Abstract
The quality difference of six varieties Ganoderma lucidum with different origins was investigated in this study by comparing the contents of ganoderic acid A and B, polysaccharide, and triterpenoids. The contents of ganoderic acid A and B in G. lucidum were analyzed by ultra performance liquid chromatography (UPLC). There was higher content of ganoderic acid A in G. lucidum of Dabie Mountain and Longquan. The G. lucidum from Longquan has the highest content of ganoderic acid B. The content of polysaccharide was determined by Anthrone–sulfuric acid method. The highest of polysaccharide content is G. lucidum from Liaocheng. The content of triterpenoid in G. lucidum was quantified by ultraviolet spectrophotometer at 548.1 nm using Ursolic acid as standard. The G. lucidum from Dabie Mountain has the highest content of triterpenoids. In summary, the content of ganoderic acid A and B, polysaccharide, and triterpenoids in G. lucidum with different origins are remarkably different, which may be caused by the conditions of cultivation and geographic environment.
Keywords: Ganoderma lucidum, ganoderic acid A; ganoderic acid B; polysaccharide; triterpenoids; content determination
Introduction
Ganoderma lucidum Karst a medicinal fungus, belonging to Basidiomycetes, Aphyllophorales, Ganodermataceae, is widely used in Oriental medicine to maintain health. With both edible and medicinal value, it has more than 2000 years of history in China. And the annual output of G. lucidum is over 10000 tons. G. lucidum has the function of anti-aging, enhancing immunity, radioprotective, and liver detoxification as well as inhibiting malignant tumor growth (Zhao et al., 1999; Lin, 2007; Lü et al., 2011). The chemical composition of G. lucidum is complex, which contains 11 categories of active substances, such as polysaccharides, triterpenoids, fats and oils, organic germanium, inorganic ions, and sterols. These ingredients are closely related to their pharmacological activity (el-Mekkawy et al., 2007). Polysaccharides and triterpenoids are considered to be its main medicinal components (Wang and Sun, 1990; Zhao et al., 2002; Lin, 2007). The quality of G. lucidum is evaluated though the content of polysaccharide in “Chinese Pharmacopeia,” but Ganoderic acid in Japan (Zhang and Yang, 2006). Ganoderic acid belongs to triterpenoids, which has a wide range of pharmacological active components. It has become a hot study subject in G. lucidum (Chen and Yu, 1990; Yang et al., 1995; Zhou et al., 2004). Ganoderic acid A and B content account for more than half of G. lucidum (Ding et al., 2009), so the determination of ganoderic acid A and B content can be used as the scientific basis for judging quality of G. lucidum.
Because wild fungus resources are limited and artificial cultivation of G. lucidum is affected by origin, cultivation, harvesting conditions, and so on. These factors lead to different quality productions of G. lucidum. We tested the G. lucidum samples from some main producing areas in Shandong Liaocheng, Jiangsu Nantong, Fujian Wuyi Mountain Zhejiang Longquan, Jilin Changbai Mountain, and Anhui Dabie Mountain. The test is focused on the contents of polysaccharide, triterpenoid, and ganoderic acid A and B. The result is to provide the basis of procurement for using G. lucidum as main raw materials.
Materials and Methods
Materials
Ganoderma lucidum karst is used for this experiment which is respectively from the Shandong Liaocheng, Jiangsu Nantong, Fujian Wuyi Mountain, Zhejiang Longquan, Jilin Changbai Mountain, and Anhui Dabie Mountain. In addition to G. lucidum from Shandong Liaocheng cultured on cotton seed, others are wood cultured.
Instruments and reagent
Waters Acquity ultra performance liquid chromatography (UPLC), KQ-250E type ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.), AB265-S/100000 electronic balance (Mettler Toledo). TU-1900 type ultraviolet–visible spectrophotometer (Beijing Purkinje General Instrument Co., Ltd.). Standard ganoderic acid A, ganoderic acid B (purity >98%) provided by Shanghai Tong Tian Biotechnology Co., Ltd. Urolic acid reference substance provided by the national institute for the control of pharmaceutical and biological products. Acetonitrile (chromatographic grade), double distilled water, phosphoric acid, chloroform, petroleum ether, ethyl acetate, methanol, and other reagents are all analytically pure.
