Abstract
There is an inherent conflict between interpreting ultrasound imaging, which generates rich, highly textured complex representations of 3-dimensional space, and the rendering of these images into discrete measures for statistical analysis Reducing images to a small set of numbers, by necessity, ignores vast amounts of useful data, but the process is a prerequisite for generating objective information that can be shared by the clinical community. This article illustrates how ultrasound imaging is used in quantitative ways. The goal is not to supplant or diminish the importance of descriptive findings but to make the field amenable to statistical methods and standards for making diagnoses and designing therapeutic trials of new interventions. The discussion focuses first on muscle ultrasonography, a technique that was developed more than a decade earlier than nerve ultrasound, to illustrate key elements in determining how to best extract quantitative information from complex images. The discussion then applies some of these same principles to the study of 2 common nerve disorders.
Keywords: Neuromuscular ultrasound, Amyotrophic lateral sclerosis, Muscle hypertrophy, Muscle atrophy, Nerve enlargement, Carpal tunnel syndrome, Leprosy
MUSCLE ULTRASONOGRAPHY
Size Measurements
Conceptually, the simplest way to identify muscle is to measure its size. All ultrasound instruments are equipped with electronic calipers so that once a muscle is imaged, its boundaries can be identified and measured. However, there are several caveats1–6:
In the relaxed state, the muscle is compressible and even slight pressure on the transducer can lead to significant changes in measured thickness. A commonly used effective strategy is to place the transducer on the muscle with ample gel, optimize the image, and then use the minimal pressure required to not lose transducer contact with the skin surface.
Muscle dimensions vary with location, so selecting a reliable and consistent site for measurement is a prerequisite for obtaining normative data or for performing serial measurements over time. Bony landmarks, and fixed or proportional distances from them, are commonly used.
Careful transducer management is required to ensure that the probe is held precisely in a parallel or transverse manner toward the muscle of interest so as for optimal size measurements and to ensure that the angle of insonation is perpendicular to the long dimensions of the muscle to ensure optimal echogenicity measurements.
Muscle dimensions change with contraction/relaxation, so it is critical to ensure that the subject is cooperating for either full relaxation or contraction.
Muscle blood flow increases significantly with even low levels of exertion, and this can influence measured size of the muscle5; typically patients are imaged after a brief period of rest.2 Muscle mass is largely composed of water (75%),6 and, because muscle represents a large portion of the body water reserve (30%), it may change with dehydration or fluid overload.
Several clinical studies, discussed later, have shown that the technical issues of muscle size measurements can be managed and that the technique is capable of providing useful information about muscle function and dysfunction. Using a series of simple questions, the following sections address the evidence that supports the value of ultrasound measurements of muscle size.
Are ultrasound measurements of muscle size reliable?
The studies that have looked at the test-retest reliability of muscle thickness and cross-sectional area show that there is a correlation coefficient of 0.98 to 0.99 and a correlation of 0.99 with magnetic resonance imaging (MRI) measurements of muscle.7–11 Because of the way ultrasonography processes speed of sound, which is slightly faster in muscle than average human tissue, the technique may consistently underestimate muscle thickness by a few percentage points, but, because this underestimate is invariant and directly related to measures obtained by MRI, its significance is moot.12
Can ultrasonography accurately quantify changes in muscle thickness with activation?
There are several simple ways to evaluate the ability of ultrasonography to measure variations in muscle thickness. The simplest is to show that it can measure changes in muscle size and shape in response to muscle contraction. This has been well documented13–16 in published studies and is easily demonstrated by imaging any muscle in the relaxed and contracted state (Fig. 1).
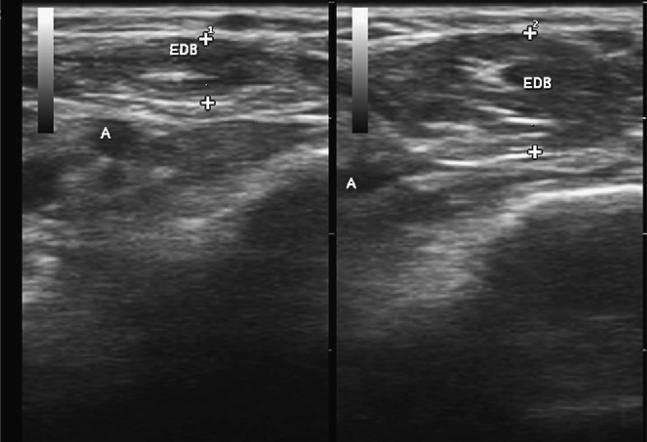
Fig. 1.
Two identical cross-sectional images of the extensor digitorum brevis (EDB). On the left, the muscle (EDB), which is just above a small artery (A), is fully relaxed, and the crosses show the thickness at 2.8 mm. On the right, the muscle is fully contracted, displacing the artery, with a thickness of 5.1 mm.
Is ultrasonography capable of demonstrating an increase in muscle size with exercise?
A somewhat greater test of the utility of ultrasonography as a tool for measuring muscle size derives from studies that evaluate the effect of exercise. Ultrasonography proves to be a reliable and valid method for measuring the hypertrophy of muscles in the extremities, pelvic floor, and diaphragm that results from formal exercise programs.17–27 Four illustrative studies (Fig. 2) are worth reviewing in more depth. Downey and colleagues24 studied the response of diaphragm thickness to inspiratory muscle training exercises in healthy young adults. After 8 weeks of training, which involved deep breathing against large inspiratory loads, diaphragm thickness increased by 10% and inspiratory muscle strength increased 25%. Enright and colleagues25,26 showed a similar improvement in diaphragm thickness in healthy controls and also showed an almost 20% increase in diaphragm thickness in adults with cystic fibrosis after this same type of training. In healthy controls, even an exercise program of biceps curls and sit-ups can lead to significant increases in diaphragm thickness.27 In all studies, there was improvement in a variety of other measured parameters of pulmonary function in tandem with the changes seen on ultrasonography.
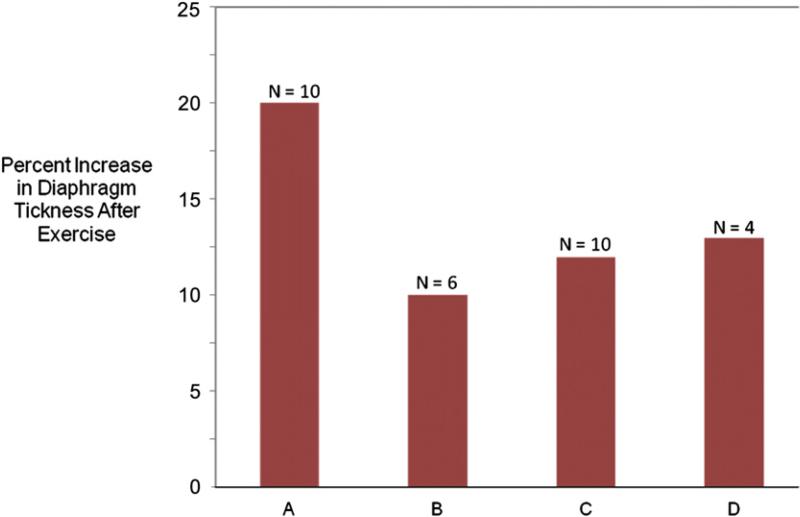
Fig. 2.
