Abstract
Muscular dystrophies (MDs) are caused by genetic mutations in over 30 different genes, many of which encode for proteins essential for the integrity of muscle cell structure and membrane. Their deficiencies cause the muscle vulnerable to mechanical and biochemical damages, leading to membrane leakage, dystrophic pathology, and eventual loss of muscle cells. Recent studies report that MG53, a muscle-specific TRIM-family protein, plays an essential role in sarcolemmal membrane repair. Here, we show that systemic delivery and muscle-specific overexpression of human MG53 gene by recombinant adeno-associated virus (AAV) vectors enhanced membrane repair, ameliorated pathology, and improved muscle and heart functions in δ-sarcoglycan (δ-SG)-deficient TO-2 hamsters, an animal model of MD and congestive heart failure. In addition, MG53 overexpression increased dysferlin level and facilitated its trafficking to muscle membrane through participation of caveolin-3. MG53 also protected muscle cells by activating cell survival kinases, such as Akt, extracellular signal-regulated kinases (ERK1/2), and glycogen synthase kinase-3β (GSK-3β) and inhibiting proapoptotic protein Bax. Our results suggest that enhancing the muscle membrane repair machinery could be a novel therapeutic approach for MD and cardiomyopathy, as demonstrated here in the limb girdle MD (LGMD) 2F model.
Introduction
Functional deficiencies of proteins involved in the maintenance of sarcolemmal membrane integrity or repair are commonly observed in muscular dystrophies (MDs), which are resulted from genetic mutations. For example, mutations of dystrophin gene in Duchenne and Becker MDs or sarcoglycan genes in limb girdle MDs (LGMDs) lead to muscle sarcolemma destabilization and fragility. The myofibers become highly susceptible to contraction-induced injures, which are manifested by elevated muscle creatine kinase (CK) activity in the serum and muscle degeneration. No effective treatment is currently available for any form of MD. A majority of the current experimental therapeutic strategies focus on the restoration of the specific muscle protein encoded by the individual gene that is mutated.1,2,3,4,5 Since many MDs share similar pathological consequences from sarcolemma damage, in this study, we have investigated a gene therapy strategy that aims at repairing the leaky cell membranes rather than targeting specific genetic mutations. Abnormal extracellular Ca2+ influx was found in recent studies sufficient to induce dystrophic phenotypes, whereas sequestration of the influxed Ca2+ improved dystrophic muscle pathology and functions.6,7 We reason that enhanced membrane repair should be beneficial by resealing the leaky membrane, preventing Ca2+ influx and maintaining cellular homeostasis.
Muscle membrane repair is a conserved, multicomponent process and involves Ca2+-triggered intracellular vesicles exocytosis toward the injury site, followed by vesicle fusion at membrane lesions.8 A Ca2+-dependent muscle-specific fusogen, dysferlin, is one of the first proteins identified in the membrane repair machinery in both skeletal and cardiac muscle. Dysferlin-deficiency causes LGMD 2B.8,9,10 Gene delivery of dysferlin restored membrane repair in a dysferlin-deficient mouse model.5 However, dysferlin is not necessary for the recruitment of vesicles to the membrane injury sites, although it is a key player in membrane repair.8,9,10,11
MG53, a muscle-specific tripartite motif family protein (TRIM72), was recently shown to be an essential component of the membrane repair system necessary for vesicle trafficking to the injury sites.12 MG53-mediated intracellular vesicle trafficking acts upstream of dysferlin movement to the damaged sarcolemma.13 Additional experiments demonstrated that MG53 interacts with dysferlin and caveolin-3, another muscle-specific protein, to facilitate skeletal muscle membrane repair upon influx of extracellular Ca2+ at the injury sites.11 Given the pivotal role of MG53, increasing its expression to enhance muscle membrane repair prior to the downstream events of myofiber necrosis and apoptosis represents a novel approach for in vivo therapeutic studies in animal models of MDs. However, overexpression of MG53 in vivo has not been achieved before.
In the present study, we used the δ-sarcoglycan (δ-SG)-deficient MD and cardiomyopathy TO-2 hamster model to investigate the therapeutic efficacy of human MG53 (hMG53) gene transfer and overexpression by systemic adeno-associated virus 8 (AAV8) delivery. We previously showed that systemic delivery of human δ-SG gene by AAV8 achieved global and long-term therapeutic effects in TO-2 hamsters.3,14,15 Here, we showed that MG53 gene transfer in both neonates and adults achieved efficient sarcolemma repair in vivo and in vitro, resulting in systemic amelioration of myopathies and functional rescue of skeletal muscle and heart. Therapeutic efficacy and safety were further improved by a combination of a muscle-specific promoter and a liver microRNA (miRNA) target sequence to assure heart- and muscle-specific transgene expression. MG53 gene therapy also increased dysferlin expression and facilitated its trafficking to muscle membrane through participation of caveolin-3. Furthermore, phosphorylation of several survival kinases, such as Akt, ERK1/2, and glycogen synthase kinase (GSK), were elevated, while the proapoptotic Bcl-2 molecules, such as Bax, were reduced in gene therapy treated TO-2 skeletal muscle and heart.
Results
Systemic and long-term gene transfer of MG53 in dystrophic TO-2 hamsters by AAV8 vectors
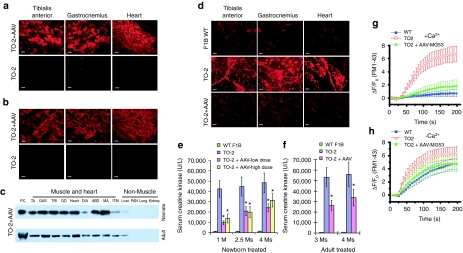
Since MG53 is a muscle-specific protein, we generated AAV8 vectors carrying muscle-specific CK promoter to control the gene expression of a myc-tagged human MG53. Efficient gene transfer in the heart and skeletal muscles was achieved by vector injection either intraperitoneally in 10-day-old TO-2 neonates (1 × 1013 vector genomes/kg bodyweight, vg/kg) or intravenously in 2-month-old TO-2 adults (2 × 1013 vg/kg). When examined at 2 months postinjection by myc-tag immunofluorescent staining, expression of myc-MG53 was observed in the treated muscles, but no staining appeared in the muscle of control hamsters (Figure 1a,b). Muscle-specific myc-MG53 expression was further confirmed by western blot (Figure 1c). These results indicated that systemic delivery of MG53 was robust and stable in the heart and skeletal muscle of both TO-2 neonates and adults following intraperitoneal or intravenous treatment.