Methods
Ganoderic acid A and ganoderic acid B content determination
Chromatographic conditions
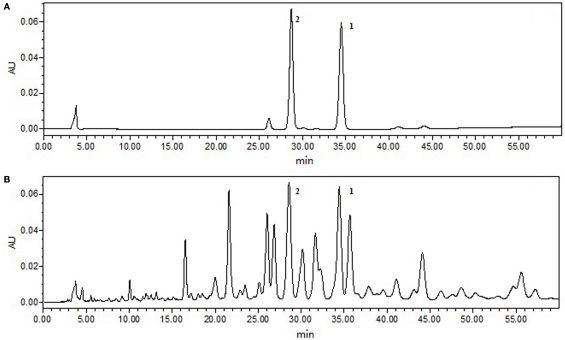
The chromatographic column was Waters X-BridgeC18 (4.6 mm × 150 mm, 3.5 μm); the detection wavelength was set at 252 nm; gradient elution, liquid phase gradient ratio, and time relationships as shown in Table 1; the column temperature was kept at 40°C, and the flow rate was 0.4 ml/min; the injection volume was 10 μl. In these chromatographic conditions, ganoderic acid A and ganoderic acid B Mixed reference substance, and G. lucidum extraction UPLC spectrum diagram as shown in Figure 1.
Table 1.
Effect of different time of elution gradient ratio.
| Time (min) | Acetonitrile | 0.03% phosphoric acid aqueous solution |
|---|---|---|
| 0 | 30 | 70 |
| 5 | 30 | 70 |
| 50 | 35 | 65 |
| 60 | 60 | 40 |
Figure 1.
Mixed reference substance (A) and Ganoderma lucidum extraction (B) UPLC spectra.
Preparation of standard solution and calibration curves
Precisely weighing amount of each reference substance and then put it into a 10-ml volumetric flask respectively, adding methanol to dissolve and to the constant volume, reaching concentrations of 1.802 mg/ml of ganoderic acid A and 1.020 mg/ml of ganoderic acid B. Precisely weigh the liquid reserves each 8.0 ml in 25 ml volumetric flask, add methanol to scale, and shake to make mixed standard stock solution of ganoderic acid A and ganoderic acid B whose concentration are 0.577 and 0.326 mg/ml. Precisely weigh standard stock solution 2.0–10 ml volumetric flask, dilute to the mark with methanol, and then shake to get mixed standard solution of ganoderic acid A and B whose mass concentration are 0.100 and 0.065 mg/ml.
According to the method of Zhao et al. (2009), precisely measure mixed control solution 0.5, 1, 2, 4, 6, 8.0 to 10 ml volumetric flask, dilute to the mark with methanol, and shake to get a series of standard solution. Respectively take a 10-μl sample of the mixed standard solution to analyze under the chromatographic conditions. Draw a standard curve and make regression calculation with mass concentration of the reference as abscissa, peak area as the ordinate, the results show that, the regression equation of ganoderic acid A is Y = 1.9E + 07X-122000 and ganoderic acid B is R2 = 0.9996; Y = 2.0E + 07X + 139583, R2 = 0.9991. Ganoderic acid A and ganoderic acid B respectively in 28.85–400.8, 16.30–260.8 μg/ml are in good linear relation.
Preparation of sample solution
According to the method of Liu (2008), the accurately weighed powder sample (250 mg) was extracted with 100 ml chloroform by the heating reflux for 1 h. The extract was filtered with filter paper which washed by methanol. After evaporating chloroform to dryness by a rotary evaporator, residue was dissolved in methanol in a 5-ml flask, and then filtered through a 0.45-μm membrane. Ten microliters of sample solution were injected into the UPLC system for analyzing.
Precision test
According to the chromatographic conditions, taking ganoderic acid A and ganoderic acid B mixed reference solution to successive injection six time, and recording the peak area. The RSD of peak area of ganoderic acid A and ganoderic acid B was 1.6 and 2.4% respectively.
Repetitive test
Accurately weighed G. lucidum samples of six from Dabie Mountain, according to methods of above, the RSD of contents of ganoderic acid A and ganoderic acid B is 3.1 and 1.8% respectively. The result shows that this method has a good repetitiveness.
