Abstract
Alpha-1 acid glycoprotein (AGP) is a highly glycosylated plasma protein that exerts vasoprotective effects. We hypothesized that AGP's N-linked glycans govern its rate of clearance from the circulation, and followed the disappearance of different forms of radiolabeled human AGP from the plasma of rabbits and mice. Enzymatic deglycosylation of human plasma-derived AGP (pdAGP) by Peptide: N-Glycosidase F yielded a mixture of differentially deglycosylated forms (PNGase-AGP), while the introduction of five Asn to Gln mutations in recombinant Pichia pastoris-derived AGP (rAGP-N(5)Q) eliminated N-linked glycosylation. PNGase-AGP was cleared from the rabbit circulation 9-fold, and rAGP-N(5)Q, 46-fold more rapidly than pdAGP, primarily via a renal route. Pichia pastoris-derived wild-type rAGP differed from pdAGP in expressing mannose-terminated glycans, and, like neuraminidase-treated pdAGP, was more rapidly removed from the rabbit circulation than rAGP-N(5)Q. Systemic hyaluronidase treatment of mice transiently decreased pdAGP clearance. AGP administration to mice reduced vascular binding of hyaluronic acid binding protein in the liver microcirculation and increased its plasma levels. Our results support a critical role of N-linked glycosylation of AGP in regulating its in vivo clearance and an influence of a hyaluronidase-sensitive component of the vessel wall on its transendothelial passage.
1. Introduction
Alpha-1 acid glycoprotein (AGP; also known as orosomucoid) is a 44 kDa, heavily glycosylated protein synthesized primarily by hepatocytes and secreted into the plasma, where it circulates under normal conditions in humans at 8–30 μM [1, 2]. During the acute phase response, circulating levels of human AGP are elevated over baseline values by 2- to 7-fold [3]; in rats, rabbits, and mice, the elevation of either circulating AGP or hepatic AGP mRNA ranges from 10- to 200-fold [4–8]. The biological function of AGP is unclear. Suggested roles for this plasma protein include the modulation of the immune response [9]; the stabilization of plasminogen activator inhibitor-1 (PAI-1) functional activity [10]; and the maintenance of normal capillary permeability and selectivity [11].
Evidence from perfusion or cell culture systems supports a role for AGP in maintaining capillary permselectivity. Perfusion studies using isolated rat skeletal muscle [12] or kidneys [13] or frog mesenteric preparations [14] initially showed that the rate of negatively charged protein loss from the perfusate (e.g., albumin or lactalbumin) was reduced in the presence of AGP. These findings lead to the hypothesis that AGP binds to the capillary vessel wall and increases its negative charge [14]. AGP binding to endothelial cells in culture has also been demonstrated [15–17]. The protein has furthermore been suggested to form part of the glycocalyx, a dynamic endothelial surface layer of glycosaminoglycans, proteoglycans, and adsorbed plasma proteins deduced by indirect examination, for instance as a red cell exclusion zone in intravital microscopy [18]. Most recently, transfused human AGP was shown to exert renal effects by restoring normal glomerular filtration rates in rats rendered nephrotic by puromycin aminoglycoside administration [19].
It has long been known that enzymatic removal of terminal sialic acid residues, and exposure of terminal galactose moieties, converts AGP into a high-affinity ligand of the asialoglycoprotein receptor, leading to its rapid removal from the mammalian circulation [20–23]. Relatively few studies, however, have examined the in vivo clearance of the unmodified plasma glycoprotein [24–26], and none have examined the consequences of complete removal of glycans on the clearance and catabolism of AGP. Since AGP is a relatively small protein of approximately 43 kDa, with 45% of its mass being contributed by its carbohydrates, we hypothesized that removal of its sugars would render it filterable in the kidneys and accelerate its clearance from the circulation[27]. In the current study, we tested this hypothesis and in addition investigated the consequences to AGP clearance of systemic disruption of the glycocalyx.
2. Materials and Methods
2.1. Materials
Affi-Gel Blue chromatography resin and Bio-Gel HT hydroxyapatite resin were purchased from Bio-Rad Laboratories (Hercules, CA). Concanavalin A (Con A), Sepharose 4B, and T4 DNA ligase were from GE Healthcare (Piscataway, NJ). Reverse transcription reagents RNEasy and Sensiscript RT kits and heat-stable HotStarTaq DNA polymerase were from Qiagen (Chatsworth, CA). Neuraminidase, peptide N-glycosidase : F (PNGase F), and Phusion heat-stable DNA polymerase were purchased from New England BioLabs (Ipswich, MA). Streptomyces hyaluronidase, commercial AGP, goat antihuman AGP antibodies, and alkaline phosphatase conjugated anti-goat IgG were from Sigma (St. Louis, MO). A Glycan Differentiation kit was purchased from Roche Applied Science (Mannheim, Germany). Biotinylated hyaluronic acid binding protein was from Associates of Cape Cod (East Falmouth, MA). Alexa Fluor 488 labeling kits, streptavidin conjugated Alexa Fluor 647, Alexa Fluor 488-Griffonia simplicifolia lectin-1B4 (GS-1B4), Pichia pastoris strain X-33 and Zeocin, ThermalAce high fidelity DNA polymerase, the pcDNA 3.1 vector, and E. coli DH5α cells were all purchased from Invitrogen Corporation (Carlsbad, CA). Restriction and DNA modification enzymes were supplied by either Fermentas Life Sciences (Burlington, ON), or Fisher Scientific (Unionville, ON). HepG2 (human hepatocellular carcinoma) cells were obtained from the American Type Culture Collection (Manassas, VA). Na125I for radioiodination was from Perkin Elmer Life Sciences (Boston, MA).
2.2. Experimental Animals
Experiments involving animals were carried out under the terms of Animal Utilization Protocols approved by the Animal Research Ethics Board of the Faculty of Health Sciences of McMaster University. Rabbits (New Zealand White, 2.1 to 2.8 kg, male and female, specific pathogen-free) were purchased from Charles River (St. Constant, Quebec), as were C57 Black 6 (C57Bl/6) male mice of 20–30 g body weight.
2.3. Purification of AGP from Human Plasma
AGP was purified exactly as described by Hervé et al. [28] from citrated plasma obtained from healthy volunteer donors, collected with informed consent using a protocol approved by the Hamilton Health Sciences Research Ethics Board. The plasma pool was combined, using equal volumes from each donor, aliquoted and frozen at −80°C until use; the same five donors (4 Caucasian, 1 Black) were used for all preparations employed in this study.
2.4. Enzymatic Modification of AGP
The terminal sialic acid chains of purified AGP were removed by treatment with Clostridium perfringens neuraminidase, using 5 units of enzyme per μg of AGP in 50 mM sodium citrate, pH 6.0, overnight at 37°C. Entire N-linked glycan chains were removed from AGP using Peptide: N-Glycosidase F (PNGase F). For electrophoretic use, AGP (0.02 mg) was denatured by boiling in 0.5% SDS, 80 mM dithiothreitol for ten minutes, then reacted with 2500 units PNGase F at 37° for one hour, after dilution into 1% NP-40, 50 mM sodium phosphate pH 7.5. For radiolabeling and in vivo use, 0.2 mg AGP in 5.0 M urea was incubated for one hour at room temperature, then diluted 1 : 1 with 10,000 units of PNGase F in 0.1 M sodium phosphate pH 7.5, and incubated overnight at 37°C, prior to dialysis against phosphate buffered saline.
