Abstract
Myxoma virus (MYXV) is a novel oncolytic virus that has been shown to replicate in pancreatic cancer cells, but its efficacy in animal models of pancreatic cancer has not been determined. The efficacy of MYXV as monotherapy or in combination with gemcitabine was evaluated in intraperitoneal dissemination (IPD) models of pancreatic cancer. The effects of an intact immune system on the efficacy of MYXV therapy was tested by comparing immunodeficient versus immunocompetent murine models and combination therapy with gemcitabine was also evaluated. In cell culture, MYXV replication was robust in a broad range of pancreatic cancer cells and also showed increased oncolysis in combination with gemcitabine. In animal models, MYXV treatment conferred survival benefits over control or gemcitabine-treated cohorts regardless of the cell line or animal model used. MYXV monotherapy was most effective in an immunocompetent IPD model, and resulted in 60% long-term survivors. In Pan02 engrafted immunocompetent IPD models, sequential treatment in which MYXV was administered first, followed by gemcitabine, was the most effective and resulted in 100% long-term survivors. MYXV is an effective oncolytic virus for pancreatic cancer and can be combined with gemcitabine to enhance survival, particularly in the presence of an intact host immune system.
Introduction
Pancreatic cancer was reported as the fourth cause of cancer related deaths in the United States in 2010 by the American Cancer Society.1 The prognosis for patients with pancreatic cancer remains poor regardless of treatments. After initial diagnosis, only 6% of patients survive past 5 years and most die within the first year. Most patients are diagnosed at stages of locally advanced or metastatic disease and even in cases when tumors are determined to be resectable, the 5-year survival rate is only 22%.1 The current treatments for pancreatic cancer are limited and usually consist of radiation and/or chemotherapy with gemcitabine (Gemzar; Eli Lilly, Indianapolis, IN), a nucleoside analog. Since its introduction in the late nineties, gemcitabine has been considered the standard of care for this cancer, but provides only marginal survival benefits compared to untreated patients.2,3,4 Resistance to gemcitabine and common chemotherapy agents is frequently a problem in pancreatic cancer5,6 and also contributes to poor prognosis. Clearly, new therapies or new combination therapies with the currently approved drugs are needed to efficiently treat and prolong the survival of these patients. Unfortunately, most clinical trials exploring the use of gemcitabine in combination with other drugs have failed to show any significant added survival benefits over gemcitabine alone.7,8
Oncolytic virotherapy proposes the use of live viruses with selective tropism for cancer cells as a novel anticancer therapeutic approach for many types of cancers, including pancreatic cancer.9,10,11,12,13 In addition, many of these cancer-selective viruses or oncolytic viruses (OVs) have the potential to be combined with currently available therapies resulting in increased therapeutic benefits compared to single agent therapies in preclinical cancer models.14,15 Myxoma virus (MYXV) is a novel OV candidate with a selective tropism for a wide spectrum of human cancer cells.16,17 MYXV is a Leporipoxvirus with a very restrictive rabbit-specific tropism in nature and is apathogenic and safe to all non-lagomorphs tested, including mice, rats and humans18,19,20,21,22, thus supporting its potential clinical use as an OV. In animal models, MYXV infects and provides therapeutic benefits in several animal cancer models including xenograft models of human gliomas, medulloblastomas and rhabdoid tumors as well as immunocompetent murine melanoma and racine glioma models.22,23,24,25,26 The molecular basis of MYXV tropism in human cancer cells depends in part on the activation of the cellular serine/threonine kinase Akt27,28,29 and to impaired innate antiviral immune responses.30 Human pancreatic cancer cells have been reported to be susceptible to MYXV infection in vitro,31 but its efficacy in animal models of pancreatic cancer in vivo has not been previously studied.
In this report, we investigate the susceptibility of pancreatic cancer cells to MYXV oncolysis in vitro as either a single agent therapy or in combination with gemcitabine, the current standard of care for pancreatic cancer patients. In addition, the efficacy of MYXV virotherapy alone or in combination with gemcitabine was determined in both immunodeficient and immunocompetent murine models of pancreatic disseminated cancer.
Results
MYXV replicates in both murine and human pancreatic cancer cells in vitro and in vivo
A panel of five pancreatic cancer cell lines (Panc-1, Hs766T, AsPC-1, BxPC-3 and Pan02) was tested for their permissiveness to MYXV infection. This panel included pancreatic cancer cell lines with mutated K-ras, such as Panc-1, Hs766T and AsPC1 as well as the BxPC3 cell line with a wildtype K-ras as reported by the Sol Goldman Pancreatic Cancer Research Center at John Hopkins (http://pathology2.jhu.edu/pancreas/geneticsweb/profiles.htm). This panel also contained cell lines with different gemcitabine sensitivities, such as Hs766T which is highly resistant and Pan02 which is highly sensitive to the drug (Figure 3b). Figure 1 showed that MYXV productively infected and spread (albeit with varying efficiencies) in all the pancreatic cancer cell lines tested. Early and late MYXV gene expression was observed in all infected cells tested such as Pan02 and Hs766T (Figure 1a). MYXV also spread from cell-to-cell in monolayers of pancreatic cancer cells as shown by an increase in the percent of infected cells expressing MYXV-encoded green fluorescent protein (GFP) with time (Figure 1b) and by the formation of viral foci (Figure 1c). MYXV was able to spread very robustly in Pan02 cells where the percent of virally encoded GFP was similar to that observed for cell lines of rabbit origin such as RK-13. In other pancreatic cell lines such as Panc-1 and Hs766T MYXV spread was less efficient. However even in these cell lines with slow MYXV spread, (i.e., Hs766T), MYXV still formed foci by 4 days post infection (p.i.) (Figure 1c). When progeny virus yields were determined, differences in the ability to produce infectious progeny virus were also noted, but all cell lines were determined to be permissive for MYXV replication (Figure 1d). Murine Pan02 cells were highly permissive to MYXV replication. In this cell line MYXV achieved high viral titers comparable to those obtained in rabbit RK-13 cells with approximately a 3-log titer increase at 72 hours p.i. Other pancreatic cancer cell lines such as AsPC-1 and Hs766T were also permissive for MYXV infection with approximately a 2-log increase in viral titer at 72 hours p.i. From the panel of cell lines tested, Panc-1 cells were the least susceptible. In this cell line, MYXV replicated but achieved lower titers when compared to other cell lines (Figure 1d). Taken together, these results show that productive MYXV replication occurs in a variety of different pancreatic cancer cells.