The percentage increase in diaphragm thickness after a dedicated exercise program in (A) 10 patients with cystic fibrosis,25 (B) 6 healthy controls,24 (C) 10 healthy controls,26 and (D) 4 healthy controls.27 Although the N is small, the results from different studies show good concordance.
Fujiwara and colleagues18 showed that heel-raising exercises in elderly women significantly increased gastrocnemius (6%) and soleus (12%) thickness, and McNee21 showed that strength training in children with cerebral palsy increased the volume of the medial and lateral gastrocnemius muscles by about 15%. As such, it is clear that exercise training, in healthy young and old controls and diseased individuals, leads to increased muscle thickness and improves measures of strength and function. However, studies have yet to confirm significant effects of exercise on neuromuscular disease outcomes. So, although ultrasonography is a valid way to measure direct effects of exercise on muscle size, establishing this measure as a marker of clinical benefit requires additional studies. The link seems likely, and, given its ease of use and availability, confirmatory studies can be performed without undue expense or delay.
Can muscle ultrasonography measure loss of muscle size with disuse?
Another way to evaluate the usefulness of muscle ultrasonography is its ability to assess loss of muscle mass in nonneuromuscular conditions commonly associated with atrophy.19,23,28 Kawakami and colleagues19 used ultrasonography to show that muscle mass in the lower extremities decreased significantly (3%–4%) after 20 days of strict bed rest in healthy controls. Reid and colleagues23 showed that muscle thickness decreases even more rapidly in critically ill patients, 1.6% per day. Triandafilou and Kamper28 showed that ultrasonography sensitively detects the loss of muscle mass in the affected hands of patients who have had a stroke and that this loss significantly exceeds the difference of muscle sizes expected between dominant and nondominant hands.
Is ultrasonography capable of measuring the size differences of muscles between populations of children, seniors, and adult men and women?
Another test of the utility of muscle ultrasonography measures involves how well it assesses differences in muscle size in large populations of individuals. As adults grow older, there is an age-specific decline in muscle size, particularly in the quadriceps. This effect is muted in those who remain physically active in old age.8,29–32 Ultrasonography has proven to be a robust tool for demonstrating this effect in a more cost-effective manner than MRI. Similarly, ultrasonography confirms differences in the thickness of the dominant versus nondominant upper extremity and between men and women. Ultrasonography also shows the increase in muscle mass in children that occurs with maturation.
Is it possible to track progressive loss of muscle mass in chronic neuromuscular disorders?
A more direct test of the clinical relevance of muscle ultrasonography is its ability to track changes in muscle size in progressive neuromuscular diseases. Studies in large groups of patients with adult and childhood motor neuron disease and muscular dystrophy have used ultrasound to show that those with longer duration or severity of symptoms have smaller muscle size.11,33–40 Pseudohypertrophy of muscles occurs in some disorders such as Duchenne muscular dystrophy, a finding ultrasonography also readily quantifies.10 In addition to cross-sectional disease population studies, there are 2 recent smaller studies that show ultrasonography can be used to serially measure muscle size changes over time in patients with amyotrophic lateral sclerosis (ALS).33,34 Lee and colleagues33 showed that there was a statistically significant progression of muscle atrophy over time in patients with motor neuron disease in a sample of 3 muscles; however, in a larger sample of muscles in a greater number of patients, Arts and colleagues34 were unable to show a significant progression of atrophy in patients with ALS. Taken together, these studies provide evidence that ultrasonography has the potential for being a useful biomarker in neuromuscular disease, but, in ALS at least, further studies are needed to determine if this technique can be used as a surrogate end point for treatment outcomes. Ultrasonography can measure muscle size and its change over time, but the question remains if muscle loss in ALS is sufficiently distributed in multiple muscles and linearly progressive to be amenable to standard statistical calculations for estimating functional disability and disease severity.
Can ultrasonography measure the effect of drugs on causing either loss or enhancement of muscle thickness?
A final test of the usefulness of ultrasound measurements of muscle size is to assess its responsiveness to drug treatment. Hamjian and Walker,41 in a series of healthy controls, showed that muscle ultrasonography of the extensor digitorum brevis was a sensitive indicator of atrophy after small doses of botulinum toxin. In hemiplegic patients admitted to an intensive care unit, Moukas and colleagues42 showed that serial ultrasound measurements of muscle size could demonstrate an enhanced rate of atrophy in those treated with steroids and neuromuscular junction blockers. Candow and colleagues,43 in healthy exercising adults, showed that creatine supplementation significantly enhanced muscle thickness. Cardiac ultrasonography, a technique of considerable similarity to skeletal muscle ultrasonography, has been used to detect changes in heart muscle size in health and disease for many years.44 This technique is responsive to reductions in cardiac wall thickness in heart transplant recipients treated with sirolimus and to increases in wall thickness in patients undergoing hemodialysis treated with growth hormone.45 These findings show that ultrasonography is a measure that can detect bidirectional changes in muscle size in response to a variety of pharmacologic interventions in even fairly small samples of patients.
How promising are ultrasound measurements of muscle size as potential surrogate end points of disease severity?
It seems clear that ultrasonography is a useful technique for measuring muscle size and that this property has immediate diagnostic implications and the potential to be a useful tool for clinical decision making. This technique meets the requirements of a useful surrogate end point of neuromuscular disease as listed, in a simplified form, in Box 1. Of 5 key variables that characterize an ideal surrogate end point, the cost and convenience of ultrasonography is self-evident in that it is noninvasive, painless, safe, and routinely available with just a modest amount of training. It is clearly valid from both a technical and clinical perspective. The technique is simple, reliable, and well standardized as a measure of tissue size. From a clinical perspective, atrophy is a robust manifestation of most neuromuscular diseases. Muscle size is known to correlate strongly with strength, a variable of clinical significance. Furthermore, normalization of atrophied muscles is almost a necessity for therapeutic intervention to work in a neuromuscular disorder; put another way, it would be difficult to envision a mechanism of action that would not involve increase in muscle size. Although not discussed in detail, the measurement precision of ultrasonography is quite good, with known standards of biological variation in the populations studied and straightforward image acquisition and measurement techniques. These are objective and amenable to central oversight if needed. Perhaps of greatest significance is that the studies demonstrate bidirectional responsiveness and sensitivity of ultrasound measurements of muscle size to a variety of diseases and the outcomes of diverse therapeutic interventions. However, to become fully acceptable as a surrogate end point, muscle ultrasonographic measurements of muscle size need to be validated in a pivotal clinical trial of therapeutic benefit. Because there are few currently meaningful therapies for progressive neuromuscular disorders, such validation likely will have to await the emergence of newer interventions.