Figure 1.
Muscle membrane integrity improvement in TO-2 skeletal and cardiac muscles via systemic delivery of AAV8-MG53.(a) Immunofluorescent (IF) staining of myc-tagged-MG53 expression in muscles of neonatally treated TO-2 hamsters at vector dose of 1 × 1013 vg/kg. Samples were collected 2 months later. Age-matched untreated TO-2 served as controls (n = 5 per group; bar = 100 µm). (b) The same as in (a) except that a vector dose of 2 × 1013 vg/kg was delivered in 2-month-old TO-2 hamsters. Samples were collected at 2 months after injection (n = 5 per group; bar = 100 µm). (c) Western analysis of myc-tagged-MG53 in muscle and nonmuscle tissues in both neonate-treated and adult-treated TO-2 hamsters. Samples collected were same as described in (a) and (b). Muscles included tibialis anterior (TA), gastrocnemius (GAS), triceps (TRI), quadriceps (QD), heart, diaphragm (DIA), abdominal muscle (ABD), masseter (MA), and intercostal muscle (ITM). Nonmuscle tissues included liver, pancreas (PAN), kidney, and lung. (d) Improvement in muscle membrane integrity as revealed by exclusion of Evan blue dye (red fluorescence) in muscle and heart at 2 month after neonatal injection (1 × 1013 vg/kg). (e) Reduction of serum CK levels after delivery of AAV8-MG53 in newborn hamsters. Low dose: 1 × 1013 vg/kg; high dose: 1 × 1014 vg/kg. *P < 0.05 versus the untreated TO-2. (f) Reduction of serum CK level after delivery of AAV8-MG53 (2 × 1013 vg/kg) in adult hamsters. *P < 0.05 versus the untreated TO-2. (g) Time-course of FM1-43 accumulation at injury sites of the isolated single myofibers following UV-laser induced membrane damage in the presence of 2 mmol/l Ca2+ in solution. Values represent mean ± SD. P < 0.01 when AAV-MG53 treated (1 × 1013 vg/kg) versus the untreated TO-2 myofibers. (h) The same as in (g) except the absence of extracellular Ca2+ (plus 0.5 mmol/l EGTA). Values represent mean ± SD. AAV, adeno-associated virus.
Restoration of TO-2 muscle cell integrity by MG53-mediated membrane repair
δ-SG deficiency in TO-2 hamsters leads to chronic damage to the myofiber and leaky sarcolemma that is a major cause of pathology in these dystrophic muscles. We examined whether MG53 gene transfer could improve muscle membrane repair and integrity at both cellular and bodywide levels. In vivo examination of membrane integrity by intravenous injection of Evans Blue, a sarcolemma-impermeable dye, was performed 2 months after AAV treatment. Muscle thin sections showed that MG53 overexpression largely prevented the dye from entering muscle cells of the tibialis anterior (TA), gastrocnemius (GAS), and heart in TO-2 hamsters neonatally treated with AAV8-MG53 (1 × 1013 vg/kg). As expected, pronounced muscle membrane leakage in the untreated TO-2 hamsters was found, whereas the wild-type (WT) F1B control hamsters showed no leakage (Figure 1d). Additional evidence of sarcolemma integrity improvement was the diminution of the elevated serum CK levels at 1, 2.5, and 4 months after neonatal treatment with both low and high vector doses (1 × 1013 and 1 × 1014 vg/kg) compared to the untreated age-matched TO-2 hamsters (Figure 1e). In addition, vector-treatment in the 2-month-old adult TO-2 hamsters, which already displayed dystrophic pathology, also significantly decreased CK activities when examined at 1 and 2 months after vector injection (2 × 1013 vg/kg) (Figure 1f). Together, both histological and biochemical results suggest that AAV8-mediated MG53 systemic gene transfer increased membrane resealing in dystrophic muscle and heart, partially restored membrane integrity, and ameliorated muscle pathology. Interestingly, in the neonatally treated TO-2 hamsters, more beneficial effects were observed in the groups with lower vector dose (1 × 1013 vg/kg), suggesting that an optimal vector dose threshold has been reached.
To directly evaluate the effect of MG53 gene transfer on muscle membrane repair, we performed a single-myofiber membrane repair assay by measuring the entry of FM1-43 fluorescent dye following UV-laser induced membrane damage.8 Single myofibers of flexor digitorum brevis muscle were isolated respectively from age-matched WT F1B, untreated TO-2 and TO-2 hamsters that were treated 3 months earlier at the neonatal age (1 × 1013 vg/kg). In the presence of 2 mmol/l extracellular Ca2+, the WT F1B myofibers effectively and quickly resealed sarcolemmal membranes (Figure 1g, Supplementary Figure S1, top panel and Supplementary Video S1) but remarkable FM1-43 dye entry into untreated TO-2 myofibers was observed due to compromised sarcolemmal membrane integrity (Figure 1g, Supplementary Figure S1, middle panel and Supplementary Video S2). In contrast, myofibers from AAV-MG53-treated TO-2 effectively and quickly resealed the membrane and excluded the entry of FM1-43 dye following UV-laser damage to an extent indistinguishable from the WT F1B myofibers (Figure 1g, Supplementary Figure S1, bottom panel and Supplementary Video S3). Since MG53-mediated membrane repair is triggered by influx of Ca2+,12 identical FM1-43 dye entry experiments were repeated but in the absence of extracellular Ca2+. Marked FM1-43 dye entry after UV-induced wounding was seen in the myofibers of all three groups of hamsters regardless (Figure 1h). The above results demonstrated that overexpression of MG53 is responsible for the rescue of membrane repair in the δ-SG deficient TO-2 hamsters.