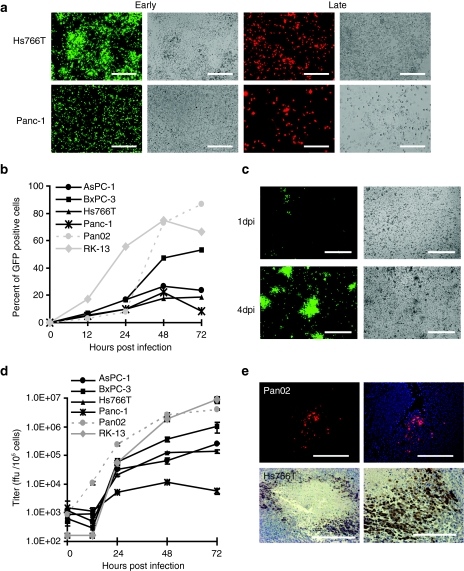
Figure 1.
MYXV replicates in pancreatic cancer cells. (a) Pancreatic cancer cells infected with MYXV express early and late markers of viral gene expression. Hs766T or Panc-1 cells were infected with vMyx-GFP at MOI 10 or vMyx-RFP at MOI 5. Fluorescence images and their respective bright field images were taken 24 hours p.i. Bar = 250 µm. (b, c) MYXV spreads in monolayers of pancreatic cancer cells. (b) Cells were infected with vMyx-GFP at MOI 0.1, collected at the indicated time points and analyzed by flow cytometry to determine the percent of infected GFP positive cells. (c) Hs766T cells were infected with vMyx-GFP at MOI 0.1 and analyzed for GFP expression by direct fluorescence 1 day post infection (dpi) and 4 dpi. Bar = 250 µm. (d) MYXV productively infects pancreatic cancer cells. Cells were infected with vMyx-GFP at MOI 0.1, collected at the indicated time points and lysed to determine viral titers. Titers for each sample were performed in triplicate and error bars shown are the mean plus/minus one standard deviation (mean ± SD). (e) MYXV infects pancreatic cancer tumors in vivo. Pan02 (top panels) and Hs766T (lower panels) derived subcutaneous tumors were injected IT with vMyx-tdTr and analyzed for expression of viral reporter genes. Pan02 tumors were excised 3 dpi and analyzed for tdTr expression by direct fluorescence. DAPI was used as a contrast stain. Bar = 200 µm. Hs766T were excised 7 dpi and analyzed for the presence of virus by immunostaining for GFP. Bar = 100 µm. GFP, green fluorescent protein; MOI, multiplicity of infection; MYXV, Myxoma virus; p.i., post infection; RFP, red fluorescent protein; tdTr, tandem dimer tomato red fluorescent protein.
MYXV also disseminated in subcutaneous pancreatic tumors. Subcutaneous Hs766T-derived tumors engrafted into immunodeficient nude mice and injected intra-tumorally (IT) with MYXV expressing GFP (vMYX-GFP) stained positive for GFP expression 7 days after virus injection (Figure 1e). Subcutaneous Pan02-derived tumors engrafted into C57Bl6 immunocompetent mice and injected IT with MYXV expressing tomato red fluorescent protein (vMyx-tdTr) showed tdTr fluorescence 3 days after virus injection (Figure 1e), but not at 7 days after virus injection (data not shown), suggesting a transient replication of MYXV in immunocompetent animals.
MYXV infection reduces the viability of pancreatic cancer cells in vitro
Colony forming assays (Figure 2a) and MTT assays (Figure 2b) were used to determine if infection with MYXV resulted in classic oncolysis of pancreatic cancer cells. The highly MYXV-susceptible murine cell line Pan02 as well as two less susceptible human cell lines (Hs766T and Panc-1) were tested. Colony forming assays showed that infection with MYXV reduced the ability of the cultured cells to divide and form colonies from individual cells (Figure 2a). MTT assays on MYXV-infected cells, showed between 40 and 60% reductions in cellular mitochondrial function when compared to mock treated cells. Of the three cell lines tested Pan02 showed the greatest reduction in the percent of live cells (~60%) compared to the other two cells lines, which showed about a 40% reduction (Figure 2b). Thus, MYXV infection of cultured pancreatic cancer cells lead to significant reductions in cell viability and suggests that MYXV treatment of these types of cancers may provide therapeutic benefits in vivo.
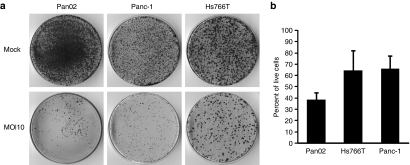
Figure 2.
MYXV infection reduces the viability of pancreatic cancer cells. (a) Colony forming assays. Pancreatic cancer cells were infected with vMyx-GFP at MOI 10 or mock infected. Twenty-four hours p.i. cells were re-seeded in 10 cm dishes and 2 weeks later colonies were stained using crystal violet. (b) MTT assays. Pancreatic cancer cells were infected with vMyx-GFP at MOI 10 and analyzed for cell viability 72 hours p.i. MTT assays were performed in triplicate and error bars shown are the mean plus/minus one standard deviation (mean ± SD). GFP, green fluorescent protein; MOI, multiplicity of infection; MYXV, Myxoma virus; p.i., post infection.
Effects of gemcitabine on MYXV replication and virus-drug combination treatments in pancreatic cancer cells in vitro
The potential use of MYXV in combination with gemcitabine may provide a novel combination treatment of clinical interest. A dose-dependent inhibitory effect on MYXV early gene expression (as determined by GFP expression) in vitro was observed when cells were infected in the presence of gemcitabine (Figure 3a), suggesting that treatments involving the simultaneous administration of MYXV and gemcitabine may not be advantageous. Therefore, combination treatments involving the sequential administration, and not the simultaneous administration, of these two therapies were evaluated in vitro.
