Abstract
Introduction: Despite the importance of dopamine neurotransmission in schizophrenia, very few studies have addressed anomalies in the mesencephalic dopaminergic neurons of the substantia nigra/ventral tegmental area (SN/VTA). Tyrosine hydroxylase (TH) is the rate-limiting enzyme for the production of dopamine, and a possible contributor to the anomalies in the dopaminergic neurotransmission observed in schizophrenia. Objectives: In this study, we had three objectives: (1) Compare TH expression (mRNA and protein) in the SN/VTA of schizophrenia and control postmortem samples. (2) Assess the effect of antipsychotic medications on the expression of TH in the SN/VTA. (3) Examine possible regional differences in TH expression anomalies within the SN/VTA. Methods: To achieve these objectives three independent studies were conducted: (1) A pilot study to compare TH mRNA and TH protein levels in the SN/VTA of postmortem samples from schizophrenia and controls. (2) A chronic treatment study was performed in rodents to assess the effect of antipsychotic medications in TH protein levels in the SN/VTA. (3) A second postmortem study was performed to assess TH and phosphorylated TH protein levels in two types of samples: schizophrenia and control samples containing the entire rostro-caudal extent of the SN/VTA, and schizophrenia and control samples containing only mid-caudal regions of the SN/VTA. Results and Conclusion: Our studies showed impairment in the dopaminergic system in schizophrenia that could be mainly (or exclusively) located in the rostral region of the SN/VTA. Our studies also showed that TH protein levels were significantly abnormal in schizophrenia, while mRNA expression levels were not affected, indicating that TH pathology in this region may occur posttranscriptionally. Lastly, our antipsychotic animal treatment study showed that TH protein levels were not significantly affected by antipsychotic treatment, indicating that these anomalies are an intrinsic pathology rather than a treatment effect.
Keywords: neuropsychiatric disorders, postmortem studies, human brain, rat brain, western-blot
Introduction
Tyrosine hydroxylase (TH) is a crucial enzyme in the production of dopamine and other catecholamines. In the substantia nigra/ventral tegmental area (SN/VTA), dopamine is the final product of cells that express TH, and these cells are the largest source of dopamine in the brain. Studies of TH in the SN/VTA of schizophrenia subjects are almost anecdotal, with just one study that addressed enzymatic activity (Toru et al., 1988), and two studies that addressed mRNA levels (Ichinose et al., 1994; Mueller et al., 2004). However, no studies have assessed TH protein levels in the SN/VTA in schizophrenia. In addition, recent studies on the synthesis of TH have shown that the regulation of the production of TH is very different in the SN/VTA than in other catecholaminergic neurons (Wong and Tank, 2007; Chen et al., 2008; Tank et al., 2008). These studies also showed that posttranscriptional regulations have a crucial role in the synthesis of TH in the SN/VTA (Chen et al., 2008; Tank et al., 2008). Posttranscriptional modulation occurs after the production of mRNA is completed, and includes the stabilization of the mRNA, the attachment to polyribosomes, and the regulation of the efficacy of translation into protein (Tank et al., 2008; Lenartowski and Goc, 2011). For all these reasons, we believe that is necessary to study TH protein levels in the SN/VTA in schizophrenia, to assess the possibility of pathologies that could be located in posttranscriptional steps in the synthesis of TH, rather than at the transcription level.
In addition to its production, TH protein activity in the SN/VTA is modulated by the phosphorylation of certain serine sites in the first 40 amino acids of the N-terminus of the TH protein (Haycock, 1990; Nakashima et al., 2009). Of these phosphorylation sites, serine 40 has been shown to be the most important in the regulation of TH activity in the brain (Nakashima et al., 2009). The phosphorylation of this site is a complex process that requires proper conservation of the amino acid residues flanking this serine residue, and phosphorylation of this site produces the disinhibition of the catalytic subunit of TH, thus allowing the production of dopamine (Nakashima et al., 2009).
Genetic association studies in schizophrenia have produced somewhat inconclusive results (Meloni et al., 1995; Wei et al., 1995; Jonsson et al., 1996, 1998; Thibaut et al., 1997; Burgert et al., 1998; Ishiguro et al., 1998; Kunugi et al., 1998; Kurumaji et al., 2001; Ota et al., 2001; Chao and Richardson, 2002; Pae et al., 2003; Hoogendoorn et al., 2005; Jacewicz et al., 2008; Talkowski et al., 2008; Andreou et al., 2009). However, some of these studies have shown consistent data in different cohorts of patients, indicating an association of certain mutations in the first intron of the TH gene with a higher risk of schizophrenia (Meloni et al., 1995; Wei et al., 1995; Kurumaji et al., 2001; Pae et al., 2003; Jacewicz et al., 2008). Interestingly this first intron of the TH gene has been postulated to be a modulatory region for the synthesis of TH (Pae et al., 2003; Jacewicz et al., 2008).
Previous studies assessing anomalies of the dopaminergic system in schizophrenia have heavily focused their attention on different aspects of the dopamine neurotransmission at the postsynaptic level, and studies of molecules involved in the transport and degradation of dopamine (see as reviews, Abi-Dargham et al., 2010; Perez-Costas et al., 2010; Tost et al., 2010). Another important aspect of the dopaminergic neurotransmission, which originates in neurons in the SN/VTA, is the topographical organization of the dopamine-producing cells in this complex, which defines the preferential terminal target of their projections (e.g., the mesolimbic, mesocortical, or nigro-striatal pathways; Fallon and Moore, 1978a,b; Fallon et al., 1978a,b; Porrino and Goldman-Rakic, 1982; Gaspar et al., 1992; McRitchie et al., 1995; Haber and Fudge, 1997; Haber and Gdowski, 2004; Halliday, 2004). Some studies have found anomalies in TH in the terminals of the nigro-striatal pathway in schizophrenia (Roberts et al., 2009) and in the terminal fields of the mesocortical pathway (Akil et al., 1999, 2000), but none of theses studies have assessed the cells of origin of the dopaminergic inputs to these brain regions. This brought our attention to the importance of assessing regional anomalies in TH synthesis within the SN/VTA in schizophrenia.
Materials and Methods
Ethics statement
All the human brain samples used in this study were obtained from the Maryland Brain Collection (Maryland Psychiatric Research Center, University of Maryland School of Medicine) with permission from the Maryland Brain Collection Steering Committee. All experimental procedures were also approved by the University of Alabama at Birmingham Institutional Review Board (protocol # N110505002) and in accordance with The Code of Ethics of the World Medical Association.
All the animal experiments were conducted at the University of Maryland following a protocol approved by the Institutional Animal Care and Use Committee (protocol # 0705010), in accordance with National Institutes of Health guidelines regarding the care and use of animals for experimental procedures.
Postmortem human brain samples
For all the postmortem human cases used in this study, two independent senior psychiatrists established DSM-IV diagnoses based on the review of all available medical records and the data obtained from the Structured Clinical Interview for DSM-IIIR (Spitzer et al., 1992) and DSM-IV Axis I disorders with the next of kin. Control cases had enough information from next of kin and medical records to discard any major neurological or neuropsychiatric disorders.
Two types of samples were used in this study: samples containing the entire rostro-caudal extent of the SN/VTA (pilot study and second postmortem study), and samples containing only mid-caudal SN/VTA (second postmortem study). In both cases samples were frozen on dry ice and stored at −80°C until use. Samples were sectioned on a cryostat at −20°C obtaining five parallel series of 16 μm thick sections. Four parallel series were collected on superfrost slides, while the fifth series was collected in a tube for protein extraction. In all cases, series #1 was stained with thionin (Nissl stain) to assess possible morphological anomalies. Only cases that presented good preservation of the SN/VTA region were included in the study. The parameters used in this first inspection under the microscope included discarding any tissue sample that presented extensive autolysis of cells, cell shrinkage, and general bad morphological preservation. In addition, all the cases that were preselected after this microscopic assessment were also checked for abnormally low actin levels (as an indicator of poor protein preservation). Rostral and caudal limits for the SN/VTA were determined using the Paxinos and Huang (1995) brainstem atlas. For samples containing only mid-caudal regions the rostral limit was set at the starting point of the substantia nigra compacta (SNc) pars ventralis as defined by McRitchie et al. (1995).
Two different sets of experiments were performed with human brain samples: (a) a pilot study in which TH mRNA and TH protein levels were assessed in samples from the same cases (see Table 1); (b) a second postmortem study of total TH protein levels and phosphorylated TH (pTH) levels in samples containing the entire extent or only mid-caudal regions of the SN/VTA (see Table 2). In both tables information on antipsychotic medication taken at time of death is provided. However, it is likely that during the duration of illness most of the schizophrenia cases were exposed to multiple antipsychotic therapies. In addition, in Table 2 there are two schizophrenia cases labeled as “unknown medication at time of death.” This refers to the fact that at time of death, it was not known if they were actively taking any antipsychotic medication, although they did not meet the criteria to be classified as “off-drugs” (i.e., more than 3 or 6 months reportedly not taking antipsychotics).
Table 1.