2.5. DNA Manipulations
A human AGP cDNA was obtained by reverse transcription/PCR of HepG2 cell mRNA, employing RNEasy and Sensiscript RT kits (Qiagen) and oligonucleotide primers ML13047 (5′-GAATGGATCC AAGGTGACTG CACCCTGC-3′) and ML13048 (5′-ATCGAATTCG GTACACATGT CGGGTTGG-3′). The product was subcloned into pUC19, yielding plasmid pUC19-AGP. The latter plasmid was amplified using Phusion polymerase under conditions recommended by the manufacturer (New England Biolabs), and sense oligonucleotide primer 07-1495 (5′ ACGTCTCGAGA AAAGACAGAT CCCATTGTGT GCCAACC-3′) and antisense oligonucleotide primer 07-1493 (5′ CAGTGAATTC CTAGTGATGG TGATGGGATTCCCCCTCCTC CTGTTT-3′). The resulting PCR product was restricted with XhoI and EcoRI and inserted between these sites in yeast expression vector pPICZ9ssamp [29] to form pPICZ9ssAGPH6. This plasmid was modified by PCR in site-directed mutagenesis reactions that altered 5 Asn (N) codons 15, 38, 54, 75, and 85 to Gln (Q), forming pPICZ9ssAGP-N(5)Q-H6. All DNA constructs were verified by DNA sequencing (MOBIX, McMaster University) prior to expression and utilization of recombinant AGP.
2.6. Expression and Purification of Recombinant AGP
Plasmids pPICZ9ssAGPH6 and pPICZ9ssAGP-N(5)Q-H6 were separately used to transform Pichia pastoris X33 yeast to Zeocin resistance, and resulting cell lines were cultured and induced with methanol; conditioned media was neutralized and secreted recombinant proteins were purified by nickel affinity chromatography, as previously described [30].
2.7. In Vivo Clearance of AGP in Rabbits
Purified plasma-derived, enzymatically treated, or recombinant forms of AGP were iodinated using the Iodogen method [31] and injected into the marginal ear vein of rabbits, as previously described [32]. At timed intervals (0.083, 0.5, 1, 2, 4, 6, 8, 24, 48, 72, 96, and 168 hours or until the recovered radioactivity declined lower than 0.1% of the injected dose) blood samples were taken from the marginal vein of the other ear, centrifuged to obtain plasma, and the trichloroacetic acid-precipitable radioactivity was determined by γ counting. For each rabbit, semilogarithmic substrate disposition graphs of residual plasma radioactivity against time were generated, and analyzed using PK Solutions version 2.0 software (Summit Research Services, Montrose, CO, USA). Briefly, the software was employed to fit the data using noncompartmental (area) methods based on the trapezoid rule. For this purpose, radioactive doses were converted into pmoles using the measured specific activity of radiolabeling, and molecular weights of 43, 33, and 23 kDa for pdAGP, PNGase-AGP, and rAGP N(5)Q, respectively. The specific activity of radiolabeling was similar for all three proteins, ranging from 3.94 to 7.05 X 106cpm/μg. In some experiments, the tissue distribution of radioactivity 30 minutes after intravenous injection of radioiodinated AGP or derivatives was measured as described [33]; briefly, organs were excised, rinsed in ice cold saline, weighed, and either a portion or the entire organ, depending on its size, was γ counted. Results were expressed as a fraction of the total radioactive dose injected. In other experiments, either 4.0 g of α-methyl-D-mannopyranoside (α-methyl-mannoside) in 20 mL sterile saline, or 0.54 g of D-galactose in 10 mL sterile saline, was injected intraperitoneally immediately prior to intravenous injection of radioiodinated protein in an effort to block glycan-dependent uptake via hepatic receptors.
2.8. In Vivo AGP Clearance in Mice
Clearance of radiolabeled human AGP in C57Bl/6 mice was followed as previously described [30, 34] by measuring acid-precipitable plasma radioactivity in plasma samples. Plasma radioactivity was reported as percentage of the injected dose, using as a dilution factor the plasma volume divided by the sample volume. Plasma volume was calculated as the product of the weight of the mouse in grams X 0.078 mL blood volume per gram body weight X (1-the hematocrit), using a hematocrit of 0.44 (US NIH reference values from http://oacu.od.nih.gov/ARAC/). In some experiments the mice were treated with Streptomyces hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA) by intravenous injection of 47 U (to deliver 30 U/mL estimated plasma volume) of the enzyme in 0.1 mL sterile saline coinjected with iodinated human AGP.
2.9. Intravital Microscopy
Anesthetized mice (20–30 g body weight) were cannulated via the right jugular vein, the abdomen opened, and a lobe of the liver placed on the heated Plexiglas stage of a Leica DMI 6000 B confocal microscope. The liver microvasculature was photographed using a Hamamatsu C9100-12 back-thinned EMCCD camera attached to a Leica DMI 6000 B confocal microscope using the 63X objective. Either 15 μg biotinylated bovine hyaluronic acid binding protein (bHABP) complexed to streptavidin-conjugated Alexa Fluor 647 (Associates of Cape Cod, Falmouth, MA, USA) or 40 μg Griffonia simplicifolia 1B4 (GS-1B4,) lectin directly conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) was injected through the cannula into the vasculature. Baseline images were obtained over the next 15 minutes, then hyaluronidase was injected as described above, and images were acquired for an additional hour. In some experiments purified human AGP (165 mg/kg body weight) was injected after bHABP:streptavidin Alexa Fluor 647 administration and baseline image capture and 15 minutes prior to hyaluronidase treatment. Fluorescent intensity was quantified using ImageJ software (http://rsb.info.nih.gov/ij/).
2.10. Ligand Blotting
The ability of various forms of AGP to react with digoxigenin-labeled lectins was assessed following SDS-PAGE and electrical transfer of proteins to nitrocellulose; blots were analyzed using a DIG Glycan Differentiation Kit as suggested by the manufacturer (Roche Diagnostics, Mississauga, ON, Canada).
2.11. Statistical Analysis
Data are reported as the mean ± the standard deviation in all cases. Statistical analysis was performed using GraphPad Instat version 3.06 software (GraphPad Software Inc, San Diego, CA), using one-way Analysis of Variance (ANOVA) for multiple comparisons and Student's t-tests for paired comparisons. Nonparametric methods were employed if data did not pass tests of normality and equivalence of standard deviation; further details are provided in Figures 1–6 and Table 1.
Figure 1.