Stability experiment
Take sample solution from Anhui Dabie Mountain for the test, which is in 0, 2, 4, 6, 8, 10, 12, 24 h at room temperature and record peak area. The results show that the sample solution has good stability in 24 h and the RSD of peak area of ganoderic acid A and ganoderic acid B were 0.9 and 1.4%.
Recovery rate test
Take nine portions of G. lucidum (0.25 g) from the Wuyishan which the content of ganoderic acid A and ganoderic acid B are known. Divide the portions into three groups and add the control solution of low, middle, high concentrations of ganoderic acid A and ganoderic acid B to each portion, then calculate the recovery rate follow the method above. The result is as shown in Table 2.
Table 2.
The recovery rate test (n = 3).
| Composition | Label | Sample quantity (mg) | Adding amount (mg) | Recovery rate (%) | RSD (%) |
|---|---|---|---|---|---|
| Acid A | 1 | 0.5385 | 0.2481 | 95.4 | 1.41 |
| 2 | 0.5293 | 0.2417 | 97.6 | ||
| 3 | 0.5236 | 0.2352 | 94.4 | ||
| 4 | 0.5427 | 0.5228 | 97.7 | 1.02 | |
| 5 | 0.5328 | 0.5173 | 95.9 | ||
| 6 | 0.5343 | 0.5284 | 98.6 | ||
| 7 | 0.5422 | 0.7362 | 98.1 | 1.28 | |
| 8 | 0.5375 | 0.7340 | 100.1 | ||
| 9 | 0.5432 | 0.7274 | 99.4 | ||
| Acid B | 1 | 0.1745 | 0.0936 | 93.7 | 1.28 |
| 2 | 0.1715 | 0.0915 | 94.2 | ||
| 3 | 0.1697 | 0.0867 | 96.0 | ||
| 4 | 0.1759 | 0.1864 | 95.3 | 1.75 | |
| 5 | 0.1726 | 0.1735 | 96.9 | ||
| 6 | 0.1731 | 0.1726 | 98.7 | ||
| 7 | 0.1757 | 0.2619 | 97.1 | 1.19 | |
| 8 | 0.1742 | 0.2700 | 98.6 | ||
| 9 | 0.176 | 0.2623 | 99.4 |
Determination of polysaccharide
Sample preparation
Accurately weighing 2.0 g of power sample, extracted by Soxhlet extractor with 90 ml water in the round-bottom flask, and heated under reflux for 6 h, then transfer the extract to a 100 ml flask, add water to the scale. Precisely measured 10 ml extract, added ethanol 150 ml, placed for 12 h at 4°C. The extract separated by centrifugal precipitation, the precipitate is dissolve in water in a 50-ml flask as the sample solution.
Preparation of standard curve
d-Glucose anhydrous (25 mg) is accurately weighed and then dissolved in 25 ml of double distilled water, 1 ml solution is drawn to dilute 100 times with double distilled water to produce corresponding stock standard solution (0.01 mg/ml). Accurately draw glucose control solution 0.2, 0.4, 0.6, 0.8, 1, 1.2 ml to the 10 ml test tube, add water to the volume of 2.0 ml, precisely add anthrone–sulfuric acid [1.0 g of anthrone was dissolved in sulfuric acid (80%) in a 100 ml flask] 6 ml, heated for 15 min, then remove and put in ice-water to cool for 15 min, with the corresponding reagent as control. Determine the absorbance in the 625 nm wavelength and make it as the ordinate, concentration as abscissa to establish a standard curve.
Precisely measure the sample solution 2 ml, put it into 10 ml test tube, Follow the method of establishing the standard curve, as the “precisely add anthrone–sulfuric acid 6 ml” begin to determine absorbance. Then calculate the content of the polysaccharide according to the standard curve.
Triterpenoid determination
Preparation of standard curve
Accurately weigh 1.15 mg of the ursolic acid, dissolve in 10 ml ethyl acetate to produce corresponding stock standard solution. Take 0, 0.10, 0.20, 0.40, 0.60, 0.80, 1, and 1.20 ml control solution to dryness in a water bath at 100°C. Then add 0.40 ml 5% vanillin–acetic acid solution and 1 ml perchloric acid, at 60°C water bath heating for 15 min then move it into ice-water bath, add 5 ml acetic acid, place it at room temperature for 15 min. Determine its absorbance in the 548.1 nm.
