Abstract
Maternal mood disorders such as depression and chronic anxiety can negatively affect the lives of not only mothers, but also of partners, offspring, and future generations. Chronic exposure to psychosocial stress is common in postpartum mothers, and one of the strongest predictors of postpartum depression is social conflict. The objective of the current study was to evaluate the effects of chronic social stress (CSS) during lactation on the maternal behavior (which consists of maternal care and aggression toward a novel conspecific) of lactating rats, as well as on the growth of the dams and their offspring. It was hypothesized that chronic daily exposure to a novel male intruder would alter the display of maternal behavior and impair growth in both the dam and offspring during lactation due to the potentially disruptive effects on maternal behavior and/or lactation. The data indicate that CSS during lactation attenuates maternal care and the growth of both dams and pups, and increases self-grooming and maternal aggression toward a novel male intruder. These results support the use of CSS as a relevant model for disorders that impair maternal behavior and attenuate growth of the offspring, such as postpartum depression and anxiety.
Keywords: Anxiety, depression, maternal aggression, maternal care, offspring development, social behavior
Introduction
Maternal mood disorders such as depression and chronic anxiety can negatively affect the lives not only of mothers, but also of partners, offspring, and future generations. Although the overall prevalence of postpartum depression has been reported as 12–15% (O’Hara and Swain 1996; Segre et al. 2007), this may be a conservative estimate due to the negative stigma associated with postpartum depression, and variation due to socioeconomic and cultural differences (Halbreich and Karkun 2006; Benoit et al. 2007). Reviews of the effects of postpartum depression on child behavior indicate that the offspring of depressed mothers suffer from increases in crying intensity, distractibility, and antisocial behavior, and may have impaired cognitive and emotional development (Beck 1998; Grace et al. 2003). Mood disorders during lactation have also been associated with impaired child growth and development (Patel et al. 2003; Surkan et al. 2008). These effects are often mediated through maternal behavior (Goodman 2007). Despite the negative effects of mood disorders on families, most studies of depression only include male subjects, and few include maternal females.
A current focus of depression research is the role of chronic stress in the development of these disorders. Chronic exposure to psychosocial stress is common in humans, especially in mothers, and one of the strongest predictors of depression in mothers is social conflict (Westdahl et al. 2007). Several clinical studies have linked pre- or postpartum depression with exposure to various stressors during pregnancy or the postpartum period (Paykel et al. 1980; O’Hara et al. 1983; O’Hara 1986; Seguin et al. 1995; Beck 1996, 2001; O’Hara and Swain 1996; Robertson et al. 2004; Westdahl et al. 2007; Klier et al. 2008). Furthermore, postpartum mood disorders have been correlated with high levels of social conflict (within the family and/or workplace) in combination with low levels of social support (O’Hara et al. 1983; Seguin et al. 1995). The use of animal models of stress-induced depression and anxiety allows researchers to study this relationship in greater detail and provides a medium for the testing of novel treatments. To date, postpartum depression has not been convincingly modeled in rodents, and here we focus on testing whether maternal behavior is adversely affected by repeated social conflict in ways that can accompany postpartum depression.
Although there are several animal models of altered maternal behavior (which consists of maternal care and aggression toward a novel conspecific) using chronic stress, most have substantial physiological components which may not pertain to chronic psychosocial stressors commonly experienced by humans. Chronic restraint stress during pregnancy in rats increases maternal care and anxiety and decreases maternal aggression in some studies (Maestripieri et al. 1991), but decreases maternal care in others (Smith et al. 2004). Restraint stress is a potent physical stressor (King et al. 2005), and some reports are inconsistent with the attenuated maternal care observed in human mothers with postpartum depression (Goodman 2007). Prenatal ultramild stress does not affect pup care, but does reduce maternal aggression (Pardon et al. 2000). Ultramild stress paradigms use a variety of randomized stressors (such as 30 degree cage tilt, reversed light cycle, confinement, overnight illumination, and soiled cage) over several days, and the importance of individual components is unclear. It is likely that reversed light cycle and overnight illumination directly affect maternal care independent of any stress effects, as maternal care in rats has a circadian rhythm (Stern and Levin 1976). Exposure to wet bedding during lactation was reported to decrease nursing frequency during the dark period, but it is possible that there was no difference in the duration of nursing between the control and wet bedding groups if the stressed females had longer nursing bouts (Léonhardt et al. 2007). Furthermore, this study reported increases in the frequencies of nursing and pup grooming in the light period during exposure to wet bedding. Exposure to wet bedding is a significant thermal stress to the pups, resulting in pup mortality in less than 24 h in 1–7-day-old litters (personal observations). In an effort to reduce the physiological component of the stressor used and generate consistent behavioral and physiological results, the current study focused on the use of social stress.