Pilot study: demographic table and clinical data in schizophrenia and controls.
| Case # | Age (years) | Race | gender | COD | PMI (hours) | pH | SZ subtype | APD type | TD |
|---|---|---|---|---|---|---|---|---|---|
| SCHIZOPHRENIA (N = 6) | |||||||||
| SZ1 | 53 | C | M | ASCVD | 14 | 6.01 | Undifferentiated | Quetiapine (A) | Yes |
| SZ2 | 48 | AA | M | Narcotic intoxication | 24 | 7.07 | NOS | Quetiapine (A) | un |
| SZ3 | 83 | C | M | Electrocution | 18 | 6.98 | NOS | Quetiapine (A) | un |
| SZ4 | 46 | C | F | DVT | 25 | 6.18 | NOS | Clozapine (A) | un |
| SZ5 | 54 | C | M | Suicide (bleeding) | 19 | 6.58 | NOS | Quetiapine (A) | No |
| SZ6 | 42 | AA | F | ASCVD/MI | 13 | 5.86 | Chronic paranoid | Fluphenazine and Quetiapine (T + A) | Yes |
| Mean | 54.33 | 18.80 | 6.45 | ||||||
| SD | 14.73 | 4.96 | 0.51 | ||||||
| CONTROLS (N = 5) | |||||||||
| C1 | 49 | AA | F | Asthma | 19 | 6.19 | |||
| C2 | 51 | AA | M | ASCVD | 19 | 7.18 | |||
| C3 | 45 | C | F | Cardiac arrhythmia | 16 | 6.69 | |||
| C4 | 62 | C | M | Cardiac arrest | 19 | 6.48 | |||
| C5 | 67 | AA | M | ASCVD | 17 | 6.68 | |||
| Mean | 54.80 | 18.0 | 6.64 | ||||||
| SD | 9.28 | 1.41 | 0.36 | ||||||
A, atypical antipsychotic; AA, African American; APD, antipsychotic drug; ASCVD, arteriosclerotic cardiovascular disease; C, Caucasian; COD, cause of death; DVT, deep vein thrombosis; F, female; M, male; MI, myocardial infarction; NOS, not otherwise specified; PMI, postmortem interval; SD, standard deviation; SZ subtype, schizophrenia subtype; T, typical antipsychotic; TD, tardive dyskinesia; un, unknown.
Table 2.
Demographic table and clinical data second postmortem study.
| Case # | Age (years) | Race | Gender | COD | PMI (hours) | pH | SZ subtype | APD type | TD |
|---|---|---|---|---|---|---|---|---|---|
| SCHIZOPHRENIA: ROSTRO-CAUDAL SAMPLES (N = 8) | |||||||||
| SZ1 | 53 | C | M | ASCVD | 14 | 6.01 | Undifferentiated | Quetiapine (A) | Yes |
| SZ2 | 48 | AA | M | Narcotic intoxication | 24 | 7.07 | NOS | Quetiapine (A) | un |
| SZ3 | 83 | C | M | Electrocution | 18 | 6.98 | NOS | Quetiapine (A) | un |
| SZ4 | 46 | C | F | DVT | 25 | 6.18 | NOS | Clozapine (A) | un |
| SZ5 | 54 | C | M | Suicide (bleeding) | 19 | 6.58 | NOS | Quetiapine (A) | No |
| SZ6 | 42 | AA | F | ASCVD/MI | 13 | 5.86 | Chronic paranoid | Fluphenazine and Quetiapine (T + A) | Yes |
| SZ7 | 25 | C | M | Suicide (hanging) | 20 | 5.90 | Chronic paranoid | Risperidone (A) | Yes |
| SZ8 | 77 | C | M | ASCVD | 17 | 6.84 | CUT | Risperidone (A) | un |
| Mean | 53.50 | 18.75 | 6.43 | ||||||
| SD | 18.70 | 4.27 | 0.50 | ||||||
| SCHIZOPHRENIA MID-CAUDAL SAMPLES (N = 8) | |||||||||
| SZ9 | 42 | AA | M | Suicide (stabbing) | 10 | 7.10 | Chronic paranoid | Haloperidol (T) | Yes |
| SZ10 | 69 | AA | M | ASCVD | 14 | 6.71 | CUT | Haloperidol (T) | No |
| SZ11 | 48 | C | F | Respiratory arrest | 21 | 7.80 | CUT | Clozapine (A) | No |
| SZ12 | 42 | AA | M | Pulmonary embolism | 16 | 7.10 | Chronic paranoid | Haloperidol and Chlorpromazine (T) | Yes |
| SZ13 | 77 | AA | F | ASCVD | 17 | 6.20 | NOS | UNK | un |
| SZ14 | 67 | AA | M | ASCVD | 7 | 7.17 | CUT | UNK | un |
| SZ15 | 47 | AA | M | ASCVD | 20 | 6.95 | Chronic paranoid | Fluphenazine (T) | No |
| SZ16 | 37 | C | M | Suicide (electrocution) | 14 | 6.68 | Chronic paranoid | Risperidone and Fluphenazine (T + A) | No |
| Mean | 53.60 | 14.90 | 6.96 | ||||||
| SD | 15.00 | 4.73 | 0.46 | ||||||
| CONTROLS: ROSTRO-CAUDAL SAMPLES (N = 6) | |||||||||
| C2 | 51 | AA | M | ASCVD | 19 | 7.18 | |||
| C3 | 45 | C | F | Cardiac arrhythmia | 16 | 6.69 | |||
| C4 | 62 | C | M | Cardiac arrest | 19 | 6.48 | |||
| C5 | 67 | AA | M | ASCVD | 17 | 6.68 | |||
| C6 | 77 | C | M | ASCVD | 16 | 6.80 | |||
| C7 | 33 | AA | M | Drowning | 21 | 7.20 | |||
| Mean | 55.80 | 18.00 | 6.84 | ||||||
| SD | 16.00 | 2.00 | 0.29 | ||||||
| CONTROLS: MID-CAUDAL SAMPLES (N = 6) | |||||||||
| C8 | 28 | C | M | Gunshot wound | 7 | 6.82 | |||
| C9 | 35 | C | M | MVA | 4 | 6.70 | |||
| C10 | 41 | AA | F | Cardiac disease | 21 | 6.73 | |||
| C11 | 48 | C | M | Aneurysm | 19 | 6.51 | |||
| C12 | 72 | C | F | MVA | 17 | 6.83 | |||
| C13 | 45 | C | F | Pulmonary embolism | 12 | 5.74 | |||
| Mean | 44.80 | 13.30 | 6.56 | ||||||
| SD | 15.10 | 6.83 | 0.42 | ||||||
A, atypical antipsychotic; AA, African American; APD, antipsychotic drug; ASCVD, arteriosclerotic cardiovascular disease; C, Caucasian; COD, cause of death; CUT, chronic undifferentiated type; DVT, deep vein thrombosis; F, female; M, male; MI, myocardial infarction; NOS, not otherwise specified; MVA, motor vehicle accident; PMI, postmortem interval; SD, standard deviation; SZ subtype, schizophrenia subtype; T, typical antipsychotic; TD, tardive dyskinesia; un, unknown; UNK, unknown medication at time of death.
Animal samples
A total of 27 adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were used in this study to test the effect of chronic treatment with antipsychotic drugs on TH protein levels. Animals were allowed to adapt to the animal room for 1 week prior to starting any treatment, and water consumption was monitored to calculate initial treatment doses. Animals were then randomly assigned to one of the three treatment groups (n = 9 per group): haloperidol (1.5 mg/kg/day), olanzapine (6 mg/kg/day), or control. Antipsychotics were administered for 3 weeks in drinking water, adjusting the doses to body weight and daily liquid consumption as previously described in Perez-Costas et al. (2008). At endpoint, animals were decapitated and trunk blood was collected to monitor plasma drug levels. Brains were immediately removed from the skull, frozen in dry ice, and stored at −80°C until use. Four parallel series at the level of the SN/VTA were collected on superfrost slides, while the fifth series was collected in a tube for protein extraction. For all animals, series #1 was stained with thionin to assess possible morphological anomalies. No morphological anomalies were found in any of the samples used.
In situ hybridization
In situ hybridization was performed for the postmortem SN/VTA samples included in the pilot study (Table 1). Slides containing sections at the level of the rostral, medial, and caudal SN/VTA were selected from each case to perform this technique. To select the sections, series #1 (stained with thionin) was used to accurately identify matching rostral, medial, and caudal areas of the SN/VTA for each case. A 48-mer antisense oligonucleotide probe that hybridizes to nucleotides 32–79 of the human TH mRNA sequence was used. This sequence is common to all three mRNA transcript variants identified for the human TH (GenBank accession numbers: NM_199292; NM_000360; NM_199293). Probe labeling, hybridization, and post-hybridization rinses, were performed as described in Gao et al. (1998). Briefly, oligonucleotide probes were labeled with 35S-dATP at the 3′-end using terminal transferase. The sections were hybridized overnight at 37°C with an hybridization buffer containing 50% deionized formamide, 4× sodium saline citrate (SSC), Denhardt’s solution, 10× dextran sulfate, yeast tRNA (250 μg/ml), single strand salmon DNA (500 μg/ml), and 100 mM dithiothreitol (DTT). After hybridization, the sections were sequentially rinsed in 1× SSC containing 10 mM DTT at 55°C, and 1× SSC at room temperature. Finally, the sections were gradually dehydrated in ethanol, air dried, and autoradiograms were generated by exposing Kodak BioMax MR films (Kodak, Rochester, NY, USA) along with [14C] microscale standards (Amersham Biosciences, Little Chalfont, UK) for 14 days. Films were developed using Kodak D-19 developer and fixer, scanned at 600 dpi, and optical density was measured using Image Pro-Plus 6.2 software (Media Cybernetics, Bethesda, MD, USA). For each case, two sections from each rostro-caudal region of the SN/VTA (rostral, mid, caudal), were randomly selected for analysis (i.e., six sections total were analyzed per case for the entire rostro-caudal SN/VTA).