Box 1 Characteristic of surrogate markers.
Validity (technical/clinical)
Responsiveness to disease and treatment
- Measurement precision
- Biological variation
- Acquisition process
Convenience; for example speed of use, portability, patient tolerance
Cost
An ideal surrogate marker should identify a physiologic process on a direct pathway between treatment and outcome.
Echogenicity Measurements of Muscle
Another useful parameter to study muscle is ultrasound echogenicity.2,3,46 Healthy muscle tissue is normally echolucent or dark. Given the highly organized structure of muscle, sound mostly is transmitted through the tissue with reflections occurring primarily at sites of fibrous structures in the muscle, such as the vasculature, perimysium, and epimysium. However, in myopathies and neurogenic disorders, muscle tissue undergoes atrophy, necrosis, inflammation, fatty infiltration, and fibrosis. These changes set up multiple new planes of sound reflection in muscle, and, as a result, diseased muscle becomes progressively more echogenic, with loss of the normal heterogeneity of healthy muscle and its supporting fibrous stroma.
Measuring muscle echo intensity, however, is technically more complex than measuring muscle size. There is no set convention among ultrasound instrument manufacturers as to how to best display brightness, so the relationship between the brightness displayed on a screen and the actual intensity of reflected echoes varies somewhat from instrument to instrument, depending on proprietary software used by each manufacturer.2,3 In fact, there is no set standard for image contrast or brightness in the field. Sonographers may differ in their opinions on optimal brightness settings for performing certain types of diagnostic studies.12 The subjectivity of brightness settings is further limited by the absence of any good correlative measurement, other than microscopic anatomy, which, at best, provides an indirect correlation.46 Although muscle size as measured by ultrasonography is subject to comparisons of size as measured by other techniques, echo intensity measurements are only directly apparent by ultrasonography.
Further complicating the display of echogenicity is the nature of human visual perception.12 In general, the brightness of most objects depends largely on the extent of ambient illumination and fairly little with respect to the object perceived. An object's perceived size, in contrast, is constant regardless of illumination. Studies of human perception have shown that size is cognitively coded in a linear manner. As such, even if a subject cannot tell the relative height in inches of 2 adjacent objects, they can give accurate estimates of their relative size. In contrast, brightness is coded cognitively in an exponential manner47; as such, the relative brightness of 2 objects is nonlinear and not easily appreciated in a quantitative manner. Using a standard oscilloscope display, amplitude mode (A-mode) ultrasonography can display the amplitude of an ultrasound signal precisely but only for the display of a single element from the multiple elements in a linear array transducer.12 Such 1-dimensional displays are of limited use and are impractical in modern imaging. Although necessary for tissue imaging, the use of brightness mode (B-mode) ultrasonography requires mapping amplitude as brightness. However, something is lost in translation because this conversion, from a perceptual perspective, involves displaying amplitude information on a log scale instead of a linear scale. Although difficult to characterize the impact of this translational shift, Fig. 3 helps to show the difference of expressing something on a linear versus a log scale. This subject is discussed in more detail elsewhere.12
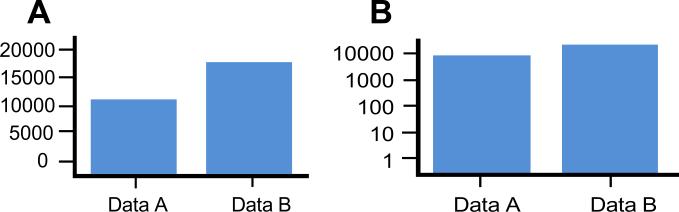
Fig. 3.
Bar graph using a (A) standard size scale and (B) a log scale. Note that the log scale adjustment significantly diminishes the apparent magnitude of the difference. Use of B-mode imaging, by displaying amplitude as brightness, tends to reduce perceived echo intensity differences because of the different way the human brain processes size versus illumination.
Further complicating the interpretation of ultrasound brightness is the number of operator-controlled parameters that alter echogenicity of a tissue. These parameters include time gain compensation, the angle of incidence of the transducer, and power and gain settings. Fortunately, these are manageable through careful technique, which includes standardized instrument settings, transducer positioning, and using the same instrument. However, such settings may be not be equivalent on different instruments, given the differences in proprietary display parameters.2,11,29,34,36 Alternatively, a somewhat more complex process involving backscatter analysis48–51 allows for comparison of data from different instruments. In either case, technical factors can be managed effectively to obtain useful data on echogenicity. One factor, however, which is a patient variable that is more difficult to control, is the variability of the sound-absorbing characteristics of superficial layers of tissue on the echogenicity of deeper layers. In practice, this variability does not seem to be particularly problematic in that most muscle imaging involves muscles fairly close to the surface and the variation present in most superficial tissue is minimal. However, excess subcutaneous edema, superficial fat, or hypertrophic skin disorders can distort echogenicity measurements and may play a significant role in the evaluation of certain groups of patients (eg, critically ill patients with pronounced edema).
As would be expected, there are fewer studies that have measured echogenicity in neuromuscular diseases than studies that have measured muscle thickness. Nonetheless, increased echogenicity was recognized early as a key parameter in the diagnosis of chronic neuromuscular disease (Fig. 4). The finding has been validated numerically using digital technology, descriptively and by qualitative rating scales scored by blinded investigators.8,10,11,29,34–37,51–59 At least as a diagnostic parameter, ultrasound echogenicity measurements of muscle provide added value.
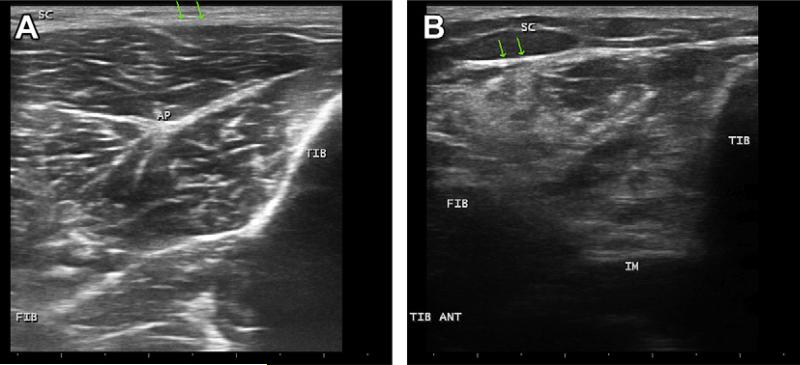
Fig. 4.
