Abstract
Noninvasive intranasal drug administration has been noted to allow direct delivery of drugs to the brain. In the present study, the therapeutic efficacy of intranasal small interfering RNA (siRNA) delivery was investigated in the postischemic rat brain. Fluorescein isothiocyanate (FITC)-labeled control siRNA was delivered intranasally in normal adult rats using e-PAM-R, a biodegradable PAMAM dendrimer, as gene carrier. Florescence-tagged siRNA was found in the cytoplasm and processes of neurons and of glial cells in many brain regions, including the hypothalamus, amygdala, cerebral cortex, and striatum, in 1 hour after infusion, and the FITC-fluorescence was continuously detected for at least 12 hours. When siRNA for high mobility group box 1 (HMGB1), which functions as an endogenous danger molecule and aggravates inflammation, was delivered intranasally, the target gene was significantly depleted in many brain regions, including the prefrontal cortex and striatum. More importantly, intranasal delivery of HMGB1 siRNA markedly suppressed infarct volume in the postischemic rat brain (maximal reduction to 42.8 ± 5.6% at 48 hours after 60 minutes middle cerebral artery occlusion (MCAO)) and this protective effect was manifested by recoveries from neurological and behavioral deficits. These results indicate that the intranasal delivery of HMGB1 siRNA offers an efficient means of gene knockdown-mediated therapy in the ischemic brain.
Introduction
Drug delivery to the brain is needed to treat diseases of the central nervous system, but delivery efficiencies are limited by the blood-brain barrier, which separates brain tissues from the systemic circulating system and central interstitial fluid. In particular, the blood-brain barrier effectively prevents the passages of hydrophilic and large molecular weight drugs. Accordingly, many different methods of administrating pharmacologic drugs have been devised to circumvent this barrier and achieve therapeutic or diagnostic benefits.
The intranasal delivery route is not obstructed by the blood-brain barrier, and thus, allows the direct delivery of drugs to the central nervous system.1 In addition, this direct route between the nasal cavity and the brain also circumvents drug elimination by the liver and gastrointestinal tract, filtration through the kidney, and degradation in serum. Moreover, the nose-brain route offers enhanced patient compliance for drugs administered via a parenteral route. Direct deliveries of various proteins, for example, insulin,2,3,4 insulin-like growth factor,5,6 nerve growth factor,7,8 and cholecystokinin,9 have been reported. In fact, the intranasal method has been proposed for direct delivery of insulin to the brain to treat Alzheimer's disease.2 Improvements in memory and cognition have been reported in Alzheimer's patients who were received insulin intranasally.10,11 Recently, Doyle et al. (2008)12 reported that the nasal administration of osteopontin peptide mimetics confers neuroprotection in rat stroke model, and Hashizume et al. (2008)13 found that the intranasal delivery of an oligonucleotide telomerase inhibitor suppresses brain tumor growth and increased survival in rats. In addition, Danielyan et al. (2009)14 reported the intranasal deliveries of mesenchymal stem cells and glioma cells to the rat brain.
In the present study, we investigated the therapeutic potential of intranasal small interfering RNA (siRNA) delivery in a rat model of focal cerebral ischemia using high mobility group box 1 (HMGB1) as a target gene. HMGB1 functions as an endogenous danger signaling molecule,15 which is released by necrotic cells into the extracellular milieu and is also actively secreted by macrophages and monocytes to induce inflammation.16,17,18,19 In a previous report, we showed that HMGB1 is massively released during excitotoxicity-induced acute damaging process in the postischemic brain, and that its release then aggravates brain damage by, for example, inducing microglia activation and apoptotic neuronal death.20,21 In addition, we also found that HMGB1 knockdown by intraparenchymally-administered siRNA significantly reduces infarct volumes, thus confirming the crucial role played by HMGB1 in the postischemic brain.20,21 The purpose of this study was to examine the efficiency of intranasal delivery of siRNA to various brain regions, to investigate target gene knockdown efficiency in those regions, and to evaluate the therapeutic potential of intranasally delivered HMGB1 siRNA in the postischemic rat brain.
Results
Localization of siRNA in the rat brain after intranasal delivery
The efficiency of intranasal siRNA delivery was examined in normal rat brain by triple fluorescent labeling using fluorescein isothiocyanate (FITC)-labeled control siRNA, cell-type specific immunostaining, and DAPI staining (Figure 1c). FITC-labeled transfection control siRNA was delivered using e-PAM-R as a gene carrier. e-PAM-R is a biodegradable PAMAM dendrimer, which shows maximum siRNA delivery efficiency at weight ratio of 5.0 (e-PAM-R/siRNA) in primary cortical neuronal cultures and in the postischemic rat brain.21 When we examined zeta potentials of polymer/siRNA complexes at weight ratios of 1.2, 2.5, 5.0 and 10.0, the zeta potential increased with increasing weight ratios (Supplementary Figure S1), wherein 22.3 mV was obtained at weight ratio 5.0. In addition, the particle size was decreased to 188.7 ± 1.9 nm at weight ratio: 5.0 (n = 3), which was remarkably smaller than 367.7 ± 40.4 nm of polymer without siRNA (n = 3, P < 0.01). These results indicated that e-PAM-R/siRNA complex at weight ratio: 5.0 was efficiently condensed and might be able to penetrate into the cells. We used 2 µg of HMGB1 siRNA/e-PAM-R complexes at weight ratio of 5.0 for all the experiments unless indicated otherwise.
Figure 1.
Intranasal small interfering RNA (siRNA) delivery to the normal rat brain. Fluorescein isothiocyanate (FITC)-labeled transfection indicator siRNAs (2 µg) were administered intranasally. (a) Whole brains were isolated from normal rats and positioned ventral sides upward. (b) Cresyl violet staining of coronal brain sections at the levels indicated in a. (c–p) Localizations of FITC-labeled cells were examined by triple fluorescent staining with FITC, DAPI, and cell-type specific antibodies (anti-NeuN for neuron, anti-GFAP for astrocyte, anti-Iba 1 for microglia) in coronal brain sections prepared at 1, 3, or 12 hours after siRNA administration. (c–j) The superior prefrontal cortex, (k,n) striatum, (l,o) amygdala, and (m,p) hypothalamus are indicated in b as Ct, St, Am, and Hy, respectively. Insets in d–f are high magnification images. (n–p) High magnification images of each brain region (white box) at 3 hours postadministration. (c, g, i, k, l, m) Brain sections from phosphate-buffered saline (PBS)-treated control animals were also included. Arrows indicate transfection indicator siRNA-positive cells. Bar = 50 µm.