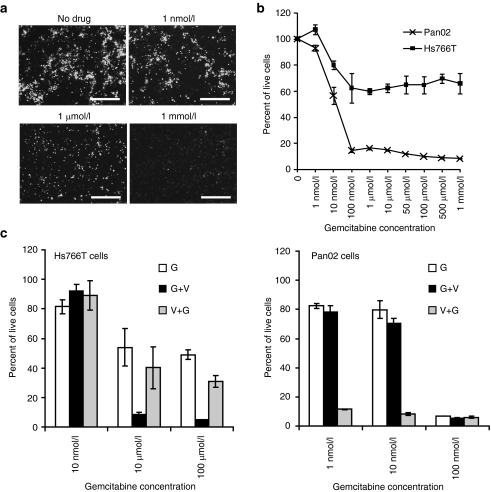
Figure 3.
Effects of gemcitabine on MYXV replication and oncolysis. (a) Hs766T cells were treated with the indicated concentrations of gemcitabine for 24 hours or left untreated and then infected with vMyx-GFP at MOI 10 in the presence of drug for 24 hours. Infected GFP-expressing cells were detected by direct fluorescence 24 hours p.i. Bar = 250 µm. (b) Gemcitabine sensitivity of pancreatic cancer cells. Hs766T cells and Pan02 cells treated with gemcitabine were analyzed for cell viability by MTT assays. MTT assays were performed in triplicate. (c) MYXV in combination with gemcitabine treatment enhances oncolysis. Hs766T cells or Pan02 cells were treated as indicated: Gemcitabine treatment followed by virus infection (G+V); virus infection followed by gemcitabine treatment (V+G); gemcitabine only (G). MTT assays were performed in duplicate or triplicate. Error bars for all MTT assays represent the mean plus/minus one standard deviation (mean ± SD). MOI, multiplicity of infection; MYXV, Myxoma virus; p.i., post infection.
To evaluate the potential use of MYXV as an adjuvant treatment for gemcitabine resistant tumors, we first determined the relative gemcitabine susceptibility of the highly MYXV-susceptible Pan02 cells and of the less permissive Hs766T cell line (Figure 3b). As reported previously,32 Hs766T cells were highly resistant to gemcitabine treatment. Even at high concentrations of the drug (1 mmol/l) 60% of the cells were still viable by MTT assays. By contrast, Pan02 cells were more sensitive to the drug when compared to Hs766T cells and concentrations of 100 nmol/l or higher produced more than 80% cell death by MTT assays (Figure 3b).
After establishing the relative gemcitabine sensitivities of these two cell lines, MYXV-induced oncolysis was compared in these cell lines in combination with gemcitabine. For one combination treatment, cells were first treated with gemcitabine followed by MYXV infection (G+V). A second combination treatment was also compared in which MYXV infection was followed by gemcitabine treatment (V+G). For the resistant Hs766T cell line, two concentrations of gemcitabine (10 and 100 µmol/l) which do not show a dose-dependent decrease in the percent of live cells by MTT assays (Figure 3b,c) were used to determine if combination treatment with MYXV could further reduce the percent of viable cells. For the gemcitabine sensitive Pan02 cells, two suboptimal concentrations of gemcitabine 1 and 10 nmol/l were used. At these concentrations of drug ~80% and 55% of the cells are still alive by MTT assays (Figure 3b,c) and therefore any further reduction in viability caused by MYXV-driven oncolysis would be detected.
Figure 3c shows that Hs766T cells (left panel) treated with the sequential treatment in which drug was administered first (G+V) dramatically decreased the viability (<10%) of these cells at both 10 µmol/l and 100 µmol/l compared to single treatments (~50–60% for gemcitabine and 60% for MYXV single therapies) (Figures 3c and 2b). By contrast the V+G treatment did not show this robust decrease in cell viability even at the highest concentration of the drug (Figure 3c). These results suggest that treatment of gemcitabine resistant tumors with drug followed by MYXV may provide a therapeutic benefit in vivo.
The same regimens were also tested in the Pan02 drug-sensitive cell line (Figure 3c, right panel). For this cell line the treatment combination in which MYXV was administered before drug treatment (V+G) induced a dramatic decrease in cell viability even at the lowest concentration of drug (1 nmol/l). At this concentration, less than 20% of the cells treated with the V+G regimen were viable compared to single treatments (80% and 40% for gemcitabine and MYXV single therapies, respectively) (Figures 3c and 2b). On the other hand, the administration of suboptimal doses of drug first followed by MYXV infection (G+V) failed to increase the oncolysis of these cells compared to gemcitabine alone (Figure 3c).
Taken together, these results showed that MYXV can be successfully combined with gemcitabine in vitro to increase oncolysis, in two different cell lines (Pan02 and Hs766T), which vary in their sensitivity to gemcitabine as well as in their permissiveness to MYXV infection. In addition, the susceptibility of these cells to oncolysis with the combination treatments tested varied depending on the order in which the treatments were sequentially administered. This suggests that the optimal order of treatment administration is cell type dependent.
MYXV treatment reduces the tumor burden of xenografted mice bearing intraperitoneal human tumors and prolongs survival
The efficacy of MYXV oncolysis was tested in an intraperitoneal dissemination (IPD) model using Hs766T cells expressing firefly luciferase in NOD/SCID mice to determine if MYXV could provide therapeutic benefits in the case of gemcitabine resistant pancreatic tumors. Using this model, the effect of MYXV treatment on tumor burden was determined (Figure 4). After initial tumor burden detection in the IP cavity, virus treatment was started and tumor progression was monitored in mice utilizing bioluminescence imaging (Figure 4a). In this model, the tumor burden (as a measure of radiance) was significantly less in MYXV-treated mice compared to untreated mice starting at 35 days after cell engraftment (Figure 4a,b).
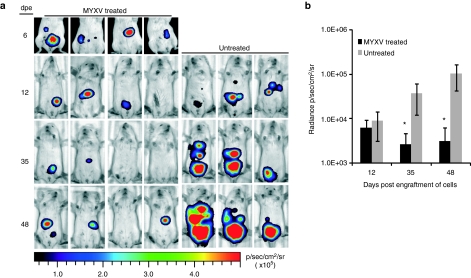
Figure 4.