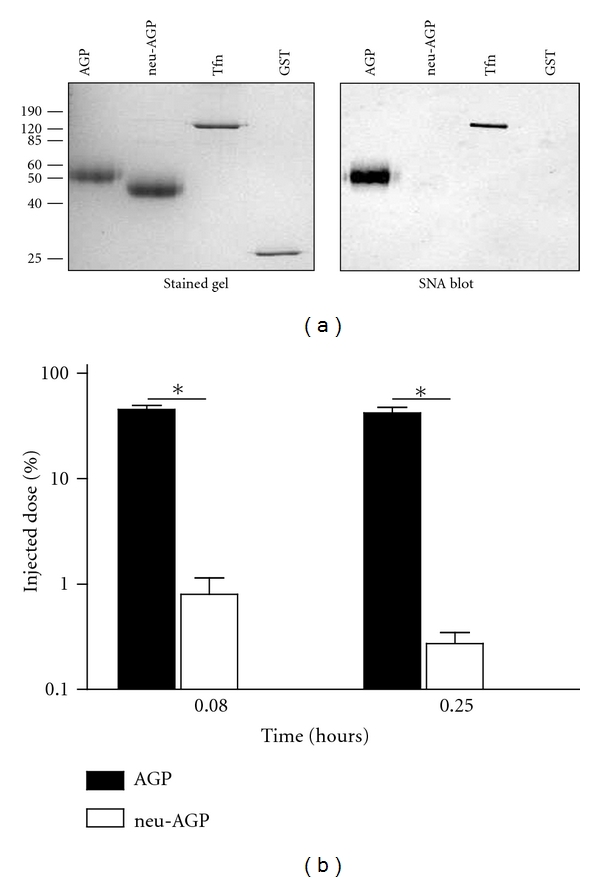
Neuraminidase treatment of human AGP increases its clearance from the rabbit circulation. Plasma-derived AGP with (neu-AGP) or without (AGP) neuraminidase treatment was examined on a 12% SDS reducing gel, either by Coomassie Blue staining ((a), left, stained gel) or by ligand blotting with SNA ((a), right, SNA blot). The position of molecular mass markers is shown in kDa, at left. Tfn: transferrin; GST: glutathione sulfotransferase. (b) Shows the recovery (% injected dose) of acid-precipitable radioactivity in plasma at different times following injection of radioiodinated AGP (solid bar) or neu-AGP (open bar) into rabbits. *Indicates P < 0.05 versus corresponding AGP value by unpaired t-test, Welch corrected. The mean of 7 determinations ± SD is shown.
Figure 6.
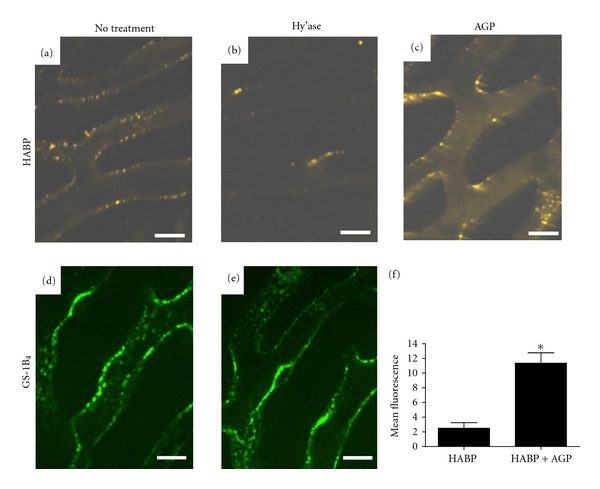
Either systemic hyaluronidase treatment or AGP administration reduces HABP binding to the vessel wall of mouse liver sinusoids in vivo. Mice were treated with hyaluronidase (Hy'ase; (b) and (e)) or without hyaluronidase (no treatment; (a) and (d)) after injection of either fluorescent HABP complex or fluorescent GS-1B4 and visualization of liver sinusoids by confocal intravital microscopy. (c) Is an image of an experiment in which AGP intravenous injection was substituted for hyaluronidase. (f) (Mean of n = 3 ± SD) quantifies the intrasinusoidal HABP fluorescence in the experiment shown in (c) and in three additional replicates; each replicate is the mean of four intravascular samples within microscopic fields similar to that seen in (c); *signifies P < 0.05 by unpaired t-test, Welch corrected.
Table 1.
Pharmacokinetic parameters relating to AGP clearance in rabbits.
| Form of AGP | |||
|---|---|---|---|
| pdAGP | PNGase-AGP (PNGase-treated) | rAGP N(5)Q | |
|
| |||
| Area under the curve (AUC 0-∞) (in pmol-hr/mL) | 11 ± 1 | 5.8 ± 0.9 | 0.74 ± 0.07*** |
| Mean residence time (MRT; in hr) | 73 ± 6 | 57 ± 3 | 23 ± 6*** |
| Volume of distribution (Vdiss; in mL/kg) | 190 ± 20 | 700 ± 100*** | 6000 ± 1000*** |
| Steady state volume of distribution (Vss; in mL/kg) | 160 ± 20 | 550 ± 90*** | 2000 ± 500*** |
| Clearance (CL; mL/hr/kg) | 2.23 ± 0.03 | 11 ± 2*** | 100 ± 10*** |
| Half-life calculated from Vdiss and CL (hours) | 58 ± 4 | 50 ± 3 | 42 ± 9** |
The mean ± the standard deviation is shown (n = 6). Derivatives of AGP were compared to the corresponding AGP value using one-way ANOVA with Tukey's post-test; **indicates P < 0.01, ***indicates P < 0.001.
3. Results
The in vivo clearance of AGP treated with neuraminidase to remove the terminal sialic acid residues has been widely investigated, to a greater extent than that of the unmodified protein [21, 24, 35, 36]. We first ensured that our purified AGP behaved as expected following this enzymatic modification. As shown in Figure 1(a), neuraminidase treatment increased the electrophoretic mobility of AGP and eliminated its recognition by Sambucus nigra agglutinin (SNA), which binds to sialic acid linked to galactose. Five minutes after injection, 45.5 ± 4.0% of the injected AGP was detected in plasma, compared to 0.77 ± 0.36% of neuraminidase-treated AGP, a difference of 82-fold. By fifteen minutes, the proportion of AGP remaining in the circulation was unchanged for native AGP (42.0 ± 5.5%) but had further declined to 0.25 ± 0.5% in the case of the neuraminidase-treated form (Figure 1(b)).
Enzymatic and recombinant DNA methods were used to reduce or eliminate the number of N-linked glycan chains attached to AGP. Denaturation of AGP was required to obtain any significant reduction in its extent of glycosylation using PNGase F, which removes glycan chains in their entirety at their point of attachment to asparagine residues on the polypeptide backbone of AGP. Enzymatic deglycosylation following denaturation of AGP with urea or SDS was compared. As shown in Figure 2, transient denaturation with urea allowed the partial removal of 0–5 glycans per AGP molecule; full denaturation with SDS removed all glycans. That the most rapidly migrating PNGase F reaction product of AGP was fully deglycosylated was indicated by its comigration both with SDS and PNGase F-treated AGP, and with a form of recombinant AGP mutated to abrogate N-linked glycosylation (rAGP N(5)Q) (Figure 1(a)), and by the loss of recognition of all of these putatively deglycosylated species with SNA lectin (data not shown). In keeping with its intermediate state of deglycosylation, PNGase F-treated AGP exhibited more rapid clearance in rabbits than unmodified AGP, but was retained in the circulation to a greater extent than rAGP N(5)Q; these differences were apparent as early as 30 minutes after injection (Figure 2(b)). Electrophoresis and autoradiography of postinjection plasma samples revealed that the most rapidly migrating deglycosylated forms of AGP (e.g., “1” versus “2” in Figure 3(b)) were lost from plasma more rapidly than less rapidly migrating forms (e.g., “3” or “4” in Figure 3(b)). In terms of organ distribution, both PNGase F-treated AGP and rAGP N(5)Q were found in the kidney to a greater extent than native AGP, the effect being more pronounced for the fully deglycosylated form (Figure 3(d)).