At 1 hour after intranasal delivery, FITC-labeled cells were found in the frontal cortex (pre-motor cortex) (Ct; Figure 1b) at the level indicated in Figure 1a. The FITC-label was localized in the cytoplasm of NeuN- or DAPI-positive cells (Figure 1d). The number of FITC-labeled cells was maintained at 3 and 12 hours after delivery, though intensities appeared to be increased (Figure 1e,f). Triple labeling with FITC-labeled control siRNA, DAPI, and anti-GFAP or anti-Iba-1 antibody revealed that siRNA was also delivered to astrocytes (Figure 1h) or microglia (Figure 1j), respectively. Similarly, FITC-labeled cells were found in other brain regions, including striatum (St; Figure 1k,n), amygdala (Am; basolateral and central nuclei) (Figure 1l–o), and hypothalamus (Hy; ventro-medial nucleus) (Figure 1m,p). FITC-fluorescence was detected mainly in cytoplasm, but it was also observed in neuritic processes in all brain regions examined (insets in Figure 1d–f, n–p). In contrast, animals administered e-PAM-R alone showed hardly any FITC-fluorescence in the brain at any time point examined (data not shown).
HMGB1 knockdown by intranasal siRNA delivery in the olfactory bulb
HMGB1 siRNA (2 µg)/e-PAM-R or nonspecific siRNA (2 µg)/e-PAM-R complexes were delivered intranasally and HMGB1 expression levels in the olfactory bulb (Figure 2a–c) were examined by double immunohistochemical analysis using anti-HMGB1 and anti-NeuN (a neuronal marker) antibodies. In normal brain, HMGB1 was present in almost all NeuN-positive cells and also in some NeuN-negative cells (Figure 2d), demonstrating that HMGB1 is expressed in neurons and probably in glial cells. Three hours after HMGB1 siRNA delivery, HMGB1 immunoreactivity was decreased in mitral cells and also in cells located in the inferior surface of the olfactory bulb (Figure 2e) compared to that in controls (Figure 2d), and this decrease was maintained at 12 hours after HMGB1 siRNA delivery (Figure 2f). Similar levels of decrease were observed in the medial and superior surfaces (data not shown). However, in animals administered nonspecific siRNA/e-PAM-R, HMGB1 expression was detected in the nuclei of all NeuN-positive cells in the olfactory bulb (Figure 2g). Furthermore, in those animals, no visible change in cell morphology was observed (Figure 2g), thus alleviating concerns regarding the possible toxic effects of e-PAM-R. These results indicate that the intranasal delivery of HMGB1 siRNA effectively downregulate HMGB1 expression in the olfactory bulb, the brain region adjacent olfactory epithelium.
Figure 2.
High mobility group box 1 (HMGB1) knockdown in the olfactory bulb by intranasal delivery of HMGB1 small interfering RNA (siRNA). HMGB1 siRNA (2 µg)/e-PAM-R complex was intranasally administered to normal animal. (a) Whole brains were isolated from normal rats and positioned ventral side upward. (b) Cresyl violet staining of coronal sections of the olfactory bulb at the level indicated in a. (c) High magnification image of the indicated region (black box) in b. (d–g) Localizations of HMGB1 in neurons in the indicated region (black box) in c of the olfactory bulb are determined by double immunostaining with anti-HMGB1 and anti-NeuN antibodies. Fluorescence photographs were prepared (e) 3 or (f,g) 12 hours after (e,f) HMGB1 siRNA/e-PAM-R or (g) nonspecific siRNA/e-PAM-R complex administration. Arrows indicate HMGB1-negative cells and arrow-heads indicate HMGB1-positive/Neu N-negative cells. Epl, external plexiform layer; Gl, glomerular layer; Mi, mitral cell layer. d–g, bar = 50 µm.
HMGB1 knockdown by intranasal siRNA delivery in the amygdala and hypothalamus
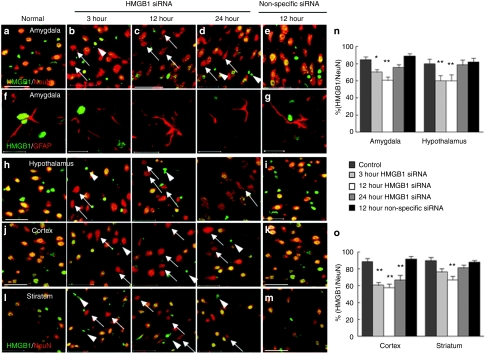
Next, we examined the efficiency and temporal profile of target gene knockdown obtained by intranasal HMGB1 siRNA in the amygdala and hypothalamus (brain regions associated with the olfactory pathway) (indicated in Figure 1a,b). HMGB1 siRNA (2 µg)/e-PAM-R or nonspecific siRNA/e-PAM-R complexes were delivered intranasally. In normal brain, almost all NeuN-positive cells in the amygdala were HMGB1-positive (Figure 3a). However, 3 hours after the intranasal delivery of HMGB1 siRNA/e-PAM-R, HMGB1-negative/NeuN-positive cells were detected (Figure 3b,n: n = 6, P < 0.05), and the number of these cells was further increased at 12 hours postdelivery (Figure 3c,n: n = 6, P < 0.01). Although, the percentage of HMGB1-positive cells recovered at 24 hours postdelivery (Figure 3d,n: n = 6), our results indicated that the intranasal delivery of HMGB1 siRNA effectively depletes HMGB1 expression in the amygdala. In addition to neurons, HMGB1 knockdown was also observed in astrocytes (Figure 3f). In the hypothalamus, HMGB1-positive cells were similarly reduced after siRNA administration (Figure 3h,n: n = 6, P < 0.01). In contrast, HMGB1 suppression did not occur in nonspecific siRNA-treated animals both in the amygdala (Figure 3e,g,n: n = 4) and in the hypothalamus (Figure 3i,n: n = 4). These results indicate that intranasally administered siRNA/e-PAM-R allows access to the central nervous system, at least to brain regions along the olfactory pathway.
Figure 3.