Western-blot
Western-blot assays were performed for human postmortem SN/VTA protein samples (pilot study and second postmortem study; see Tables 1 and 2), and for rat SN/VTA protein samples (animal study). In all cases the protein extraction procedure and sample handling were identical. One entire series of the SN/VTA from each case was collected on a tube. This series contained sections (80 μm apart each) from the entire mid-caudal regions of the SN/VTA (mid-caudal cases for the second postmortem study), or from the entire rostro-caudal extent of the SN/VTA (all the other samples). The only differences were the antibodies used for the detection of TH. For the human postmortem studies a mouse monoclonal anti-TH antibody (Sigma-Aldrich, St. Louis, MO, USA) diluted 1:10,000 was used, while for the animal study a mouse monoclonal anti-TH antibody (Millipore, Billerica, MA, USA) diluted 1:20,000 was used. In addition, a rabbit polyclonal anti-phospho-Ser40 TH antibody (PhosphoSolutions, Aurora, CO, USA) diluted 1:4,000 was used in human samples (second postmortem study) for the detection of pTH. Finally, reblots for actin were performed in all the western-blot experiments (human and rat) using a mouse monoclonal anti-actin antibody (Millipore) diluted 1: 40,000.
Antibodies specificity
In this work, we used two different types of antibodies to detect TH, one type to detect total content of tyrosine hydroxylase, and another type to detect the active (phosphorylated) form of this enzyme. In addition, an antibody against actin was used as an internal control for the accuracy of the experiments performed (e.g., protein loading and tissue preservation control).
For the detection of total tyrosine hydroxylase, two different antibodies were used: For the human studies, a mouse monoclonal anti-TH manufactured by Sigma-Aldrich (clone TH-16, catalog # T2928) was used. As per information provided in the data sheet by the manufacturer, this antibody is raised against purified rat tyrosine hydroxylase and recognizes an epitope in the N-terminal region between amino acids 40 and 152 of human TH. For the rat study, we used an anti-TH antibody manufactured by Millipore (clone 2/40/15, catalog # MAB5280). As per information provided by the manufacturer, this antibody is raised against purified tyrosine hydroxylase from a rat pheochromocytoma and its specificity is routinely assessed by western-blot by the manufacturer.
For the detection of phosphorylated (active) tyrosine hydroxylase, a rabbit polyclonal anti-phospho-ser40 antibody by Phosphosolutions (catalog # p1580-40) was used. As per information provided by the manufacturer, this antibody is raised against a phosphopeptide corresponding to amino acid residues surrounding the phosphor-ser40 of rat tyrosine hydroxylase. The antibody is purified by affinity purification via sequential chromatography on phospho and dephosphopeptide affinity columns. The antibody specificity was also tested by western-blot, and has been shown to recognize exclusively pTH.
For the detection of actin, a mouse monoclonal anti-actin antibody by Millipore (catalog # MAB1501, clone C4) was used. As per information provided by the manufacturer, this antibody is raised against purified chicken gizzard actin, and recognizes an epitope located at the N-terminal two-thirds of the protein near amino acids 50–70. This antibody reacts against actin from all vertebrates.
Protein extraction
Tissue collected for western-blot was diluted 1:5 in lysis buffer containing Tris–HCl pH 8.0, sodium chloride, sodium dodecyl sulfate, disodium EDTA, and a protease inhibitor cocktail (Sigma-Aldrich; P8340). Samples were homogenized with a sonicator, and the homogenate was centrifuged at 13,000 rpm for 15 min at 4°C. The supernatant was recovered, and the total protein concentration was measured using a modified Lowry kit (Bio-Rad; 500-0113, 500-0114).
Gel electrophoresis and western-blot
Protein extracts with total protein concentrations of 30 (rat), or 60 μg (human), were loaded onto 10% polyacrylamide gels along with a molecular weight marker (Bio-Rad, Hercules, CA, USA; 161-0324). Proteins were resolved at 150 V, and then transferred at 30 V overnight onto PVDF membranes (Bio-Rad; 162-0174). The following day, the membranes were blocked in Tris-buffered saline (TBS) containing 5% non-fat powdered milk and 0.1% Tween-20 for 1 h at room temperature. TH or pTH were detected by incubating the membranes with the appropriate primary antibodies (see above) diluted in TBS containing 1% non-fat powdered milk and 0.1% Tween-20 (TBSTm) for 21 h at 4°C. After rinsing in TBSTm, the membranes were incubated for 1 h at room temperature with a goat anti-mouse alkaline phosphatase-coupled secondary antibody (Millipore; AP124A) diluted 1:15,000, or a goat anti-rabbit alkaline phosphatase-coupled secondary antibody (Vector Laboratories, Burlingame, CA, USA; AP-1000) diluted 1:1,000. The membranes were then rinsed in TBS, and the bands were visualized using a chemiluminescent system (Bio-Rad; 170-5018), exposing Kodak BioMax XAR films. In all cases, the membranes were reblotted and re-incubated with an antibody against actin (see details above). Films were scanned at 600 dpi using a flatbed scanner, and optical density was measured using Image Pro-Plus 6.2 software (Media Cybernetics).
Statistical analysis
All data sets (outcome measures and possible covariates) were assessed for normality using the Kolmogorov–Smirnov test. For this and subsequent statistical tests, categorical variables (i.e., gender and race) were assessed using a “dummy coding” scheme. Power analyses were performed entering the outcome measures (i.e., TH protein levels in schizophrenia and control groups) from the first postmortem study (pilot study) in G power 3.1 software (Faul et al., 2007). For subsequent postmortem studies (i.e., regional expression of TH and pTH protein) an “a priori” test to compute sample size for t-test comparison was used. For the animal study, an “a priori” test to compute sample size for one-way ANOVA was used. The effect of possible covariates in the outcome measures of interest (i.e., mRNA TH levels, TH protein, and pTH protein levels) for postmortem studies was assessed using a multiple regression model, in which the effect of the possible covariates was tested independently for each of the outcome measures of interest. The covariates included in the multiple regression analyses were carefully selected based on their possible influence on the expression of TH (mRNA or protein) or pTH (protein). The tested covariates included age, race, gender, postmortem interval (PMI), pH, and actin levels. PMI and pH are directly related with the quality of the tissue preservation (Stan et al., 2006), while actin levels are directly related with the accuracy on the processing of the samples (i.e., loading of the samples on the electrophoresis gels). The model of multiple regression analysis used also included the assessment of possible multicollinearity. For the animal study, the only covariate assessed was actin levels, and this was done by linear regression analysis. For postmortem human studies the statistical analysis of the outcome measures was performed by using the appropriate t-test (i.e., Student’s t-test, or Welch’s t-test) for data that were normally distributed, or a non-parametric test (Mann–Whitney U-test), when one or both of the data sets were not normally distributed. One-way ANOVA was used in the case of animals treated with antipsychotics. The statistical analysis was performed using InStat 3.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Demographic features
Postmortem human cases were carefully selected for each study, trying to match as much as possible the cases in the control and schizophrenia groups by their age, gender, race, PMI, and brain pH (see Tables 1 and 2). In addition, statistical analysis was performed for each of these demographic features to ensure that the control and schizophrenia groups were not significantly different.
Pilot study
Age, gender, race, and PMI were not normally distributed (Kolmogorov–Smirnov Normality test) for one or both groups, therefore a Mann–Whitney U-test was used to compare each of these demographic features between the control and schizophrenia groups (Age: U = 13.00; P = 0.79. Race: U = 11.00; P = 0.46. Gender: U = 14.00; P = 0.91. PMI: U = 14.50; P > 0.99). Brain pH data were normally distributed for both groups, therefore a standard unpaired t-test was performed [t(9) = 0.72; P = 0.49]. In summary, all the demographic features were not significantly different between the control and schizophrenia groups for the pilot study (Table 1).