While chronic social defeat is effectively used as a model for social stress-induced depression in male rodents (Kudryavtseva and Avgustinovich 1998), this is not an ecologically relevant behavioral model for many maternal rodents if male intruders are used. A single social defeat of a maternal rodent usually results in cannibalism of the litter by the male intruder. Acute exposure to a novel male intruder elicits robust behavioral and endocrine stress responses in lactating females (Neumann et al. 2001; Douglas et al. 2007; Nephew and Bridges 2008; Nephew et al. 2009). There is also evidence that chronic stress during lactation, including exposure to a male intruder, directly impairs milk production (Lau 2001; Lau and Simpson 2004), which may impact pup growth. These results in maternal rats are supported by studies in virgin females, where chronic social instability (varying isolation and crowding) increases hypothalamic–pituitary–adrenal axis activity (Baranyi et al. 2005; Herzog et al. 2009), alters body temperature regulation, reduces sucrose and food intake, and has been proposed as an animal model for female depression (Herzog et al. 2009).
The objective of this investigation was to evaluate the effects of chronic social stress (CSS) during lactation on the maternal behavior of lactating rats, as well as on the growth of the dams and their offspring. It was hypothesized that chronic daily exposure to a novel male intruder would reduce the display of maternal behavior, specifically resulting in decreased pup grooming and nursing and increased aggression due to social instigation (Potegal 1992; de Almeida and Miczek 2002). Self-grooming, which has been associated with anxiety (Lazosky and Britton 1991; Spruijt et al. 1992), was expected to increase in response to CSS (Kalueff et al. 2007; Denmark et al. 2010). It was further hypothesized that the exposure to CSS would impair growth in both the dam and offspring during lactation due to the potentially disruptive effects on maternal behavior and/or lactation.
Methods
Animals
Animals in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee.
Twenty female Sprague Dawley (Crl:CD[SD]BR) rats (175–200 g) were obtained from Charles River Laboratories (Wilmington, MA, USA) and maintained in temperature (21–25°C) and light (14h:10h light: dark cycle; lights on at 05:00 h) controlled rooms. Food and water were available ad libitum throughout the studies. Females were mated by placing two females with one male; the presence of a sperm plug indicated successful mating, and the females were then housed communally (three per cage). One day prior to the day of parturition (lactation day 1), each female was housed singly. Maternal behavior was observed in all rats on the day of birth (retrieval of all pups, cleaning and grooming of pups, and nursing), and dams were then randomly assigned to the control or CSS group. The numbers of pups and weights of the litters were recorded, and litters were culled to eight pups (four males/four females) on day 1. Pups were sexed using anogenital distance. All group sizes for the control and CSS dams were 10, with the exception of the day 16 behavior and growth data. One CSS dam’s pups were attacked by an intruder male following the aggression testing on day 9, so the numbers of dams for the CSS groups on days 9 and 16 for maternal care and aggression were 9. To assess the growth of the female offspring when they were 70 days old, three females were randomly selected from each of eight litters on lactation day 21 (weaning) and subsequently weighed on day 70 (n = 24 for control and CSS groups).
The male intruders consisted of a group of 20 Sprague Dawley males (240–285 g at the start of the study). These males were rotated among the resident females so that each female was always presented with a novel male. Each male was placed in the cage of a resident female every third day on average.
Behavioral testing
Maternal care and maternal aggression were assessed on days 2, 9, and 16 of lactation between 09:00 and 12:00 h in all dams. A digital video camera (Panasonic PV-GS180) allowed for behavioral observation without human interference. Maternal care testing consisted of the re-introduction of all eight pups to the home cage after a 30-minute removal, and behavior was then video recorded for 30 min. The latencies to retrieve pups back to the next and initiate nursing, and frequencies and durations of pup retrieval, pup grooming, nursing, nesting, self-grooming, and general locomotor activity were scored by an observer who was blind to the treatment using the ODLog behavioral analysis software (Macropod Inc., USA). The ODLog software records continuous data in 5 s bins, and also generates frequency and duration summaries for all behavioral measures over the 30-minute observation period. Frequency data represent the total number of bouts for a specific behavior during the observation period. Details of the maternal care variables are in Supplementary Table I (online version only). Following the maternal care testing, the pups were left in the cage, and a novel male intruder was introduced for 30 min and maternal aggression was video recorded. The latency to initiate aggression and the frequency and durations of attacking (boxing or tackling), biting, kicking, pinning, self-grooming, locomotor activity, and maternal care (with the exception of retrieval) were scored. The mean duration of an aggressive bout was also calculated by dividing the total aggression time by the total number of aggressive interactions. Details of the maternal aggression variables are given in Supplementary Table II (online version only).