High mobility group box 1 (HMGB1) knockdown in the amygdala, hypothalamus, cortex, and striatum after the intranasal delivery of HMGB1 small interfering RNA (siRNA). (a–d,f,h,j,l) HMGB1 siRNA/e-PAM-R complex or (e,g,i,k,m) nonspecific siRNA/e-PAM-R complex was administered intranasally to normal rats and coronal brain sections (indicated in Figure 1b) were prepared 3, 12, or 24 hours after HMGB1 the administration. Localizations of HMGB1 in neurons and astrocytes were determined by double immunostaining with (a–e and h–m) anti-HMGB1 and anti-Neu N antibodies or (f,g) with anti-HMGB1 and anti-GFAP antibodies, respectively. The images shown were obtained from (a–g) amygdala (Am), (h,i) hypothalamus (Hy), (j,k) prefrontal cortices (Ct) and (l,m) striatum (St) as indicated in Figure 1b. Arrows indicate HMGB1-negative cells and arrow-heads indicate HMGB1-positive/NeuN-negative cells. (l,o) Percentages of HMGB1-positive cells among Neu N-positive cells in indicated areas (230 µm × 230 µm) of each brain region (Figure 1b) were determined by counting 10 randomly selected regions in each of 4–6 independent experiments. Data represent means ± SEMs. *P < 0.05, **P < 0.01. a–e, h–m, bar =50 µm; f, g, bar = 20 µm.
Knockdown of HMGB1 expression in the superior prefrontal cortex and striatum by intranasal siRNA
We next examined whether intranasal HMGB1 siRNA causes target gene knockdown in brain regions other than those associated with the olfactory nerve pathway, such as, prefrontal cortex and striatum (as indicated in Figure 1b,c). Three hours after HMGB1-siRNA/ePAM-R treatment, HMGB1 knockdown was evident in the superior prefrontal cortex (Figure 3j), and HMGB1-positive cell percentages were at 56.3 (n = 6, P < 0.01), 58.1 (n = 6, P < 0.01), and 62.5% (n = 6, P < 0.01) at 3, 12, and 24-hours postdelivery, respectively (Figure 3o). In the striatum (lateral), HMGB1-positive cell percentages were reduced to 77.8% (n = 6) and 69.7% (n = 6, P < 0.01) at 3 and 12 hours postdelivery, respectively (Figure 3l,o). These results demonstrate robust HMGB1 knockdown in the superior prefrontal cortex in a protracted manner. Furthermore, HMGB1 depletion was also detected in other parts of the brain, for examples, in pons and hippocampus, but levels of depletion varied (data not shown).
Marked reductions in the levels of HMGB1 protein and RNA in the superior prefrontal cortex and striatum by intranasal siRNA
HMGB1 knockdown in the frontal cortex and striatum by intranasal HMGB1 siRNA was further confirmed by immunoblot analysis and real-time PCR. HMGB1 siRNA/e-PAM-R or nonspecific siRNA/e-PAM-R complexes were delivered intranasally and protein samples were purified from the regions indicated in Figure 4a, which included the area subjected to immunohistochemical analysis in the previous section. It was found that the HMGB1 protein level was significantly decreased in the frontal cortex at 3 hours after siRNA administration (n = 6, P < 0.01) (Figure 4b), and further decreased at 12 hours postadministration (n = 6, P < 0.01) and then maintained until 24 hours (n = 6, P < 0.01) (Figure 4b). In striatum, HMGB1 protein levels were also reduced at 3 hours postdelivery (n = 6, P < 0.05), and further decreased at 12 hours (n = 6, P < 0.01) and then maintained at 24 hours (n = 6, P < 0.01) (Figure 4c). On the other hand, α-tubulin levels were unchanged both in cortex and striatum at all time points examined (n = 6) (Figure 4b,c). Notable reductions in HMGB1 RNA were also observed by real-time PCR analysis in both cortex (n = 4, P < 0.05 or P < 0.01) (Figure 4d) and striatum (n = 4, P < 0.01) (Figure 4e), supporting the immunoblot analysis results. However, in nonspecific siRNA-administered animals, neither HMGB1 nor α-tubulin levels were changed (n = 4) (Figure 4b–e). These results corroborate the notion that intranasal siRNA delivery provides a highly efficient means of achieving target gene knockdown in various brain regions.
Figure 4.
Levels of high mobility group box 1 (HMGB1) protein and RNA in cortex and striatum after intranasal HMGB1 small interfering RNA (siRNA) delivery. HMGB1 siRNA/e-PAM-R complex or nonspecific siRNA/e-PAM-R complex was administered intranasally to normal rats and immunoblotting and real-time PCR were carried out 3, 12, and 24 hours later. (a) Cresyl violet staining of coronal sections of the brains. (b,d) Protein and RNA samples were prepared from cerebral cortices and (c,e) striatum as indicated in a. α-tubulin was used as a negative control. Quantitative data are presented as means ± SEMs (n = 4–6). *P < 0.05, **P < 0.01.
HMGB1 reduction was not detectable in peripheral tissues
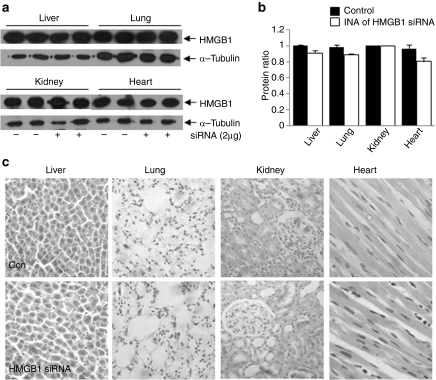
In contrast to the brain, HMGB1 reduction was not detectable in various peripheral tissues, such as, lung, liver, kidney, and heart, at 12 hours after intranasal siRNA delivery (Figure 5a,b). In particular, changes in the HMGB1 levels were not detected in the lung, an organ directly connected with nasal cavity through pharynx and upper and lower respiratory passages. In addition, no morphological differences were detected in those peripheral tissues (Figure 5c), suggesting that intranasal delivery of HMGB1 siRNA does not cause any detrimental effects to these tissues, at least at this level.
Figure 5.
Effects of intranasal high mobility group box 1 (HMGB1) small interfering RNA (siRNA) delivery on the levels of HMGB1 protein and morphology of peripheral tissues. HMGB1 siRNA/e-PAM-R complex or nonspecific siRNA/e-PAM-R complex was administered intranasally to normal rats. Protein samples and tissue sections were prepared from liver, lung, kidney, and heart, and (a,b) immunoblotting and (c) H/E staining were carried out 12 and 24 hours after siRNA administration, respectively. α-tubulin was used as a control in immunoblotting. Quantitative data are presented as means ± SEMs (n = 4–6).
