Abstract
The adaptive immune response to viral vectors reduces vector-mediated transgene expression from the brain. It is unknown, however, whether this loss is caused by functional downregulation of transgene expression or death of transduced cells. Herein, we demonstrate that during the elimination of transgene expression, the brain becomes infiltrated with CD4+ and CD8+ T cells and that these T cells are necessary for transgene elimination. Further, the loss of transgene-expressing brain cells fails to occur in the absence of IFNγ, perforin, and TNFα receptor. Two methods to induce severe immune suppression in immunized animals also fail to restitute transgene expression, demonstrating the irreversibility of this process. The need for cytotoxic molecules and the irreversibility of the reduction in transgene expression suggested to us that elimination of transduced cells is responsible for the loss of transgene expression. A new experimental paradigm that discriminates between downregulation of transgene expression and the elimination of transduced cells demonstrates that transduced cells are lost from the brain upon the induction of a specific antiviral immune response. We conclude that the anti-adenoviral immune response reduces transgene expression in the brain through loss of transduced cells.
Introduction
Immune responses against adenoviral vectors challenge the use of such vectors for gene therapy of the brain. Transgene expression in the absence of an antiadenoviral immune response has been shown to last up to 12 months.1,2 However, once a systemic antiadenoviral immune response is induced, transgene expression is eliminated from the brain within 30–60 days.3 The cellular and molecular mechanisms by which the immune response eliminates transgene expression from the central nervous system (CNS) remain poorly understood. Given the clinical use of first-generation adenoviral vectors for gene therapy of brain diseases,4,5,6,7,8,9,10,11,12 understanding the cellular and molecular basis of brain immune responses as well as their consequence for brain structure and function are critical elements of clinical gene therapy in neurology using viral vectors.
Especially, whether the immune response blocks transgene expression or actually kills transduced cells needs to be determined, as only functional inhibition of transgene expression would be transient and reversible. Immune-mediated killing of infected brain cells would represent an unacceptable consequence and potentially limit clinical gene therapy.13,14,15
Recently, we demonstrated that upon the systemic immunization against adenovirus, antiviral CD8+ T cells form close anatomical appositions, i.e., immunological synapses, with target adenovirally transduced astrocytes.16,17 During this process, transgene expression is lost from ~50% of infected cells, 85% of which are reactive astrocytes.18 Additionally, T-cell activation leads to the production and secretion of IFNγ, perforin, and TNFα.19,20,21
Much research has also been done on the mechanisms by which the immune system clears infection from the brains of animals infected with Lymphocytic Choriomeningitis Virus (LCMV),22 Sindbis Virus,23 measles virus, West Nile virus,24 Borna virus,25 Murine Cytomegalovirus,26 Theiler's Virus, Semliki Forest virus, mouse hepatitis virus (MHV),27 or herpes simplex virus type 1 (HSV1).28 Cytotoxic T cells, especially CD8+ T cells, IFNγ, perforin, and TNFα have all been shown to be necessary to various degrees to clear or control viral infections in the brain. However, bona fide killing of infected brain cells has only ever been demonstrated using in vitro paradigms, but never in vivo.29 It is thought that clearing of virus from the brain occurs without physical damage to the structure of the CNS.30
We now describe results using a novel reporter system to discriminate whether immune responses to adenoviral vectors are functional and reversible or cytotoxic. ROSA26 transgenic mice that encode within the ROSA locus a STOP sequence flanked with loxP sites upstream of the transcriptional start site of the lacZ gene. In this mouse strain, genomic β-galactosidase is only expressed after Cre-mediated excision of loxP-flanked STOP sequence.31 We used an adenoviral vector–expressing Cre recombinase to infect the brains of ROSA26 mice.32 Upon systemic immunization, functional downregulation of Ad-mediated transgene expression should result in loss of Cre expression, without loss of genomic β-galactosidase expression; loss of both Cre—expressed from the adenoviral vector—and β-galactosidase—expressed from the genome of infected cells—would be the result of killing of Ad-infected brain cells.30
Previous studies have shown that adeno-associated virus (AAV) and lentiviral vector–mediated expression of Cre in neurons of ROSA26 transgenic mice induce long-term expression of β-galactosidase from the recombined ROSA26 locus,33 thereby supporting the feasibility of our reporter system. In the liver, Wang et al.34 have used AAV-Cre to demonstrate that instability of newly formed AAV dsDNA is responsible for low rAAV transduction efficacy. These authors observed that upon administration of AAV-expressing Cre recombinase to ROSA26 transgenic mice, liver expression of recombined lacZ remains high and stable, while expression of AAV-encoded alkaline phosphatase is modest; although dsAAV are formed in most infected cells, they are rapidly lost. The instability of a large proportion of AAV dsDNA precludes their use in our paradigm which requires continued, comparable, stable, and long-term expression of genomic recombined lacZ and transgenes encoded by episomally-located Ad vectors' genomes.