Regional expression of TH and pTH study
This study was performed in two different types of samples that were analyzed independently: Cases containing all the rostro-caudal extent of the SN/VTA, and cases containing only the mid-caudal region of the SN/VTA. The demographic features for control and schizophrenia cases for each of these two types of samples were statistically analyzed. In rostro-caudal samples, age, PMI, and brain pH were normally distributed for the control and schizophrenia groups, therefore an unpaired t-test was performed [Age: t(12) = 0.15; P = 0.89. PMI: t(12) = 0.40; P = 0.70. Brain pH: t(12) = 1.79; P = 0.1]. On the other hand, race and gender for the rostro-caudal samples were not normally distributed at least for one of the groups, therefore a Mann–Whitney U-test was performed (Race: U = 18.00; P = 0.39. Gender: U = 22.00; P = 0.79). In summary, no demographic features were significantly different between the control and schizophrenia groups for the cases containing all the rostro-caudal extent of the SN/VTA. In mid-caudal samples, age, PMI, and pH were normally distributed for the control and schizophrenia groups, therefore an unpaired t-test was performed [Age: t(12) = 1.08; P = 0.30. PMI: t(12) = 0.50; P = 0.63; Brain pH: t(12) = 1.70; P = 0.11]. For mid-caudal samples race and gender were not normally distributed and a Mann–Whitney U-test was performed (Race: U = 10.00; P = 0.045. Gender: U = 18.00; P = 0.39). In summary, race was the only demographic feature that presented significant differences between the schizophrenia and control groups in mid-caudal samples. This significant difference was further assessed (see below, second postmortem study results) as a possible predictor for the main outcome measures (TH and pTH protein levels; Table 2).
Pilot study: TH mRNA and protein expression in human postmortem schizophrenia and control cases
TH mRNA and TH protein levels were tested in SN/VTA samples from the same cases (see Table 1). In this pilot study a total of 11 cases were used (n = 5 controls; n = 6 schizophrenia).
TH mRNA levels
Multiple regression analysis with the main outcome measure (TH mRNA levels) as the dependent variable, and the possible covariates as independent variables was used to test if the covariates (i.e., age, race, gender, PMI, pH) significantly affected the TH mRNA levels regardless of diagnoses (schizophrenia or control). The results of this test were non-significant (R2 = 0.38; P = 0.70). After that, TH mRNA data in the schizophrenia and control groups were tested for normality using the Kolmogorov–Smirnov test. For both groups the TH mRNA data were normally distributed (Control group: KS = 0.23; P > 0.10. Schizophrenia group: KS = 0.23; P > 0.10). Since the data were normally distributed, an unpaired two-tailed t-test assuming equal variances was performed to test for differences in TH mRNA levels between the controls and schizophrenia groups, which yielded a non-significant result [t(9) = 1.74; P = 0.12]. See also Figure 1A.
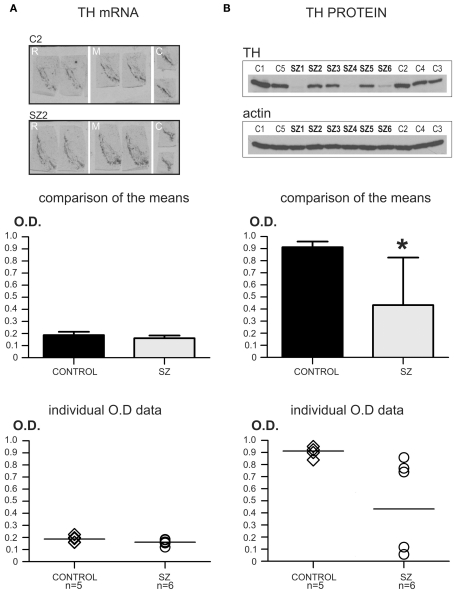
Figure 1.
Pilot study: TH mRNA and protein levels in schizophrenia and control cases. *P < 0.05. (A) TH mRNA levels in control (n = 5) and schizophrenia (n = 6) cases. The top panel shows representative images of TH mRNA in situ hybridization in rostral (R), medial (M), and caudal (C) sections of the SN/VTA of a control case (C2), and a schizophrenia case (SZ2). Comparison of the mean values for TH mRNA OD is shown in the bar graph (middle panel). Mean optical density (OD) values for TH mRNA levels in control and schizophrenia (SZ) cases did not differ significantly (P = 0.12; OD mean ± SD: control = 0.187 ± 0.027, schizophrenia = 0.161 ± 0.023). The bottom panel shows a scatter plot for the individual TH mRNA OD values. The horizontal bar in the scatter plot indicates the mean OD value for each group. Note the dense clustering of all the OD values for both groups. (B) TH protein levels in the same control and schizophrenia cases used for the mRNA study. The top panel shows representative images of the same western-blot membrane, first blotted for TH protein, and then reblotted for actin. This membrane contained all the control (C1–C5) and schizophrenia (SZ1–SZ6) cases used in this pilot study. Note the conspicuous difference in blot signal among the different samples in the TH western-blot image, while actin expression is uniform among all cases. The comparison of the means for TH protein levels in controls and schizophrenia is shown in the mid panel (bar graph). OD values for TH protein were significantly different (*) between the two groups (P = 0.031; OD mean ± SD: control = 0.912 ± 0.046, schizophrenia = 0.433 ± 0.393). The bottom panel shows a scatter plot for the individual TH protein OD values. The horizontal bar in the scatter plot indicates the mean OD value for each group. Note the dense clustering of the OD values for the control group, while in the schizophrenia group there is a large scattering of the data. However, note that in the lower TH expression cluster, two cases presented the same exact TH OD value and are represented in the scatter plot as a single circle.
TH protein levels
Multiple regression analysis with TH protein as the main outcome measure (dependent variable) and age, race, gender, PMI, pH, and actin levels as possible covariates (independent variables) revealed a non-significant result (R2 = 0.60; P = 0.52). Kolmogorov–Smirnov normality test revealed that TH protein levels data in the control and schizophrenia groups were normally distributed (Control group: KS = 0.21; P > 0.10. Schizophrenia group: KS = 0.29; P > 0.10). An unpaired two-tailed t-test assuming unequal variances (Welch’s correction) was used since the SDs between the two groups were significantly different (F = 72.75; P = 0.001). The t-test with Welch’s correction revealed significant differences in TH protein levels between the control and schizophrenia groups [t(5) = 2.96; P = 0.031]. See also Figure 1B.
In summary, this pilot study showed that TH protein levels were significantly different between the schizophrenia and control groups, while TH mRNA levels did not differ between the two groups. These results prompted us to design new experiments to test: (a) if chronic antipsychotic treatment could significantly affect the levels of TH protein (animal study), and (b) if the differences observed in TH protein expression were regionally specific within the SN/VTA, and if these differences were also present in the active form of TH (pTH), which were tested in a subsequent postmortem human study.
Animal study: Antipsychotic treatment effect on TH protein levels
Power analysis
For this study, an “a priori” power analysis was performed to determine the minimum adequate sample size for a one-way ANOVA with a minimum power of 0.80. Taking into account the results of the pilot study for TH protein levels in postmortem human tissue, we used the following assumption: if changes in TH protein levels can be explained as an effect of the antipsychotic medication, these changes have to be large to explain the differences observed in the pilot study between the control and schizophrenia groups. For this reason, we modeled the study to expect a large effect size (0.80). With these assumptions, the power analysis determined that a minimum sample size of n = 21 (n = 7 per group) was necessary to obtain the minimum power required. Since in our study the sample size was n = 27 animals (n = 9 per group), a post hoc power analysis using the actual effect size obtained (f = 0.64) revealed that the actual power of our study was 0.804 (critical F = 3.40; α = 0.05).
TH protein levels in animals chronically treated with APDs
The only covariate to be assessed for this study was actin protein levels, which is a measurement of the accuracy of the experiment. The effect of this possible covariate on TH protein levels was analyzed by linear regression, which revealed a non-significant result (r = 0.15; P = 0.44). The Kolmogorov–Smirnov test showed that TH protein levels were normally distributed for the three groups (Control group: KS = 0.22; P > 0.10; Haloperidol group: KS = 0.17; P > 0.10. Olanzapine group: KS = 0.26; P = 0.08). However, a Kruskal–Wallis non-parametric ANOVA was used, since a Bartlett’s test for homogeneity of variances showed very significant differences among the three groups (Bartlett statistic = 10.37; P = 0.006). The Kruskal–Wallis ANOVA revealed non-significant differences among the three groups for TH protein levels (KW = 1.79; P = 0.41). See also Figure 2.
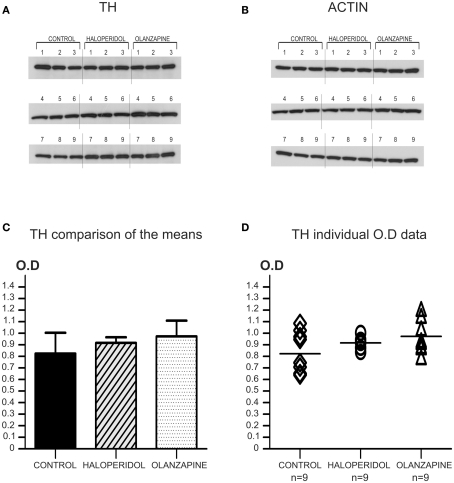
Figure 2.