Suppression of infarct formation by intranasal HMGB1 siRNA in the postischemic brain
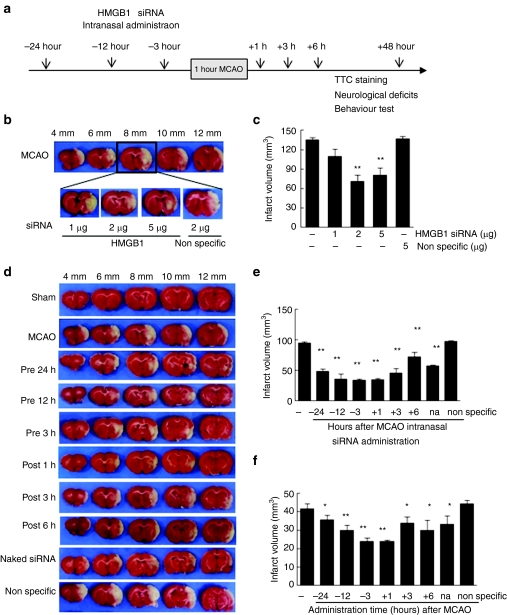
The superior and lateral prefrontal cortices and striatum are supplied by the middle cerebral artery (MCA). Knockdown of HMGB1 in the prefrontal cortex and striatum by intranasal siRNA (Figures 3 and 4) prompted us to investigate the potential beneficial effects of this delivery method on animal models of MCA occlusion (MCAO), a focal cerebral ischemia model. Various amounts of HMGB1 siRNA were complexed with e-PAM-R at weight ratio of 1:5 and administered intranasally at 12 hours prior to MCAO (Figure 6b). TTC staining revealed that the administrations of 2 or 5 µg of HMGB1 siRNA/e-PAM-R suppressed infarct volumes to 47.9 ± 5.4% (n = 4, P < 0.01) and 52.8 ± 3.6% (n = 4, P < 0.01), respectively, of that of the phosphate-buffered saline (PBS)-treated MCAO control (Figure 6b,c). When we examined infarct suppression efficacy of HMGB1 siRNA (2 µg)/e-PAM-R complexes at various weight ratios (2.5, 5, or 10), complex with weight ratio 5 showed a maximum infarct reduction (Supplementary Figure S2). Significant infarct volume suppression was evident when HMGB1 siRNA (2 µg)/e-PAM-R complexes (weight ratio 5) were administered at 24, 12, or 3 hours pre-MCAO or at 1, 3, or 6 hours post-MCAO (Figure 6a), with maximal reduction to 42.8 ± 5.6% (n = 6, P < 0.01) at 1-hour post-treatment (Figure 6d–f). It is notable here that HMGB1 siRNA administration at 6 hours post-MCAO significantly reduced infarct volume to 73.8 ± 7.2% of that of the PBS-treated MCAO control (n = 5, P < 0.05) (Figure 6d,e). The administration of naked siRNA was also found to confer protective effects, but its efficiency was significantly lower than that of siRNA/e-PAM-R (Figure 6d–f). However, the administration of nonspecific siRNA even at 12 hours pre-MCAO had no suppressive effect on infarct formation (Figure 6d,e). We also confirmed that pH, PaO2, PaCO2, and blood glucose levels were similar in HMGB1 siRNA-treated and untreated animals, which demonstrated that physiological parameters were not influenced by intranasal siRNA delivery (Table 1).
Figure 6.
Suppression of infarct formation by intranasal high mobility group box 1 (HMGB1) small interfering RNA (siRNA) in the postischemic brain. (a) HMGB1 siRNA/e-PAM-R complex or nonspecific siRNA/e-PAM-R complex was administered intranasally at various times before or after MCAO and infarction volumes were assessed at 2 days post-MCAO by TTC staining. (b–c) HMGB1 siRNA (0.5, 1, 2, or 5 µg)/e-PAM-R complex (weight ration 1:5) was administered intranasally 12 hours prior to 60 minutes of MCAO and infarction volumes were assessed at 2 days post-MCAO. (d–f) HMGB1 siRNA (2 ug)/e-PAM-R complex (weight ration 1:5) or nonspecific siRNA/e-PAM-R (weight ration 1:5) complex was administered intranasally 24, 12, or 3 hours prior to or 1, 3, or 6 hours after MCAO and infarction volumes were assessed at 2 days post-middle cerebral artery occlusion (MCAO) by TTC staining. Representative pictures showing suppressed infarct formation are presented in b and d; the values above the pictures represent distances from the frontal pole of the brain. (c) Quantitative infarction volumes in whole ischemic hemisphere or in the cortex and (e,f) striatum of ischemic hemispheres are presented as means ± SEMs (n = 4–6). na., naked. *P < 0.05, **P < 0.01.
Table 1. Physiological parameters.

Intranasal delivery of HMGB1-siRNA improved neurological deficits in rats that underwent MCAO
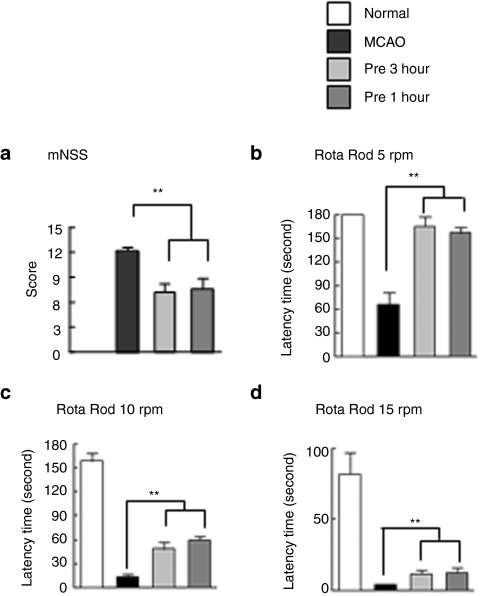
Next, we examined the beneficial effects of intranasal HMGB1-siRNA delivery on ischemic brains using behavioral tests. When HMGB1 siRNA was administered at 3 hours pre- or 1 hour post-MCAO, mean modified Neurological Severity Scores at 2 day post-MCAO were 6.8 ± 0.9 (n = 8, P < 0.01) and 7.2 ± 1.1 (n = 8, P < 0.01), respectively, which were significantly lower than that of rats in the PBS-treated MCAO group (12.3 ± 0.5) (n = 9, P < 0.01) (Figure 7a). Motor activities were assessed using the rota-rod test at 5 rpm and the mean time spent on the rota-rod by PBS-treated MCAO control animals at 2 days post-MCAO was 65.3 ± 16.4 seconds (n = 9) (Figure 7b), whereas for animals treated with HMGB1 siRNA intranasally, rota-rod performance was markedly improved (171.0 ± 12.8 and 165.0 ± 11.9 seconds) (n = 8, P < 0.01) (Figure 7b). Furthermore, these HMGB1 siRNA-treated animals had higher rota-rod scores than PBS-treated MCAO controls at 10 and 15 rpm (with an hour interval between tests) (Figure 7c,d). Together these results show that intranasal HMGB1-siRNA/e-PAM-R has neuroprotective effects on the postischemic brain, which were manifested by improvements in motor impairments and neurological deficits.