CSS protocol and growth
The CSS group had a novel male placed in their home cage for 1 h each day from days 2–16 of lactation (randomized introductions between 09:00 and 15:00 h), whereas the control group was exposed to the intruder for 30 min only on behavioral testing days 2, 9, and 16 of lactation. Following the introduction of the novel male intruder, the resident dam investigated the intruder and then attacked. Initial confrontations usually consisted of boxing or tackling, and aggression was always initiated by the dam. Intruders ended up either on their backs, or crouching in a corner away from the nest, evidently trying to defend themselves from the dam. After the initial attack, or series of attacks, the female returned to the nest area and observed the male or cared for the pups. During the remainder of the aggression test or one-hour exposure, the female periodically attacked the intruder whether or not he continued to approach the nest area. As in previous studies of maternal aggression, the use of smaller males ensured consistently submissive behavior from the males throughout the study. Although the pups were left unprotected during the aggression tests and CSS protocol, interaction between the intruder male and pups was rare due to the aggressive responses of the female. The one-hour exposure for the CSS dams on lactation days 2, 9, and 16 included the 30-minute aggression test, after which the males were left in the maternal female’s cage for an additional 30 min.
Body weights of the dams and pups were recorded on the day of birth and each behavioral testing day to assess if the chronic maternal exposure to an intruder had an overall effect on dam or offspring growth. The weights for 24 control and 24 CSS female offspring were also assessed on day 70. The rationale for not including an additional group that was not exposed to an intruder was that the response to an intruder on days 2, 9, and 16 of lactation would have necessitated individual groups of rats and introduced a novelty confound into the group comparisons of maternal aggression. In addition, this no-intruder group would not have been stress free, as cage change is a significant stressor to female rodents (Sharp et al. 2003).
Statistics and data presentation
Initial statistical testing for the behavioral data consisted of a two-way repeated-measures ANOVA with lactation days (2, 9, and 16) as the repeated factor. There were no overall effects of the CSS treatment on any behavioral variables, but there were several variables significantly affected by lactation day. The behaviors of the control and stressed groups were further compared on each lactation day with one-tailed t-tests to assess the effects of the CSS on individual lactation days. Growth data on days 2, 9, and 16 of lactation, as well as when the offspring were 70 days old, were compared with one-tailed t-tests on weight (in grams) on day 2 or the percentage growth relative to day 2 (day 9 or 16 weight/day 2 weight × 100). As previous studies have already established that maternal care and aggression change over the course of lactation and the focus of the current study was on the effects of the chronic stress on maternal behavior, the effect of lactation day (time) was not a primary focus of the current report. All graphical results are presented as group means + SEM, and the level of statistical significance was p ≤ 0.05.
Results
Maternal care
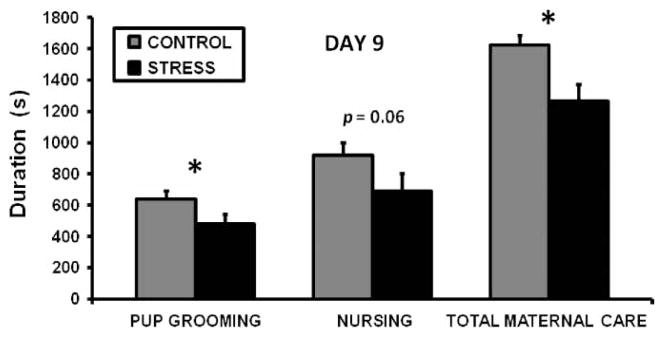
There were no differences between the control and CSS groups in any measure of maternal care at the start of the study on lactation day 2 (details in Supplementary Table I (online version only)). As the groups were treated identically up to this point, this was expected. On day 9 of lactation, the latency to initiate nursing was longer in the CSS dams (493 ± 82.4 vs. 299 ± 47.4 s, t(18) = 2.1, p < 0.05), and the durations of pup grooming (t(18) = 1.9, p < 0.05) and total maternal care (t(18) = 2.3, p < 0.05) were lower in the CSS dams (Figure 1). Locomotor activity duration was elevated in the CSS group on day 9 during the attenuated display of maternal care (243 ± 36.0 vs. 134 ± 12.1 s, t(18) = 2.9, p < 0.01). On day 16 of lactation, the expression of maternal care was similar in both groups.
Figure 1.
Mean + SEM duration values for maternal care on day 9 of lactation during a 30-minute observation for control and CSS (STRESS) dams (n = 10 per group). *Indicates a significant difference between treatments (t-test p ≤ 0.05).