Draw standard curve based on the determination result. Standard weight in 0–0.14 mg range showed a good linear relationship with the absorbance value, the linear regression equation was Y = 0.2158X − 0.001 8, correlation coefficient r = 0.9991.
Extraction of triterpenoids
Triterpenoid extracts were prepared by 95% alcohol extraction as described before (Hou and Liu, 2010). Accurately weigh 200 g of dry G. lucidum powder for extraction. Take G. lucidum extracts about 10 mg to dissolve in 10 ml ethyl acetate, and determine its absorbance following the method above.
Results
Content determination of different origin of ganoderic acid A and ganoderic acid B
The content of ganoderic acid A and ganoderic acid B is as shown in Table 3. The content of ganoderic acid A of Dabie Mountain is the highest (7.254 mg/g); the followed behind is Longquan (6.658 mg/g), Shandong (1.959 mg/g). Ganoderic acid B content for Longquan (4.574 mg/g) is the highest.
Table 3.
Six kinds of ganoderic acid A and ganoderic acid B content.
| Category | Dabie mountain (mg/g) | Longquan (mg/g) | Nantong (mg/g) | Changbai mountain (mg/g) | Wuyi mountain (mg/g) | Liaocheng (mg/g) |
|---|---|---|---|---|---|---|
| Ganoderic acid A | 7.254 | 6.658 | 3.563 | 2.618 | 2.154 | 1.959 |
| Ganoderic acid B | 0.337 | 4.574 | 0.574 | 1.450 | 0.698 | 2.078 |
Content determination of polysaccharide
The content of polysaccharide has significant differences. The highest content of G. lucidum polysaccharides is in Shandong, followed by Wuyi Mountain (7.38%); the lowest (1.85%) is in Dabie Mountain (see Table 4).
Table 4.
Polysaccharide content of Ganoderma lucidum in different regions.
| Sample | Mass fraction (%) |
|---|---|
| Dabie mountain | 1.85 |
| Longquan | 5.88 |
| Nantong | 3.30 |
| Changbai mountain | 6.03 |
| Wuyi mountain | 7.38 |
| Liaocheng | 9.08 |
| The SDs | 2.64 |
Content determination of triterpenoid
The content of triterpenoid from different origins has been shown in Table 5. The highest content of triterpenoid of G. lucidum is cultivated in Dabie mountain (5.38%), the lowest is in Longquan (2.07%). The difference between them is significant.
Table 5.
Triterpenoid content of Ganoderma lucidum in different regions.
| Sample | Mass fraction (%) |
|---|---|
| Dabie mountain | 5.38 |
| Longquan | 2.07 |
| Nantong | 3.95 |
| Changbai mountain | 3.06 |
| Wuyi mountain | 4.33 |
| Liaocheng | 3.51 |
| The SDs | 1.13 |
Discussion
Triterpenoid and polysaccharide are as the basis of quality of Ganoderma product. Triterpenoid has significant effect in immune regulation and antitumor (Morigiwa et al., 1986; Ceng and Bao, 2004; Huang and Xiao, 2008); its content decides the antitumor effect of G. lucidum products. Polysaccharide has the function of improving immunity, antitumor effects, removing free radical, hypoglycemic, lipid-lowering (Xu and Xu, 2003), and other functions. In recent years, the study has attracted many researchers (Lin et al., 2002; Cao and Lin, 2004). Ganoderic acid B and lucidenic acid A have the inhibitory activity against HIV-1 protease (Min et al., 1998). For the reason of above, we got the main medical ingredients of G. lucidum from six origins.
Through this experimental data, both the triterpenoid and ganoderic acid B are the highest in G. lucidum from Dabie mountain. But the highest polysaccharide of artificial Cultivation G. lucidum is from Liaocheng. There are several reasons impacting the accumulation of polysaccharides and triterpenoids. First, the same species of G. lucidum from different origins due to the culture medium, the growth environment, different stages of growth, and covered soil or not, will have different polysaccharide content (Li et al., 1997; Ding et al., 1999; Wei et al., 2006; Chen et al., 2009; Ye et al., 2010). Second, the content of polysaccharide and triterpenoid is different in varieties of G. lucidum (Liu et al., 1999; Xing and Jiang, 2001; Xing et al., 2004; Zheng et al., 2007). Third, some research suggests that the different drying methods have some effect in the content of polysaccharide of G. lucidum. The own drying for G. lucidum is significantly higher than the direct drying in polysaccharide content, which is considered to be hydrolysis, induced by hydrolytic enzymes (Xing and Jiang, 2001). The content of polysaccharide is higher in asporogenous G. lucidum than in sporiparous G. lucidum. The different parts of G. lucidum have different content of polysaccharide (Shi et al., 2010). Maybe due to the superior cultivation environment in Dabie Mountain, the triterpenoid content is highest in G. lucidum. There maybe some relations between the high polysaccharide content and the culture medium in Liaocheng where the only G. lucidum were cultured on cotton seed.