Figure 7.
Amelioration of motor deficit by intranasal high mobility group box 1 (HMGB1) small interfering RNA (siRNA) delivery in the postischemic brain. (a) HMGB1 siRNA (2 µg)/e-PAM-R complex (weight ration 1:5) was administered intranasally at 3 hours prior to or 1 hour post-middle cerebral artery occlusion (MCAO) and neurological deficits were evaluated using modified neurological severity scores at 2 days post-MCAO. (b) The rota-rod test was performed at 5, 10 and 15 rpm at 2 days post-MCAO. Sham, sham-operated group; MCAO, saline-treated MCAO group; MCAO+pre 3 hours, intranasal HMGB1 siRNA-administered (3 hours prior to) MCAO group; MCAO+post 1 hour, intranasal HMGB1 siRNA-administered (1 hour after) MCAO group. Data are presented as means ± SEM (n = 8–12) **P < 0.01.
Discussion
The present study demonstrates the successful delivery of siRNA and subsequent target gene knockdown in various brain regions, that is, in cerebral cortex, striatum, amygdala, and hypothalamus. Furthermore, the intranasal delivery of HMGB1 siRNA was found to markedly reduce infarct volumes and alleviate neurological and behavioral deficits in the postischemic rat brain. It has been reported that intranasally administered insulin-like growth factor-I reduced infarction volume and recovered behavioral defects in a rat MCAO model.5 In addition, intranasally delivered brain-derived neurotrophic factor has been reported to protect brain from ischemic insult via modulating local inflammation in rats.22 Similarly, protective effect of intranasal delivery of ginsenoside Rb1 has been reported in stroke animal model.23 Recently, Doyle et al. (2008)12 reported that intranasal administration of osteopontin peptide confers neuroprotection in a rat MCAO model. The present study adds siRNA to the growing list of neuroprotectants delivered intranasally to stroke animal model and provides the first report of marked alleviation of ischemic damage by intranasally delivered siRNA.
In terms of therapeutic potencies, intranasal delivery appears to be superior to intraparenchymal delivery in several important respects. In our previous report, we showed that the maximum reduction of infarct volume was by 34% by intraparenchymal siRNA administration, which was obtained by injecting 500 ng of HMGB1 siRNA into the cortex penumbra 18 hours before MCAO.21 However, in the present study we found that although 2 µg of HMGB1 siRNA was administered, infarct volumes were reduced by 57.2 % by intranasal delivery 1 hour after MCAO (Figure 6d–f). Considering the administration time, intranasal delivery conferred greater benefit in our stroke model, because the therapeutic window was extended to 6 hours post-treatment (Figure 6d–f), which was far delayed from 24 and 12 hours prior to MCAO for intraparenchymal injection.21 The greater protection afforded by intranasal delivery might be explained by the fact that intranasally administered siRNA seems to be delivered within an hour and distributed widely in the brain (Figure 1). In particular, the remarkable HMGB1 depletion (Figures 3 and 4) and infarct volume reduction (by 64.8%; Figure 6e) observed in the cerebral cortex demonstrated that cerebral cortex could be the greatest beneficiary of intranasal administration. Therefore, it would appear that intranasal drug delivery could be particularly beneficial for cortex-related diseases, such as, Alzheimer's disease.2,10,11,24,25 In addition to the knowledge regarding the routes to different brain regions, additional study is required to determine whether target gene depletion differences are due to intrinsic cellular differences in different brain regions.
The persistency of target gene knockdown observed in the present study was also notable (from 3 to 24 hours post-siRNA delivery), and was confirmed by immunohistochemistry, immunoblot analysis, and real-time PCR (Figures 3 and 4). The protracted effect of intranasal siRNA delivery was also corroborated by the protective effect observed when animals were treated at 24 hours prior to MCAO (Figure 6d,e). These effects might be attributed to multiple routes for intranasally delivered siRNA. Initially it has been reported that intranasal delivery occurs within minutes along the olfactory and trigeminal nerves.6,26,27 Then, fast transport via cerebrospinal fluid and subsequent slow delivery via intracellular transport and even simple diffusion have been reported as extracellular routes.28 Danielyan et al. (2009)14 claimed that in addition to the migration path involving the olfactory bulb to other parts of the brain, the cerebrospinal fluid route is operative at least for the delivery of mesenchymal stem cells. They claimed that intranasally delivered mesenchymal stem cells were present in cerebrospinal fluid and that they migrated along the surface of the cortex and into brain parenchyma. In addition to these routes, a perivascular cell trafficking route has also been suggested for olfactory bulb neuroblast migration.29 In the present study, the efficient knockdown of HMGB1 in various brain regions at 3 hrs after intranasal siRNA delivery (Figures 2–4) and the significant improvements observed in pathological and neurological symptoms when administered at 1 hr post-MCAO (Figures 6 and 7) suggests that HMGB1 siRNA is dispersed via a route that accesses many different brain regions quickly, which in turn suggests that this route might involve the cerebrospinal fluid and vascular system.
We cannot exclude the possibility that persistent effect might be due, at least in part, to e-PAM-R, which is a biodegradable polycationic PAMAM ester, in which arginine is ester bound to PAMAM-OH functionalized dendrimer,30,31. e-PAM-R has been reported to be gradually degraded under physiological conditions (pH 7.4, 37 °C), for example, Nam et al. (2009)30 found that more than 50% of the grafted amino acids were hydrolyzed within 5 hours, and that this degradation caused a slow and protracted release of HMGB1 siRNA. It is interesting to note that neither siRNA/e-PAM-R complex nor naked siRNA exerts neuroprotective effects when delivered intravenously (Supplementary Figure S3), suggesting that they cannot penetrate the blood-brain barrier and that the effects observed in the present study might be due to direct nose to brain delivery.