Our results indicate that, upon systemic antiadenoviral immunization, CD8+ and CD4+ T cells, IFNγ, perforin, and TNFα are all necessary to reduce transgene expression from the brain. In addition, immune suppression fails to restitute transgene expression. Finally, both expression of Cre and β-galactosidase were reduced by >80% in our ROSA26 paradigm. We conclude that transgene expression from adenoviral vectors in the brain is eliminated by the killing of virally infected brain cells.
Results
Immune cells infiltrate the brains of Ad-transduced mice, establish contacts with transduced brain cells, and reduce transgene expression for up to 120 days
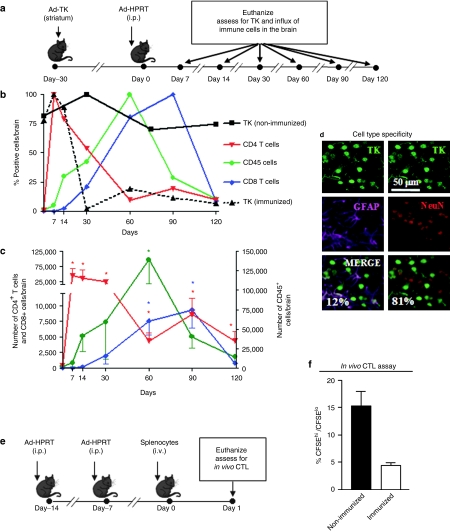
We examined the immune cell types infiltrating the brain parenchyma during the elimination of Ad-mediated transgene expression from the brain. Naive C57Bl/6 mice were injected in the right brain striatum with first-generation adenoviral vectors encoding herpes simplex type 1 thymidine kinase (Ad-TK) as a marker transgene. Thirty days later, animals were immunized systemically with a first-generation adenoviral vector encoding an unrelated transgene (Ad-HPRT) (Figure 1a). Expression of TK is reduced following immunization, remaining at very low levels for up to 120 days after immunization (Figure 1b). Figure 1b (%) and Figure 1c (total numbers) indicate that CD4+ T cells infiltrate the brain as early as 7 days post immunization and remain in the brain at significant levels up to 120 days after immunization. CD45+ cells, a marker representing all bone marrow–derived cells, infiltrate the mouse brain as early as 14 days post immunization, with peak levels obtained at 60 days after immunization and remain in the brain at significant levels up to 90 days post-immunization; at early time points, when CD4+ cell counts are high, the comparable numbers of CD45+ cell numbers obtained are most likely reflecting the influx of T cells; however, from 30 days onwards, as T-cell numbers decrease, CD45+ cells most likely indicate the presence of macrophages/microglia in the brain; to avoid any confusion in later experiments to detect intercellular interactions, we used the antibody F4/80 to label only macrophages/microglia. CD8+ T cells infiltrate the mouse brain later, with peak levels obtained at 90 days after immunization. To determine the cell type transduced, brain sections were double labeled with antibodies to the transgene HSV1-TK, and the neuronal nuclear marker (NeuN) or the astrocyte marker glial fibrillary acidic protein (GFAP; Figure 1d). More than 80% of transduced cells were neurons, while ~12% were astrocytes. The remaining cells were not characterized in detail. Analysis of in vivo cytotoxic T lymphocyte activity reveals that systemic immunization with Ad-HPRT generates systemic circulating cytotoxic T lymphocytes specific and cytotoxic for target cells presenting adenovirus epitopes (Figure 1e,f).
Figure 1.