MYXV reduces the tumor burden in vivo. (a) NOD/SCID mice engrafted with intraperitoneal Hs766T-FFluc tumors were treated with four doses of 108 ffu of vMyx-tdTr intraperitoneally starting at 6 days post engraftment (dpe) of cells or left untreated. Tumor burden was monitored at the indicated time points using bioluminescence imaging and D-luciferin as substrate. (b) Radiances from the peritoneal area of mice in a were calculated. *P < 0.05 between untreated and MYXV-treated groups. Error bars shown represent the mean plus/minus one standard standard deviation (Mean ± SD). ffu, foci forming units; FFluc, firefly luciferase; MYXV, Myxoma virus.
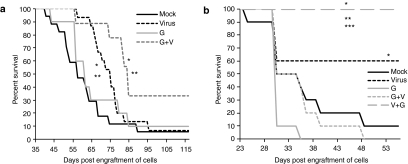
Furthermore, to determine if this reduction in tumor growth would result in prolonged survival of mice, the survival curves of Hs766T-engrafted NOD/SCID mice treated with MYXV as a single therapy were compared to those of mock and gemcitabine only treated cohorts. In this Hs766T xenografted immunodeficient model, gemcitabine therapy (at the doses given in this study) did not significantly improve the survival of mice when compared to mock treated mice (P = 0.4092) (Figure 5a), in agreement with the gemcitabine resistance of this cell line in vitro (Figure 3a). In contrast, treatment with MYXV as a single therapy in this same model resulted in a significant prolongation of survival compared to mock treatment (P = 0.0306), with a median survival of 75 days compared to 57 days for the mock treatment (Figure 5a). However, no long-term survivors were observed in this xenograft model.
Figure 5.
MYXV prolongs survival of mice engrafted with intraperitoneal tumors. (a) NOD/SCID mice engrafted with intraperitoneal Hs766T-FFluc tumors were divided into four treatment groups: MYXV-treated (virus) cohort received 5–6 doses of 108 ffu; gemcitabine-treated mice (G) received four doses of 50 mg/kg of gemcitabine; the combination treatment cohort received gemcitabine followed by virus (G+V); mock treated mice received PBS. (b) C57Bl6 mice engrafted with intraperitoneal Pan02-FFluc tumors were treated with PBS (mock), MYXV only (virus), gemcitabine only (G) or with combination treatments involving gemcitabine followed by virus (G+V) or virus followed by gemcitabine (V+G). All treatments were administered IP and started after confirmation of tumor burden by bioluminescence imagining with D-luciferin 5–6 days after injection of cells into the IP cavity. *P < 0.05 between mock and MYXV treated groups; **P < 0.05 between MYXV and gemcitabine-treated groups; ***P < 0.05 between MYXV and V+G treated groups. ffu, foci forming units; MYXV, Myxoma virus; PBS, phosphate-buffered saline.
When Hs766T tumors from mice treated with vMyx-tdTr were excised at end point, no MYXV replication (as evidenced by tdTr fluorescence) was observed (data not shown). This suggests that MYXV infection within the tumors is transient and/or that the input virus delivered into the IP cavity did not reach all the tumors. Thus, the effect of MYXV treatment in this model may be hampered by a lack of complete intra- or inter-tumoral viral spread resulting only in transient replication of MYXV in some but not all of the tumors. However, despite these limitations, MYXV monotherapy in this immunodeficient model significantly prolonged the survival of mice when compared to both mock and gemcitabine only treated cohorts.
MYXV treatment of syngeneic, immunocompetent mice bearing intraperitoneal murine tumors results in long-term survivors
To determine the oncolytic efficacy of MYXV in the presence of an intact immune system, C57Bl6 mice were engrafted with Pan02 cells and treated with MYXV or gemcitabine as single therapies and their survival was compared to mock treated mice. Despite the high gemcitabine sensitivity of Pan02 cells observed in vitro (Figure 3b), mice treated with gemcitabine failed to survive significantly longer than mock treated mice. A tendency towards lower survival times were noted in this group when compared to mock treated mice, but this tendency did not reach statistical significance (P < 0.062) compared to mock treated cohorts. This tendency in survival was not due to toxicity of the drug since: (i) all deaths in this cohort were related to tumor burden and/or the formation of severe ascites; and (ii) two other cohorts (G+V and V+G cohorts) also received the same number of doses at the same concentration and did not exhibit this tendency.
In contrast to gemcitabine single therapy, MYXV monotherapy in this model significantly prolonged the survival of mice compared to mock treatment (P = 0.0334) despite the presence of an intact immune system (Figure 5b). At the end point of this study (i.e., 55 days after engraftment of cells), 60% of mice treated with MYXV were still alive, showed no overt clinical signs of tumor burden and were considered long-term survivors. The results from these two animal models showed that even in the presence of an intact immune response, MYXV treatment alone provided a better therapeutic benefit than gemcitabine and that this survival benefit is observed regardless of the predicted in vitro gemcitabine sensitivity of the cell lines used to generate the tumors (Figures 3b and 5b).
Furthermore, these results also suggested that MYXV therapy was most effective in the presence of an intact immune response. To determine the contribution of the presence of an intact immune system on the efficacy of MYXV oncolysis, MYXV therapy was also tested in a Pan02-NOD/SCID model in which the same Pan02 cells were engrafted in immunodeficient NOD/SCID mice (Supplementary Figure S1). In this immunodeficient model, even though MYXV therapy was still able to prolong survival no long-term survivors were observed as was the case in the immunocompetent model. This suggests that an intact immune response is a critical factor involved in the increased survival benefit observed after MYXV therapy in immunocompetent tumor bearing mice.
MYXV in combination with gemcitabine enhanced the survival of mice compared to single treatments with drug or virus
MYXV therapy was combined sequentially with gemcitabine chemotherapy and these combination therapies were then compared to the single agent therapies in both Hs766T and Pan02 IPD models.