Since the proportions of intranasally administered drugs transported to the brain are minimal,32 various strategies have been examined to improve delivery efficiencies. The application of drugs encapsulated into particulate vectors, like synthetic nanoparticles, to olfactory epithelium could potentially improve central nervous system delivery. In addition, hyaluronidase pretreatment has been reported to improve intranasal delivery efficiencies.14,33 Furthermore, Cardoso et al. (2007 and 2008)34,35 reported that siRNA delivery efficiency is enhanced by conjugating siRNA with targeting moieties, such as, transferrin-associated liposome and transferrin-associated lipoplex, which were found to enhance delivery to neuronal cells and the brain, respectively. On the other hand, the present study demonstrates the usefulness of e-PAM-R as a delivery carrier for siRNA. It is evident that formulational modifications and possible systemic side effects of nasal delivery require future study.
Materials and Methods
Animals. Male Sprague–Dawley rats (The Orient, Seongnam, South Korea) were used throughout, and randomly assigned to HMGB1 siRNA-delivered experimental group and nonspecific siRNA-delivered or vehicle (PBS)-treated control groups. At the start of the experiment, animals weighed 280–320 g (10 weeks old) were housed separately under a 12:12 hours light: dark cycle with free access to food and water.
Preparation of the siRNA/e-PAM-R complex. HMGB1 siRNA (ON-TARGETPplus SMARTpool siRNA DH-2081024, accession No. NM_012963; Dharmacon, Lafayette, CO), FITC-labeled transfection indicator siRNA (siGLO Green Transfection Indicator D-001630, Dharmacon), and nonspecific siRNA (on-TARGETplus non-targeting pool D-001810, Dharmacon) were complexed with e-PAM-R using 2 µg of siRNA in Opti-MEM (20 µl, e-PAM-R/siRNA weight ratio 5.0), and then incubated for 30 minutes at room temperature before intranasal administration. Production procedure for e-PAM-R was described in previous reports.21,30 The ζ-potential and the size of the dendrimers/siRNA complexes were measured using a Malvern Zetasizer 3000 HAs (Malvern Instruments, Worcestershire, UK) using PCS 1.61 software. About 1 ml of polyplex solutions at various weight ratios was prepared to a final concentration of 2 µg/ml siRNA in HBSS (10 mmol/l HEPES, 1 mmol/l NaCl, pH 7.4).
Intranasal siRNA delivery. All animal experiments were carried out in accordance with “The Guidelines for Animal Research” issued by Inha University School of Medicine. Rats were anesthetized with an intramuscular ketamine/xylazine hydrochloride injection (3:1) (100–150 µl/100 g body weight). Body temperatures were maintained at 37 °C using a heating pad. The animals were randomly divided into three groups; animals in the first group were administered HMGB1 siRNA, those in the second group were administered nonspecific siRNA, and animals in the third group were administered vehicle (PBS). The nasal administration procedure was carried out generally as previously described by Thorne et al. (2004),6 but with significantly larger drop size. Briefly, a nose drop containing siRNA suspension (2 µg/20 µl) or PBS (20 µl) was carefully placed on one nostril of an anesthetized animal (supine 90° angle) using a sterile 26-G Hamilton microsyringe (80330; Hamilton Company, Reno, NV) and allowed to be delivered to the nasal cavity. Then the rest of the suspension was administered similarly in an alternating manner with a 2-minute interval between applications.
Surgical procedures for MCAO. MCAO was carried out as previously described.20 In brief, male Sprague–Dawley rats (280–320 g) were initially anesthetized with 5% isoflurane in a 30% oxygen/70% nitrous oxide gas mixture, and anesthesia was maintained using 0.5% isoflurane in the same gas mixture throughout the procedure. Occlusion of the MCA was maintained for 1 hour using a nylon suture, and this was followed by reperfusion. The left femoral artery was cannulated during the procedure to obtain blood samples and to monitor pH, PaO2, PaCO2, and blood glucose concentration (I-STAT; Sensor Devices, Waukesha, WI). Regional cerebral blood flow was monitored using a laser Doppler flowmeter (Periflux System 5000; Perimed, Jarfalla, Sweden), and a thermo-regulated heating pad and heating lamp were used to maintain a rectal temperature of 37 ± 0.5 °C. Animals were randomly divided into three treatment groups: untreated MCAO control, HMGB1 siRNA-treated MCAO group, and nonspecific siRNA-treated MCAO group. For HMGB1 siRNA-treated MCAO group, HMGB1 siRNA was intranasally administered 24, 12, or 3 hours prior to or 1, 3, or 6 hours post-MCAO. TTC staining and behavior studies were conducted at 2 days post-MCAO. Animals allocated to the sham group underwent an identical procedure with the exception of MCAO.
Infarct volume assessments. Rats were decapitated at 2 days post-MCAO, and whole brains were dissected coronally into 2-mm brain slices using a metallic brain matrix (RBM-40000; ASI, Springville, UT). Slices were immediately stained by immersion in 2% 2,3,5-triphenyl tetrazolium chloride (TTC) at 37 °C for 15 minutes and then fixed in 4% paraformaldehyde. The areas of infarcted regions in cortex and striatum were measured using the Scion Image program (Frederick, MD). To account for cerebral edema and differential shrinkage resulting from tissue processing, areas of ischemic lesions were determined by subtracting areas in ipsilateral hemispheres from those of contralateral hemispheres. Infarct volumes were calculated (in mm3) by multiplying summed section infarct areas by section thickness.
Modified neurological deficit severity scores. Neurological deficits were evaluated using modified Neurological Severity Scores at 2 days post-MCAO. The modified Neurological Severity Scores system consists of motor, sensory, balance, and reflex tests, all of which are graded using a scale of 0–18 (normal: 0, maximal deficit: 18).36 Motor scores were determined by summing the results of two tests. The first involved suspending a rat by its tail and allocating scores of zero or one to each of the following; flexion of forelimbs, flexion of hindlimbs, and head movement by >10 ° with respect to the vertical axis within 30 seconds. The second test involved placing a rat on the floor and allocating scores as follows; 0 for normal walking, 1 for an inability to walk straight, 2 for circling toward the paretic side, and 3 for falling on the paretic side. Sensory tests included a placing test (score 0–1) and a proprioceptive test (score 0–1). A beam balance test was used to test balance and scores of zero to six were allocated as follows; 0 for balance with a steady posture, 1 for grasping the side of the beam, 2 for hugging the beam with one limb off the beam, 3 for hugging the beam with two limbs off the beam or spun on the beam for over 60 seconds, 4 for attempting to balance on the beam but falling off within 40 seconds, to 60 seconds, 5 for attempted to balance on the beam but falling off within 20 seconds, and 60 seconds for making no attempt to balance or hang on to the beam. Reflex test scores were determined by awarding scores to the following four items (total score 0–4): pinna reflex, 0–1; corneal reflex, 0–1; startle reflex, 0–1; and seizures, myoclonus or myodystony, 0–1.