Amelioration of TO-2 muscle pathology by systemic MG53 gene delivery
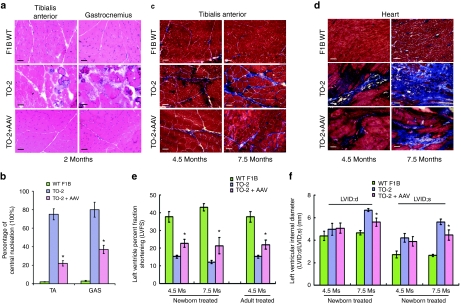
We further examined whether improved membrane repair and subsequent restoration of sarcolemma integrity in AAV-treated TO-2 hamsters led to systemic amelioration of muscle histopathology. Hematoxylin and eosin staining of the TA and GAS muscle cross-sections from WT F1B hamsters showed normal histology, while the untreated TO-2 hamster muscles displayed fibrosis, focal necrosis, and extensive central nucleation. The AAV-MG53-treated TA and GAS muscle collected 2 months after neonatal treatment (1 × 1013 vg/kg) displayed more uniform myofiber sizes, less mononuclear cell infiltration, and a significant decrease in the percentage of centrally localized nuclei in TA (22.0 ± 3.0%) and GAS (37.0 ± 5.0%) when compared to those of age-matched untreated hamsters (75.0 ± 6.0% and 80.0 ± 9.0%, respectively) (Figure 2a,b). Furthermore, Masson's trichrome staining revealed a significant reduction of fibrosis in the neonatally treated TO-2 skeletal muscle (TA) (Figure 2c). Tissue hydroxyproline content, a more quantitative index of fibrosis, was also significantly decreased in the AAV-MG53-treated muscle at the ages 4.5 and 7.5 months (1.0 ± 0.2 and 1.7 ± 0.3 µg/mg dry tissue) compared to the untreated groups (2.2 ± 0.4 and 3.5 ± 0.6 µg/mg dry tissue, respectively) (Supplementary Figure S2a). These data demonstrated that MG53 indeed rendered significant therapeutic efficacy in TO-2 muscles at the histopathological level.
Figure 2.
Amelioration of muscle and heart degeneration and fibrosis and functional improvement in dystrophic TO-2 hamsters by systemic MG53 gene transfer. (a) Histological improvement as assessed by hematoxylin and eosin (H&E) staining. TO-2 hamsters treated with AAV-MG53 (1 × 1013 vg/kg) were analyzed 2 months after neonatal injection. Bar = 100 µm. (b) Decrease in central nucleation (CN) of treated muscle fibers described in (a). Displayed are the percentages of CN in muscle myofibers for the groups above. Values represent mean ± SD. *P < 0.01 versus the untreated TO-2 (n = 5 per group with 300 fibers analyzed per muscle). (c,d) Reduction of fibrosis in skeletal and cardiac muscle as evidenced by Masson's trichrome staining. Samples were collected at 4.5 and 7.5 months of age after neonatal treatment (1 × 1013 vg/kg). Bar = 100 µm. (e) Cardiac function improvement as revealed by increase in left ventricle percent fraction shortening (% LVFS) in TO-2 hamsters treated as neonates (1 × 1013 vg/kg) or adults (2 × 1013 vg/kg) (Ms, months). *P < 0.05 versus the untreated TO-2 (n = 5). (f) Cardiac function improvement as revealed by reduction in left ventricular end-systolic and end-diastolic internal diameter (LVID;s and LVID;d)) in TO-2 treated as neonates (1 × 1013 vg/kg) (Ms, months). *P < 0.05 versus the untreated TO-2 (n = 5). Values represent mean ± SD. All hamsters were male. AAV, adeno-associated virus.
Improvement of TO-2 cardiac histology and function by systemic MG53 gene transfer
δ-SG-deficient TO-2 hamsters also manifest severe cardiac dysfunction and dilated congestive heart failure. Given that robust gene transfer of MG53 was observed in the heart (Figure 1a–c) that resulted in substantial reduction of cardiac sarcolemmal leakage (Figure 1d) and fibrosis (Figure 2d and Supplementary Figure S2b), we next investigated whether MG53 gene transfer could improve heart function in TO-2 hamsters. The noninvasive echocardiography was performed at 4.5 and 7.5 months of age in both neonatal (1 × 1013 vg/kg) and 2-month-old adult (2 × 1013 vg/kg) treated hamsters. Compared to the age-matched untreated TO-2 hamsters, both treatment groups showed a significant increase in left ventricular percentage fractional shortening, an index of systolic function (Figure 2e). Significant reduction in left ventricular end-systolic and end-diastolic internal diameters (LVID;s and LVID;d) were also observed at the age of 7.5 months, at which time overt cardiac dilation occurred in TO-2 hamsters (Figure 2f). These results indicated MG53-mediated membrane repair offered partial but very significant therapeutic efficacies.