One regimen which consisted in the administration of MYXV after gemcitabine treatment (G+V) was tested in the human Hs766T IPD model to determine if the increase in oncolysis with this treatment observed in vitro (Figure 3c) could result in increased survival in an Hs766T IPD model. When the G+V treatment was administered in this model, mice survived significantly longer compared to mock treatment (P = 0.0068) and to MYXV (P = 0.0373) and gemcitabine (P = 0.0480) single treatments (Figure 5a). With this model, in the absence of a host adaptive immune response, the G+V combination treatment resulted in an increase in long term survivors (~30%) when compared to mock treated and single treatment groups (under 10%). These results are in agreement with the oncolysis observed in vitro and suggest that MYXV given after gemcitabine chemotherapy may provide a therapeutic benefit in cases where chemotherapy has failed and drug-resistant tumors develop in immune compromised patients.
The syngeneic Pan02 IPD model was used to compare two drug-virus combination regimens that differed in the time of administration of virus to determine if this is an important factor affecting the efficacy of gemcitabine and MYXV combination therapy in vivo. As observed in vitro (Figure 3c), the G+V combination treatment in this model failed to improve survival of mice (Figure 5b). This may be explained by the inhibitory effect of gemcitabine on MYXV replication or on a timing effect since in this cohort, virus was administered at later time points when the tumor burden was considerably higher than in the MYXV-only group. In contrast, using this same syngeneic model, combination treatment in which MYXV was administered first followed by gemcitabine (V+G) resulted in 100% of mice presenting as long-term survivors (Figure 5b). This dramatic increase in survival was statistically significant when compared across all treatment groups (P < 0.0001 when compared to mock, gemcitabine only or G+V groups; and P = 0.0293 when compared to virus-only treatment) and is also in agreement with the increased oncolysis observed for this treatment in Pan02 cell in vitro (Figure 3c). In contrast, when this regimen was tested in the immunodeficient Pan02-NOD/SCID model, this sequential treatment failed to provide an enhanced survival compared to MYXV only treated mice (Supplementary Figure S1). Thus the V+G regimen was able to increase the rate of long term survivors compared to MYXV monotherapy only in immunocompetent mice bearing Pan02-derived tumors but not in immunodeficient mice bearing tumors derived from the same Pan02 cells. Taken together, these results suggest that an intact immune response is required for the observed enhancement of survival conferred by the V+G treatment in immunocompetent mice.
Discussion
We report for the first time that MYXV is an effective OV with significant therapeutic activity in immunodeficient and immunocompetent pancreatic cancer models of late stage intraperitoneal disseminated (IPD) disease. MYXV monotherapy provided benefits in both models but produced more dramatic therapeutic effects in the immunocompetent Pan02 animal model. Several factors may have contributed to this difference in efficacy. In vitro, MYXV replicated to higher titers in murine Pan02 cells compared to human Hs766T cells, which may account in part for the differences in MYXV oncolysis in vivo. But perhaps more importantly, an intact host immune system in the syngeneic Pan02 model likely played a key role in enhancing the overall antitumor effects during virotherapy. The failure of MYXV treatment to provide long term survival in an immunodeficient model compared to 60% long-term survival in an immunocompetent model engrafted with the same Pan02 cell line supports this conclusion (Figure 5b and Supplementary Figure S1). Antiviral immune responses during oncolytic virotherapy have usually been considered a disadvantage, particularly if it restricts the effective lifetime of the virus in the tumor beds. MYXV treatment has been previously shown to induce an early antiviral response that appears to restrict active viral replication in tumors to within the first week of virus injection in immunocompetent murine melanoma and racine glioma.22,26 The use of immunosuppressive drugs, such as cyclophosphamide and rapamycin, has been evaluated in many models and for many OVs, including MYXV, in an attempt to suppress the immune responses to the virus and enhance the inter- and/or intra-tumoral replication and spread of the virus.22,33,34,35
On the other hand, even though an active immune response may restrict the time window of MYXV replication in tumors, it may also produce a beneficial long lasting antitumoral immune response. A potential antitumor immune response, stimulated by MYXV replication within tumors, may provide long-term oncolysis of tumor cells even after MYXV infection is cleared from them. In the Hs766T xenograft model, no long-term survivors were observed following MYXV monotherapy suggesting again that an enhanced immune response against the tumor generated during MYXV virotherapy is likely responsible for the improvement in survival observed in the syngeneic Pan02 model.
Furthermore, a comparison between MYXV versus gemcitabine as monotherapies in both Hs766T and Pan02 IPD models tested showed that MYXV treatment provided a more potent therapeutic benefit than gemcitabine treatment (at the dose used in this study). Even in the Pan02 model, established with cells shown to be relatively sensitive to gemcitabine in cell culture, the gemcitabine-alone cohort failed to demonstrate a therapeutic benefit. The dose of gemcitabine used in this study was approximately half of the maximal tolerated dose for mice. It is possible that in the models tested this dose resulted in a suboptimal concentration of drug that was not effective in prolonging survival or that some characteristics of tumor sensitivity or tumor accessibility to the drug in vivo are not reproduced in cell culture resulting in a poor correlation between in vitro and in vivo drug sensitivities. Nevertheless, these results warrant the further optimization of gemcitabine and MYXV doses that may results in the maximal therapeutic effects.
Since gemcitabine is considered the current standard of care for pancreatic patients, the possibility of combining this chemotherapy with MYXV is of particular clinical interest. The inhibitory effects on MYXV gene expression observed in gemcitabine-treated cells in vitro was not unexpected, given that the drug is a nucleoside analogue that inhibits DNA synthesis. Other OVs with double stranded DNA genomes such as herpersviruses36 and single stranded DNA genomes such as rat parvovirus37 have also reported inhibitory effects of gemcitabine on virus replication in vitro, but in most cases they still provide synergistic interaction in animal models when used in combination therapies. To avoid these potentially inhibitory effects, a sequential virus/drug treatment strategy was used, similar to regimens proposed for parvoviruses.37
In vitro and in vivo studies showed that MYXV and gemcitabine therapies can be combined sequentially to improve the overall survival of mice bearing IPD pancreatic tumors, but that the timing of virus administration is cell type-dependent and critical in order to maximize the therapeutic effects of this combination therapy. For Hs766T cells the best oncolysis is achieved when drug is administered first, while in Pa02 cells the best oncolysis is achieved when virus is administered as first therapy. The different responses to combination therapy with gemcitabine and MYXV observed in this two cell lines may depend on specific factors such as sensitivity to the drug, mechanism(s) of resistance and MYXV permissiveness among other factors. However, the specific factor and/or mechanisms involved in oncolysis driven by gemcitabine and MYXV combination treatments remain to be determined.