Rota-rod test. Twenty-four hours before MCAO, rats were conditioned on a rota-rod unit at a constant 3 rpm until they were able to remain on the rotating spindle for 180 seconds. Two days post-MCAO, each rat was subjected to rota-rod testing at 5 rpm, and residence times on the rota-rod at 10 and 15 rpm were measured with an inter-trial interval of 1 hour.
Immunohistochemistry. Normal animals were randomly divided into three groups and administered with PBS, HMGB1 siRNA, or nonspecific siRNA. After 3, 12, or 24 hours after the administration, they were subjected to double immunohistochemistry. Animals were sacrificed after being anesthetized. Brains were isolated and fixed with 4% paraformaldehyde by transcardial perfusion and post-fixed in the same solution overnight at 4 °C. Coronal brain sections (20 µm) were prepared using a vibratome (Leica, Solms, Germany), and immunological staining was performed using a previously described floating method.37 The following primary antibodies were diluted 1:500; anti-HMGB1 (ab18956-100; Abcam, Cambridge, UK), anti-Neu N (MAB377; Chemicon, Temecula, CA), anti-GFAP (556327, BD Pharmingen, Franklin Lakes, NJ), and anti-Iba-1 (Wako Pure Chemicals, Osaka, Japan). FITC-labeled anti-rabbit IgG (Jackson ImmunoRes Lab, West Grove, PA) was used as a secondary antibody for anti-HMGB1. After washing stained sections with PBS containing 0.1% Triton X-100, sections were incubated with Rhodamine-conjugated anti-mouse IgG (Jackson ImmunoRes Lab) for anti-Neu N, anti-GFAP, or anti-Iba-1 antibodies in PBS for 30 minutes at room temperature. After rinsing three times for 10 minutes in PBS, the sections were incubated with the nuclear marker DAPI (4′-6-diamidino-2-phenylindole dilactate) (D3571, Invitrogen, Carlsbad, CA) for 10 minutes at room temperature, washed three times with PBS, and then observed under a confocal imaging system (Radiance 200; Bio-Rad, Hertfordshire, UK). The representative pictures were presented and quantification results were presented as percentages of the number of HMGB1-positive cells in NeuN-positive cells counted in 10 randomly selected regions (230 µm x 230 µm, indicated in Figure 1b) in 4–6 independent experiments.
Real-time PCR. Normal animals were randomly divided into three groups: they were administered with PBS, HMGB1 siRNA, or nonspecific siRNA. After 3, 12, or 24 hours after the administration, brain tissues were prepared from indicated areas in Figure 4a and total RNA was purified from with TRI reagent (Sigma-Aldrich, St Louis, MO) according to the manufacturer's instructions. RNA from each group was treated with DNase 1 to avoid genomic contamination. First-strand cDNA was synthesized using the Takara RNA PCR kit (Takara Bio, Otsu, Japan) in a total volume of 20 µl containing 1 µg of total RNA. PCR was performed in a final volume of 20 µl containing 10 µl of 2x iQ SYBR Green supermix (Takara Bio), 1 µl each of 5 pmol/µl forward and reverse primers, and 5 µl of cDNA (1/100 dilution) using the Mini-Opticon Real-Time PCR System Detector (Bio-Rad, Richmond, CA). PCR was performed using: 5 minutes at 95 °C, followed by 40 cycles of 30 seconds at 95 °C, 30 seconds at 57 °C, and 30 seconds at 72 °C. The annealing temperature was increased to 60 °C for HMGB1 amplification. Specificity of amplification was determined by DNA melting curve analysis using the built-in software for automatic fluorescence data captured during temperature increments (0.5 °C) from 55 °C to 95 °C. Differences in amplification fold were calculated based on real-time PCR amplification of the target gene versus GAPDH (reference) using the built-in Gene Expression Analysis software in an iCycler iQ Real-Time RCR Detection System (Bio-Rad). The following primers sets were used; 5′-GGCTGACAAGGCTCGTTATG-3′ and 5′-GGGCGGTACTCAGAACAGAA-3′ for HMGB1; 5′-TCATTGAC CTCAACTACATGGT-3′ and 5′-CTAAGCAGTTGGTGGTGCAG-3′ for GAPDH. Real-Time PCR was performed four times per group for each brain region (cortex, striatum, hypothalamus, and amygdale).
Cresyl violet staining. For cresyl violet staining, normal rat brains were embedded in paraffin using a standard procedure. Sections (8 µm) were then cut coronally using a microtome at the level of the dorsal hippocampus and stained with 1% cresyl violet.
H&E staining. HMGB1 siRNA (2 µg) or nonspecific siRNA was administered intranasally to normal rats. At 24 hours after siRNA administration, rats were deeply anesthetized and fixed with 4% paraformaldehyde by transcardial perfusion. Liver, lung, heart, and kidney were removed quickly and post-fixed with 4% paraformaldehyde overnight at 4 °C. The organs were embedded in paraffin using a standard procedure. Tissues sections were cut 8 µm thick, stained with hematoxylin and eosin, and dehydrated in ethanol. The slides were cleared using xylene and mounted with cover slips.
Immunoblotting. Normal animals were randomly divided into three groups: they were administered with PBS, HMGB1 siRNA, or nonspecific siRNA. After 3, 12, or 24 hours after the administration, brain homogenates were prepared from indicated areas in Figure 4a and immunoblotted as described previously.20 Primary antibodies for anti-HMGB1 (2600-1; Epitomics, Burlingame, CA) and anti-α-tubulin (Calbiochem, San Diego, CA) were diluted 1:1000 and detected using a chemiluminescence kit (Roche, Basel, Switzerland) using anti-rabbit HRP-conjugated secondary antibody (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis. Statistical analysis was performed by analysis of variance followed by the Newman–Keuls test. All data are presented as averages ± SEMs and statistical significance was accepted at the 5% level.
SUPPLEMENTARY MATERIAL Figure S1. The zeta potential values of polymer/siRNA complexs. Figure S2. Suppression of infarct formation by intranasal HMGB1 siRNA/e-PAM-R in the postischemic brain. Figure S3. Suppression of infarct formation by intranasal or intravenous HMGB1 siRNA in the postischemic brain.