Elimination of Ad-mediated transgene expression occurs concomitantly with a biphasic influx of anti-adenovirus-specific immune cells into the mouse brain. (a) Experimental design. C57BL/6 mice were injected with Ad-TK into the striatum. Thirty days later, mice were immunized i.p. with Ad-HPRT, or saline as a control (nonimmunized). Mice were euthanized at 7, 14, 30, 60, 90, and 120 days post immunization. Brain sections were assessed by immunohistochemistry with antibodies specific for TK, CD4+ T cells, CD8+ T cells, and CD45+ cells. The number of immunoreactive cells was quantified by quantitative stereology at each time point. (b) The dynamics of immune cell influx into the brain are shown as percentages of the maximum value of each immune cell population over time. The percentage of cells expressing TK in immunized and nonimmunized mice is also shown. (c) The total number of immune cells in the brain is shown at each time point; *P < 0.05 compared to all other time points, two-way ANOVA followed by Tukey's test. (d) Brain sections from immunized mice were double labeled with antibodies specific for TK (transgene expression, green) and neurons (red, NeuN), or astrocytes (magenta, GFAP). Immunofluorescence was analyzed by confocal microscopy colocalization of transgene expression and neurons or astrocytes. The percentage of double labeled cells is shown. (e) Experimental design of in vivo cytotoxic T lymphocyte assay is shown. 14 and 7 days before adoptive transfer, C57BL/6 mice were immunized with Ad-HPRT, or saline as a control (i.p.). Before adoptive transfer, splenocytes were labeled with either 2 µM CFSE (CFSEhi) or with 0.2 µM CFSE (CFSElo). CFSEhi splenocytes were also pulsed with adenovirus fiber peptide and heat-inactivated Ad-HPRT. A 1:1 mixture of each cell population was adoptively transferred into immunized mice. 18 hours later, animals were euthanized and splenocytes were assessed for CFSE fluorescence by flow cytometry. (f) The ratio of CFSEhi:CFSElo splenocytes is shown. A reduction in the population of CFSEhi indicates antigen-specific in vivo cytotoxic T lymphocyte activity.
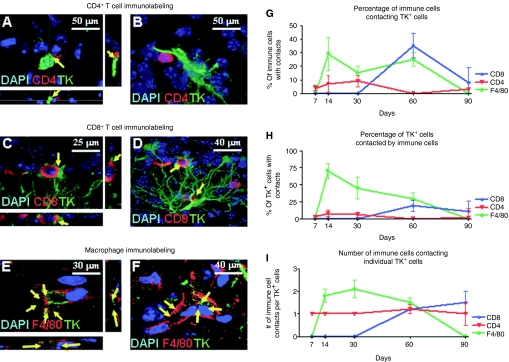
Confocal microscopy was used to quantitate the existence of close anatomical contacts between Ad-transduced cells and either CD4+ T cells (Figure 2a,b), CD8+ T cells (Figure 2 c,d), or F4/80+ macrophages/activated microglia (Figure 2 e,f). The detailed kinetics of immune cell contacts with transduced TK-expressing brain cells is shown in Figure 2g,h,i. Infiltrating T cells were not detected in nonimmunized animals (data not shown).
Figure 2.
Quantitative analysis of the interactions between Ad-transduced brain cells and CD4+ and CD8+ T cells and macrophages. Representative confocal images of brain sections from immunized mice depicting close anatomical contacts between (a,b) CD4+ T cells (CD4+, red) and Ad-infected cells (TK, green), (c,d) CD8+ T cells (CD8+, red) and Ad-infected cells (TK, green), (e,f) macrophages/activated microglia (F4/80, red) and Ad-infected cells (TK, green). In all images, nuclei are stained with DAPI (blue). Stereological quantification of CD4+ T cells, CD8+ T cells, and macrophages over time in the brains of Ad-immunized animals depicting (g) the percentage of each immune cell contacting TK-expressing cells, (h) the percentage of TK cells with immune cell contacts, and (i) the number of immune cell contacts per TK-expressing cell. TK, thymidine kinase; DAPI, 4',6-diamidino-2-phenylindole.