Figure 2.
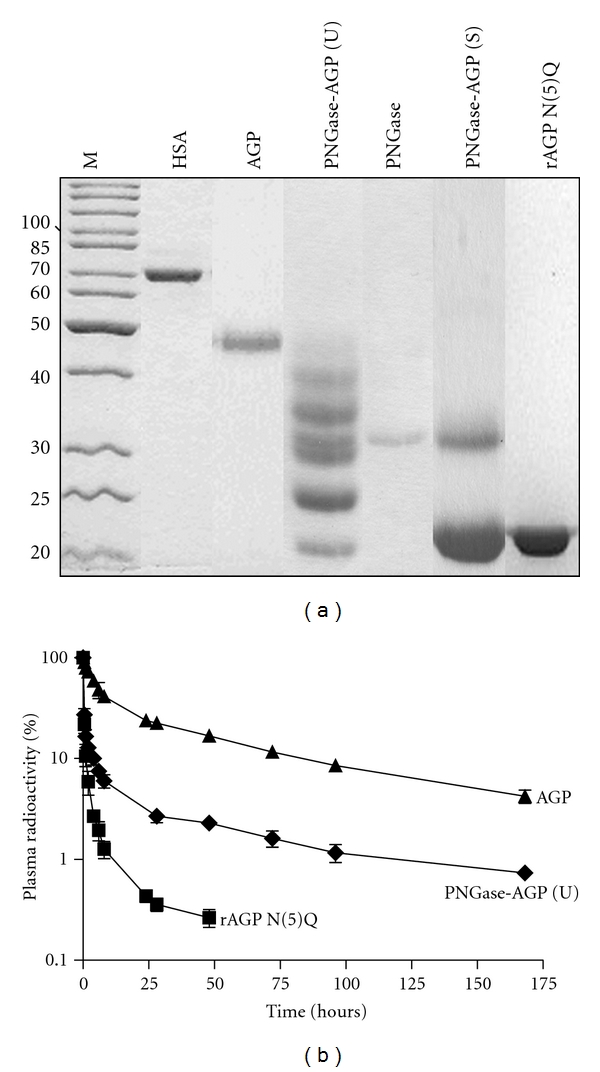
Forms of AGP with reduced or absent N-linked glycosylation are cleared from the rabbit circulation more rapidly than native AGP following intravenous injection. (a) Shows a Coomassie blue-stained 12% SDS reducing gel loaded with 5–10 μg per lane of human serum albumin, HSA; AGP; AGP treated with PNGase F (PNGase) in the presence of either urea (PNGase-AGP (U) or SDS (PNGase-AGP (S); or recombinant yeast-derived nonglycosylated AGP (rAGP N(5)Q). (b) Depicts the residual acid-precipitable radioactivity remaining in the plasma of rabbits after intravenous injection of the indicated radiolabeled proteins as a function of time; for each protein, plasma radioactivity is presented as the percentage of the injected radioactive dose recovered in plasma at the indicated time after injection. Each point is the mean ± SD of 6 determinations; in most instances error bars are smaller than the symbol that denotes the mean.
Figure 3.
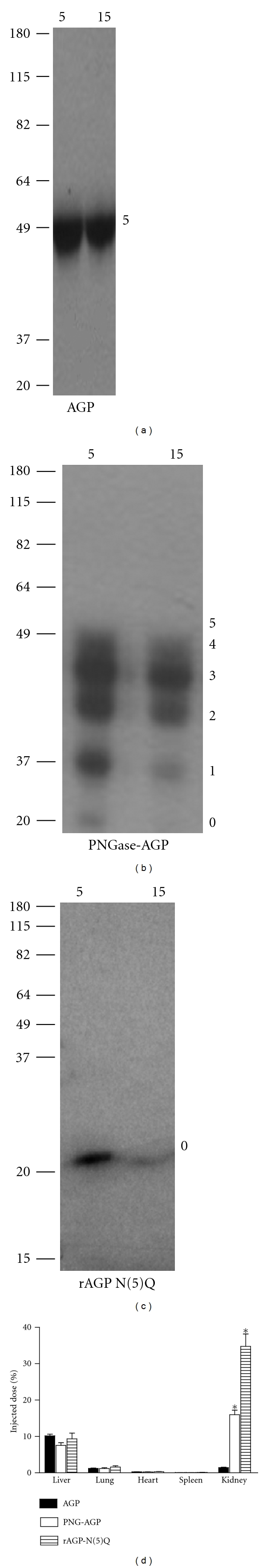
Forms of AGP with reduced glycosylation are cleared from the rabbit circulation and distribute to the kidneys more rapidly than native AGP. Plasma from rabbit injected with radioiodinated proteins (identified below (a)–(c)) was electrophoresed on reduced SDS-PAGE gels; autoradiograms of samples taken 5 and 15 minutes after injection are shown. Molecular mass markers in kDa are shown at left, and the deduced number of N-linked glycan chains at right. (d) Shows the percentage of the total radioactive dose of injected radioactivity found in the organs (identified below the panels) 30 minutes after injection of AGP (solid bars) or PNGase F-treated AGP (PNGase-AGP, open bars) or rAGP-N(5)Q (stippled bars). The mean of 6 determinations ± SD is shown. *Indicates P < 0.001 versus AGP value for the other two proteins within each organ sampled; all other comparisons to AGP values within organs are not significant.
The differences in clearance of the three forms of AGP were apparent not only on substrate disposition versus time graphs, such as those shown in Figure 2, in which the proteins were compared on the basis of residual radioactivity, but also following pharmacokinetic analysis, in which the proteins were compared based upon their dose in pmoles and their residual plasma concentration in pmol/mL (Table 1). PNGase-AGP and rAGP N(5)Q exhibited decreased areas under the clearance curve, decreased mean retention times, and decreased half-lives, but the decreases were only statistically significant for rAGP N(5)Q. The volumes of distribution and clearance rates were significantly increased for both altered forms (e.g., mean CL was increased 4.9-fold for PNGase-AGP and 45-fold for rAGP N(5)Q, P < 0.001 versus pdAGP in both cases).