Maternal aggression
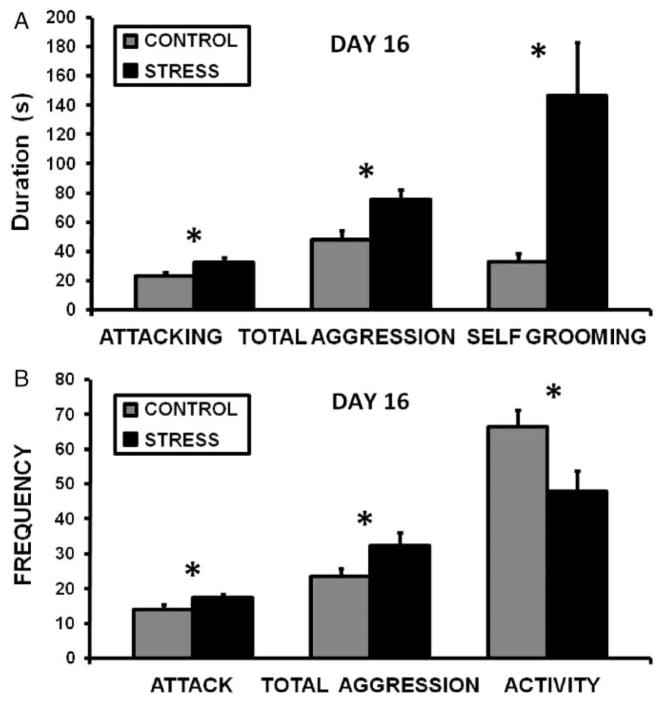
There were no differences between the control and CSS groups in any measure of maternal aggression at the start of the study on lactation day 2 (details in Supplementary Table II (online version only)). As the groups were treated identically up to this point, this was expected. There was a significant effect of time on total aggression due to the presence of high levels of aggression on days 2 and 9 and a decrease on day 16 (RM ANOVA F2,56 = 7.1, p < 0.01). On day 9, the CSS dams had shorter aggression latencies (17.8 ± 3.0 vs. 30.5 ± 4.5 s, t(18) = 2.3, p < 0.05) and longer average aggressive bouts (3.2 ± 0.4 vs. 2.3 ± 0.2 s, t(18) = 2.0, p < 0.05). On day 16, attack (t(17) = 2.3, p < 0.05), total aggression (t(17) = 3.1, p < 0.01), and self-grooming (t(17) = 3.3, p < 0.01) durations were elevated in the CSS group (Figure 2A). The frequencies of both attacks (t(17) = 1.8, p < 0.05), and total aggression bouts (t(17) = 2.1, p < 0.05), were significantly increased by the CSS treatment (Figure 2B). As on day 9, average aggressive bout length was elevated in CSS dams on day 16 (2.4 ± 0.2 vs. 2.0 ± 0.1, t(17) = 1.8, p < 0.05). Although the number of activity bouts was lower in the CSS dams on day 16 (t(17) = 2.5, p < 0.05, Figure 2B), there was no effect of CSS on the duration of locomotor activity.
Figure 2.
A–B Mean + SEM duration (A) and frequency (B) values for maternal aggression on day 16 of lactation during a 30-minute observation for control (n = 10) and CSS (STRESS, n = 9). *Indicates a significant difference between treatments (t-test p ≤ 0.05).
Growth
There were no differences between the groups in initial litter size (control = 15.8 ± 1.2, CSS = 13.2 ± 1.9), dam weight on day 2 (control = 314 ± 9.3 g, CSS = 309 ± 6.1 g), or mean pup weight on day 2 (control = 6.7 ± 0.3 g, CSS = 7.0 ± 0.3 g). The CSS dams had a significantly attenuated increase in weight by day 9 (t(18) = 3.4, p < 0.01), but this difference was not significant on day 16 (t(17) = 1.7, p = 0.06, Figure 3A). Growth of the offspring of dams chronically exposed to intruder males was attenuated on both day 9 (t(18) = 2.1, p < 0.05) and day 16 (t(17) = 1.8, p < 0.05) of lactation (Figure 3B). The final assessment of female offspring body weight at 70 days of age revealed that the control offspring were 25 grams heavier on average than the adult CSS offspring (293 ± 6.3 vs. 268 ± 4.3 g, n = 24 per group, t(46) = 3.3, p < 0.01).
Figure 3.
A–B Mean + SEM of percentage body weight gain for control and CSS (STRESS) dams (A) and pups (B) on days 9 and 16 of lactation (n = 9–10 per group). *Indicates a significant difference between treatments (t-test p ≤ 0.05).
Discussion
The present study found that CSS during lactation attenuates maternal care and the growth of both dams and pups, and increases anxiety-related behavior and the expression of maternal aggression toward a novel male intruder. Although behavioral tests of depression-like behavior commonly used in males were not administered, maternal care, a robust reward-mediated behavior (Lee et al. 1999; Mattson et al. 2001; Ferris et al. 2005), was decreased in response to CSS. It is postulated that these effects on maternal care are indicative of anhedonia, similar to findings with the use of the sucrose/saccharin preference test (Katz 1982; Willner et al. 1992). The current results support the use of the CSS paradigm as an effective model to study chronic stress-induced disorders that affect maternal care and infant growth, such as postpartum depression and anxiety, although the paradigm has not yet been shown to induce a depressed state by other measures. Compared to other chronic stress protocols which include stressors with substantial physiological components, social stress is more ethologically and clinically relevant as a model for human disorders during lactation, as human mothers are more likely to suffer from exposure to chronic social stressors than physical stressors (Westdahl et al. 2007).