Conclusion
This study gives a comprehensive assessment of the G. Lucidum in terms of its efficacy and material, it provides shallow datum for the G. Lucidum quality from different areas. The result of the experiment indicated that there was no distinction correlation between polysaccharide and triterpenoid contents. Because of the difference in active ingredient from different origins, we can choose the G. lucidum according to our purposes.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank professor Ding Zimian and his laboratory members for the help of this research, the companies provided the free samples and Beijing Hengjitang Medical Technology Development Co., Ltd.
References
- Cao Q. J., Lin Z. B. (2004). Antitumor and anti-angiogenic activity of Ganoderma lucidum polysaccharides peptide. Acta Pharmacol. Sin. 25, 833–838 [PubMed] [Google Scholar]
- Ceng X. L., Bao H. Y. (2004). Advances of researches on triterpene constituents and pharmacology of Ganoderma lucidum. J. Fungal Res. 2, 68–77 [Google Scholar]
- Chen R. Y., Yu D. Q. (1990). The research advance on the constituents of Ganoderma lucidum. Yao Xue Xue Bao 25, 940–953 [PubMed] [Google Scholar]
- Chen Z. L., Wen L., Jia Z. H. (2009). Effect of some trace elements and vitamins on contents of polysaccharide and acid of Ganoderma lucidum. J. Anhui Agric. Sci. 37, 2041–2043 [Google Scholar]
- Ding P., Qiu J. Y., Liang Y. J., Wang H. L. (2009). Chromatographic fingerprints of triterpenoid constituents of Ganoderma lucidum. Zhongguo Zhong Yao Za Zhi 34, 2356–2359 [PubMed] [Google Scholar]
- Ding P., Zeng Y. E., Lai X. P., Xu H. H. (1999). Effect of places and stages on the contents of Ganoderma lucidum. Chin. Herb. Med. 22, 271–272 [PubMed] [Google Scholar]
- el-Mekkawy S., Meselhy M. R., Nakamura N. (2007). Anti – HIV-1 and anti – HIV-1 protease substances from Ganoderma lucidum. Phytochemistry 49, 1651–1657 10.1016/S0031-9422(98)00254-4 [DOI] [PubMed] [Google Scholar]
- Hou M. N., Liu J. (2010). Ganoderma triterpenoids extraction and determination of the total triterpenoid. Res. Pract. Chin. Med. 24, 70–71 [Google Scholar]
- Huang Y. J., Xiao G. L. (2008). The progress of pharmacology on Ganoderma triterpene. Cuiding J. Tradit. Chin. Med. Pharm. 14, 87–97 [Google Scholar]
- Li X. H., He Y. Q., Li R. Z. (1997). Determination of content of polysaccharides in different habitats. Zhongguo Zhong Yao Za Zhi 22, 83–84 [Google Scholar]
- Lin L., Fang X. H., Wu D. (2002). A survey on effective constituents of Ganoderma lucidum. Chin. Tradit. Pat. Med. 24, 293–296 [Google Scholar]
- Lin Z. B. (2007). Modern Research of Ganoderma. Beijing: Medical University Press [Google Scholar]
- Liu C. (2008). Studies on the Chemical Constituents of Ganoderma Sinense & Ganoderma Tsugae and the Content Determinations of Triterpene Acids in Ganoderma Specimens, Master’s thesis. Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College; 12 [Google Scholar]
- Liu Y. P., Cai H. J., Lin L. L. (1999). Determination of polysaccharides in different kinds of culture of Ganoderma lucidum. J. Guangzhou Univ. Tradit. Chin. Med. 1, 54–55 [Google Scholar]
- Lü C. T., Yao X. Y., Sun C. (2011). Progress of researches on main active substances and pharmacology of Ganoderma Lucidum. J. Anhui Agric. Sci. 17, 50–51 [Google Scholar]