We produced recombinant nonglycosylated AGP N(5)Q in a Pichia pastoris yeast expression system [29]. The disappearance of injected radiolabeled rAGP-WT from the rabbit circulation, which like rAGP-N(5)Q, was C-terminally hexahistidinylated to facilitate purification, but which unlike the mutated AGP, retained all five sites of N-linked glycosylation, was also examined. As shown in Figure 4(a), rAGP-WT was much more rapidly removed from the circulation than its mutant rAGP-N(5)Q counterpart. Thirty minutes after injection of equal doses of either radiolabeled rAGP-WT or rAGP N(5)Q, the residual plasma concentration of the latter exceeded the former by more than 10-fold (Figure 4(a); 2.2 ± 0.5% versus 26 ± 3%, P < 0.0001 by unpaired t-test, Welch corrected). Coadministration of α-methylmannoside, but not galactose, significantly reduced the loss of rAGP-WT from the circulation (to 6 ± 1%, P < 0.05 by Kruskal-Wallis test); neither sugar significantly altered the concentration of rAGP N(5)Q in the circulation, suggesting that the yeast glycans contained terminal mannose residues (Figure 4(a)). Electrophoresis showed that rAGP-WT migrated more rapidly than pdAGP, consistent with its modification with shorter N-linked glycans than in plasma-derived AGP (Figure 4(b)), and ligand blotting showed that rAGP-WT, but not plasma-derived human AGP, bound Galanthus nivalis agglutinin (GNA), which is a lectin specific for glycans terminating in mannose residues (Figure 4(b)). In contrast, rAGP-N(5)Q was unreactive with all lectins, consistent with its intended lack of glycosylation (data not shown).
Figure 4.

Recombinant AGP glycosylated in Pichia pastoris yeast is rapidly removed from the rabbit circulation via mannose receptors. (a) Shows the radioiodinated rAGP-WT or rAGP N(5)Q remaining in the plasma of rabbits 30 minutes after injection, without (no sugar) or with coadministered α-methylmannoside (+α MM) or D-galactose (+ Galactose). The mean and SD of 5–7 determinations are shown; *indicates P < 0.05 versus the corresponding “no sugar” condition by Kruskal-Wallis test. The proportion of the two proteins remaining in plasma in the absence of added sugar was also compared; ***indicates P < 0.001 by unpaired t-test, Welch corrected. (b) (Gel) shows a Coomassie blue-stained 12% SDS reducing gel loaded with 5–10 μg per lane of AGP (plasma-derived); rAGP-WT; carboxypeptidase Y (CPY); and glutathione sulfotransferase (GST). Molecular mass marker positions, shown at left, correspond to: 100; 90; 80; 70; 60; 50; 40; 30; 25; and 20 kDa. (c) (GNA blot) is a blotted replicate of the gel shown in (b), probed with GNA lectin.
Intravenous administration of hyaluronidase prior to injection of radiolabeled AGP changed both its recovery and distribution in mice for several hours after treatment. Initially, the recovery of AGP rose from 80.2 ± 5.8 in the absence of hyaluronidase to 96.8 ± 6.0 (P = 0.0007 by two-tailed unpaired t-test); as seen in Figure 5, the elevation of AGP in the plasma in hyaluronidase-treated mice remained detectable until 3 hours after treatment. The area under the observed curve (t = 0 to t = 6 hours) was increased from 250 ± 30%-hr to 300 ± 30%-hr (P = 0.037 by two-tailed unpaired t-test) by hyaluronidase treatment. We next sought independent confirmation that hyaluronidase was having its intended effect on the murine vasculature, using intravital confocal microscopy of livers of mice treated with or without hyaluronidase. Treatment with the enzyme reduced sinusoidal vessel staining with fluorescently labeled HABP but left vascular binding by fluorescent GS-1B4 unchanged (Figure 6, compare (a) to (b) and (d) to (e)). The distribution of HABP was also altered by administration of 3.3 mg intravenous human AGP (10-20 times endogenous AGP levels) without hyaluronidase treatment. This intervention left 5- to 6-fold more HABP in the plasma column than in the absence of AGP treatment (Figures 6(c) and 6(f)). Although hyaluronidase treatment altered the clearance and distribution of AGP, and AGP injection increased HABP retention in plasma, AGP did not bind to immobilized hyaluronan in vitro in microtiter plates, unlike HABP, which demonstrated avid binding (data not shown).
Figure 5.
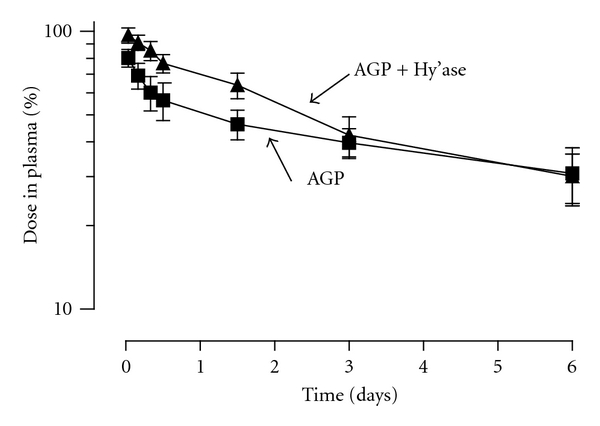
The disappearance of injected AGP from the plasma of mice following intravenous injection is altered by systemic hyaluronidase treatment. Radioiodinated AGP was injected into mice with (AGP + hy'ase) or without (AGP) concurrent hyaluronidase treatment, and acid-precipitable radioactivity, shown as the percentage of the dose remaining in plasma, was followed over time, n = 6 ± SD.
4. Discussion
In this study we explored the effects of altered, diminished, or absent glycosylation of AGP on its in vivo clearance. Although neuraminidase-treated AGP (also called asialoAGP or asialoorosomucoid) has been widely employed for many years as an injectable ligand and competitor for the hepatic asialoglycoprotein receptor in vivo [21, 24, 35, 36], the clearance of glycosylated AGP has been less extensively examined. Regoeczi et al. [24] reported a terminal catabolic half-life of 68.5 hours for iodinated human AGP injected into a single 3.8 kg rabbit; this value is similar to our finding of 59 ± 4 hours (mean ± SD, n = 6, range 53–65 hours, weight range 2.52–2.79 kg). Some of the difference may derive from our use of noncompartmental modeling and pharmacokinetic software, as opposed to the compartmental model and manual calculation employed in the older report; alternatively, our use of lighter rabbits may also be a contributing factor. In this regard, others employing noncompartmental analysis of AGP clearance in rats reported steady state weight-adjusted volumes of distribution similar to that observed in rabbits in this study. For pdAGP in rabbits, we observed 160 ± 20 mL/kg, while others reported 150 mL/kg [37] and 90 ± 30 mL/kg [26]. Taken together, these comparisons suggest that our clearance findings with respect to pdAGP are reasonable in light of previous reports in the literature and form a sound basis for comparison to the novel deglycosylated forms of AGP that we generated.