Inhibition of ectopic MG53 expression in liver by miRNA-mediated transgene silencing diminished toxicity and enhanced efficacy
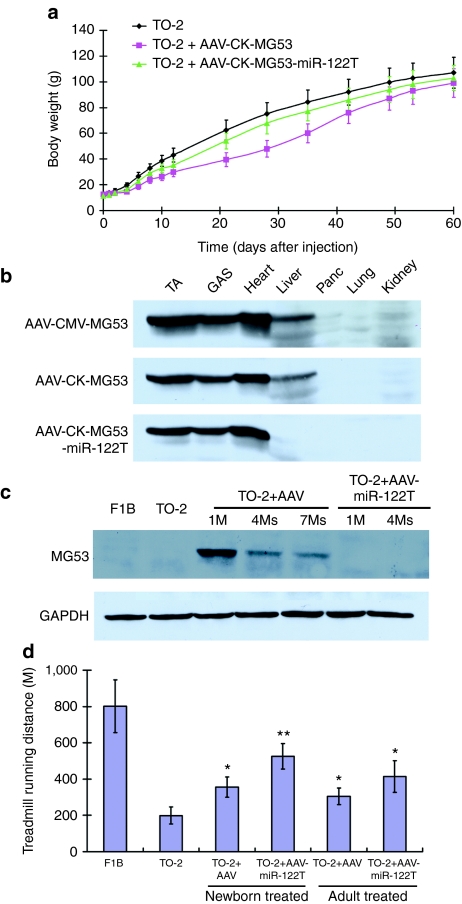
Our initial pilot experiments using the nonspecific cytomegalovirus promoter to drive MG53 expression resulted in severe growth retardation of the TO-2 neonates, suggesting potential toxicity due to overexpression in nonmuscle tissues, especially the liver. Switching to the muscle-specific CK promoter dramatically reduced this toxicity.16 However, since AAV8 has a strong hepatic tropism and delivers high copy numbers of vectors to the liver,3,15,17,18 leaky expression could still occur even with the CK promoter.19 Indeed a slight growth delay between 3 and 6 weeks postinjection in the neonatal-treated TO-2 hamsters was found (Figure 3a). Western blot analysis of muscle and liver samples collected 2 weeks after injection confirmed leaky MG53 expression in the liver (Figure 3b), which resulted in elevation of serum alanine transaminase levels 1 month after injection (Supplementary Figure S3a). However, liver expression decreased progressively post neonatal injection due to dilution of vector DNA after liver growth (Figure 3c). Since AAV8 also transduces the pancreas,18 serum amylase activity was examined but no difference was found between treatment and TO-2 control group (Supplementary Figure S3b).The lack of detectable MG53 gene expression by western blot (Figures 1c and 3b) confirmed the inactivity of the CK promoter in the pancreas.
Figure 3.
Attenuation of MG53 overexpression-induced liver toxicity by the microRNA-122 targeting sequence (miR-122T)-mediated transgene silencing strategy. (a) Inclusion of the liver-specific miR-122T in AAV vector prevented the growth retardation observed in the AAV-CK-MG53-treated hamsters (1 × 1013 vg/kg). Values represent mean ± SD. *P < 0.05, AAV-CK-MG53-treated versus the untreated TO-2 hamsters. (b) Efficient inhibition of ectopic MG53 expression in nonmuscle tissues by CK promoter and miR-122T. Western blot of MG53 was performed for muscle and nonmuscle tissues collected 2 weeks after neonatal injection with different vectors as indicated (1 × 1013 vg/kg). (c) Efficient inhibition of ectopic MG53 expression in the AAV-CK-MG53-treated TO-2 hamster liver by miR-122T. Western blot of MG53 was performed to detect leaky activities of CK promoter in the liver samples collected at different time points after neonatal vector injection (1 × 1013 vg/kg). GAPDH blot was used as a loading control. (d) Improved treadmill running distances (meters, mean ± SD) of 4-month-old WT F1B, untreated TO-2, and TO-2 treated with different AAV vectors as indicated (1 × 1013 vg/kg for neonates and 2 × 1013 vg/kg for 2-month-old adults). Values represent mean ± SD. *P < 0.01 versus the untreated TO-2; **P < 0.05 versus TO-2 AAV-MG53 neonatal treatment (n = 5 per group). AAV, adeno-associated virus.
To further minimize MG53 expression in the liver, we used a miRNA-mediated transgene silencing strategy by adding the liver-specific miRNA-122 targeting sequence (miR-122T) to the 3′ untranslated region of MG53 cDNA, an approach we previously found effective in reducing reporter gene expression in liver.20 Injection of the TO-2 neonates with AAV-CK-MG53-miR-122T (1 × 1013 vg/kg) did not cause appreciable delay in body weight gain (Figure 3a). MG53 became undetectable in the liver by sensitive western blot analysis (Figure 3b,c). Serum alanine transaminase activity remained the same as seen in the untreated control TO-2 hamsters (Supplementary Figure S3a). Liver-silenced vector also allowed for better functional recovery of whole-body strength and endurance as evaluated by treadmill running capacity tested at 4 months of age. While the running distances of AAV-CK-MG53-treated TO-2 hamsters were greater than those of the age-matched untreated TO-2 in both neonatal and adult treatment groups (Figure 3d), additional improvement was achieved in the AAV-CK-MG53-miR-122T-treated TO-2 hamsters (Figure 3d). These results demonstrated that the microRNA transgene silencing strategy effectively diminished ectopic MG53 overexpression and toxicity and further improved overall function of TO-2 hamsters.
Elevation of dysferlin and caveolin-3 levels in MG53-mediated TO-2 membrane repair
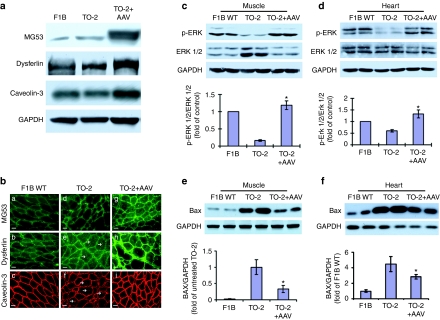
Abnormal localization of dysferlin was found in dystrophic myocytes in LGMDs.21 Our recent studies show that MG53 could interact with dysferlin and caveolin-3 to regulate sarcolemmal membrane repair in skeletal muscle.11 We wished to examine whether overexpression of MG53 could increase dysferlin and caveolin-3. Western analysis indeed showed higher quantity of dysferlin and caveolin-3 proteins in the MG53-treated TO-2 muscles, compared with both WT F1B and untreated TO-2 muscle (Figure 4a). Immunofluorescent staining of muscle thin sections also showed intracellular location of MG53, dysferlin, and caveolin-3. Higher levels of these three proteins were observed in the AAV-MG53-treated muscles (Figure 4b, right panels), consistent with western blot results. Interestingly, extensive cytoplasmic accumulation of both dysferlin and caveolin-3 in untreated dystrophic TO-2 muscle was observed as punctuated dots of vesicles, indicative of retarded membrane repair (Figure 4b, panels e and f, marked by arrow head). However, AAV-MG53 treatment and overexpression normalized and redistributed dysferlin and caveolin-3 vesicles to the sarcolemmal membrane (Figure 4b, panels h and i), as similarly seen in the WT F1B muscle (Figure 4b, panels b and c). These results demonstrated that AAV-mediated MG53 overexpression not only increased the quantity of dysferlin and caveolin-3 but also facilitated their trafficking to and retention at the muscle membrane, hence improved the membrane repair capacity of the dystrophic muscle.