In vivo, the sequential regimen in which MYXV was administered before gemcitabine (V+G) proved to be the most optimal treatment in the Pan02 cell line generating 100% of long-term survivors, but only in immunocompetent mice. To explain the improved oncolytic effects observed in immunocompetent animals treated with MYXV followed by gemcitabine, we propose a working model in which MYXV infection of tumors: (i) Triggers an adaptive immune response to both viral and tumor antigens and these antitumor immune responses continue the oncolytic process started by the virus; and/or (ii) Sensitizes the cancer cells to the cytotoxic effects of gemcitabine. However, further studies are needed to determine if these proposed molecular mechanism(s) are indeed responsible for the observed enhancement in MYXV-based oncolytic therapy as a single therapy or in combination with gemcitabine in immonocompetent models.
The molecular basis for the susceptibility of pancreatic cancer cells to MYXV remains to be determined. The presence of dysfunctional or deleted tumor suppressor genes such as Rb, ATM and p53 contribute to the in vitro susceptibility of cancer cells to MYXV.38 Akt signaling also regulates the cancer cell tropism of MYXV and drugs that upregulate this pathway can enhance viral replication and spread.23,27,39 The role of other signaling pathways, in particular those that are commonly dysregulated in pancreatic cancer (such as Ras) and the use of MYXV in combination with drugs that target these pathways would provide insights into the molecular determinants of MYXV susceptibility within pancreatic tumors in vivo. With further knowledge about these determinants and with the ability to easily manipulate the MYXV genome, second generation recombinant MYXVs can be engineered to improve the efficacy of MYXV in pancreatic cancer models or to sensitize these cancers to the effects of chemotherapy. This would be particularly advantageous for the treatment of gemcitabine resistant tumors. In conclusion, the results presented show that sequential MYXV/gemcitabine strategies can dramatically improve the clearance of disseminated pancreatic cancer in an immunocompetent host.
Materials and Methods
Cell lines. The human pancreatic cancer cell lines Panc-1, AsPC-1, BxPC3 and Hs766T were kindly provided by Dr Fong (Memorial Sloan-Kettering Cancer Center, New York, NY). The murine pancreatic cancer cell line Pan02 was provided by Dr Scott (University of Florida, Gainesville, FL). The monkey kidney cell line BSC40 was kindly provided by Dr Condit (University of Florida). Rabbit RK-13 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, CCL-37). All cells were grown at 37 °C and 5% CO2. Panc-1 cells were grown in Iscove's Modified Dulbecco's Media (Gibco, Grand Island, NY). AsPC-1, BxPC-3 and Pan02 were grown in RPMI-1640 media (Gibco) while Hs766T, RK-13 and BSC40 cells were grown in Dulbecco's Modified Eagle Media (Invitrogen, Grand Island, NY). All media were supplemented with 10% fetal bovine serum (Gibco), 2 mmol/l glutamine (Invitrogen) and 100 µg/ml penicillin-streptomycin (Invitrogen).
Virus and drug treatments. The recombinant MYXV's tagged with different fluorescent reporter genes used in this study have been described elsewhere.40,41 Briefly, vMyx-GFP and vMyx-tdTr are MYXV constructs expressing the enhanced GFP or the tandem dimer (td)-Tomato red fluorescent protein (tdTr), respectively, from an intergenic location (between the M135 and M136 genes) under the control of a poxvirus early/late synthetic promoter. vMyx-RFP is a MYXV expressing the red fluorescent protein (RFP) under the control of a poxviral late (p11) promoter. Virus stocks were grown and titered in BSC40 or BGMK cells as previously described.42 Virus stocks for animal studies were purified through a sucrose cushion (36% sucrose in 10 mmol/l Tris-HCl pH 8) and resuspended in 10 mmol/l Tris-HCl pH 8. Virus stocks for animal injections were diluted in phosphate-buffered saline (PBS) prior to injection. For viral growth curves, cells were infected with MYXV at a multiplicity of infection (MOI) 0.1 and collected by trypsinization or scraping. Virions were released from the cells by three freeze-thaw cycles and titered on BSC40 cells. Gemcitabine (Santa Cruz Biotechnology, Santa Cruz CA) was resuspended at 40 mg/ml in PBS and was further diluted in PBS for animal experiments or in growth media for in vitro experiments.
Microscopy. Fluorescence images and bright field images were taken using an inverted fluorescent Leica DMI 6000B microscope or with a Leica DM 2500 fluorescent upright microscope and Leica fluorescence imaging software packages.
Cell viability assays. For colony forming assays cells were infected with MYXV at a MOI 10. At 24 hours p.i., cells were trypsinized and 1 × 104 cells were seeded in 10 cm plates and allowed to form colonies for 2 weeks. Colonies were stained using crystal violet (0.02% in 20% ethanol). MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays (Promega, Madison, WI) were performed according to the manufacturer's instructions. Cells were infected with vMyx-GFP at a MOI 10 or treated with gemcitabine and MTT assays were performed 72 hours p.i. To determine gemcitabine sensitivity, 1 µmol/l of drug was used as the cut off concentration. Cell lines with greater than or equal to 50% of viable cells at this concentration were considered resistant. Consequently, cell lines with <50% of viable cells at this concentration were considered sensitive. For cell viability assays involving combination treatments with gemcitabine MTT assays were performed 6 days after the start of treatments. For the G+V treatment cells were treated with the indicated concentrations of gemcitabine for 72 hours, left 24 hours in media without drug and then infected with vMyx-GFP at MOI 10. For the V+G treatment, cells were first infected with virus for 72 hours followed by gemcitabine treatment for 72 hours. For all MTT assays, the values for infected and/or drug treated samples are reported as percent of live cells compared to mock treated samples, which were considered 100% viable. MTT values reported are the average of duplicate or triplicate samples. Results reported for these assays are representative of at least two independent experiments.