Animal study: antipsychotic treatment effect on TH protein levels. (A) Western-blot images of TH protein for all the animals of the three treatment groups. All the samples (n = 9 per treatment group) were incubated and developed together on the same film to accurately measure OD. Note the homogeneity of expression of TH for all cases independently of the treatment group. (B) The same western-blot membrane shown in A was reblotted for the detection of actin protein. (C) Comparison of the mean OD for TH protein levels showed no significant differences among the three groups (P = 0.41; OD mean ± SD: control = 0.824 ± 0.181, haloperidol = 0.917 ± 0.049, olanzapine = 0.973 ± 0.136). (D) Scatter plot graph showing the individual TH protein OD values for the three groups. The horizontal bar in the scatter plot indicates the mean OD value for each group.
In summary, this study showed that chronic treatment with antipsychotic medications (typical or atypical) did not produce significant differences in TH protein levels when compared with untreated controls.
Second postmortem study: Regional expression of TH and phosphorylated TH
Power analysis
For this study, an “a priori” power analysis was performed to determine the minimum adequate sample size for a two-tailed t-test analysis with a minimum power of 0.80. To calculate this, data obtained from the TH protein levels in the pilot study were entered to calculate the effect size. This analysis yielded that a sample size of n = 14 cases (schizophrenia n = 8; control n = 6) would produce an actual power of 0.83 (t critical = 2.18; α = 0.05). In mid-caudal samples, an outlier was present in the schizophrenia group (case SZ11). This case was detected as an outlier using a combination of different factors: first, the pH of this sample was highly basic (pH = 7.80, see Table 2); second, TH levels were highly different from any other case in the schizophrenia or control groups, and a Grubb’s test was performed using Quick Calcs Online calculator (GraphPad Software Inc.). This test showed that case SZ11 was a significant outlier for TH levels (Z = 2.94; P < 0.05). For these reasons, this case was removed from the subsequent statistical analysis of mid-caudal TH and pTH expression. After removing this case for the analysis, a two-tailed t-test “compromise” power was calculated, entering the data obtained for TH protein levels in the pilot study to calculate the effect size. This analysis yielded a power of 0.80 (critical t = 2.20; α = 0.05) for the mid-caudal analysis with a sample size of n = 13 (control n = 6; schizophrenia n = 7).
TH protein levels in rostro-caudal SN/VTA samples
Multiple regression analysis with TH protein as the main outcome measure (dependent variable) and age, race, gender, PMI, pH, and actin levels as possible covariates (independent variables) revealed a non-significant result (R2 = 0.74; P = 0.06). Kolmogorov–Smirnov normality test revealed that TH protein levels in the control and schizophrenia groups were normally distributed (Control group: KS = 0.31; P = 0.07. Schizophrenia group: KS = 0.17; P > 0.10). An unpaired two-tailed t-test assuming unequal variances (Welch’s correction) was used since the SDs between the two groups were significantly different (F = 32.97; P = 0.001). The t-test with Welch’s correction revealed significant differences in TH protein levels between the control and schizophrenia groups [t(7) = 2.59; P = 0.036]. See also Figure 3A.
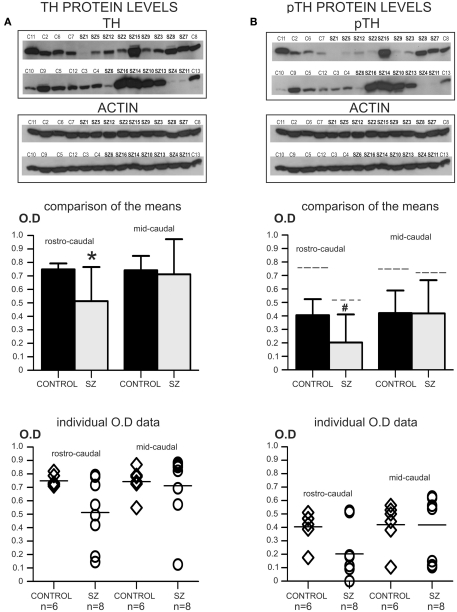
Figure 3.
Second postmortem study: regional distribution of TH and pTH. *P < 0.05; #P > 0.05 < 0.06. (A) The top panel shows images of two western-blots for TH that were developed on the same film, containing all the cases used in this study. Note that rostro-caudal and mid-caudal cases were run together in the same gels. In addition, reblots for actin of the same membranes are shown immediately below. The graph bar (mid panel) shows the comparison of the mean OD values for TH protein in rostro-caudal and mid-caudal samples for controls and schizophrenia. Only the rostro-caudal samples showed significant differences (*) between schizophrenia and control cases (P = 0.036; OD mean ± SD: control rostro-caudal = 0.749 ± 0.044, schizophrenia rostro-caudal = 0.513 ± 0.252; control mid-caudal = 0.743 ± 0.107, schizophrenia mid-caudal = 0.712 ± 0.261). The bottom panel shows a scatter plot for the individual TH OD data. The horizontal bar in the scatter plot indicates the mean OD value for each group. (B) The same membranes in (A) were reblotted for the detection of pTH (top panel). In addition, reblots for actin of the same membranes are shown immediately below. The graph bar in the mid panel shows the comparison of the mean OD values for pTH in rostro-caudal and mid-caudal samples. Even when there were no significant differences in pTH OD values between controls and schizophrenia in rostro-caudal samples, a trend (#) toward significance was observed (P = 0.056; OD mean ± SD: control rostro-caudal = 0.405 ± 0.120, schizophrenia rostro-caudal = 0.203 ± 0.208; control mid-caudal = 0.421 ± 0.168, schizophrenia mid-caudal = 0.419 ± 0.246). For comparison, the dashed line on top of each of the individual bars indicates the mean level of total TH for each group. The ratio of pTH versus total TH for each group was as follows: control rostro-caudal = 54.07%, schizophrenia rostro-caudal = 39.57%; control mid-caudal = 56.66%, schizophrenia mid-caudal = 58.85%. The bottom panel shows a scatter plot for the individual pTH OD data. The horizontal bar in the scatter plot indicates the mean OD value for each group.
TH protein levels in mid-caudal SN/VTA samples
Multiple regression analysis with TH protein as the main outcome measure and age, race, gender, PMI, pH, and actin levels as possible covariates revealed a non-significant result (R2 = 0.75; P = 0.11). In addition, the multiple regression analysis showed that, even when race was detected as a significantly different demographic feature between the schizophrenia and control groups for mid-caudal cases (see demographic features analysis above), this was not a significant contributor (predictor) of TH levels outcome (t ratio = 0.75; P = 0.48). Kolmogorov–Smirnov Normality test revealed that TH protein levels were normally distributed for the control group but not for the schizophrenia group (Control group: KS = 0.27; P > 0.1. Schizophrenia group: KS = 0.31; P = 0.047). Since data were not normally distributed for one of the groups, a non-parametric Mann–Whitney U-test was used. This test revealed non-significant differences in TH protein levels between the control and schizophrenia groups (U = 13.0; P = 0.29). See also Figure 3A.
Phosphorylated TH protein levels in rostro-caudal SN/VTA samples
Multiple regression analysis with pTH protein levels as the main outcome measure, and age, race, gender, PMI, pH, and actin levels as possible covariates revealed a non-significant result (R2 = 0.51; P = 0.39). Kolmogorov–Smirnov normality test revealed that pTH protein levels data in the control and schizophrenia groups were normally distributed (Control group: KS = 0.27; P > 0.10. Schizophrenia group: KS = 0.27; P = 0.09). An unpaired two-tailed t-test assuming unequal variances yielded no significant differences [t(12) = 2.12; P = 0.056], although the P value was low enough to indicate a trend toward significance. See also Figure 3B.
Phosphorylated TH protein levels in mid-caudal SN/VTA samples
Multiple regression analysis with pTH protein as the main outcome measure and age, race, gender, PMI, pH, and actin levels as possible covariates revealed a non-significant result (R2 = 0.73; P = 0.13). In addition, the multiple regression analysis showed that, even when race was detected as a significantly different demographic feature between the schizophrenia and control groups for mid-caudal cases (see demographic features analysis above), this was not a significant contributor (predictor) of pTH levels outcome (t ratio = 0.36; P = 0.73). Kolmogorov–Smirnov normality test revealed that pTH protein levels were normally distributed for the control group but not for the schizophrenia group (Control group: KS = 0.24; P > 0.1. Schizophrenia group: KS = 0.35; P = 0.01). Since data were not normally distributed for one of the groups, a non-parametric Mann–Whitney U-test was used. This test revealed non-significant differences in TH protein levels between the control and schizophrenia groups (U = 12.5; P = 0.25). See also Figure 3B.
In summary, this regional postmortem study showed that TH protein levels in samples containing the entire rostro-caudal extent of the SN/VTA were significantly different between the schizophrenia and control groups, while no significant differences were detected in samples that only contained mid-caudal regions. In addition, no significant differences were detected for pTH protein levels for samples containing either the entire rostro-caudal, or only mid-caudal regions of the SN/VTA. However, a trend toward significance (P = 0.056) was observed for pTH levels in samples containing the entire rostro-caudal extent of the SN/VTA.