Acknowledgments
This work was financially supported by a Research Grant (2011-0002160) funded by National Research Foundation of Korea (NRF) and a Research Grant from Inha University for J.-K. L.
Supplementary Material
The zeta potential values of polymer/siRNA complexs.
Suppression of infarct formation by intranasal HMGB1 siRNA/e-PAM-R in the postischemic brain.
Suppression of infarct formation by intranasal or intravenous HMGB1 siRNA in the postischemic brain.
REFERENCES
- Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Frey WH II.2001Method for administering insulin to the brain.US Patent 6,313,093.
- Sigurdsson P, Thorvaldsson T, Gizurarson S, Gunnarsson E. Olfactory absorption of insulin to the brain. Drug Deliv. 1997;4:195–200. [Google Scholar]
- Kern W, Born J, Schreiber H., and, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes. 1999;48:557–563. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Thorne RG, DeFor TA., and, Frey WH.2nd (2001Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage J Neurol Sci 18791–97. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V., and, Frey WH.2nd (2004Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration Neuroscience 127481–496. [DOI] [PubMed] [Google Scholar]
- Frey WH 2nd, Liu J, Chen X, Thorne RG, Fawcett JR, Ala TA.et al. (1997Delivery of 125I-NGF to the brain via the olfactory route Drug Deliv 487–92. [Google Scholar]
- Chen XQ, Fawcett JR, Rahman YE, Ala TA., and, Frey II WH. Delivery of Nerve Growth Factor to the Brain via the Olfactory Pathway. J Alzheimers Dis. 1998;1:35–44. doi: 10.3233/jad-1998-1102. [DOI] [PubMed] [Google Scholar]
- Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A., and, Wray S. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci. 2004;24:4737–4748. doi: 10.1523/JNEUROSCI.0649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA.et al. (2008Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults J Alzheimers Dis 13323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B.et al. (2008Intranasal insulin improves cognition and modulates beta-amyloid in early AD Neurology 70440–448. [DOI] [PubMed] [Google Scholar]
- Doyle KP, Yang T, Lessov NS, Ciesielski TM, Stevens SL, Simon RP.et al. (2008Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke J Cereb Blood Flow Metab 281235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume R, Ozawa T, Gryaznov SM, Bollen AW, Lamborn KR, Frey WH.2ndet al. (2008New therapeutic approach for brain tumors: Intranasal delivery of telomerase inhibitor GRN163 Neuro-oncology 10112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielyan L, Schäfer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T.et al. (2009Intranasal delivery of cells to the brain Eur J Cell Biol 88315–324. [DOI] [PubMed] [Google Scholar]
- Bianchi ME., and, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J.et al. (1999HMG-1 as a late mediator of endotoxin lethality in mice Science 285248–251. [DOI] [PubMed] [Google Scholar]
- Abraham E, Arcaroli J, Carmody A, Wang H., and, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T., and, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A.et al. (2003Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion EMBO J 225551–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW.et al. (2006HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain J Neurosci 266413–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ID, Lim CM, Kim JB, Nam HY, Nam K, Kim SW.et al. (2010Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain J Control Release 142422–430. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wei N, Lu T, Zhu J, Xu G., and, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- Lu T, Jiang Y, Zhou Z, Yue X, Wei N, Chen Z.et al. (2011Intranasal ginsenoside Rb1 targets the brain and ameliorates cerebral ischemia/reperfusion injury in rats Biol Pharm Bull 341319–1324. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Gray AJ, Hirata-Fukae C, Minami SS, Waterhouse EG, Mattson MP.et al. (2007Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer's disease at early pathological stage J Mol Neurosci 31165–170. [DOI] [PubMed] [Google Scholar]
- Hanson LR, Frey WH 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;10:S3–S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerusalmi A, Morris-Downes MM, Sheahan BJ., and, Atkins GJ. Effect of intranasal administration of Semliki Forest virus recombinant particles expressing reporter and cytokine genes on the progression of experimental autoimmune encephalomyelitis. Mol Ther. 2003;8:886–894. doi: 10.1016/j.ymthe.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR., and, Frey WH.2nd (2004Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis J Neuroimmunol 15166–77. [DOI] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR., and, Frey WH.2nd (2009Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system J Pharm Sci 982501–2515. [DOI] [PubMed] [Google Scholar]
- Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T., and, Puche AC. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HY, Nam K, Hahn HJ, Kim BH, Lim HJ, Kim HJ.et al. (2009Biodegradable PAMAM ester for enhanced transfection efficiency with low cytotoxicity Biomaterials 30665–673. [DOI] [PubMed] [Google Scholar]
- Nam HY, Hahn HJ, Nam K, Choi WH, Jeong Y, Kim DE.et al. (2008Evaluation of generations 2, 3 and 4 arginine modified PAMAM dendrimers for gene delivery Int J Pharm 363199–205. [DOI] [PubMed] [Google Scholar]
- Illum L. Is nose-to-brain transport of drugs in man a reality. J Pharm Pharmacol. 2004;56:3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- Zwijnenburg PJ, van der Poll T, Florquin S, van Deventer SJ, Roord JJ., and, van Furth AM. Experimental pneumococcal meningitis in mice: a model of intranasal infection. J Infect Dis. 2001;183:1143–1146. doi: 10.1086/319271. [DOI] [PubMed] [Google Scholar]
- Cardoso AL, Simões S, de Almeida LP, Pelisek J, Culmsee C, Wagner E.et al. (2007siRNA delivery by a transferrin-associated lipid-based vector: a non-viral strategy to mediate gene silencing J Gene Med 9170–183. [DOI] [PubMed] [Google Scholar]
- Cardoso AL, Simões S, de Almeida LP, Plesnila N, Pedroso de Lima MC, Wagner E.et al. (2008Tf-lipoplexes for neuronal siRNA delivery: a promising system to mediate gene silencing in the CNS J Control Release 132113–123. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE.et al. (2001Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats Stroke 322682–2688. [DOI] [PubMed] [Google Scholar]
- Kim JB, Lim CM, Yu YM., and, Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res. 2008;86:1125–1131. doi: 10.1002/jnr.21555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The zeta potential values of polymer/siRNA complexs.
Suppression of infarct formation by intranasal HMGB1 siRNA/e-PAM-R in the postischemic brain.
Suppression of infarct formation by intranasal or intravenous HMGB1 siRNA in the postischemic brain.