(A) Normal cross section through the tibialis anterior of a healthy adult showing the tibia (TIB), a faint outline of the fibula (FIB), and a prominent central aponeurosis (AP) in the muscle. At the top of the image is a very thin layer of subcutaneous tissue (SC) with arrows pointing to the upper edge of the muscle. (B) Cross section of the tibialis anterior from a young adult with hereditary motor and sensory neuropathy. The subcutaneous tissue (SC) layer is thicker with the muscle edge deeper (arrows), the fibula (FIB) and interosseous membrane (IM) that connects the fibula and tibia (TIB) show markedly reduced depth of the muscle. The muscle is distinctly more echogenic, which tends to obscure the bone edge of the tibia, with a sort of moth-eaten appearance more typical of neurogenic than myopathic change.
Does increased echogenicity serve as a useful measure of disease severity?
In cross-sectional studies of groups of patients, there is some evidence to suggest that greater echogenicity is seen in more advanced forms of motor neuron disease or at least in disease of longer duration.34–36 The change of echogenicity over time has not been as well studied in human muscular dystrophy. However, in dystrophic mice, there is evidence to suggest that it might be a useful progressive disease biomarker.60,61 There is also evidence that increased echogenicity of muscle inversely correlates with survival in ALS. This effect is most pronounced when estimating the rate of change of echogenicity from baseline based on normal values and the known duration of symptoms.36 When looked at serially over 6 months in a group of 31 patients with ALS, however, the change in echo-intensity over time was too variable and inconsistent to track disease severity. As a marker of the presence of muscle disease, echogenicity is quite useful; however, as a marker of disease evolution or regression, the limited data available are unconvincing.
One potential problem with ultrasound echogenicity is its predicted responsiveness to disease therapy. There are concerns that changes in echogenicity may not be reversible even should patients improve from their underlying disorder.36 Because increased echogenicity results from fibrosis and fatty changes in muscle, it may be that changes in echogenicity are chronic and may not necessarily repair with clinical improvement. For example, old polio patients may have markedly asymmetric recovery from paraplegia. Ultrasound images from affected limbs, one that is flaccid and one that shows normal strength, may show virtually equal echogenicity.62 Further improvements in techniques for quantifying echogenicity may help in determining if ultrasonography can detect a reduction in preexisting echogenicity or forestall the progression of echogenicity in neuromuscular disorders undergoing effective therapy.
Muscle Dynamics
By its very nature, measuring movement is typically more complex than measuring findings on a still image, because movement involves measurements across multiple images. However, certain techniques can simplify such measurements. Motion mode (M-mode) imaging can capture movement on a single still image, such as the duration of a compound muscle action potential or fasciculation (Fig. 5).4 Ultrasonography records the duration of a mechanical event, such as muscle contraction, whereas electromyography records the duration of a compound surface membrane potential, an electrical event that is far shorter.4,12
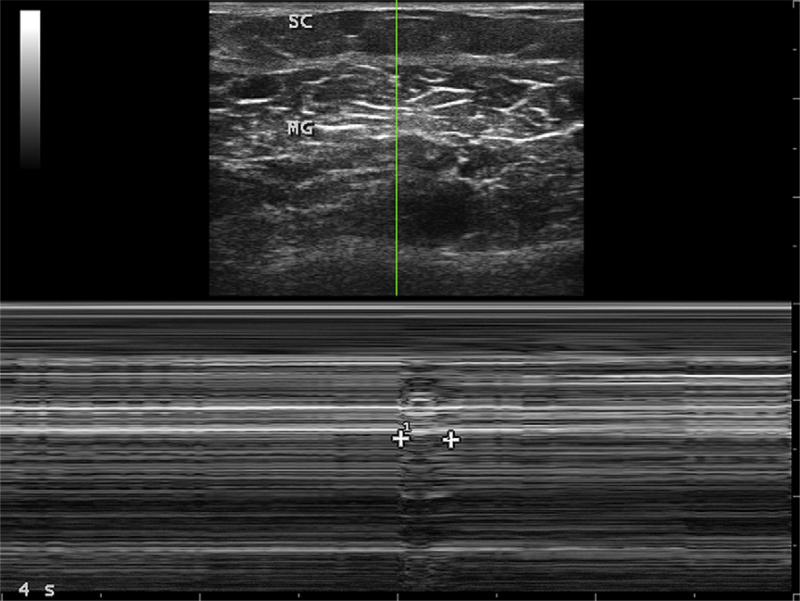
Fig. 5.
Cross-sectional image of the median gastrocnemius muscle (MG) with a thin layer of subcutaneous tissue (SC) above it. The vertical line through the center of the muscle is displayed below, using M-mode (motion mode) ultrasonography. The 1 line of ultrasound data, from a single transducer element from a linear array of multiple elements, is displayed below as it changes over 4 seconds. At about half way during this 4-second epoch, a fasciculation occurs in the muscle, marked by a focal perturbation that lasts 253 milliseconds. Both contraction (thickening) and relaxation is seen during this transient motor unit discharge. The duration exceeds that of electrically recorded fasciculations because ultrasonography captures the mechanical behavior of muscle, whereas electrodiagnostic measurements only capture the brief change in muscle membrane potential.
Perhaps the simplest measure of muscle movement is the degree of thickening on contraction of muscle. This has been measured in the diaphragm24–27 and is easily measured in other muscles. To date, it has not been proven that this measurement adds value to the measurement of muscle thickness alone, but it has not been looked at rigorously. Another common muscle movement of interest is fasciculations. Benign fasciculations are quite common, and a recent study by Daube and Kumar63 suggests that, at least after vigorous exercise, these fasciculations occur in virtually 100% of normal individuals in the abductor hallucis muscle. Ultrasonography can also be used to count fasciculations in a given area of muscles.64 Such findings can be of considerable diagnostic use in early forms of the disease,65,66 but they do not seem to be particularly useful in quantifying severity of disease in ALS.34 This is not surprising in that one would anticipate that fasciculation frequency would have a U-shaped relationship with severity of symptoms in motor neuron disease, with a gradual increase in frequency as the disease goes through early stages and a gradual decrease in frequency in later stages of the disorder concomitant with significant lower motor neuron loss.
Another area of potential interest in quantification is muscle blood flow. Muscle blood flow is known to increase dramatically after even small amounts of exercise, and this effect can contribute to measured muscle volume.67 Little, however, is known about muscle blood flow in primary neuromuscular disorders other than that increased blood flow is seen in inflammatory myopathies.68 In animal models of muscular dystrophy, abnormal blood flow regulation is seen in relation to changes in nitric oxide function,69 but the significance of this finding in human disease is unknown. Access to ultrasonography may make it possible for clinicians to more actively study blood flow changes in a variety of pathologic states.