We used two strategies to reduce or eliminate entire N-linked glycans from AGP: enzymatic treatment with PNGase F, or mutation of the five asparagine residues to which the glycans are ordinarily attached. The enzymatic strategy was complicated by the resistance of AGP to deglycosylation in the absence of treatment with SDS. We elected to employ urea, since it could be removed by dialysis, promoting refolding, as opposed to SDS, which binds tightly to proteins and cannot readily be removed. PNGase F treatment was only partially effective in altering AGP clearance, likely due to the presence of some unaltered, multiply glycosylated AGP in the mixture; however, the least glycosylated forms of AGP in that mixture were most rapidly removed from the circulation. A more convincing difference between native AGP and nonglycosylated AGP was achieved via the mutational strategy; nonglycosylated rAGP-N(5)Q exhibited a 15-fold decrease in area under the clearance curve (extrapolated to infinity) and a 45-fold increase in clearance rate compared to plasma-derived AGP. Although it was not possible to control the rAGP-N(5)Q experiments with wild-type recombinant AGP made in the same experimental system, due to the high mannose-type glycosylation typical of yeast, our results were consistent with previous data. These data were obtained by investigators who employed metabolic labeling of rat hepatocytes in the presence of an inhibitor of N-linked glycosylation (tunicamycin) and immunoaffinity purification to obtain nonglycosylated rat AGP [38]. Although the difficulty in obtaining radiolabeled AGP by this route prevented replication and kinetic analysis in this study, the 50% dose retention time was noted to decrease from 100 minutes to 5 minutes for nonglycosylated AGP, as compared with native AGP, consistent with our findings, as was increased renal clearance of nonglycosylated AGP [39].
Having shown that appropriately terminated N-linked glycans keep AGP from exhibiting accelerated clearance either via glomerular filtration or via hepatic receptors specific for abnormal glycosylation, we turned to the issue of AGP interaction with the vessel wall. Intravenous administration of hyaluronidase to hamsters led to a decrease in the FITC-Dextran 70 permeation zone defined using intravital microscopy [40], one consistent with degradation of the glycocalyx, a surface layer of glycosaminoglycans, proteoglycans, and adsorbed plasma proteins [18, 41] thought to contain AGP [42, 43]. While hyaluronidase treatment of rabbits would have been ideal in the context of this study, we found such treatment impractical in this larger laboratory animal. In mice, we found that hyaluronidase treatment decreased the initial clearance of AGP, an effect consistent with an AGP-glycocalyx action as a normal part of its distribution. Hyaluronidase treatment appeared to be specific in that HABP binding to liver sinusoidal vessels was reduced, but that of GS-1B4, an α-galactose-specific lectin that mainly binds to animal endothelial cells [44], was unaffected. AGP administration also increased the amount of HABP found in the plasma column within liver sinusoids. This increase could have occurred due to AGP binding to hyaluronan in the glycocalyx, or its binding to a component of the glycocalyx that affected the access of HABP to hyaluronan. We favour the second explanation because we were unable to detect AGP-hyaluronan binding in vitro; nevertheless, we cannot exclude the possibility that this interaction occurs in vivo. Although our primary reason for examining the liver microcirculation was technical in that this organ is amenable to intravital microscopy [45], it has also been shown that this microcirculatory bed is greatly enriched in hyaluronan as opposed, for instance, to those of lung or heart [46]. Our results support the suggestion that AGP is a component of the glycocalyx, as previously suggested [17, 42, 47, 48].
From a biotechnological point of view, our results also provide novel information with respect to targeting of recombinant AGP to either renal or hepatic destinations. AGP is a member of a protein family known as the lipocalins [49]. Lipocalins bind many drugs and metabolites within a central binding pocket of the polypeptide. Interestingly, in this regard lipocalin libraries have been described with engineered binding properties [50]. Our biological distribution results raise the possibility that engineered AGPs with defined ligand binding specificities could be directed to renal or hepatic destinations using either the N(5)Q mutant, or mannosylated rAGP-WT produced in yeast. In the latter instance, producing AGP (e.g., for conjugation to DNA, as has been previously reported for gene targeting [51, 52]) or an AGP fusion protein in Pichia pastoris could provide hepatic targeting in a single step, without the need for neuraminidase treatment.
This study had several limitations. It would have been preferable to use homologous rabbit AGP or murine AGP in rabbits and mice, respectively, but our efforts to isolate rabbit AGP by adapting a protocol designed for the purification of rat AGP [53] were not successful, and it was impractical to purify sufficient quantities of murine AGP from murine plasma for our purposes. Our study was also limited to human AGP purified from the plasma of healthy individuals, and clearance was similarly investigated in healthy animals. As such, our results do not address the possibility that AGP glycosylation or clearance properties are altered during inflammation, as has been suggested [54–56]. Our use of plasma purified from a small pool of healthy donors to purify AGP was aligned with the concept that this material might be a future therapeutic product of plasma fractionation [57, 58].
Our results show that N-linked glycosylation of AGP is a critical contributor to its plasma residency, in that reducing the number of glycans accelerated clearance; this was most clearly demonstrated for a mutant AGP completely lacking N-linked glycans. In this regard, AGP resembles antithrombin, in which mutation of any one of four sites of N-linked glycosylation has been shown to lead to accelerated clearance in vivo in rabbits [59], but not albumin, in which either naturally occurring [60] or engineered sites of N-linked glycosylation accelerate the clearance of the plasma protein [33]. Glycosylation thus contributes to maintaining a circulating pool of AGP for exchange into the glycocalyx, where it may contribute to the maintenance of vascular permeability.
Acknowledgments
The authors thank Dr. Mark Hatton, Professor Emeritus, McMaster University, for helpful discussions during the course of this work. The authors declare that they have no competing financial interests in any aspect of the work described in this paper.