Discussion
As far as we are aware, this is the first study to analyze TH protein levels in the SN/VTA of schizophrenia compared to controls, and only two previous studies have assessed TH mRNA levels in schizophrenia (Ichinose et al., 1994; Mueller et al., 2004).
Pilot study: TH mRNA and protein expression in human postmortem schizophrenia and control cases
The most remarkable finding of our first postmortem study (pilot study) was the fact that in some of the schizophrenia cases (three out of the six cases studied) TH protein levels were profoundly altered, while in the same brain samples TH mRNA levels were similar to control cases. It was also remarkable the large variability presented in the schizophrenia group, with some cases presenting levels in the same range than controls, while others presented very low TH protein levels. The regulation of the transcription and translation of the TH gene is a highly complex process that is also specific to the type of TH expressing cell (i.e., dopaminergic versus adrenergic or noradrenergic neurons). Tank and collaborators have shown that while in adrenergic and noradrenergic cell populations of the locus coeruleus and adrenal medulla TH is highly regulated at the transcription level, this type of regulation is minimal in the case of dopaminergic cells of the SN/VTA (Chen et al., 2008; Tank et al., 2008). In the locus coeruleus and adrenal medulla, most types of stress can induce short-term and long-term upregulation of TH mRNA transcription, that is well correlated with upregulation of TH protein levels (Alterio et al., 2001; Wong and Tank, 2007; Tank et al., 2008). This is also correlated with changes in transcription factors that are known to regulate the TH gene promoter (Wong and Tank, 2007; Tank et al., 2008). However, in the case of the TH expressing cells of the SN/VTA it has been shown that induction of the transcription for TH mRNA does not always lead to an upregulation of TH protein levels (Chen et al., 2008; Tank et al., 2008). The application of drugs that are known to affect the transcription rate of TH mRNA in the adrenal medulla and the locus coeruleus (Alterio et al., 2001; Wong and Tank, 2007; Tank et al., 2008), does not produce any effect in TH mRNA levels in the SN/VTA, while they produce an upregulation of TH protein levels (Chen et al., 2008; Tank et al., 2008). In midbrain slice explants containing the SN/VTA, these authors demonstrated that forskolin and cAMP analogs increase TH protein levels in a dose-dependent manner, while TH mRNA levels are unchanged. These authors proposed that in the midbrain there is a cAMP-mediated induction of TH protein by a translational mechanism that includes the formation of binding complexes with Poly(C)-binding proteins, producing the stabilization of TH mRNA, and/or increase of its association with polysomes, finally leading to increased translation (Tank et al., 2008).
The only two previous studies addressing TH mRNA levels in schizophrenia reported opposite results. One of these studies (Ichinose et al., 1994) showed that, as in our study, TH mRNA levels in schizophrenia were unchanged compared to controls. A second study (Mueller et al., 2004) showed significantly elevated levels of TH mRNA in schizophrenia. Several factors can contribute to this difference in the results obtained, including differences in the cohort of patients as well as methodological differences.
Animal study: Antipsychotic treatment effect on TH protein levels
In our study, we also addressed the possibility that the observed changes in TH protein in the SN/VTA of schizophrenia patients could be an effect of antipsychotic drug treatments. Our results showed that chronic treatment with antipsychotic medication did not produce significant changes in TH protein expression in the SN/VTA of rodents. Supporting our findings, a previous study in rodents using a different atypical antipsychotic (clozapine) has shown similar results for the SN/VTA dopaminergic cells, with no changes in TH protein levels or TH immunolabeling (Tejedor-Real et al., 2003). In addition, it has been shown that chronic treatment with haloperidol does not produce changes in TH axonal density in the prefrontal and entorhinal cortex of monkeys, while lamina-specific changes in TH axonal density are present in schizophrenia patients on antipsychotic medication (Akil et al., 1999, 2000). Two laboratories have reported changes in cell morphology and TH immunolabeling in the SN/VTA after chronic treatment with haloperidol (Levinson et al., 1998; Mazurek et al., 1998; Marchese et al., 2002). However, in these studies drugs were administered in very high doses. Mazurek and collaborators performed two studies using intraperitoneal injections of haloperidol once a day in doses ranging from 1 to 10 mg/kg/day (Levinson et al., 1998; Mazurek et al., 1998). The other group found changes in animals that were subcutaneously administered haloperidol twice a day at a dose of 1 mg/kg (Marchese et al., 2002). The doses used by these investigators are between 10- and 100-fold the dose necessary to achieve receptor occupancies equivalent to therapeutic conditions (Kapur et al., 2003), which can lead to cellular toxicity. Finally, another chronic rodent study using haloperidol or combinations of haloperidol and quetiapine did not find any morphological anomalies in the dopaminergic neurons of the SN/VTA (Zhang et al., 2007).
Second postmortem study: Regional expression of TH and phosphorylated TH
Our second postmortem study addressed the regional expression of TH and pTH protein using two different types of samples: One set of cases contained the entire rostro-caudal extent of the SN/VTA, and another set contained only mid-caudal regions. Compared to controls, schizophrenia rostro-caudal samples presented significant differences in TH protein levels, while for pTH there was a trend toward significance. However, in the case of samples containing only mid-caudal regions there were no differences detected between schizophrenia and controls for TH or pTH protein levels. These results are especially interesting because there is a regional segregation on the projections from the SN/VTA complex to cortical and subcortical regions. The rostral to mid-regions of the SN/VTA complex contain the rostral part of the dorsal tier of the SNc and the adjacent VTA. Both of these neuronal groups project preferentially to the prefrontal, entorhinal, and piriform cortex, as well to the ventral striatum and the amygdala (Fallon and Moore, 1978a,b; Fallon et al., 1978a,b; Porrino and Goldman-Rakic, 1982; Gaspar et al., 1992; McRitchie et al., 1995; Haber and Fudge, 1997; Halliday, 2004). The mid to caudal regions of the SN/VTA complex contain the remainder of the dorsal tier, as well as the entire ventral tier of the SNc that projects preferentially to the dorsal striatum (Fallon and Moore, 1978a,b; Fallon et al., 1978b; McRitchie et al., 1995; Haber and Fudge, 1997; Joel and Weiner, 2000; Halliday, 2004). Our findings indicate that the tyrosine hydroxylase anomalies are located preferentially (or exclusively) in the rostral areas of the SN/VTA complex. This finding suggests that TH protein anomalies could be preferentially located in the mesocortical and mesolimbic pathways. Supporting this, anomalies in TH immunolabeling have been found in specific layers of the prefrontal and entorhinal cortex in schizophrenia (Akil et al., 1999, 2000; Lewis and Gonzalez-Burgos, 2006).
Although TH protein levels have not been assessed before, a previous study in a cohort of Japanese patients addressed TH enzymatic activity in the SN/VTA of schizophrenia compared to controls (Toru et al., 1988). TH enzymatic activity is linked with the portion of the TH protein that is active, which in our study we assessed by measuring the levels of pTH. In brain tissue, it has been shown that TH can be phosphorylated at several serine residues, including serine 19, 31, and 40, all located in the first 40 amino acids of the N-terminus (Haycock, 1990). Among these serine phosphorylation sites, serine 40 appears to be the most relevant for the activation of TH (Haycock, 1990; Nakashima et al., 2009), and the positively charged amino acids flanking this serine play an important role in the proper phosphorylation of this site (Nakashima et al., 2009). This serine phosphorylation area is considered a modulatory region for the activity of the catalytic subunit of the TH protein, since phosphorylation in this area produces an enhancement of the activity of TH (Haycock, 1990; Nakashima et al., 2009). Our study of pTH levels in schizophrenia showed a trend toward a significant difference compared to controls in the samples containing the entire rostro-caudal extent of the SN/VTA, while no differences where observed for the samples containing mid-caudal regions. Toru et al. (1988) in their work assessing TH enzymatic activity reported a significant increase in TH activity in the SN/VTA of schizophrenia samples. In our study, pTH levels presented a very heterogeneous profile: For the samples containing the entire rostro-caudal extent of the SN/VTA, pTH levels were lower or equal than in control samples, while for the cases containing only mid-caudal regions, pTH levels ranged from higher than controls to lower (see scatter plot graphs in Figure 3B). The discrepancy between our results and the previous study by Toru et al. (1988) may be due to several factors. This includes differences in the area selected for study (e.g., their dissection included only the substantia nigra, while we included in our study the substantia nigra and the VTA as a dopaminergic producing complex), differences in the cohort of patients, and the use of different techniques.