QUANTIFICATION OF NERVE ULTRASONOGRAPHY
The most common types of nerve disorders, entrapment neuropathies, tend to show changes that are the inverse of the changes seen on muscle imaging of chronic neuro-muscular disease. Although chronically diseased muscles become atrophic and hyperechoic, chronically compressed nerves tend to become enlarged and hypoechoic. In both cases, the changes are clearly of diagnostic usefulness. For example, currently, there are more than 250 published studies of nerve ultrasonography in carpal tunnel syndrome showing that measured nerve enlargement and loss of echo-genicity are hallmark diagnostic features of the disorder. However, it is unclear if these changes are useful as surrogate markers in nerve disease. This is because there is a structural limit to how much a compressed nerve can enlarge; with increasingly severe compression and axonal loss, nerves may have less swelling than in less pronounced cases, and there is a floor effect of how much echogenicity a nerve can lose. This suggests that there may be an inverted U-shaped relationship between nerve enlargement and severity of compression neuropathy and possibly a similar relationship with loss of echogenicity; as such, they may have some limitations as surrogate markers of severity of compression.
The cause of increased size and loss of echogenicity of compressed nerves is not well understood, but, in part, this may result from increased vascularity of the nerve in carpal tunnel syndrome, a finding that can be demonstrated with color flow Doppler (Fig. 6). Excess blood flow would tend to engorge the nerve and, because blood is much less echoic than tissue, would reduce nerve echogenicity.70–73 Both ultrasound-guided steroid injections and surgical treatment of carpal tunnel syndrome lead to prompt and sustained reductions in nerve size as measured by cross-sectional area.70–72,74,75 When Cartwright and colleagues70 looked at median nerve vascularity in conjunction with nerve size, both decreased in tandem in patients with carpal tunnel syndrome after ultrasound-guided steroid injections around the median nerve. Nerve echogenicity, measured by examiner rating, showed a similar time course of improvement. As such, there may be a role of ultrasound in gauging treatment effects in carpal tunnel syndrome because several measures demonstrate responsiveness to therapeutic intervention in these studies.
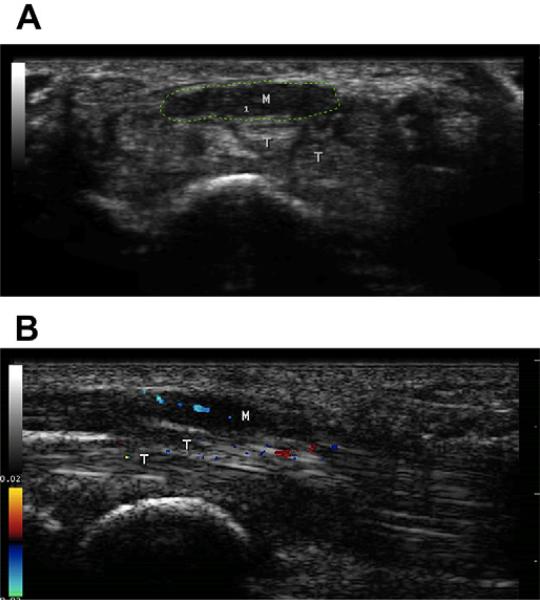
Fig. 6.
(A) Cross-sectional image of the wrist showing a markedly enlarged and somewhat flattened median nerve. The cross-sectional area of the nerve (green tracing) is 31 mm2, which is about 4 times the normal size. Note that the nerve is hypoechoic, particularly when compared with the tendons immediately below it that are much brighter. (B) Sagittal view of the median nerve using color flow Doppler. Note the linear blue elements within the nerve, which demonstrate a stable pulsating blood flow within it, a finding not seen in normal healthy nerves. There is random transient noise in nearby structures such as the tendons (T).
Is Quantitative Ultrasonography Useful in Infectious Neuropathy?
If carpal tunnel syndrome is the most common treatable focal neuropathy, it seems likely that leprosy may be the most common curable generalized polyneuropathy, at least from a global perspective.76 Leprosy's worldwide prevalence and the potential for ultrasonography to significantly affect its management warrant its inclusion in this review. Two major categories of leprosy include lepromatous, which is a diffuse infection of skin and nerves caused by reduced cell-mediated immunity in the disorder, and tuberculoid, in which there is an active immunologic response to the disease, restricting its distribution to relatively few areas of skin and nerve.76 In up to 25% of patients undergoing antibiotic therapy, there are treatment reactions, usually in lepromatous forms of the disease.76 These reactions can be mild but can also be associated with significant inflammatory impairment of nerve function. Increased blood flow in nerves, detected by color Doppler ultrasonography, provides evidence of such treatment reactions.77
Ultrasound measurements showing increased nerve size are a sensitive indicator of the presence of neuropathy in leprosy.78,79 This is not surprising because it is well known that nerves are often palpably enlarged in leprosy, particularly in areas where they are superficial and in tissues that are typically cooler than core body temperature, for example, the ulnar nerve at the elbow, the tibial nerve at the ankle, the fibular nerve at the fibular head, and the greater auricular nerve.76
Another study of leprosy, at least one of semiquantitative impact, is the use of vaso-motor blood flow studies in the distribution of the ulnar nerve. Wilder-Smith and colleagues80 showed that color Doppler measurements of blood flow in the ulnar artery by ultrasonography were sensitive and specific in identifying small fiber autonomic dysfunction in 12 patients with leprosy compared with 20 healthy controls. The study is of interest because it demonstrates not only the diagnostic utility of ultra-sound in this disorder, in which small fiber dysfunction is significant in terms of tissue injury and digit loss, but also the potential of ultrasound to diagnose other disorders of small fibers controlling the autonomic nervous system.
Leprosy is an example of a disorder in which nerve ultrasonography may prove to be a useful technique for screening, diagnosis, and assessment of the extent of the disease. However, of even greater importance, leprosy is treatable, and, as such, it can help establish the responsiveness of ultrasound measures of nerve disease in this disorder to antibiotic therapy. Because a percentage of treated patients develop treatment reactions, it is also likely that ultrasonography can contribute to their recognition and management. In this context, leprosy could prove to be a model disease for studying how a low-cost portable imaging technology can alter the diagnosis, treatment, and management of nerve disease prevalent in populations with limited access to sophisticated electrophysiologic testing.
SUMMARY
Ultrasonography, first used to study muscle in 1980 and nerve in 1991, has evolved into a valid technique for the diagnosis of nerve and muscle disease.56,81 Recent studies have suggested an additional role for neuromuscular ultrasonography as a measure of disease severity and distribution. Improved understanding of how to analyze and quantitate the complex findings provided by ultrasound imaging is helping to build a case for the use of ultrasound measurements as potential surrogate end points of neuromuscular disease and its response to treatment. By including ultra-sound assessment in ongoing and future clinical trials of therapeutic interventions in neuromuscular disease, it will be possible to determine how the technique can be used to facilitate clinical trials and the evaluation of new promising therapies.