Figure 4.
Identification of mechanisms involved in MG53-mediated therapeutic effects. (a) Western blot analysis of dysferlin and caveolin-3 in muscle [gastrocnemius (GAS)] tissues collected from wild-type (WT) F1B, untreated TO-2, and AAV-MG53 neonatally treated TO-2 (1 × 1013 vg/kg) at 4 months of age. Rabbit anit-MG53 antibody was used. GAPDH was loading control. (b) Immunofluorescent (IF) staining shows overexpression of MG53 in TO-2 hamster normalized and redistributed dysferlin and caveolin-3 vesicles to the sarcolemma membrane. Samples collected were same as described in (a). Note cytoplasmic accumulation of both dysferlin and caveolin-3 in untreated TO-2 muscle (arrow head). (c,d) Activation of cell survival kinase ERK and an increase in the ratio of p-ERK/ERK in muscle and heart of AAV-MG53-treated TO-2 hamsters. Representative immunoblots and statistical data of phosphorylated and total ERK1/2 in (c) tibialis anterior (TA) muscle and (d) heart were from 4-month-old F1B, TO-2, and TO-2 treated with AAV (1 × 1013 vg/kg) at neonatal age. GAPDH was loading control. Values represent mean ± SD. *P < 0.01 versus the untreated TO-2 (n = 5 hamsters per group). (e,f) Decreased proapoptotic factor Bax expression in muscle and heart of AAV-MG53-treated TO-2 hamsters. Samples collected were same as described in (c) and (d). Values represent mean ± SD. *P < 0.01 versus the untreated TO-2 (n = 5 hamsters per group). AAV, adeno-associated virus.
Activation of cellular prosurvival pathways in MG53-treated TO-2 muscle and heart
Finally, to further elucidate the mechanisms of MG53-mediated therapeutic effects, we investigated the status of key survival kinases, including Akt, extracellular signal-regulated kinases (ERK1/2) and GSK-3β, as well as the Bcl-2-family proapoptotic molecules, such as Bax, in muscle tissues.22 In vector-treated TO-2 hamsters, we found strong activation of muscle ERK1/2 and a much higher ratio of p-ERK1/2/ERK1/2 compared to the untreated muscle (1.18 ± 0.13 versus 0.16 ± 0.02, Figure 4c). Furthermore, the heart of MG53-treated TO-2 also demonstrated significant elevation of p-ERK1/2/ERK1/2 ratio (1.33 ± 0.17 versus 0.59 ± 0.05, Figure 4d), the p-Akt/Akt ratio (0.91 ± 0.11 versus 0.58 ± 0.08, Supplementary Figure S4a), and p-GSK-3β/GSK-3 ratio (0.70 ± 0.16 versus 0.22 ± 0.04, Supplementary Figure S4b) relative to the untreated TO-2 heart, suggesting that MG53 offers cardiac protection in TO-2 hamsters.24
Multiple lines of evidence have indicated apoptotic cell-death mechanisms in neuromuscular disorders and the antiapoptosis therapy benefits MD.25,26 Bax is a proapoptotic Bcl-2 family protein and initiates apoptotic response in the mitochondrial pathway of apoptosis. Western blot analysis demonstrated marked decrease of Bax in MG53-treated versus untreated muscles (0.34 ± 0.11 versus 1.00 ± 0.23, Figure 4e) and heart (2.83 ± 0.29 versus 4.48 ± 0.98, Figure 4f and Supplementary Figure S5). Thus, the therapeutic effects of MG53 on TO-2 skeletal muscle and heart are associated with activation of Akt, ERK1/2, and GSK-3β pathways and attenuation of the proapoptotic Bcl-2 family molecule Bax.
Discussion
Recent reports showed that MG53 facilitates plasma membrane repair through intracellular vesicle translocation to the injury site for fusion to form a repair patch.12 Here, we investigated the therapeutic potential of MG53-mediated membrane repair by gene delivery in the δ-SG-deficient TO-2 hamsters, which display severe muscle cell membrane damage and manifest both muscle pathology and dilated congestive heart failure. Previously, we have successfully tested gene replacement therapy in the TO-2 model by AAV8-δ-SG gene delivery.3,14 In the present study, we used the same animal model and AAV8 vector but delivered the MG53 gene for efficient systemic and long-term gene transfer. We found that enhanced membrane repair by MG53 overexpression improved membrane integrity in both skeletal and cardiac muscles in the TO-2 hamsters and resulted in global therapeutic efficacies for MD and heart failure. To obtain muscle-specific transgene expression and minimize the side effects due to ectopic expression, especially in the liver, we utilized a muscle-specific promoter and liver-specific miRNA-122 target sequence in the AAV-MG53 expression vector cassette. These significantly increased specificity of transgene expression in the muscle and heart and dramatically reduced ectopic expression and side effects in the liver. In addition, we found that MG53 overexpession elevated the levels of other important components of the membrane repair machinery such as dysferlin and caveolin-3. It also enhanced a number of key cell survival signaling pathways and inhibited proapoptotic molecules. Together, these favorable molecular mechanisms contributed to the beneficial effects of enhanced membrane repair by MG53 gene delivery in MD animal model.