References
- 1.Blain PG, Mucklow JC, Rawlins MD. Determinants of plasma α1-acid glycoprotein (AAG) concentrations in health. British Journal of Clinical Pharmacology. 1985;20(5):500–502. doi: 10.1111/j.1365-2125.1985.tb05107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochimica et Biophysica Acta. 2000;1482(1-2):157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 3.Kremer JMH, Wilting J, Janssen LHM. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacological Reviews. 1988;40(1):1–47. [PubMed] [Google Scholar]
- 4.Ricca GA, Hamilton RW, McLean JW. Rat α1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. Journal of Biological Chemistry. 1981;256(20):10362–10368. [PubMed] [Google Scholar]
- 5.Cooper R, Papaconstantinou J. Evidence for the existence of multiple α1-acid glycoprotein genes in the mouse. Journal of Biological Chemistry. 1986;261(4):1849–1853. [PubMed] [Google Scholar]
- 6.Ray BK. Molecular cloning and nucleotide sequence of complementary DNA encoding rabbit α1-acid glycoprotein. Biochemical and Biophysical Research Communications. 1991;178(2):507–513. doi: 10.1016/0006-291x(91)90136-u. [DOI] [PubMed] [Google Scholar]
- 7.Baumann H, Gauldie J. The acute phase response. Immunology Today. 1994;15(2):74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 8.Clawson GA, Button J, Woo CH, Liao YC, Smuckler EA. In vitro release of α1-acid glycoprotein RNA sequences shows fidelity with the acute phase response in vivo. Molecular Biology Reports. 1986;11(3):163–172. doi: 10.1007/BF00419737. [DOI] [PubMed] [Google Scholar]
- 9.Hochepied T, Berger FG, Baumann H, Libert C. α1-acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine and Growth Factor Reviews. 2003;14(1):25–34. doi: 10.1016/s1359-6101(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 10.Smolarczyk K, Gils A, Boncela J, Declerck PJ, Cierniewski CS. Function-stabilizing mechanism of plasminogen activator inhibitor type 1 induced upon binding to α1-acid glycoprotein. Biochemistry. 2005;44(37):12384–12390. doi: 10.1021/bi050690p. [DOI] [PubMed] [Google Scholar]
- 11.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochimica et Biophysica Acta. 2000;1482(1-2):157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 12.Haraldsson B, Rippe B. Orosomucoid as one of the serum components contributing to normal capillary permselectivity in rat skeletal muscle. Acta Physiologica Scandinavica. 1987;129(1):127–135. doi: 10.1111/j.1748-1716.1987.tb08047.x. [DOI] [PubMed] [Google Scholar]
- 13.Haraldsson BS, Johnsson EKA, Rippe B. Glomerular permselectivity is dependent on adequate serum concentrations of orosomucoid. Kidney International. 1992;41(2):310–316. doi: 10.1038/ki.1992.43. [DOI] [PubMed] [Google Scholar]
- 14.Curry FE, Rutledge JC, Lenz JF. Modulation of microvessel wall charge by plasma glycoprotein orosomucoid. American Journal of Physiology. 1989;257(5):H1354–H1349. doi: 10.1152/ajpheart.1989.257.5.H1354. [DOI] [PubMed] [Google Scholar]
- 15.Schnitzer JE, Pinney E. Quantitation of specific binding of orosomucoid to cultured microvascular endothelium: role in capillary permeability. American Journal of Physiology. 1992;263(1):H48–H55. doi: 10.1152/ajpheart.1992.263.1.H48. [DOI] [PubMed] [Google Scholar]
- 16.Predescu D, Predescu S, Mcquistan T, Palade GE. Transcytosis of α1-acidic glycoprotein in the continuous microvascular endothelium. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6175–6180. doi: 10.1073/pnas.95.11.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sörensson J, Ohlson M, Björnson A, Haraldsson B. Orosomucoid has a cAMP-dependent effect on human endothelial cells and inhibits the action of histamine. American Journal of Physiology. 2000;278(5 47-5):H1725–H1731. doi: 10.1152/ajpheart.2000.278.5.H1725. [DOI] [PubMed] [Google Scholar]
- 18.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Archiv European Journal of Physiology. 2000;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 19.Hjalmarsson C, Lidell ME, Haraldsson B. Beneficial effects of orosomucoid on the glomerular barrier in puromycin aminonucleoside-induced nephrosis. Nephrology Dialysis Transplantation. 2006;21(5):1223–1230. doi: 10.1093/ndt/gfk050. [DOI] [PubMed] [Google Scholar]
- 20.Pricer WE, Jr., Hudgin RL, Ashwell G, Stockert RJ, Morell AG. A membrane receptor protein for asialoglycoproteins. Methods in Enzymology. 1974;34(C):688–691. doi: 10.1016/s0076-6879(74)34090-6. [DOI] [PubMed] [Google Scholar]
- 21.Regoeczi E, Debanne MT, Hatton MWC, Koj A. Elimination of asialofetuin and asialoorosomucoid by the intact rat. Quantitative aspects of the hepatic clearance mechanism. Biochimica et Biophysica Acta. 1978;541(3):372–384. doi: 10.1016/0304-4165(78)90196-4. [DOI] [PubMed] [Google Scholar]
- 22.Pardridge WM, Van Herle AJ, Naruse RT. In vivo quantification of receptor-mediated uptake of asialoglycoproteins by rat liver. Journal of Biological Chemistry. 1983;258(2):990–994. [PubMed] [Google Scholar]
- 23.Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annual Review of Biochemistry. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 24.Regoeczi E, Hatton MWC, Charlwood PA. Carbohydrate mediated elimination of avian plasma glycoprotein in mammals. Nature. 1975;254(5502):699–701. doi: 10.1038/254699a0. [DOI] [PubMed] [Google Scholar]
- 25.Hervé F, D'Athis P, Tremblay D, Tillement J-P, Barré J. Glycosylation study of the major genetic variants of human α 1-acid glycoprotein and of their pharmacokinetics in the rat. Journal of Chromatography B. 2003;798(2):283–294. doi: 10.1016/j.jchromb.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 26.Parivar K, Tolentino L, Taylor G, Oie S. Elimination of non-reactive and weakly reactive human α1-acid glycoprotein after induction of the acute phase response in rats. Journal of Pharmacy and Pharmacology. 1992;44(5):447–450. doi: 10.1111/j.2042-7158.1992.tb03643.x. [DOI] [PubMed] [Google Scholar]
- 27.Sheffield WP. Modification of clearance of therapeutic and potentially therapeutic proteins. Curr Drug Targets Cardiovasc Haematol Disord. 2001;1(1):1–22. doi: 10.2174/1568006013338150. [DOI] [PubMed] [Google Scholar]
- 28.Hervé F, Millot M-C, Eap CB, Duché J-C, Tillement J-P. Two-step chromatographic purification of human plasma α1-acid glycoprotein: its application to the purification of rare phenotype samples of the protein and their study by chromatography on immobilized metal chelate affinity adsorbent. Journal of Chromatography B. 1996;678(1):1–14. doi: 10.1016/0378-4347(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 29.Sheffield WP, McCurdy TR, Bhakta V. Fusion to albumin as a means to slow the clearance of small therapeutic proteins using the Pichia pastoris expression system: a case study. Methods in Molecular Biology. 2005;308:145–154. doi: 10.1385/1-59259-922-2:145. [DOI] [PubMed] [Google Scholar]
- 30.Sheffield WP, Eltringham-Smith LJ, Gataiance S, Bhakta V. A long-lasting, plasmin-activatable thrombin inhibitor aids clot lysis in vitro and does not promote bleeding in vivo. Thrombosis and Haemostasis. 2009;101(5):867–877. [PubMed] [Google Scholar]