Acknowledgments
Dr Cartwright has funding from the NIH/NINDS (1K23NS062892) to study neuromuscular ultrasound.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Disclosures: None.
REFERENCES
- 1.Walker FO, Cartwright MS, Weisler ER, et al. Ultrasound of nerve and muscle. Clin Neurophysiol. 2004;115:495–507. doi: 10.1016/j.clinph.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Pillen S, van Alfen N, Zwarts M. Ultrasound of muscle. In: Walker FO, Cartwright MS, editors. Neuromuscular ultrasound. Elsevier; Philadelphia: 2011. pp. 37–57. [Google Scholar]
- 3.Zaidman CM. Ultrasound of muscular dystrophies, myopathies, and muscle pathology. In: Walker FO, Cartwright MS, editors. Neuromuscular ultrasound. Elsevier; Philadelphia: 2011. pp. 131–50. [Google Scholar]
- 4.Walker FO. Neuromuscular ultrasound as a complement to the electrodiagnostic evaluation. In: Aminoff MJ, editor. Aminoff's Electrodiagnosis in Clinical Neurology. 6th edition Elsevier; 2012. [Google Scholar]
- 5.Walker FO. Normal neuromuscular sonography. In: Tegeler CH, Babikian VL, Gomez CR, editors. Neurosonology. Mosby-Year-Book; New York: 1996. pp. 397–405. [Google Scholar]
- 6.Sarvazyan A, Sarvazyan A. Acoustical assessment of body water balance. J Acoust Soc Am. 2011;130(4):2539. [Google Scholar]
- 7.Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91:116–8. doi: 10.1007/s00421-003-0961-9. [DOI] [PubMed] [Google Scholar]
- 8.Reimers CD, Harder T, Saxe H. Age-related muscle atrophy does not affect all muscles and can partly be compensated by physical activity: an ultrasound study. J Neurol Sci. 1998;59:60–6. doi: 10.1016/s0022-510x(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 9.Sanada K, Kearns CF, Midorikawa T, et al. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol. 2006;96:24–31. doi: 10.1007/s00421-005-0061-0. [DOI] [PubMed] [Google Scholar]
- 10.Reimers CD, Schlotter B, Eicke BM, et al. Calf enlargement in neuromuscular diseases: a quantitative ultrasound study in 350 patients and review of the literature. J Neurol Sci. 1996;143:46–56. doi: 10.1016/s0022-510x(96)00037-8. [DOI] [PubMed] [Google Scholar]
- 11.Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37(6):679–93. doi: 10.1002/mus.21015. [DOI] [PubMed] [Google Scholar]
- 12.Walker FO. Basic principles of ultrasound. In: Walker FO, Cartwright MS, editors. Neuromuscular ultrasound. Elsevier; Philadelphia: 2011. pp. 1–23. [Google Scholar]
- 13.Pinto RZ, Ferreira PH, Franco MR, et al. Effect of two lumbar spine postures on transversus abdominis muscle thickness during a voluntary contraction in people with and without low back pain. J Manipulative Physiol Ther. 2011;34:164–72. doi: 10.1016/j.jmpt.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Jhu JL, Chai HM, Jan MH, et al. Reliability and relationship between 2 measurements of transversus abdominis dimension taken during an abdominal drawing-in maneuver using a novel approach of ultrasound imaging. J Orthop Sports Phys Ther. 2010;40:826–32. doi: 10.2519/jospt.2010.3000. [DOI] [PubMed] [Google Scholar]
- 15.Delaney S, Worsley P, Warner M, et al. Assessing contractile ability of the quad-riceps muscle using ultrasound imaging. Muscle Nerve. 2010;42:530–8. doi: 10.1002/mus.21725. [DOI] [PubMed] [Google Scholar]
- 16.Kubo K, Kawata T, Ogawa T, et al. Outer shape changes of human masseter with contraction by ultrasound morphometry. Arch Oral Biol. 2006;51:146–53. doi: 10.1016/j.archoralbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Ronei PS, Gomes N, Radaelli R, et al. Effect of range of motion on muscle strength and thickness. J Strength Cond Res. 2011 doi: 10.1519/JSC.0b013e31823a3b15. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara K, Toyama H, Asai H, et al. Effects of regular heel-raise training aimed at the soleus muscle on dynamic balance associated with arm movement in elderly women. J Strength Cond Res. 2011;25:2605–15. doi: 10.1519/JSC.0b013e3181fb4947. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami Y, Akima H, Kubo K, et al. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol. 2001;84:7–12. doi: 10.1007/s004210000330. [DOI] [PubMed] [Google Scholar]
- 20.Krentz JR, Farthing JP. Neural and morphological changes in response to a 20-day intense eccentric training protocol. Eur J Appl Physiol. 2010;110:333–40. doi: 10.1007/s00421-010-1513-8. [DOI] [PubMed] [Google Scholar]
- 21.McNee AE, Gough M, Morrissey MC, et al. Increases in muscle volume after plantarflexor strength training in children with spastic cerebral palsy. Dev Med Child Neurol. 2009;51:429–35. doi: 10.1111/j.1469-8749.2008.03230.x. [DOI] [PubMed] [Google Scholar]
- 22.Braekken IH, Majida M, Engh ME, et al. Morphological changes after pelvic floor muscle training measured by 3-dimensional ultrasonography: a randomized controlled trial. Obstet Gynecol. 2010;115:317–24. doi: 10.1097/AOG.0b013e3181cbd35f. [DOI] [PubMed] [Google Scholar]
- 23.Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23(2):273–80. doi: 10.1016/S0261-5614(03)00129-8. [DOI] [PubMed] [Google Scholar]
- 24.Downey AE, Chenoweth LM, Townsend DK, et al. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir Physiol Neurobiol. 2007;156:137–46. doi: 10.1016/j.resp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Enright S, Chatham K, Ionescu AA, et al. Inspiratory muscle training improves lung function and exercise capacity in adults with cystic fibrosis. Chest. 2004;126:405–11. doi: 10.1378/chest.126.2.405. [DOI] [PubMed] [Google Scholar]
- 26.Enright SJ, Unnithan VB, Heward C, et al. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys Ther. 2006;86(3):345–54. [PubMed] [Google Scholar]
- 27.DePalo VA, Parker AL, Al-Bilbeisi F, et al. Respiratory muscle strength training with non-respiratory maneuvers. J Appl Physiol. 2004;96(2):731–4. doi: 10.1152/japplphysiol.00511.2003. [DOI] [PubMed] [Google Scholar]
