb.125.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood RA. Direct visualization of of dynamic membrane events in cilia. J Exp Zool. 1980;213:293–295. [Google Scholar]
- Bloodgood RA. Flagella-dependent gliding motility in Chlamydomonas. Protoplasma. 1981;106:192. [Google Scholar]
- Bowser SS, Leonard ML. Properties of primary (9+0) cilia in cultured kidney epithelial cells (Abstract) Anatomical Record. 1992;232:14a. [Google Scholar]
- Cavalier-Smith T. The evolutionary origin and phylogeny of microtubules, mitotic spindles, and eurkaryote flagella. Biosystems. 1978;10:93–114. doi: 10.1016/0303-2647(78)90033-3. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The evolutionary origin and phylogeny of eukaryote flagella. Symp Soc Exp Biol. 1982;35:465–493. [PubMed] [Google Scholar]
- Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int J Syst Evol Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T, Chao EE. Phylogeny of choanozoa, apusozoa, and other protozoa and early eukaryote megaevolution. J Mol Evol. 2003;56:540–563. doi: 10.1007/s00239-002-2424-z. [DOI] [PubMed] [Google Scholar]
- Chasey D. Observations on the central pair of microtubules from the cilia of Tetrahymena pyriformis. J Cell Science. 1969;5:453–458. doi: 10.1242/jcs.5.2.453. [DOI] [PubMed] [Google Scholar]
- Christensen ST, Guerra CF, Awan A, Wheatley DN, Satir P. Insulin receptor-like proteins in Tetrahymena thermophila ciliary membranes. Curr Biol. 2003;13:R50–R52. doi: 10.1016/s0960-9822(02)01425-2. [DOI] [PubMed] [Google Scholar]
- Dubruille R, Laurencon A, Vandaele C, Shishido E, Coulon-Bublex M, Swoboda P, Couble P, Kernan M, Durand B. Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation. Development. 2002;129:5487–5498. doi: 10.1242/dev.00148. [DOI] [PubMed] [Google Scholar]
- Foster KW, Smyth RD. Light antennas in phototactic algae. Microbiol Reviews. 1980;44:572–630. doi: 10.1128/mr.44.4.572-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leick V, Bog-Hansen TC, Juhl HA. Insulin/FGF-binding ciliary membrane glycoprotein from Tetrahymena. J Membr Biol. 2001;181:47–53. doi: 10.1007/s0023200100064. [DOI] [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil. Cytoskeleton. 2003b;56:120–129. doi: 10.1002/cm.10142. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. Reconstruction of the projection periodicity and surface architecture of the flagellar central pair complex. Cell Motil Cytoskeleton. 2003a;55:188–199. doi: 10.1002/cm.10121. [DOI] [PubMed] [Google Scholar]
- Mitchell DR, Sale WS. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol. 1999;144:293–304. doi: 10.1083/jcb.144.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Yokoyama R. Structural analysis of central pair function in Chlamydomonas flagella (Abstract). Mol. Biol. Cell. 2003;14:436a. [Google Scholar]
- Nakano I, Kobayashi T, Yoshimura M, Shingyoji C. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonernes. J Cell Sci. 2003;116:1627–1636. doi: 10.1242/jcs.00336. [DOI] [PubMed] [Google Scholar]
- Olson GE, Linck RW. Observations of the structural components of flagellar axonemes and central pair microtubules from rat sperm. J Ultrastructure Res. 1977;61:21–43. doi: 10.1016/s0022-5320(77)90004-1. [DOI] [PubMed] [Google Scholar]
- Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, Witman GB. Rotation of the central pair microtubules in eukaryotic flagella. Mol Biol Cell. 1999;10:1–4. doi: 10.1091/mbc.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. TICB. 2002;12:551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9+2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–F42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoino P, Fronte P, Beltrame F, Diaspro A, Fato M, Raiteri L, Stigliani S, Usai C. Swimming behavior regulation by GABAB receptors in Paramecium. Exp Cell Res. 2003;291:398–405. doi: 10.1016/j.yexcr.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Rupp G, O’Toole E, Porter ME. The Chlamydomonas PF6 locus encodes a large alanine/proline-rich polypeptide that is required for assembly of a central pair projection and regulates flagellar motility. Mol. Biol. Cell. 2001;12:739–751. doi: 10.1091/mbc.12.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp G, Porter ME. A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J Cell Biol. 2003;162:47–57. doi: 10.1083/jcb.200303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Suetomo Y, Arikawa M, Omura G, Khan SM, Kakuta S, Suzaki E, Kataoka K, Suzaki T. Gliding movement in Peranema trichophorum is powered by flagellar surface motility. Cell Motil Cytoskeleton. 2003;55:244–253. doi: 10.1002/cm.10127. [DOI] [PubMed] [Google Scholar]
- Sale WS. The axonemal axis and calcium-induced asymmetry of active microtubule sliding in sea urchin sperm tails. J Cell Biol. 1986;102:2042–2052. doi: 10.1083/jcb.102.6.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingyoji C, Katada J, Takahashi K, Gibbons IR. Rotating the plane of imposed vibration can rotate the plane of flagellar beating in sea-urchin sperm without twisting the axoneme. J Cell Sci. 1991;98:175–181. doi: 10.1242/jcs.98.2.175. [DOI] [PubMed] [Google Scholar]
- Smith EF. Regulation of flagellar dynein by the axonemal central apparatus. Cell Motil Cytoskeleton. 2002;52:33–42. doi: 10.1002/cm.10031. [DOI] [PubMed] [Google Scholar]
- Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskeleton. 2004;57:8–17. doi: 10.1002/cm.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Shingyoji C, Katada J, Eshel D, Gibbons IR. Polarity in spontaneous unwinding after prior rotation of the flagellar beat plane in sea-urchin spermatozoa. J Cell Sci. 1991;98:183–189. doi: 10.1242/jcs.98.2.183. [DOI] [PubMed] [Google Scholar]
- Tamm SL. Flagellar development in the protozoan Peranema trichophorum (Abstract) J Exp Zool. 1967;164:163–86. [Google Scholar]
- Tamm SL, Tamm S. Ciliary reversal without rotation of axonemal structures in ctenophore comb plates. J Cell Biol. 1981;89:495–509. doi: 10.1083/jcb.89.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo MJ, McPeek MA, Smith EF. Analysis of microtubule sliding patterns in Chlamydomonas flagellar axonemes reveals dynein activity on specific doublet microtubules. J Cell Sci. 2004;117:2533–2544. doi: 10.1242/jcs.01082. [DOI] [PubMed] [Google Scholar]
- Wargo MJ, Smith EF. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc. Natl. Acad. Sci. USA. 2003;100:137–142. doi: 10.1073/pnas.0135800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB. Chlamydomonas phototaxis. Trends Cell Biol. 1993;3:403–408. doi: 10.1016/0962-8924(93)90091-e. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Shingyoji C. Effects of the central pair apparatus on microtubule sliding velocity in sea urchin sperm flagella. Cell Struct Funct. 1999;24:43–54. doi: 10.1247/csf.24.43. [DOI] [PubMed] [Google Scholar]
- Zhang H, Mitchell DR. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci. 2004 doi: 10.1242/jcs.01297. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]