Exposure to the CSS protocol depressed maternal care during mid-lactation, as care was similar in the control and stress groups late in lactation, on day 16. The overall decrease in total maternal care on day 9 was the result of the increased latency to initiate nursing and the combined decreases in nursing and pup grooming durations. In addition to these specific effects on offspring care, general locomotor activity was increased in CSS dams on day 9, indicating that the stressed dams spent more time exploring the cage and less time caring for their offspring. One potential endocrine mediator for these effects is corticosterone as there are reports of elevated HPA activity in female rodents exposed to social instability (Baranyi et al. 2005; Herzog et al. 2009). However, the exact role of corticosterone in maternal care is unclear, as exogenous administration of corticosterone increases maternal care in primiparous rats (Rees et al. 2004), but inhibits maternal care in sensitized virgin rats (Rees et al. 2006). To summarize the differences in maternal care between control and CSS dams, the CSS mothers were slower to initiate nursing, spent less time exhibiting grooming and nursing, and spent more time away from the nest. Faced with an environment of frequent threats, maternal rats invest less time in direct offspring care.
The lack of an effect of CSS on maternal care at day 16 may be due to a compensatory increase in nursing and grooming in the affected dams, possibly stimulated by increased pup vocalizations (Wellmann et al. 2010). Another potential explanation is that the maternal behavior testing paradigm was not sensitive enough to detect behavioral differences that would have been apparent through long-term daily observations of undisturbed dams. It is also possible that the lack of significant group differences in maternal care during late lactation was due to the absence of a control group that was not exposed at all to a male intruder. However, the significantly depressed growth in the pups on day 16 suggests the presence of either decreased maternal care throughout lactation, or diminished milk production and intake. Additional maternal care observations throughout lactation (including the dark period) will provide a more detailed picture of the effects of CSS on offspring care, and weighing the pups before and after maternal care testing could assess milk consumption by the pups.
Similar to the effects of social instigation on male and female aggression (Lagerspetz and Hautojärvi 1967; Potegal and TenBrink 1984; Fish et al. 1999), the repeated presentation of a male intruder increased aggression in the CSS group. Although some indices of aggression were enhanced on day 9, the most substantial effects of CSS on maternal aggression were on day 16. It is postulated that the lack of major behavioral effects on day 9 was due to a ceiling effect. Prior studies have established that rodent maternal aggression levels are high during early and/or mid-lactation, and decrease as the day of weaning approaches (Erskine et al. 1978; Mayer and Rosenblatt 1987; Nephew et al. 2009, 2010). In the current study, maternal aggression levels were high on lactation days 2 and 9, and it is possible that there was little potential for elevating aggression during this period. The relative increase in aggression toward a novel male intruder on day 16 in CSS dams was robust, with the strongest specific effects on the duration and frequency of attacking. As aggression levels were high on days 2 and 9 relative to day 16, the effect of CSS was to sustain high levels of aggression during late lactation, compared to the control dams that exhibited a more pronounced decrease in maternal aggression as weaning approached. The present data support the hypothesis that CSS dams became sensitized to the intruder presentations, as seen in response to social instigation in male and female rodents (Potegal and TenBrink 1984; Potegal 1992; de Almeida and Miczek 2002). This sensitization may be mediated by the HPA axis, as there is evidence that elevated HPA axis activity increases aggression during crowding stress (Baranyi et al. 2005). Another potentially related explanation for the increased aggression in the CSS dams is the involvement of social learning, although the current study did not assess learning.
The most substantial and significant effect of CSS on behavior during the 30-minute aggression test was on self-grooming, which was increased 400% in the CSS dams compared to the control rats. It has been suggested that grooming is an ideal behavior for studying the development of depression and anxiety disorders and assessing potential treatments for these disorders (Kalueff et al. 2007). Self-grooming has been characterized as an anxiety-related behavior in a variety of species (Spruijt et al. 1992), and in a study on the effects of chronic social defeat in mice, subordinate mice had increased anxiety and a disorganized pattern of grooming (Denmark et al. 2010). Although the current investigation did not use social defeat as the CSS, socially defeated male mice increase grooming activity during the confrontations (Bondar et al. 2009), similar to the increased grooming during aggression testing in the dams of the current study. Furthermore, chronic social contact stress increases grooming frequency in non-aggressive male mice (Veenema et al. 2003). Exposures to stress increase the central CRF activity to enhance self-grooming through a serotonin-mediated pathway (Howard et al. 2008), and it has been hypothesized that the effects of CRF on serotonin activity are involved in the development of affective and anxiety disorders (Kagamiishi et al. 2003). The increase in grooming by the stressed maternal females may be indicative of elevated anxiety in these rats, which is consistent with the possibility that the CSS paradigm is a putative model for postpartum mood disorders. In addition to these behavioral effects of CSS, growth in both the dams and their offspring was attenuated.