Chronic muscle cell membrane damage is a common pathological consequence of various genetic deficiencies seen in different forms of MDs. Widely explored therapeutic strategies include ones that specifically restore the gene products that are deficient, for example, restoration of sarcoglycans for LGMDs and dystrophin for Duchenne and Becker MDs (DMD, BMD). However, these approaches are limited to the specific disease subset and even specific patients.27 Given the very small numbers of patients in various rare MDs such as the δ-SG-deficient LGMD 2F, therapeutic strategies that improve and correct common pathological pathways instead of individual genetic defects have recently gained ground due to their broader therapeutic potentials.6,28,29,30,31 For example, influx of extracellular Ca2+ resulted from plasma membrane leakage triggers a cascade of detrimental events in the cells.7 Recent studies showed that enhancing intracellular Ca2+ handling by reducing or sequestering excessive Ca2+ in cytosol could protect and improve the functions of muscle and heart cells.6,31 MG53 renders its protective effects on the muscle cells by resealing the leaky membrane and preventing harmful extracellular Ca2+ influx, since it senses Ca2+ influx and mobilizes the membrane repair machinery. Thus, upregulation of an endogenous protein such as MG53 for the treatment of genetic diseases offers a unique advantage over gene replacement therapy. The latter produces a therapeutic protein that the host is lacking and may therefore be recognized as a neoantigen for immune responses. Another advantage of MG53 gene therapy is its broader potential for the treatment of different forms of MDs with leaky muscle cell membranes. However, since MG53 is only found in the striated muscles, tissue-specific transgene expression is highly desirable in order to avoid adverse effects of MG53 overexpression in nonmuscle tissues, especially when high copy numbers of the AAV vectors are delivered to the liver after intravenous injection. To this end, we have successfully used two layers of regulation, a muscle promoter and a liver miRNA-122 target, to attain tight control of MG53 expression in vivo. If needed, muscle- and heart-targeting AAV vectors32,33 can also be used as an additional option to further improve gene transfer specificity.
This is the first time that overexpression of MG53 has been tested in an in vivo model. We found that increased MG53 was accompanied by increased levels of dysferlin and caveolin-3, another two key components in the muscle membrane repair pathway. MG53 facilitated dysferlin's function as a fusogen for intracellular vesicle trafficking to the damaged sites to form a membrane repair patch,8,34 while caveolin-3 functions as an upstream regulator for both dysferlin and MG53 by both inhibiting endocytosis of dysferlin from sarcolemma to cytoplasma and moderating excessive membrane trafficking events induced by MG53.13,35 The long-term therapeutic effect of MG53 in dystrophic muscle and heart argues for the beneficial functions of MG53 overexpression in striated muscle membrane repair in an enhanced but balanced manner in coordination with other key factors. Additional evidence further supports the therapeutic mechanisms as we detected alteration of key survival kinases and proapoptotic pathways following AAV-MG53 treatment, namely activation of ERK1/2, Akt, and GSK-3 and inhibition of Bcl-2 family member Bax25,26 in the muscle and heart of AAV-MG53 treated dystrophic hamsters. These findings are consistent with a recent in vitro study that demonstrated the involvement of PI3K, Akt, GSK-3β, and ERK1/2 in the MG53-mediated cardiac ischemic preconditioning process.24
Finally, the limitation of surrogate gene, protein, and small molecule drug therapies for genetic diseases is that if the underlying cause of the disease is not corrected by restoration of the missing gene product, one can only anticipate partial therapeutic efficacies. MG53 gene therapy is no exception. In the δ-SG-deficient hamster model we achieved only partial nonetheless very significant therapeutic benefits, which if applied to patients could translate to a clinically meaningful therapy. In addition, MG53 gene therapy may find its way in treating ischemic heart disease and heart failure, where nonspecific cardiomyocyte membrane damage and dysfunction of Ca2+ homeostasis are commonly found. MG53 should be able to help maintain cardiomyocyte membrane integrity and repair the leaky membrane, therefore, offer an effective treatment for cardiomyopathies that arise from compromised muscle membrane integrity.36
Materials and Methods
AAV vector construction and production. Recombinant double-stranded CK promoter-driven human MG53 AAV vectors with or without the target sequence of the liver-specific miRNA-122 were constructed by the standard cloning protocols. The vector DNA was packaged into AAV8 viral particles by triple plasmid transfection of 293 cells37 and purified by polyethylene glycol precipitation followed by CsCl centrifugation.38 DNA dot blot was used to determine the titers of the purified viral stocks as vg/ml.
Animals and vector administration. All experiments using male TO-2 and F1B hamsters (Bio Breeders, Fitchburg, MA) were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill and were in accordance with National Institutes of Health Guidelines. For vector administration, neonatal TO-2 hamsters (10–12 days old, 10 g) were intraperitoneally injected with AAV8-CK-MG53 (low dose, 1 × 1013 vg/kg; high dose, 1 × 1014 vg/kg) in 0.2 ml of solution, whereas adult TO-2 hamsters (2 months old, 100 g) were intravenously injected with the same vector (2 × 1013 vg/kg) in 0.3 ml of solution via sublingual vein.
Histology, immunochemistry, and western blot analysis. Muscle and other tissues were collected at the indicated time points after vector injection. For immunofluorescent staining, hematoxylin and eosin staining, and Masson's trichrome staining, tissue samples were cryosectioned to a thickness of 7 µm and processed according to previous publications.1 Tissue hydroxyproline content was measured according to the method described1 and was normalized by dry tissue weight. For western blot, 10–30 µg of protein extract from homogenized hamster muscle was run on an 8–12% sodium dodecyl sulfate-polyacrylamide gel. The anti-hMG53 antibody was provided by Dr J.M. lab. Anti-Myc tag antibody (ab9106) and anti-GAPDH antibody (ab9485) were from Abcam (Cambridge, MA). Anti-dysferlin antibody (VP-D503) was from Vector Laboratories (Burlingame, CA). Anti-caveolin-3 antibody (sc-7665) was from Santa Cruz Biotech (Santa Cruz, CA). Anti-Akt and p-Akt antibody (#9272 and #9271), anti-ERK1/2 and p-ERK1/2 antibody (#9102 and #9101), anti-GSK-3α/β and p-GSK-3β antibody (#5676 and #9323) and anti-Bax antibody (#2772) were purchased from Cell Signaling Technology (Danvers, MA). Serum alanine transaminase and amylase levels were measured by Animal Clinical Laboratory Core Facility at UNC-Chapel Hill.