Another interesting finding was that TH protein levels were very variable in schizophrenia, while were rather constant in the control group, which suggests that the schizophrenia group has a large heterogeneity of TH expression anomalies. Based on previous studies concerning the regulation of TH expression, genetic studies of the TH gene in schizophrenia, and in our data, we hypothesize that different anomalies in the TH gene and modulatory elements in the translation of the protein may account for this heterogeneity. A large number of genetic association studies for the TH gene have found very diverse results. The most consistent association was found for intron 1 of the TH gene (TH01 locus), an area that has been postulated as a modulatory element for TH transcription (Pae et al., 2003; Jacewicz et al., 2008). In the TH01 locus, certain alleles of a DNA microsatellite sequence repeat have been linked with a significant higher risk for schizophrenia. However, this association was found in some cohorts of patients (Meloni et al., 1995; Wei et al., 1995; Kurumaji et al., 2001; Pae et al., 2003; Jacewicz et al., 2008, but not in others; Burgert et al., 1998; Jonsson et al., 1998). In addition, TH protein synthesis is a highly regulated process, with epigenetic, transcriptional, and posttranscriptional regulations (Lenartowski and Goc, 2011) that vary depending on the class of catecholaminergic neuron (i.e., dopaminergic versus adrenergic or noradrenergic; Tank et al., 2008). Our data strongly support that TH anomalies in schizophrenia occur at the posttranscriptional level, which may include anomalies in the stabilization of the mRNA, the proper attachment of mRNA to polysomes, and/or the efficacy of translation of the mRNA into protein.
Conclusion
In summary, our study shows for the first time that an anomaly in TH protein synthesis is present in the SN/VTA in schizophrenia. Since this pathology is present in the protein, but not in the mRNA, it strongly suggests that the anomalies occur during posttranscriptional processes, but this will require further investigation to identify the exact processes that are affected. Finally, our study provides the first data indicating that within the SN/VTA, the anomalies in the synthesis of TH may be restricted to specific dopaminergic neuronal populations, mostly located in the rostral regions of this complex. This suggests that anomalies in TH synthesis may be linked with pathologies in the mesocortical and/or mesolimbic dopamine pathways, but further studies will be needed to pinpoint the specific populations affected.
Conflict of Interest Statement
Dr. Robert R. Conley is currently a Distinguished Lilly Scholar at Eli Lilly and Co. However, his involvement in this work is independent from his current contractual relation with Eli Lilly and Co. None of the work reported here has been financially supported by Eli Lilly and Co., nor this company had any role in the study design, in the collection, analysis and interpretation of data, writing of the report, and submission of the paper. All the other authors have no conflict of interest to break declare.
Acknowledgments
The authors wish to thank the staff of the Maryland Brain Collection, University of Maryland School of Medicine for their help to obtain the samples used in this study. We also wish to thank Dr. Xue-Min Gao for her assistance with the in situ hybridization experiments. This work was supported by the National Institutes of Health (USA) grant RO1MH066123 to Miguel Melendez-Ferro, Emma Perez-Costas, and Rosalinda C. Roberts.
References
- Abi-Dargham A., Slifstein M., Kegeles L., Laruelle M. (2010). “Dopamine dysfuction in schizophrenia,” in Dopamine Handbook, eds Iversen L. L., Iversen S. D., Dunnett S. B., Bjorklund A. (Oxford: Oxford University Press; ), 511–519 [Google Scholar]
- Akil M., Edgar C. L., Pierri J. N., Casali S., Lewis D. A. (2000). Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol. Psychiatry 47, 361–370 10.1016/S0006-3223(99)00282-6 [DOI] [PubMed] [Google Scholar]
- Akil M., Pierri J. N., Whitehead R. E., Edgar C. L., Mohila C., Sampson A. R., Lewis D. A. (1999). Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am. J. Psychiatry 156, 1580–1589 [DOI] [PubMed] [Google Scholar]
- Alterio J., Mallet J., Biguet N. F. (2001). Multiple complexes involved in tyrosine hydroxylase mRNA stability in rat adrenal medulla, after reserpine stimulation. Mol. Cell. Neurosci. 17, 179–189 10.1006/mcne.2000.0930 [DOI] [PubMed] [Google Scholar]
- Andreou D., Saetre P., Lundmark P., Hansen T., Timm S., Melle I., Djurovic S., Andreassen O. A., Werge T., Hall H., Agartz I., Terenius L., Jonsson E. G. (2009). Tyrosine hydroxylase Val81Met polymorphism: lack of association with schizophrenia. Psychiatr. Genet. 19, 273–274 10.1097/YPG.0b013e32832a4fcd [DOI] [PubMed] [Google Scholar]
- Burgert E., Crocq M. A., Bausch E., Macher J. P., Morris-Rosendahl D. J. (1998). No association between the tyrosine hydroxylase microsatellite marker HUMTH01 and schizophrenia or bipolar I disorder. Psychiatr. Genet. 8, 45–48 10.1097/00041444-199800820-00002 [DOI] [PubMed] [Google Scholar]
- Chao H. M., Richardson M. A. (2002). Aromatic amino acid hydroxylase genes and schizophrenia. Am. J. Med. Genet. 114, 626–630 10.1002/ajmg.10606 [DOI] [PubMed] [Google Scholar]
- Chen X., Xu L., Radcliffe P., Sun B., Tank A. W. (2008). Activation of tyrosine hydroxylase mRNA translation by cAMP in midbrain dopaminergic neurons. Mol. Pharmacol. 73, 1816–1828 10.1124/mol.107.043968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon J. H., Koziell D. A., Moore R. Y. (1978a). Catecholamine innervation of the basal forebrain II: amygdala, suprarhinal cortex and entorhinal cortex. J. Comp. Neurol. 180, 509–532 10.1002/cne.901800310 [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Riley J. N., Moore R. Y. (1978b). Substantia nigra dopamine neurons: separate populations project to neostriatum and allocortex. Neurosci. Lett. 7, 157–162 10.1016/0304-3940(78)90160-X [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Moore R. Y. (1978a). Catecholamine innervation of the basal forebrain III Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J. Comp. Neurol. 180, 533–544 10.1002/cne.901800310 [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Moore R. Y. (1978b). Catecholamine innervation of the basal forebrain IV: topography of the dopamine projection to the basal forebrain and neostriatum. J. Comp. Neurol. 180, 545–580 10.1002/cne.901800310 [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A. G., Buchner A. (2007). G* power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Gao X. M., Hashimoto T., Tamminga C. A. (1998). Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. Synapse 29, 14–28 [DOI] [PubMed] [Google Scholar]
- Gaspar P., Stepniewska I., Kaas J. H. (1992). Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J. Comp. Neurol. 325, 1–21 10.1002/cne.903250102 [DOI] [PubMed] [Google Scholar]
- Haber S. N., Fudge J. L. (1997). The primate substantia nigra and VTA: integrative circuitry and function. Crit. Rev. Neurobiol. 11, 323–342 [DOI] [PubMed] [Google Scholar]
- Haber S. N., Gdowski M. J. (2004). “The basal ganglia,” in The Human Nervous System, eds Paxinos G., Mai J. K. (London: Elsevier Academic Press; ), 676–738 [Google Scholar]
- Halliday G. (2004). “Substantia nigra and locus coeruleus,” in The Human Nervous System, eds Paxinos G., Mai J. K. (London: Elsevier Academic Press; ), 449–463 [Google Scholar]
- Haycock J. W. (1990). Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J. Biol. Chem. 265, 11682–11691 [PubMed] [Google Scholar]
- Hoogendoorn M. L. C., Bakker S. C., Schnack H. G., Selten J. P. C., Otten H. G., Verduijn W., van der Heijden F. M., Pearson P. L., Khan R. S., Sinke R. J. (2005). No association between 12 dopaminergic genes and schizophrenia in a large Dutch sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 134B, 6–9 10.1002/ajmg.b.30147 [DOI] [PubMed] [Google Scholar]
- Ichinose H., Ohye T., Fujita K., Pantucek F., Lange K., Riederer P., Nagatsu T. (1994). Quantification of mRNA of tyrosine hydroxylase and aromatic L-amino acid decarboxylase in the substantia nigra in Parkinson’s disease and schizophrenia. J. Neural Transm. 8, 149–158 10.1007/BF02250926 [DOI] [PubMed] [Google Scholar]
- Ishiguro H., Arinami T., Saito T., Akazawa S., Enomoto M., Mitushio H., Fujishiro H., Tada K., Akimoto Y., Mifune H., Shiozuka S., Hamaguchi H., Toru M., Shibuya H. (1998). Systematic search for variations in the tyrosine hydroxylase gene and their associations with schizophrenia, affective disorders, and alcoholism. Am. J. Med. Genet. 81, 388–396 [DOI] [PubMed] [Google Scholar]