The effect of CSS on dam growth during lactation was consistent and robust. The observed decrease in weight gain in the dams is similar to the effects of CSS on male body weight (Blanchard and Blanchard 1989). The lack of a significant difference in growth on day 16 suggests that the stressed dams improved during the second week of the CSS protocol, but this lack of effect may have been due to increased variability in growth. Two possible mechanisms for this attenuated growth include suppressed feeding due to the intruder male presentations, or an increase in the metabolic rate of the stressed dams. A previous study reported significant increases in body temperature following acute aggressive interactions (Nephew and Bridges 2008), which may indicate an increased metabolic rate. As the males were only in the cage for 1 h each day, effects on feeding behavior and/or metabolic rate would most likely have to persist beyond the period of the CSS.
Growth of the pups was significantly attenuated on days 9 and 16 of lactation, with the greatest effect on day 16. This detrimental effect on growth may be mediated by decreased grooming of the pups and/or milk consumption. Prenatal stress attenuates nursing and time spent in the nest (Bosch et al. 2007), and the current data on lactational stress support these findings. CSS during lactation (12 h of daily exposure to a novel male for 4 days) significantly decreases milk release (Lau and Simpson 2004), so it is possible that the milk production in the CSS dams was significantly affected. The current data also support earlier conclusions that other forms of chronic stress depress maternal care (Léonhardt et al. 2007; Ivy et al. 2008), but it is not clear how chronic stress during lactation affects offspring growth. A recent study of the effects of exogenously elevated cortisol in marmoset monkey mothers found that cortisol decreases maternal care, but there were no effects on the body weights of the mothers or infants (Saltzman and Abbott 2009). An analysis of studies on gestational stress in humans suggests that low birth weight is linked to emotional disorders (Rice et al. 2007), and it is postulated that the effects of postpartum depression on emotional development could be mediated by the adverse effects of depression on growth. The use of animal models of gestational and lactational stress will allow for the controlled investigation of the potential interactions between stress physiology and behavior.
In conclusion, the current results support the use of CSS as an ethologically relevant model for postpartum emotional disorders that impair maternal behavior and attenuate offspring growth and as a model for early life stress for the offspring (Murgatroyd et al. 2010). The effects of CSS on maternal rats and their offspring parallel the effects of postpartum depression on human mothers and their children (Beck 1998; Grace et al. 2003; Patel et al. 2003), and exposure to CSS is a strong predictor for depression and anxiety disorders (Klier et al. 2008). It is hoped that other researchers will use similar models as tools in the development of improved treatments for postpartum mood disorders. Although effective treatments for depression may improve symptoms in the mother, additional focus on the relationship between the mother and infant and parenting skills may be necessary, as a positive response to depression treatment may not reduce the risk for poor child outcomes (Forman et al. 2007). Furthermore, an increased focus on the effects of social stress on both the mother and infant may lead to effective preventative measures for not only depression and anxiety, but also for other stress-related ailments, such as cardiovascular disease.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Klaus Miczek, Dr Sherryl Goodman and Dr Paula Brunton for their input on the design of the CSS protocol.
Footnotes
Declaration of Interest. The authors have no conflict of interest, and are responsible for the content and writing of this paper.