- 28.Triandafilou KM, Kamper DG. Investigation of hand muscle atrophy in stroke survivors. Clin Biomech. 2011 doi: 10.1016/j.clinbiomech.2011.10.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arts IM, Pillen S, Schelhaas HJ, et al. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010;41(1):32–41. doi: 10.1002/mus.21458. [DOI] [PubMed] [Google Scholar]
- 30.Ikezoe T, Mori N, Nakamura M, et al. Atrophy of the lower limbs in elderly women: is it related to walking ability? Eur J Appl Physiol. 2011;111(6):989–95. doi: 10.1007/s00421-010-1728-8. [DOI] [PubMed] [Google Scholar]
- 31.Scholten RR, Pillen S, Verrips A, et al. Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve. 2003;27:693–8. doi: 10.1002/mus.10384. [DOI] [PubMed] [Google Scholar]
- 32.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol. 2001;90:2070–4. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 33.Lee CD, Song Y, Peltier AC, et al. Muscle ultrasound quantifies the rate of reduction of muscle thickness in amyotrophic lateral sclerosis. Muscle Nerve. 2010;42(5):814–9. doi: 10.1002/mus.21779. [DOI] [PubMed] [Google Scholar]
- 34.Arts IM, Overeem S, Pillen S, et al. Muscle changes in amyotrophic lateral sclerosis: a longitudinal ultrasonography study. Clin Neurophysiol. 2011;122:623–8. doi: 10.1016/j.clinph.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Wu JS, Darras BT, Rutkove SB. Assessing spinal muscular atrophy with quantitative ultrasound. Neurology. 2010;75:526–31. doi: 10.1212/WNL.0b013e3181eccf8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arts IM, Overeem S, Pillen S, et al. Muscle ultrasonography to predict survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(5):552–4. doi: 10.1136/jnnp.2009.200519. [DOI] [PubMed] [Google Scholar]
- 37.Arts IM, van Rooij FG, Overeem S, et al. Quantitative muscle ultrasonography in amyotrophic lateral sclerosis. Ultrasound Med Biol. 2008;34:354–61. doi: 10.1016/j.ultrasmedbio.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Yoshioka Y, Ohwada A, Sekiya M, et al. Ultrasonographic evaluation of the diaphragm in patients with amyotrophic lateral sclerosis. Respirology. 2007;12:304–7. doi: 10.1111/j.1440-1843.2006.01029.x. [DOI] [PubMed] [Google Scholar]
- 39.Cartwright MS, Walker FO, Griffin LP, et al. Peripheral nerve and muscle ultra-sound in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:346–51. doi: 10.1002/mus.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schedel H, Reimers CD, Nägele M, et al. Imaging techniques in myotonic dystrophy. A comparative study of ultrasound, computed tomography and magnetic resonance imaging of skeletal muscles. Eur J Radiol. 1992;15(3):230–8. doi: 10.1016/0720-048x(92)90113-n. [DOI] [PubMed] [Google Scholar]
- 41.Hamjian JA, Walker FO. Serial neurophysiological studies of intramuscular botulinum-A-toxin in humans. Muscle Nerve. 1994;17:1385–92. doi: 10.1002/mus.880171207. [DOI] [PubMed] [Google Scholar]
- 42.Moukas M, Vassiliou MP, Amygdalou A, et al. Muscular mass assessed by ultra-sonography after administration of low-dose corticosteroids and muscle relaxants in critically ill hemiplegic patients. Clin Nutr. 2002;21:297–302. doi: 10.1054/clnu.2001.0532. [DOI] [PubMed] [Google Scholar]
- 43.Candow DG, Chilibeck PD, Burke DG, et al. Effect of different frequencies of creatine supplementation on muscle size and strength in young adults. J Strength Cond Res. 2011;25:1831–8. doi: 10.1519/JSC.0b013e3181e7419a. [DOI] [PubMed] [Google Scholar]
- 44.Kushwaha SS, Raichlin E, Sheinin Y, et al. Sirolimus affects cardiomyocytes to reduce left ventricular mass in heart transplant recipients. Eur Heart J. 2008;29:2742–50. doi: 10.1093/eurheartj/ehn407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen PB, Ekelund B, Nielsen FT, et al. Changes in cardiac muscle mass and function in hemodialysis patients during growth hormone treatment. Clin Nephrol. 2000;53:25–32. [PubMed] [Google Scholar]
- 46.Reimers K, Reimers CD, Wagner S, et al. Skeletal muscle sonography: a correlative study of echogenicity and morphology. J Ultrasound Med. 1993;12:73–7. doi: 10.7863/jum.1993.12.2.73. [DOI] [PubMed] [Google Scholar]
- 47.Stevens SS. To honor Feschner and to repeal his law: a power function, not a log function, describes the operating characteristic of a sensory system. Science. 1961;133:80–6. doi: 10.1126/science.133.3446.80. [DOI] [PubMed] [Google Scholar]
- 48.Zaidman CM, Holland MR, Anderson CC, et al. Calibrated quantitative ultrasound imaging of skeletal muscle using backscatter analysis. Muscle Nerve. 2008;38:893–8. doi: 10.1002/mus.21052. [DOI] [PubMed] [Google Scholar]
- 49.Zaidman CM, Connolly AM, Malkus EC, et al. Quantitative ultrasound using backscatter analysis in Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2010;20:805–9. doi: 10.1016/j.nmd.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaidman CM, Malkus EC, Siener C, et al. Qualitative and quantitative skeletal muscle ultrasound in late-onset acid maltase deficiency. Muscle Nerve. 2011;44:418–23. doi: 10.1002/mus.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heckmatt JZ, Pier N, Dubowitz V. Assessment of quadriceps femoris muscle atrophy and hypertrophy in neuromuscular disease in children. J Clin Ultrasound. 1988;16:177–81. doi: 10.1002/jcu.1870160306. [DOI] [PubMed] [Google Scholar]
- 52.Heckmatt JZ, Pier N, Dubowitz V. Measurement of quadriceps muscle thickness and subcutaneous tissue thickness in normal children by real-time ultrasound imaging. J Clin Ultrasound. 1988;16(3):171–6. doi: 10.1002/jcu.1870160305. [DOI] [PubMed] [Google Scholar]
- 53.Heckmatt JZ, Dubowitz V. Ultrasound imaging and directed needle biopsy in the diagnosis of selective involvement in muscle disease. J Child Neurol. 1987;2:205–13. doi: 10.1177/088307388700200307. [DOI] [PubMed] [Google Scholar]