Flow cytometry. Cells infected with vMyx-GFP at MOI 0.1 were trypsinized, collected at the indicated time points, fixed with 4% paraformaldehyde in PBS and analyzed for enhanced GFP expression using a BD FACSCalibur; BD Biosciences, San Jose, CA.
Development of firefly luciferase expressing cell lines. Human Hs766T or murine Pan02 pancreatic cancer cells were co-transfected with the pGL4.13[luc2/SV40] Vector (Promega) and the pcDNA3.1(+) (Invitrogen) using lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Twenty-four hours after transfection, Hs766T or Pan02 cells were split 1:10 and plated in the presence of 1 mg/ml or 0.5 mg/ml Geneticin (G418) (Invitrogen), respectively for 2–3 weeks. Geneticin resistant colonies were then screened for the expression of firefly luciferase (FFluc) by addition of D-luciferin (Caliper Life Sciences) diluted in PBS. Luciferase positive cell colonies were detected using a Xenogen IVIS 200 Spectrum bioluminescence and fluorescence imager (Caliper Life Sciences, Hopkinton, MA). Selected clones were expanded and used in animal studies.
Animal models. All animal experiments were done according to protocols approved by the Institutional Animal Care and Usage Committee at the University of Florida.
Subcutaneous tumor models: Approximately 1 × 107 Hs766T cells were injected subcutaneously into the right hind limb of 6–8-week-old female nude mice (Charles River Laboratories, Wilmington, MA). When tumors were palpable, 1 × 108 foci forming units (ffu) of vMyx-GFP were injected IT. Seven days after virus injection, tumors were excised, fixed in 10% neutral buffered formalin and paraffin embedded. Paraffin blocks were sectioned for immunoperoxidase detection of GFP using 3,3' Diaminobenzidine (DAB) as chromogen (Biocare Medical) and hematoxylin as a counterstain. Pan02 cells were used to establish subcutaneous tumors in 6-8 week old female C57Bl6 mice (Charles River Laboratories) and injected IT with vMyx-tdTr as described above. Pan02 tumors were excised 3 or 5 days after IT injection of vMyx-tdTr, fixed in neutral buffered formalin and embedded in OCT freezing medium (Sakura Finetek, Torrance, CA). OCT preserved tumors were sectioned and visualized directly for tdTr fluorescence.
Intraperitoneal dissemination models: Approximately 5 × 106 Hs766T or 1 × 107 Pan02 cells expressing firefly luciferase (Hs766T-FFLuc or Pan02-FFLuc) were injected into the peritoneal cavity of 6–8 week-old female. Hs766T-FFLuc cells were injected into NOD-SCID mice (Harlan Laboratories, Tampa, FL) while Pan02-FFLuc cells were injected into NOD-SCID and C57Bl6 mice. Five or six days after cell implantation, engraftment of luciferase expressing cancer cells was determined by bioluminescent imaging using the Xenogen Imaging System. Mice with detectable luciferase expression in the IP cavity were then randomly distributed into the various treatment cohorts. All treatment regimens started 5–6 days after cell implantation. Virus treatments consisted of four IP injections of 1 × 108 ffu of vMyx-tdTr diluted in PBS given once every other day. Gemcitabine treatments consisted of four doses of 50 mg/kg given IP once every three days. For MYXV followed by gemcitabine (V+G), gemcitabine treatment was started 24 hours after the last virus injection. When gemcitabine was administered before virus (G+V) a “rest” period of 5–6 days was given before virus treatments were started. For the Hs766T model the numbers of mice used were 17, 15, 10 and 9 for mock, virus, gemcitabine and combination treatment G+V, respectively. For the Pan02 model, 10 mice were used for each treatment group.
Bioluminescence imaging: Bioluminescent signals from tumors were detected by IP administration of D-luciferin substrate (150 mg/kg). Five minutes after substrate injection, mice were anesthetize using isofluorane and imaged 15–20 minutes after injection of substrate using the IVIS 200 Xenogen imaging system. A luciferin kinetic curve was used to determine the peak luciferase expression time for each tumor model. Data were analyzed based on radiance (p/second/cm2/sr) emitted from the IP cavity of each mice.
Statistical analysis. GraphPad Prism 5 statistical software (GraphPad Software, La Jolla, CA) was used for statistical analyses. Survival curves were generated by the Kaplan–Meier method and compared using the log-rank test in which P values <0.05 were considered statistically significant. All other reported P values were obtained using two-sided t-tests and were considered to be statistically significant at P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Survival curves of immunodeficient mice engrafted with Pan02 tumors.
Acknowledgments
We thank members of the Cell and Tissue Analysis Core (CTAC) and Molecular Pathology core at the University of Florida for expert advice and technical assistance. We also thank Dorothy Smith for technical assistance in animal experiments and Sherin Smallwood for administrative assistance in animal protocol submissions. GM's laboratory is funded by NIH R01 grants AI080607 and CA13854, R21 grant CA149869 and the Bankhead Coley Cancer Research Foundation grant 1BT02. STW received an American Cancer Society post-doctoral fellowship. All authors express no potential conflict of interests related to this report.
Supplementary Material
Survival curves of immunodeficient mice engrafted with Pan02 tumors.