References
- Baranyi J, Bakos N, Haller J. Social instability in female rats: The relationship between stress-related and anxiety-like consequences. Physiol Behav. 2005;84(4):511–518. doi: 10.1016/j.physbeh.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Beck CT. A meta-analysis of predictors of postpartum depression. Nurs Res. 1996;45(5):297–303. doi: 10.1097/00006199-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Beck CT. The effects of postpartum depression on child development: A meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: An update. Nurs Res. 2001;50(5):275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Benoit C, Westfall R, Treloar AEB, Phillips R, Jansson SM. Social factors linked to postpartum depression: A mixed-methods longitudinal study. J Mental Heal. 2007;16(6):719–730. [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103(1):70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Bondar NP, Kovalenko IL, Avgustinovich DF, Smagin DA, Kudryavtseva NN. Anhedonia in the shadow of chronic social defeat stress, or when the experimental context matters. Open Behav Sci. 2009;3:17–27. [Google Scholar]
- Bosch OJ, Müsch W, Bredewold R, Slattery DA, Neumann ID. Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: Implications for postpartum mood disorder. Psychoneuroendocrinology. 2007;32(3):267–278. doi: 10.1016/j.psyneuen.2006.12.012. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Miczek KA. Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) in mice: Inhibition by anpirtoline: A 5-HT1B receptor agonist. Neuropsychopharmacology. 2002;27(2):171–181. doi: 10.1016/S0893-133X(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Denmark A, Tien D, Wong K, Chung A, Cachat J, Goodspeed J, Grimes C, Elegante M, Suciu C, Elkhayat S, Bartels B, Jackson A, Rosenberg M, Chung KM, Badani H, Kadri F, Roy S, Tan J, Gaikwad S, Stewart A, Zapolsky I, Gilder T, Kalueff AV. The effects of chronic social defeat stress on mouse self-grooming behavior and its patterning. Behav Brain Res. 2010;208(2):553–559. doi: 10.1016/j.bbr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Meddle SL, Kroemer S, Muesch W, Bosch OJ, Neumann ID. Social stress induces hypothalamopituitary–adrenal axis responses in lactating rats bred for high trait anxiety. Eur J Neurosci. 2007;25(5):1599–1603. doi: 10.1111/j.1460-9568.2007.05380.x. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav Biol. 1978;23:206–218. doi: 10.1016/s0091-6773(78)91814-x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: Evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25(1):149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: Reduction by the 5-HT receptor agonist CP-94,253. Psychopharmacology. 1999;146(4):391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KE. Effective treatment for postpartum depression is not sufficient to improve the developing mother/child relationship. Dev Psychopathol. 2007;19(02):585–602. doi: 10.1017/S0954579407070289. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annu Rev Clin Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: A review and critical analysis of the literature. Arch Womens Ment Health. 2003;6(4):263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Karkun S. Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. J Affect Disord. 2006;91(2–3):97–111. doi: 10.1016/j.jad.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Herzog CJ, Czéh B, Corbach S, Wuttke W, Schulte-Herbrüggen O, Hellweg R, Flügge G, Fuchs E. Chronic social instability stress in female rats: A potential animal model for female depression. Neuroscience. 2009;159(3):982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Howard O, Carr G, Hill T, Valentino R, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology. 2008;199(4):569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagamiishi Y, Yamamoto T, Watanabe S. Hippocampal serotonergic system is involved in anxiety-like behavior induced by corticotropin-releasing factor. Brain Res. 2003;991(1–2):212–221. doi: 10.1016/j.brainres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model?: Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007;179(1):1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Katz RJ. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16(6):965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148(2):154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klier CM, Rosenblum KL, Zeller M, Steinhardt K, Bergemann N, Muzik M. A multirisk approach to predicting chronicity of postpartum depression symptoms. Depress Anxiety. 2008;25(8):718–724. doi: 10.1002/da.20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva NN, Avgustinovich DF. Behavioral and physiological markers of experimental depression induced by social conflicts (DISC) Agg Behav. 1998;24:271–286. [Google Scholar]
- Lagerspetz K, Hautojärvi S. The effect of prior aggressive or sexual arousal on subsequent aggressive or sexual reactions in male mice. Scand J Psychol. 1967;8(1):1–6. doi: 10.1111/j.1467-9450.1967.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Lau C. Effects of stress on lactation. Pediatr Clin North Am. 2001;48(1):221–234. doi: 10.1016/s0031-3955(05)70296-0. [DOI] [PubMed] [Google Scholar]