- Jacewicz R., Galecki P., Florkowski A., Berent J. (2008). Association of the tyrosine hydroxylase gene polymorphism with schizophrenia in the population of central Poland. Psychiatr. Pol. 42, 583–593 [PubMed] [Google Scholar]
- Joel D., Weiner I. (2000). The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96, 451–474 10.1016/S0306-4522(99)00575-8 [DOI] [PubMed] [Google Scholar]
- Jonsson E., Brene S., Geijer T., Terenius L., Tylec A., Persson M. L., Sedvall G. (1996). A search for association between schizophrenia and dopamine-related alleles. Eur. Arch. Psychiatr. Clin. Neurosci. 246, 297–304 10.1007/BF02189022 [DOI] [PubMed] [Google Scholar]
- Jonsson E. G., Geijer T., Gyllander A., Terenius L., Sedvall G. C. (1998). Failure to replicate an association between a rare allele of a tyrosine hydroxylase gene microsatellite and schizo-phrenia. Eur. Arch. Psychiatry Clin. Neurosci. 248, 61–63 10.1007/s004060050018 [DOI] [PubMed] [Google Scholar]
- Kapur S., Vanderspek S. C., Brownlee B. A., Nobrega J. N. (2003). Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J. Pharmacol. Exp. Ther. 305, 625–631 10.1124/jpet.102.046987 [DOI] [PubMed] [Google Scholar]
- Kunugi H., Kawada Y., Hattori M., Ueki A., Otsuka M., Nanko S. (1998). Association study of structural mutations of the tyrosine hydroxylase gene with schizophrenia and Parkinson’s disease. Am. J. Med. Genet. 81, 131–133 [DOI] [PubMed] [Google Scholar]
- Kurumaji A., Kuroda T., Yamada K., Yoshikawa T., Toru M. (2001). An association of the polymorphic repeat of tetranucleotide (TCAT) in the first intron of the human tyrosine hydroxylase gene with schizophrenia in a Japanese sample. J. Neural Transm. 108, 489–495 10.1007/s007020170069 [DOI] [PubMed] [Google Scholar]
- Lenartowski R., Goc A. (2011). Epigenetic, transcriptional and posttranscriptional regulation of the tyrosine hydroxylase gene. Int. J. Dev. Neurosci. 29, 873–883 10.1016/j.ijdevneu.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Levinson A. J., Garside S., Rosebush P. I., Mazurek M. F. (1998). Haloperidol induces persistent down-regulation of tyrosine hydroxylase immunoreactivity in substantia nigra but no ventral tegmental area in the rat. Neuroscience 84, 201–211 10.1016/S0306-4522(97)00447-8 [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Gonzalez-Burgos G. (2006). Pathophysiologically based treatment interventions in schizophrenia. Nat. Med. 12, 1016–1022 10.1038/nm1478 [DOI] [PubMed] [Google Scholar]
- Marchese G., Casu M. A., Bartholini F., Ruiu S., Saba P., Gessa G. L., Pani L. (2002). Sub-chronic treatment with classical but not atypical antipsychotics produces morphological changes in rat nigro-striatal dopaminergic neurons directly related to “early onset” vacuous chewing. Eur. J. Neurosci. 15, 1187–1196 10.1046/j.1460-9568.2002.01944.x [DOI] [PubMed] [Google Scholar]
- Mazurek M. F., Savedia S. M., Bobba R. S., Garside S., Rosebush P. I. (1998). Persistent loss of tyrosine hydroxylase immunoreactivity in the substantia nigra after neuroleptic withdrawal. J. Neurol. Neurosurg. Psychiatr. 64, 799–801 10.1136/jnnp.64.6.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRitchie D. A., Halliday G. M., Cartwright H. (1995). Quantitative analysis of the variability of substantia nigra pigmented cell clusters in the human. Neuroscience 68, 539–551 10.1016/0306-4522(95)00163-D [DOI] [PubMed] [Google Scholar]
- Meloni R., Laurent C., Campion D., Hadjali B. B., Thibaut F., Dollfus S., Petit M., Samolyk D., Martinez M., Poirier M. F., Mallet J. (1995). A rare allele of a microsatellite located in the tyrosine hydroxylase gene found in schizophrenic patients. C. R. Acad. Sci. III Sci. Vie 318, 803–809 [PubMed] [Google Scholar]
- Mueller H. T., Haroutunian V., Davis K. L., Meador-Woodruff J. H. (2004). Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res. Mol. Brain Res. 121, 60–69 10.1016/j.molbrainres.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Nakashima A., Hayashi N., Kaneko Y. S., Mori K., Sabban E. L., Nagatsu T., Ota A. (2009). Role of N-terminus of tyrosine hydroxylase in the biosynthesis of catecholamines. J. Neural Transm. 116, 1355–1362 10.1007/s00702-009-0227-8 [DOI] [PubMed] [Google Scholar]
- Ota M., Nakashima A., Ikemoto K., Nojima S., Tanaka M., Okuda M., Koga H., Mori K., Kaneko Y. S., Fujiwara K., Yamamoto H., Nagatsu T., Ota A. (2001). Exon 3 of tyrosine hydroxylase gene: lack of association with Japanese schizophrenic patients. Mol. Psychiatry 6, 315–319 10.1038/sj.mp.4000840 [DOI] [PubMed] [Google Scholar]
- Pae C. U., Kim J. J., Serretti A., Lee C. U., Lee S. J., Lee C., Paik I. H. (2003). VNTR polymorphism of tyrosine hydroxylase gene and schizophrenia in the Korean population. Neuropsychobiology 47, 131–136 10.1159/000070581 [DOI] [PubMed] [Google Scholar]
- Paxinos G., Huang X. F. (1995). Atlas of the Human Brainstem. San Diego: Academic Press [Google Scholar]
- Perez-Costas E., Guidetti P., Melendez-Ferro M., Kelley J. J., Roberts R. C. (2008). Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J. Neural Transm. 115, 745–753 10.1007/s00702-007-0004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Costas E., Melendez-Ferro M., Roberts R. C. (2010). Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J. Neurochem. 113, 287–302 10.1111/j.1471-4159.2010.06604.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino L. J., Goldman-Rakic P. S. (1982). Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J. Comp. Neurol. 205, 63–76 10.1002/cne.902050107 [DOI] [PubMed] [Google Scholar]
- Roberts R. C., Roche J. K., Conley R. R., Lahti A. C. (2009). Dopaminergic synapses in the caudate of subjects with schizophrenia: relationship to treatment response. Synapse 63, 520–530 10.1002/syn.20623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R. L., Williams J. B., Gibbon M., First M. B. (1992). The structured clinical interview for DSM-III-R (SCID). I: history, rationale, and description. Arch. Gen. Psychiatry 49, 624–629 10.1001/archpsyc.1992.01820080032005 [DOI] [PubMed] [Google Scholar]
- Stan A. D., Ghose S., Gao X. M., Roberts R. C., Lewis-Amezcua K., Hatanpaa K. J., Tamminga C. A. (2006). Human postmortem tissue: what quality markers matter? Brain Res. 1123, 1–11 10.1016/j.brainres.2006.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkowski M. E., Kirov G., Bamne M., Georgieva L., Torres G., Mansour H., Chowdari K. W., Milanova V., Wood J., McClain L., Prasad K., Shirts B., Zhang J., O’Donovan M. C., Owen M. J., Devlin B., Nimgaonkar V. L. (2008). A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum. Mol. Genet. 17, 747–758 10.1093/hmg/ddm347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank A. W., Xu L., Chen X., Radcliffe P., Sterling C. R. (2008). Post-transcriptional regulation of tyrosine hydroxylase expression in adrenal medulla and brain. Ann. N. Y. Acad. Sci. 1148, 238–248 10.1196/annals.1410.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor-Real P., Biguet N. F., Dumas S., Mallet J. (2003). Tyrosine hydroxylase mRNA and protein are down-regulated by chronic clozapine in both the mesocorticolimbic and the nigrostriatal systems. J. Neurosci. Res. 72, 105–115 10.1002/jnr.10555 [DOI] [PubMed] [Google Scholar]
- Thibaut F., Ribeyre J. M., Dourmap N., Meloni R., Laurent C., Campion D., Menard F. J., Dollfus S., Mallet J., Petit M. (1997). Association of DNA polymorphism in the first intron of the tyrosine hydroxylase gene with disturbances of the catecholaminergic system in schizophrenia. Schizophr. Res. 23, 259–264 10.1016/S0920-9964(96)00118-1 [DOI] [PubMed] [Google Scholar]
- Toru M., Watanabe S., Shibuya H., Nishikawa T., Noda K., Mitsushio H., Ichikawa H., Kurumaji A., Takashima M., Mataga N., Ogawa A. (1988). Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr. Scand. 78, 121–137 10.1111/j.1600-0447.1988.tb09010.x [DOI] [PubMed] [Google Scholar]
- Tost H., Hakimi S., Meyer-Lindenberg A. (2010). “Dopamine dysfunction is schizophrenia: from genetic susceptibility to cognitive impairment,” in Dopamine Handbook, eds Iversen L. L., Iversen S. D., Dunnett S. B., Bjorklund A. (Oxford: Oxford University Press; ), 558–571 [Google Scholar]
- Wei J., Ramchand C. N., Hemmings G. P. (1995). Association of polymorphic VNTR region in the first intron of the human TH gene with disturbances of the catecholamine pathway in schizophrenia. Psychiatr. Genet. 5, 83–88 10.1097/00041444-199522000-00006 [DOI] [PubMed] [Google Scholar]
- Wong D. L., Tank A. W. (2007). Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress 10, 121–130 10.1080/10253890701393529 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xu H., He J., Yang D., Jiang W., Li X., Li X. M. (2007). Quetiapine reverses altered locomotor activity and tyrosine hydroxylase immunoreactivity in rat caudate putamen following long-term haloperidol treatment. Neurosci. Lett. 420, 66–71 10.1016/j.neulet.2007.04.007 [DOI] [PubMed] [Google Scholar]