- 54.Heckmatt JZ, Dubowitz V. Detecting the Duchenne carrier by ultrasound and computerized tomography. Lancet. 1983;2:1364. doi: 10.1016/s0140-6736(83)91119-4. [DOI] [PubMed] [Google Scholar]
- 55.Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr. 1982;101:656–60. doi: 10.1016/s0022-3476(82)80286-2. [DOI] [PubMed] [Google Scholar]
- 56.Heckmatt JZ, Dubowitz V, Leeman S. Detection of pathological change in dystrophic muscle with B-scan ultrasound imaging. Lancet. 1980;1:1389–90. doi: 10.1016/s0140-6736(80)92656-2. [DOI] [PubMed] [Google Scholar]
- 57.Brockmann K, Becker P, Schreiber G, et al. Sensitivity and specificity of qualitative muscle ultrasound in assessment of suspected neuromuscular disease in childhood. Neuromuscul Disord. 2007;17:517–23. doi: 10.1016/j.nmd.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Pillen S, Verrips A, van Alfen N, et al. Quantitative skeletal muscle ultrasound: diagnostic value in childhood neuromuscular disease. Neuromuscul Disord. 2007;17:509–16. doi: 10.1016/j.nmd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Zuberi SM, Matta N, Nawaz S, et al. Muscle ultrasound in the assessment of suspected neuromuscular disease in childhood. Neuromuscul Disord. 1999;9:203–7. doi: 10.1016/s0960-8966(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 60.Ahmad N, Bygrave M, Chhem R, et al. High-frequency ultrasound to grade disease progression in murine models of Duchenne muscular dystrophy. J Ultrasound Med. 2009;28:707–16. doi: 10.7863/jum.2009.28.6.707. [DOI] [PubMed] [Google Scholar]
- 61.Laux D, Blasco H, Ferrandis JY, et al. In vitro mouse model in Duchenne muscular dystrophy diagnosis using 50-MHz ultrasound waves. Ultrasonics. 2010;50:741–3. doi: 10.1016/j.ultras.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Walker FO. Shefner J, Dashe JF, editors. Diagnostic ultrasound in neuromuscular disease. [November 28, 2011]; Available at: http://www.uptodate.com/contents/diagnostic-ultrasound-in-neuromuscular-disease?source=search_result&search=neuromuscular+ultrasound&selectedTitle=1%7E150.
- 63.Daube JR, Kumar N. Surface recorded fasciculation potential characteristics. Muscle Nerve. 2011;44:688. [Google Scholar]
- 64.Walker FO, Harpold JG, Donofrio PD, et al. Sonographic imaging of muscle contraction and fasciculations: a comparison with electromyography. Muscle Nerve. 1990;13:33–9. doi: 10.1002/mus.880130108. [DOI] [PubMed] [Google Scholar]
- 65.Misawa S, Noto Y, Shibuya K, et al. Ultrasonographic detection of fasciculations markedly increases diagnostic sensitivity of ALS. Neurology. 2011;77:1532–7. doi: 10.1212/WNL.0b013e318233b36a. [DOI] [PubMed] [Google Scholar]
- 66.Swash M, Carvalho M. Muscle ultrasound detects fasciculations and facilitates diagnosis in ALS. Neurology. 2011;77:1508–9. doi: 10.1212/WNL.0b013e318233b3c4. [DOI] [PubMed] [Google Scholar]
- 67.van Holsbeeck MT, Joseph HI. Sonography of muscle. In: van Holsbeeck MT, Joseph HI, editors. Musculoskeletal ultrasound. Mosby; St Louis (MO: 2001. pp. 23–9. [Google Scholar]
- 68.Shook SJ. Ultrasound of inflammatory myopathies. In: Walker FO, Cartwright MS, editors. Neuromuscular ultrasound. Elsevier; Philadelphia: 2011. pp. 125–31. [Google Scholar]
- 69.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–37. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 70.Cartwright MS, White DL, Demar S, et al. Median nerve changes following steroid injection for carpal tunnel syndrome. Muscle Nerve. 2011;44:25–9. doi: 10.1002/mus.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mallouhi A, Pulzl P, Trieb T, et al. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2006;186:1240–5. doi: 10.2214/AJR.04.1715. [DOI] [PubMed] [Google Scholar]
- 72.Joy V, Therimadasamy AK, Chan YC, et al. Combined Doppler and B-mode sonography in carpal tunnel syndrome. J Neurol Sci. 2011;308:16–20. doi: 10.1016/j.jns.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 73.Yayama T, Kobayashi S, Awara K, et al. Intraneural blood flow analysis during an intraoperative Phalen's test in carpal tunnel syndrome. J Orthop Res. 2010;28:1022–5. doi: 10.1002/jor.21090. [DOI] [PubMed] [Google Scholar]
- 74.El-Karabaty H, Hetzel A, Galla TJ, et al. The effect of carpal tunnel release on median nerve flattening and nerve conduction. Electromyogr Clin Neurophysiol. 2005;45:223–7. [PubMed] [Google Scholar]
- 75.Vögelin E, Nüesch E, Jüni P, et al. Sonographic follow-up of patients with carpal tunnel syndrome undergoing surgical or nonsurgical treatment: prospective cohort study. J Hand Surg Am. 2010;35:1401–9. doi: 10.1016/j.jhsa.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Rodrigues LC, Lockwood DNj. Leprosy now: epidemiology, progress, challenges, and research gaps [review]. Lancet Infect Dis. 2011;11(6):464–70. doi: 10.1016/S1473-3099(11)70006-8. [DOI] [PubMed] [Google Scholar]
- 77.Martinoli C, Derchi LE, Bertolotto M, et al. US and MR imaging of peripheral nerves in leprosy. Skeletal Radiol. 2000;29:142–50. doi: 10.1007/s002560050584. [DOI] [PubMed] [Google Scholar]
- 78.Lolge SJ, Morani AC, Chaubal NG, et al. Sonographically guided nerve biopsy. J Ultrasound Med. 2005;24:1427–30. doi: 10.7863/jum.2005.24.10.1427. [DOI] [PubMed] [Google Scholar]
- 79.Elias J, Jr, Nogueira-Barbosa MH, Feltrin LT, et al. Role of ulnar nerve sonography in leprosy neuropathy with electrophysiologic correlation. J Ultrasound Med. 2009;28:1201–9. doi: 10.7863/jum.2009.28.9.1201. [DOI] [PubMed] [Google Scholar]
- 80.Wilder-Smith EP, Wilder-Smith AJ, Nirkko AC. Skin and muscle vasomotor reflexes in detecting autonomic dysfunction in leprosy. Muscle Nerve. 2000;23:1105–12. doi: 10.1002/1097-4598(200007)23:7<1105::aid-mus14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 81.Buchberger W, Schön G, Strasser K, et al. High-resolution ultrasonography of the carpal tunnel. J Ultrasound Med. 1991;10:531–7. doi: 10.7863/jum.1991.10.10.531. [DOI] [PubMed] [Google Scholar]