- Lau C, Simpson C. Animal models for the study of the effect of prolonged stress on lactation in rats. Physiol Behav. 2004;82(2–3):193–197. doi: 10.1016/j.physbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Lazosky A, Britton D. Effects of 5-HT-1A receptor agonists on CRF-induced behavior. Psychopharmacology. 1991;104(1):132–136. doi: 10.1007/BF02244567. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar press for pups: Effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Léonhardt M, Matthews SG, Meaney MJ, Walker C-D. Psychological stressors as a model of maternal adversity: Diurnal modulation of corticosterone responses and changes in maternal behavior. Horm Behav. 2007;51(1):77–88. doi: 10.1016/j.yhbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Badiani A, Puglisi-Allegra S. Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav Neurosci. 1991;105:663–668. doi: 10.1037//0735-7044.105.5.663. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: Pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Hormonal factors influence the onset of maternal aggression in laboratory rats. Horm Behav. 1987;21:253–267. doi: 10.1016/0018-506x(87)90050-x. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Wu Y, Bockmuhl Y, Spengler D. Genes learn from stress: How infantile trauma programs us for depression. Epigenetics. 2010;5:194–199. doi: 10.4161/epi.5.3.11375. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol Biochem Behav. 2008;91(1):77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in reproductively experienced rats. Behav Neurosci. 2009;123(5):949–957. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Byrnes EM, Bridges RS. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology. 2010;58(1):102–106. doi: 10.1016/j.neuropharm.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: Correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur J Neurosci. 2001;13:1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- O’Hara HW. Social support, life events, and depression during pregnancy and the puerperium. Arch Gen Psychiatry. 1986;43(6):569–573. doi: 10.1001/archpsyc.1986.01800060063008. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risk of postpartum depression a meta-analysis. Int Rev Psychiatry. 1996;8(1):37–54. [Google Scholar]
- O’Hara HW, Rehm LP, Campbell SB. Postpartum depression: A role for social network and life stress variables. J Nerv Ment Dis. 1983;171(6):336–341. [PubMed] [Google Scholar]
- Pardon M-C, Gérardin P, Joubert C, Pérez-Diaz F, Cohen-Salmon C. Influence of prepartum chronic ultramild stress on maternal pup care behavior in mice. Biol Psychiatry. 2000;47(10):858–863. doi: 10.1016/s0006-3223(99)00253-x. [DOI] [PubMed] [Google Scholar]
- Patel V, DeSouza N, Rodrigues M. Postnatal depression and infant growth and development in low income countries: A cohort study from Goa, India. Arch Dis Child. 2003;88(1):34–37. doi: 10.1136/adc.88.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paykel E, Emms E, Fletcher J, Rassaby E. Life events and social support in puerperal depression. Br J Psychiatry. 1980;136(4):339–346. doi: 10.1192/bjp.136.4.339. [DOI] [PubMed] [Google Scholar]
- Potegal M. Time course of aggressive arousal in female hamsters and male rats. Behav Neural Biol. 1992;58(2):120–124. doi: 10.1016/0163-1047(92)90339-6. [DOI] [PubMed] [Google Scholar]
- Potegal M, TenBrink L. Behavior of attack-primed and attack-satiated female golden hamsters (Mesocricetus auratus) J Comp Psychol. 1984;98(1):66–75. [Google Scholar]
- Rees SL, Panesar S, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on maternal behavior in the postpartum rat. Horm Behav. 2004;46(4):411–419. doi: 10.1016/j.yhbeh.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Rees SL, Panesar S, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on induction of maternal behavior in the virgin female rat. Horm Behav. 2006;49(3):337–345. doi: 10.1016/j.yhbeh.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Rice F, Jones I, Thapar A. The impact of gestational stress and prenatal growth on emotional problems in offspring: A review. Acta Psychiatr Scand. 2007;115(3):171–183. doi: 10.1111/j.1600-0447.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: A synthesis of recent literature. Gen Hosp Psychiatry. 2004;26(4):289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Abbott DH. Effects of elevated circulating cortisol concentrations on maternal behavior in common marmoset monkeys (Callithrix jacchus) Psychoneuroendocrinology. 2009;34(8):1222–1234. doi: 10.1016/j.psyneuen.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre L, O’Hara M, Arndt S, Stuart S. The prevalence of postpartum depression. Soc Psychiatry Psychiatr Epidemiol. 2007;42(4):316–321. doi: 10.1007/s00127-007-0168-1. [DOI] [PubMed] [Google Scholar]
- Seguin L, Potvin L, St Denis ML, Loiselle J. Chronic stressors, social support, and depression during pregnancy. Obstet Gynecol. 1995;85(4):583–589. doi: 10.1016/0029-7844(94)00449-N. [DOI] [PubMed] [Google Scholar]
- Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci. 2003;42(1):9–18. [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. Gestational stress induces post-partum depression-like behavior and alters maternal care in rats. Psychoneuroendocrinology. 2004;29:227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, Van Hooff JARAM, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72(3):825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Stern JM, Levin R. Food availability as a determinant of the rats’ circadian rhythm in maternal behavior. Dev Psychobiol. 1976;9:137–148. doi: 10.1002/dev.420090206. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Kawachi I, Ryan LM, Berkman LF, Vieira LM, Peterson KE. Maternal depressive symptoms, parenting self-efficacy, and child growth. Am J Public Health. 2008;98(1):125–132. doi: 10.2105/AJPH.2006.108332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Meifer OC, de Kloet ER, Koolhaas JM, Bohus BG. Differences in basal and stress induced HPA regulation of wild house mice selected for high and low aggression. Horm Behav. 2003;43:197–204. doi: 10.1016/s0018-506x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Wellmann K, Lewis B, Barron S. Agmatine reduces ultrasonic vocalization deficits in female rat pups exposed neonatally to ethanol. Neurotoxicol Teratol. 2010;32(2):158–163. doi: 10.1016/j.ntt.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westdahl C, Milan S, Magriples U, Kershaw TS, Rising SS, Ickovics JR. Social support and social conflict as predictors of prenatal depression. Obstet Gynecol. 2007;110(1):134–140. doi: 10.1097/01.AOG.0000265352.61822.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 1992;16(4):525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.