- Min B. S., Nakamura N., Miyashiro H., Bae K. W., Hattori M. (1998). Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 46, 1607–1612 10.1248/cpb.46.1607 [DOI] [PubMed] [Google Scholar]
- Morigiwa A., Kitabatake K., Fujimoto Y., Ikekawa N. (1986). Angiotensin conv-eating enzyme-inhibitory triterpenes from Ganoderma lucidum. Chem. Pharm. Bull. 34, 3025–3028 10.1248/cpb.34.3025 [DOI] [PubMed] [Google Scholar]
- Shi J. Q., Zhang L. P., Yang C. Q., Wang Y. F. (2010). Study on polysaccharide content in two different variety of Ganoderma lucidum. Chin. J. Exp. Tradit. Med. Formulae 16, 104–106 [Google Scholar]
- Wang H. C., Sun S. Y. (1990). Analysis on the amino acids and trace elements in wild and artificial cultivation Ganoderma lucidum collected from Taishan. Tradit. Chin. Med. Pat. Prescript. 12, 14 [Google Scholar]
- Wei H. P., Li X. G., Pu S. C., Liu Z. Y., Liu Y. Q. (2006). Changing tendency of ganoderan content in Ganoderma lucidum during fungus growth. Guiding J. Tradit. Chin. Med. Pharm. 34, 10–12 [Google Scholar]
- Xing Z. T., Jiang H. H. (2001). Comparative study on polysaccharide contents in different Ganoderma Species. Edible Fungi 6, 4–5 [Google Scholar]
- Xing Z. T., Yu Q. H., Zhang J. S., Pan Y. (2004). Comparative study on triterpenes in different Ganoderma Species. Chin. Herb. Med. 27, 575–576 [PubMed] [Google Scholar]
- Xu L. C., Xu C. S. (2003). Determination of content of polysaccharides of 18 varieties of Ganoderma lucidum karst with different origins. World Sci. Technol. Mod. Tradit. Chin. Med. Mater. Med. 6, 57–60 [Google Scholar]
- Yang Y. Z., Su Q. H., Dong D. C. (1995). Fruiting bodies of Ganoderma triterpenoids for mice exclude the effect of acute liver disorders. Beijing Da Xue Xue Bao 24, 50–53 [Google Scholar]
- Ye M. F., Qin Y. R., Liu X. H., Qin B. S. (2010). Comparative analysis of Ganoderma lucidum polysaccharide content in several wild and artificial cultivation Ganoderma lucidum collected from northwest of Guangxi. Zhong Cao Yao 14, 186–188 [Google Scholar]
- Zhang X. Y., Yang C. Q. (2006). Chemical constituents and pharmacological activities of Ganoderma lucidum. Foreign Medical Sciences 21, 152–155 [Google Scholar]
- Zhao J., Chen X. H., Bi K. S. (2009). Simultaneous HPLC determination of four triterpenoid acids in Ganoderma lucidum. China Journal of Chinese Materia Medica 34, 2220–2222 [PubMed] [Google Scholar]
- Zhao D. X., Yang X. L., Wang B., Zhu H. (1999). Certain progress in the study of Ganoderma Lucidum. Acta Edulis Fungi 6, 59–64 [Google Scholar]
- Zhao M. W., Wang C. G., Bao P., Xing Z. T., Liang W. Q., Yu J. Q., Shang X. D., Men D. Y., Wang N., Pan Y. J. (2002). Triterpenes and polysaccharide contents of liquid-culturing mycelium of different Ganoderma lucidum. Edible Fungi China 22, 43–46 [Google Scholar]
- Zheng L. Y., Huang X. Q., Zen J., Xu X. Y., Jiang N., Luo X. (2007). Compared and analysed polysaccharides and triterpene content of different Ganoderma strains. Sichuan Da Xue Xue Bao Yi Xue Ban 44, 1121–1124 [Google Scholar]
- Zhou C. Y., Tang Q. J., Yang Y., Guo Q., Bai Y. Q., Pan Y. J. (2004). Antitumor effect of ganoderic acid from Ganoderma lucidum. Mycosystema 23, 275–279 [Google Scholar]