Treadmill. To test whole-body endurance, hamsters were placed on a motorized treadmill set at a rate of 15 m/minute at a 15° downward slope. Termination of the test occurred when the hamsters remained on the shock grid for >10 seconds without attempting to reenter the treadmill.
Membrane integrity testing. For the in vitro membrane repair assay, isolated single myofibers from flexor digitorum brevis muscle were measured for FM1-43 dye leakage as described before.12 Hamsters were euthanized by CO2 inhalation, followed by cervical dislocation. The flexor digitorum brevis muscles were surgically excised and placed in a Tyrode solution (140 mmol/l NaCl, 5 mmol/l KCl, 2.5 mmol/l CaCl2, 2 mmol/l MgCI2, 10 mmol/l HEPES, pH 7.2). Type I collagenase (2 mg/ml) was used to digest the flexor digitorum brevis muscle samples at 37 °C for 2 hours. The fibers were plated onto δTC3 glass-bottomed dishes in Tyrode solution containing either 2 mmol/l Ca2+ or 0 mmol/l Ca2+ (plus 0.5 mmol/l EGTA) and 2.5 µmol/l FM1-43 dye (Molecular Probes Eugene, OR). To induce damage to the muscle fibers, a 5 × 5 pixel area of the plasma membrane was irradiated by UV laser at maximum power (Enterprise, 80 mW, 351/364 nm) for 5 seconds using a Zeiss-LSM 510 confocal microscope equipped with a ×63 water immersion lens (N.A. 1.3). Images were captured at 5-second intervals. For each image, the mean fluorescence intensity was measured using Zeiss-LSM 510 imaging software. The fluorescence intensity was calculated in an area of about 200 µm2 directly adjacent to the injury site. Data is presented as a ratio of fluorescence intensity change relative to the value before injury (δF/F0).
For the in vivo tests of sarcolemma integrity, Evans blue (10 mg/ml in phosphate-buffered saline) was intravenously injected into hamsters at a dose of 0.1 mg/g body weight. The animals were then sacrificed 12 hours later, and cryosections of muscle tissue were analyzed. Leaky myocytes filled with Evans blue and showed strong red fluorescence. Blood (0.1 ml) was also collected from the saphenous vein for evaluation of serum CK enzyme activity, which was assayed using the CK Reagent/UV-Kinetic Method (TECO Diagnostics Anaheim, CA) according to manufacturer instructions.
Echocardiography analysis. The noninvasive echocardiography method was used to collect heart functional data.3 Echocardiographic studies in hamsters were performed at 4.5 and 7.5 months of age in the neonatal treatment group and at 4.5 months of age in the adult treatment group (AAV injection at 2 months of age). Hamsters were anesthetized with intraperitoneal injection of 2.5% Avertin (150 µl/10 g body weight), secured in a shallow left lateral decubitus position over a warming pad, and shaved over the chest. A 13.0-MHz transducer was applied to the left hemithorax. Two-dimensional-targeted M-mode imaging was obtained from the short axis immediately below the level of the mitral valve. M-mode measurements of LVID;d and LVID;s were made from recorded images by using the leading-edge convention of the American Society of Echocardiography. Left ventricular percentage fractional shortening (%) was calculated as: left ventricular percentage fractional shortening (%) = [(LVID;d-LVID;s)/LVID;d]/100.
Statistical analysis. Statistic analysis was performed with the one-way ANOVA with Bonferroni post-test. The P value was two-tailed and considered statistically significant when P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Ca2+-dependent MG53-mediated repair patch formation in TO-2 muscle fibers. Figure S2. Amelioration of fibrosis as evaluated by decreasing tissue hydroxyproline content in AAV-MG53-treated muscle and heart of TO-2. Figure S3. miRNA-mediated transgene silencing reduced liver toxicity caused by MG53 overexpression. Figure S4. Activation of prosurvival kinases in MG53-treated TO-2 heart. Figure S5. Immunoblots of proapoptotic factor Bax in hamster heart. Video S1. Representative membrane repair of single myofiber isolated from wild-type F1B. Video S2. Representative membrane repair of single myofiber isolated from TO-2 hamster. Video S3. Representative membrane repair of single myofiber isolated from AAV-MG53 treated TO-2 hamster.
Acknowledgments
This work was partially supported by a predoctoral Ruth L. Kirschstein National Research Service Award to B.H. (F31NS070540) and RO1 grants to X.X. (NIAMS) and J.M. (NIAMS, NHLBI, and NIA). Jianjie Ma and N. Weisleder are cofounders for TRIM-edicine, Inc., a university spin-off biotechnology company that is developing recombinant MG53 protein as a therapeutic reagent for regenerative medicine.
Supplementary Material
Ca2+-dependent MG53-mediated repair patch formation in TO-2 muscle fibers.
Amelioration of fibrosis as evaluated by decreasing tissue hydroxyproline content in AAV-MG53-treated muscle and heart of TO-2.
miRNA-mediated transgene silencing reduced liver toxicity caused by MG53 overexpression.
Activation of prosurvival kinases in MG53-treated TO-2 heart.
Immunoblots of proapoptotic factor Bax in hamster heart.
Representative membrane repair of single myofiber isolated from wild-type F1B.
Representative membrane repair of single myofiber isolated from TO-2 hamster.
Representative membrane repair of single myofiber isolated from AAV-MG53 treated TO-2 hamster.