- 31.Fraker PJ, Speck JC., Jr. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphenylglycoluril. Biochemical and Biophysical Research Communications. 1978;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 32.Sheffield WP, Smith IJ, Syed S, Bhakta V. Prolonged in vivo anticoagulant activity of a hirudin—albumin fusion protein secreted from Pichia pastoris. Blood Coagulation and Fibrinolysis. 2001;12(6):433–443. doi: 10.1097/00001721-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Sheffield WP, Marques JA, Bhakta V, Smith IJ. Modulation of clearance of recombinant serum albumin by either glycosylation or truncation. Thrombosis Research. 2000;99(6):613–621. doi: 10.1016/s0049-3848(00)00286-3. [DOI] [PubMed] [Google Scholar]
- 34.Begbie ME, Mamdani A, Gataiance S, et al. An important role for the activation peptide domain in controlling factor IX levels in the blood of haemophilia B mice. Thrombosis and Haemostasis. 2005;94(6):1138–1147. doi: 10.1160/TH04-03-0201. [DOI] [PubMed] [Google Scholar]
- 35.Wall DA, Wilson G, Hubbard AL. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- 36.Steer CJ, Ashwell G. Studies on a mammalian hepatic binding protein specific for asialoglycoproteins. Evidence for receptor recycling in isolated rat hepatocytes. Journal of Biological Chemistry. 1980;255(7):3008–3013. [PubMed] [Google Scholar]
- 37.Keyler DE, Pentel PR, Haughey DB. Pharmacokinetics and toxicity of high-dose human α1-acid glycoprotein infusion in the rat. Journal of Pharmaceutical Sciences. 1987;76(2):101–104. doi: 10.1002/jps.2600760203. [DOI] [PubMed] [Google Scholar]
- 38.Gross V, Steube K, Tran-Thi TA. The role of N-glycosylation for the plasma clearance of rat liver secretory glycoproteins. European Journal of Biochemistry. 1987;162(1):83–88. doi: 10.1111/j.1432-1033.1987.tb10545.x. [DOI] [PubMed] [Google Scholar]
- 39.Gross V, Heinrich PC, vom Berg D, et al. Involvement of various organs in the initial plasma clearance of differently glycosylated rat liver secretory proteins. European Journal of Biochemistry. 1988;173(3):653–659. doi: 10.1111/j.1432-1033.1988.tb14048.x. [DOI] [PubMed] [Google Scholar]
- 40.Henry CBS, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. American Journal of Physiology. 1999;277(2):H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 41.Platts SH, Duling BR. Adenosine A3 receptor activation modulates the capillary endothelial glycocalyx. Circulation Research. 2004;94(1):77–82. doi: 10.1161/01.RES.0000108262.35847.60. [DOI] [PubMed] [Google Scholar]
- 42.Sörensson J, Matejka GL, Ohlson M, Haraldsson B. Human endothelial cells produce orosomucoid, an important component of the capillary barrier. American Journal of Physiology. 1999;276(2):H530–H534. doi: 10.1152/ajpheart.1999.276.2.H530. [DOI] [PubMed] [Google Scholar]
- 43.Boncela J, Papiewska I, Fijalkowska I, Walkowiak B, Cierniewski CS. Acute phase protein α1-acid glycoprotein interacts with plasminogen activator inhibitor type 1 and stabilizes its inhibitory activity. Journal of Biological Chemistry. 2001;276(38):35305–35311. doi: 10.1074/jbc.M104028200. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein IJ, Winter HG. The Griffonia simplicifolia I-B4 isolectin. A probe for alpha-D-galactosyl end groups. Sub-Cellular Biochemistry. 1999;32:127–141. [PubMed] [Google Scholar]
- 45.Ondiveeran HK, Fox-Robichaud AE. Pentastarch in a balanced solution reduces hepatic leukocyte recruitment in early sepsis. Microcirculation. 2004;11(8):679–687. doi: 10.1080/10739680490517712. [DOI] [PubMed] [Google Scholar]
- 46.McDonald B, McAvoy EF, Lam F, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. Journal of Experimental Medicine. 2008;205(4):915–927. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Agostini AI, Watkins SC, Slayter HS, Youssoufian H, Rosenberg RD. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. Journal of Cell Biology. 1990;111(3):1293–1304. doi: 10.1083/jcb.111.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fridén V, Oveland E, Tenstad O, et al. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney International. 2011;79(12):1322–1330. doi: 10.1038/ki.2011.58. [DOI] [PubMed] [Google Scholar]
- 49.Flower DR. The lipocalin protein family: structure and function. Biochemical Journal. 1996;318(1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schönfeld D, Matschiner G, Chatwell L, et al. An engineered lipocalin specific for CTLA-4 reveals a combining site with structural and conformational features similar to antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(20):8198–8203. doi: 10.1073/pnas.0813399106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu GY, Wu CH. Receptor-mediated gene delivery and expression in vivo. Journal of Biological Chemistry. 1988;263(29):14621–14624. [PubMed] [Google Scholar]
- 52.Yang DY, Ouyang CH, Lu FG, Liu XW, Huang LQ. Targeting specificity and pharmacokinetics of asialoorosomucoid, a specific ligand for asialglycoprotein receptor on hepatocyte. Journal of Digestive Diseases. 2007;8(2):89–95. doi: 10.1111/j.1443-9573.2007.00291.x. [DOI] [PubMed] [Google Scholar]
- 53.Shibata K, Okubo H, Ishibashi H, Tsuda K. Rat α1-acid glycoprotein. Purification and immunological estimation of its serum concentration. Biochimica et Biophysica Acta. 1977;495(1):37–45. doi: 10.1016/0005-2795(77)90237-9. [DOI] [PubMed] [Google Scholar]
- 54.Brinkman-Van der Linden ECM, Mollicone R, Oriol R, Larson G, Van Den Eijnden DH, Van Dijk W. A missense mutation in the FUT6 gene results in total absence of α3-fucosylation of human α1-acid glycoprotein. Journal of Biological Chemistry. 1996;271(24):14492–14495. doi: 10.1074/jbc.271.24.14492. [DOI] [PubMed] [Google Scholar]
- 55.Jørgensen HG, Elliott MA, Priest R, Smith KD. Modulation of sialyl Lewis X dependent binding to E-Selectin by glycoforms of alpha-1-acid glycoprotein expressed in rheumatoid arthritis. Biomedical Chromatography. 1998;12(6):343–349. doi: 10.1002/(SICI)1099-0801(199811/12)12:6<343::AID-BMC760>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Rydén I, Påhlsson P, Lundblad A, Skogh T. Fucosylation of α1-acid glycoprotein (orosomucoid) compared with traditional biochemical markers of inflammation in recent onset rheumatoid arthritis. Clinica Chimica Acta. 2002;317(1-2):221–229. doi: 10.1016/s0009-8981(01)00803-8. [DOI] [PubMed] [Google Scholar]
- 57.Muchitsch E-M, Auer W, Pichler L. Effects of α1-acid glycoprotein in different rodent models of shock. Fundamental and Clinical Pharmacology. 1998;12(2):173–181. doi: 10.1111/j.1472-8206.1998.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 58.Muchitsch E-M, Schwarz HP, Ginsberg MD, Belayev L, Busto R. Beneficial effect of albumin therapy attributable to α1-acid glycoprotein? Stroke. 2003;34(1):4–5. doi: 10.1161/01.str.0000046761.84916.64. [DOI] [PubMed] [Google Scholar]
- 59.Ni H, Blajchman MA, Ananthanarayanan VS, Smith IJ, Sheffield WP. Mutation of any site of N-linked glycosylation accelerates the in vivo clearance of recombinant rabbit antithrombin. Thrombosis Research. 2000;99(4):407–415. doi: 10.1016/s0049-3848(00)00263-2. [DOI] [PubMed] [Google Scholar]
- 60.Peach RJ, Brennan SO. Structural characterization of a glycoprotein variant of human serum albumin: albumin Casebrook (494 Asp → Asn) Biochimica et Biophysica Acta. 1991;1097(1):49–54. doi: 10.1016/0925-4439(91)90023-3. [DOI] [PubMed] [Google Scholar]