REFERENCES
- Jemal A, Siegel R, Xu J., and, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Heinemann V. Gemcitabine: progress in the treatment of pancreatic cancer. Oncology. 2001;60:8–18. doi: 10.1159/000055290. [DOI] [PubMed] [Google Scholar]
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR.et al. (1997Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial J Clin Oncol 152403–2413. [DOI] [PubMed] [Google Scholar]
- Strimpakos A, Saif MW., and, Syrigos KN. Pancreatic cancer: from molecular pathogenesis to targeted therapy. Cancer Metastasis Rev. 2008;27:495–522. doi: 10.1007/s10555-008-9134-y. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M.et al. (2007Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells Br J Cancer 96457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maalouf G, Le Tourneau C, Batty GN, Faivre S., and, Raymond E. Markers involved in resistance to cytotoxics and targeted therapeutics in pancreatic cancer. Cancer Treat Rev. 2009;35:167–174. doi: 10.1016/j.ctrv.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Welch SA., and, Moore MJ. Combination chemotherapy in advanced pancreatic cancer: time to raise the white flag. J Clin Oncol. 2007;25:2159–2161. doi: 10.1200/JCO.2006.09.9788. [DOI] [PubMed] [Google Scholar]
- Mackenzie RP., and, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Rev Anticancer Ther. 2009;9:1473–1485. doi: 10.1586/era.09.109. [DOI] [PubMed] [Google Scholar]
- Kasuya H, Takeda S, Nomoto S., and, Nakao A. The potential of oncolytic virus therapy for pancreatic cancer. Cancer Gene Ther. 2005;12:725–736. doi: 10.1038/sj.cgt.7700830. [DOI] [PubMed] [Google Scholar]
- Liu TC, Galanis E., and, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Shen Y., and, Nemunaitis J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006;13:975–992. doi: 10.1038/sj.cgt.7700946. [DOI] [PubMed] [Google Scholar]
- Davis JJ., and, Fang B. Oncolytic virotherapy for cancer treatment: challenges and solutions. J Gene Med. 2005;7:1380–1389. doi: 10.1002/jgm.800. [DOI] [PubMed] [Google Scholar]
- Vähä-Koskela MJ, Heikkilä JE., and, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC., and, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Breckenridge C, Kaur B., and, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chem Rev. 2009;109:3125–3140. doi: 10.1021/cr900048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypula J, Wang F, Ma Y, Bell JC., and, McFadden G. Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol. 2004;8:103–114. [Google Scholar]
- Stanford MM., and, McFadden G. Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EW, Dorn CR, Saito JK., and, McKercher DG. Absence of serological evidence of myxoma virus infection in humans exposed during an outbreak of myxomatosis. Nature. 1966;211:313–314. doi: 10.1038/211313a0. [DOI] [PubMed] [Google Scholar]
- Fenner F. Adventures with poxviruses of vertebrates. FEMS Microbiol Rev. 2000;24:123–133. doi: 10.1016/S0168-6445(00)00027-9. [DOI] [PubMed] [Google Scholar]
- Stanford MM, Werden SJ., and, McFadden G. Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet Res. 2007;38:299–318. doi: 10.1051/vetres:2006054. [DOI] [PubMed] [Google Scholar]
- Lun X, Alain T, Zemp FJ, Zhou H, Rahman MM, Hamilton MG.et al. (2010Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin Cancer Res 70598–608. [DOI] [PubMed] [Google Scholar]
- Lun XQ, Zhou H, Alain T, Sun B, Wang L, Barrett JW.et al. (2007Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin Cancer Res 678818–8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun X, Yang W, Alain T, Shi ZQ, Muzik H, Barrett JW.et al. (2005Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas Cancer Res 659982–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lun X, Zhou H, Wang L, Sun B, Bell JC.et al. (2008Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors Clin Cancer Res 141218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MM, Shaban M, Barrett JW, Werden SJ, Gilbert PA, Bondy-Denomy J.et al. (2008Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo Mol Ther 1652–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X.et al. (2006Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor Proc Natl Acad Sci USA 1034640–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werden SJ, Barrett JW, Wang G, Stanford MM., and, McFadden G. M-T5, the ankyrin repeat, host range protein of myxoma virus, activates Akt and can be functionally replaced by cellular PIKE-A. J Virol. 2007;81:2340–2348. doi: 10.1128/JVI.01310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werden SJ., and, McFadden G. The role of cell signaling in poxvirus tropism: the case of the M-T5 host range protein of myxoma virus. Biochim Biophys Acta. 2008;1784:228–237. doi: 10.1016/j.bbapap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Bartee E., and, McFadden G. Human cancer cells have specifically lost the ability to induce the synergistic state caused by tumor necrosis factor plus interferon-beta. Cytokine. 2009;47:199–205. doi: 10.1016/j.cyto.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo Y, Kelly KJ, Stanford MM, Galanis C, Chun YS, Fong Y.et al. (2008Myxoma virus is oncolytic for human pancreatic adenocarcinoma cells Ann Surg Oncol 152329–2335. [DOI] [PubMed] [Google Scholar]
- Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL.et al. (2009Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer Cancer Res 695820–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zeng Z, Fu X., and, Zhang X. Coadministration of a herpes simplex virus-2 based oncolytic virus and cyclophosphamide produces a synergistic antitumor effect and enhances tumor-specific immune responses. Cancer Res. 2007;67:7850–7855. doi: 10.1158/0008-5472.CAN-07-1087. [DOI] [PubMed] [Google Scholar]
- Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA.et al. (2007Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide Clin Pharmacol Ther 82700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier MA, Gillespie RA, Sawtell NM, Mahller YY, Stroup G, Collins MH.et al. (2008Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide Mol Ther 16879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I, Kasuya H, Nomura N, Shikano T, Shirota T, Kanazumi N.et al. (2008Effects of tumor selective replication-competent herpes viruses in combination with gemcitabine on pancreatic cancer Cancer Chemother Pharmacol 61875–882. [DOI] [PubMed] [Google Scholar]
- Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA.et al. (2009Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV Clin Cancer Res 15511–519. [DOI] [PubMed] [Google Scholar]
- Kim M, Williamson CT, Prudhomme J, Bebb DG, Riabowol K, Lee PW.et al. (2010The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status Oncogene 293990–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MM, Barrett JW, Nazarian SH, Werden S., and, McFadden G. Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. J Virol. 2007;81:1251–1260. doi: 10.1128/JVI.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wennier S, Reinhard M, Roy E, MacNeill A., and, McFadden G. Myxoma virus expressing interleukin-15 fails to cause lethal myxomatosis in European rabbits. J Virol. 2009;83:5933–5938. doi: 10.1128/JVI.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Barrett JW, Chang W, Chung CS, Zeng W, Masters J.et al. (2003Role of the serine-threonine kinase PAK-1 in myxoma virus replication J Virol 775877–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood SE, Rahman MM, Smith DW., and, McFadden G. Myxoma virus: propagation, purification, quantification, and storage. Curr Protoc Microbiol. 2010;Chapter 14:Unit 14A.1. doi: 10.1002/9780471729259.mc14a01s17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival curves of immunodeficient mice engrafted with Pan02 tumors.