REFERENCES
- Wang B, Li J, Fu FH., and, Xiao x. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J Orthop Res. 2009;27:421–426. doi: 10.1002/jor.20781. [DOI] [PubMed] [Google Scholar]
- Wang B, Li J., and, Xiao x. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci USA. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C.et al. (2005Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer Circulation 1122650–2659. [DOI] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L.et al. (2004Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping Science 3061796–1799. [DOI] [PubMed] [Google Scholar]
- Lostal W, Bartoli M, Bourg N, Roudaut C, Bentaïb A, Miyake K.et al. (2010Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer Hum Mol Genet 191897–1907. [DOI] [PubMed] [Google Scholar]
- Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG.et al. (2011Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle J Clin Invest 1211044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ., and, Molkentin JD. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci USA. 2009;106:19023–19028. doi: 10.1073/pnas.0906591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R.et al. (2003Defective membrane repair in dysferlin-deficient muscular dystrophy Nature 423168–172. [DOI] [PubMed] [Google Scholar]
- Ellis JA. Cell biology: Patches for wounded muscle. Nature. 2003;423:129, 131. doi: 10.1038/423129a. [DOI] [PubMed] [Google Scholar]
- Han R., and, Campbell KP. Dysferlin and muscle membrane repair. Curr Opin Cell Biol. 2007;19:409–416. doi: 10.1016/j.ceb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M.et al. (2009Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin J Biol Chem 28415894–15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M.et al. (2009MG53 nucleates assembly of cell membrane repair machinery Nat Cell Biol 1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, Komazaki S.et al. (2009MG53 regulates membrane budding and exocytosis in muscle cells J Biol Chem 2843314–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M, Hayashi T, Jeon YE, Fu Z, Gu Y, Dalton ND.et al. (2011Delta-sarcoglycan gene therapy halts progression of cardiac dysfunction, improves respiratory failure, and prolongs life in myopathic hamsters Circ Heart Fail 489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J.et al. (2005Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart Nat Biotechnol 23321–328. [DOI] [PubMed] [Google Scholar]
- Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM.et al. (2007Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle Mol Ther 15320–329. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA.et al. (2006Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8 Mol Ther 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Rehman KK, Bertera S, Zhang J, Chen C.et al. (2006Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes Diabetes 55875–884. [DOI] [PubMed] [Google Scholar]
- Wang B, Li J, Fu FH, Chen C, Zhu X, Zhou L.et al. (2008Construction and analysis of compact muscle-specific promoters for AAV vectors Gene Ther 151489–1499. [DOI] [PubMed] [Google Scholar]
- Qiao C, Yuan Z, Li J, He B, Zheng H, Mayer C.et al. (2011Liver-specific microRNA-122 target sequences incorporated in AAV vectors efficiently inhibits transgene expression in the liver Gene Ther 18403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo F, Moore SA, Ford GC., and, Campbell KP. Intracellular accumulation and reduced sarcolemmal expression of dysferlin in limb–girdle muscular dystrophies. Ann Neurol. 2000;48:902–912. [PubMed] [Google Scholar]
- Feron M, Guevel L, Rouger K, Dubreil L, Arnaud MC, Ledevin M.et al. (2009PTEN contributes to profound PI3K/Akt signaling pathway deregulation in dystrophin-deficient dog muscle Am J Pathol 1741459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter AK., and, Crosbie RH. Hypertrophic response of Duchenne and limb-girdle muscular dystrophies is associated with activation of Akt pathway. Exp Cell Res. 2006;312:2580–2591. doi: 10.1016/j.yexcr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F.et al. (2010MG53 constitutes a primary determinant of cardiac ischemic preconditioning Circulation 1212565–2574. [DOI] [PubMed] [Google Scholar]
- Girgenrath M, Dominov JA, Kostek CA., and, Miller JB. Inhibition of apoptosis improves outcome in a model of congenital muscular dystrophy. J Clin Invest. 2004;114:1635–1639. doi: 10.1172/JCI22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikoso S, Ikeda Y, Yamaguchi O, Takeda T, Higuchi Y, Hirotani S.et al. (2007Progression of heart failure was suppressed by inhibition of apoptosis signal-regulating kinase 1 via transcoronary gene transfer J Am Coll Cardiol 50453–462. [DOI] [PubMed] [Google Scholar]
- Hoffman EP. Skipping toward personalized molecular medicine. N Engl J Med. 2007;357:2719–2722. doi: 10.1056/NEJMe0707795. [DOI] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L.et al. (2009Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle Nat Med 15325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C, Li J, Jiang J, Zhu X, Wang B, Li J.et al. (2008Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice Hum Gene Ther 19241–254. [DOI] [PubMed] [Google Scholar]
- Rooney JE, Gurpur PB., and, Burkin DJ. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2009;106:7991–7996. doi: 10.1073/pnas.0811599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G.et al. (2008Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy Nat Med 14442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Jiang J, Drouin LM, Agbandje-McKenna M, Chen C, Qiao C.et al. (2009A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection Proc Natl Acad Sci USA 1063946–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulicherla N, Shen S, Yadav S, Debbink K, Govindasamy L, Agbandje-McKenna M.et al. (2011Engineering Liver-detargeted AAV9 Vectors for Cardiac and Musculoskeletal Gene Transfer Mol Ther 191070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, McNeil PL.et al. (2007Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury J Clin Invest 1171805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Deviez DJ, Howes MT, Laval SH, Bushby K, Hancock JF., and, Parton RG. Caveolin regulates endocytosis of the muscle repair protein, dysferlin. J Biol Chem. 2008;283:6476–6488. doi: 10.1074/jbc.M708776200. [DOI] [PubMed] [Google Scholar]
- Wang X, Xie W, Zhang Y, Lin P, Han L, Han P.et al. (2010Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair Circ Res 10776–83. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J., and, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V.et al. (2010High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency Gene Ther 17503–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ca2+-dependent MG53-mediated repair patch formation in TO-2 muscle fibers.
Amelioration of fibrosis as evaluated by decreasing tissue hydroxyproline content in AAV-MG53-treated muscle and heart of TO-2.
miRNA-mediated transgene silencing reduced liver toxicity caused by MG53 overexpression.
Activation of prosurvival kinases in MG53-treated TO-2 heart.
Immunoblots of proapoptotic factor Bax in hamster heart.
Representative membrane repair of single myofiber isolated from wild-type F1B.
Representative membrane repair of single myofiber isolated from TO-2 hamster.
Representative membrane repair of single myofiber isolated from AAV-MG53 treated TO-2 hamster.