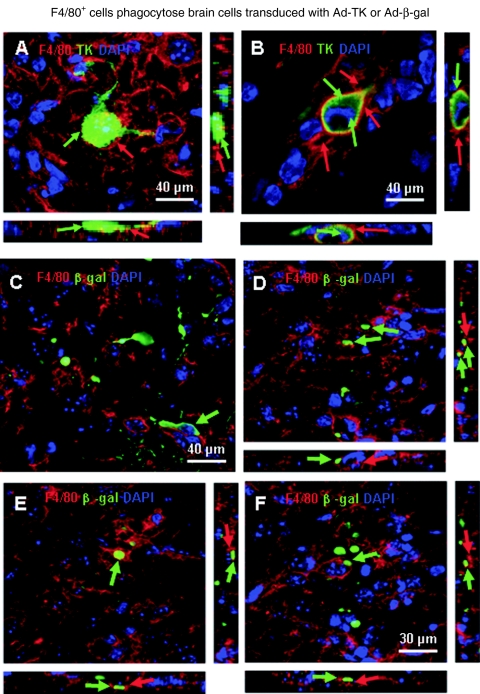
Figure 2g (percentage of immune cells contacting target cells) indicates that a maximum of 10% of CD4+ T cells contacts target cells, while >30% of CD8+ T cells or F4/80 macrophages/microglia do so; Figure 2h (percentage of transduced cells being contacted by immune cells) indicates that while only 8% of target cells are directly contacted by CD4+ T cells, 20% are contacted by CD8+ T cells, but almost 75% of target cells are in close anatomical contact with macrophages/activated microglia cells; Figure 2i (number of immune cells per target transduced cells) indicates that while 1–1.5 T cells contact target cells, 2 contacts of F4/80 microglia/macrophages per transduced cell were detected; these data suggest an important role for F4/80+ macrophages/activated microglia in the reduction of Ad-mediated transgene expression from the mouse brain, especially as phagocytosis of transduced cells was detected (Figure 3 a–f).
Figure 3.
F4/80+ cells phagocytose brain cells transduced with Ad-TK or Ad-β-gal. (a–b) Confocal microscopy analysis of immunized animals reveal macrophages (F4/80, red) which have phagocytosed an Ad-infected cell (TK, green). (a) A cell that displays strong TK immunoreactivity (green) surrounded by F4/80-immunoreactive processes from macrophages/microglia (red). (b) A final stage in which amorphous transgene immunoreactivity is still detected within F4/80-immunoreactive macrophages/microglia. Green arrows indicate transgene immunoreactivity and red arrows indicate the processes of F4/80-immunoreactive macrophages/microglia. (c–f) Confocal microscopy analysis of immunized animals in essentially identical experiments, but injected in the brain with a first-generation adenovirus expressing the transgene β-galactosidase—instead of herpes simplex virus type I thymidine kinase—reveals macrophages (F4/80, red) which have phagocytosed an Ad-infected cell (β-galactosidase, green). Note that F4/80 immunoreactive processes enclose various amounts of β-galactosidase, originally contained within the transduced brain cells. For all images, notice that the side views across the confocal stacks reveals that the transgene-immunoreactive material is indeed within macrophages, i.e., it is completely surrounded by F4/80 immunoreactive processes. Notice that macrophages are able to phagocytose adenoviral transduced cells independently of the transgene expressed by the viral vectors.
The adaptive immune response CD4+ and CD8+ T cells, and IFNγ, perforin, and TNFα, are all necessary for the elimination of transgene-expressing brain cells: results from knockout experimental models
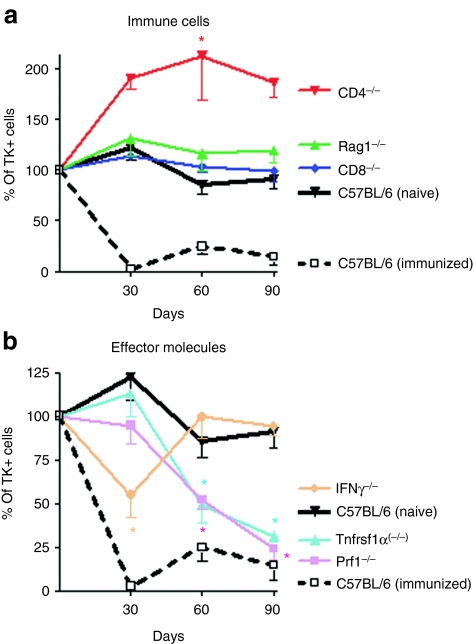
Transgene loss was absent in Rag1 knockout mice, which lack T and B cells, and mice lacking CD8+ T cells (Figure 4a). In CD4+ T cell knockout animals, transgene expression was increased, with respect to controls. These data indicate that both CD8+ T cells and CD4+ T cells play a role in the elimination of transduced cells, with CD4+ T cells playing the most prominent role.
Figure 4.
CD4+ and CD8+ T cells are required for elimination of Ad-mediated transgene expression from immunized mice; TNFα and perforin are required in the early stages and IFNγ is required throughout elimination of transgene expression. Wildtype C57BL/6 mice, or CD4−/−, CD8−/−, Rag1−/−, Prf−/−, Tnfrsf1α(−/−), or IFNγ−/− immune knockout mice were injected with Ad-TK into the striatum. Thirty days later, mice were immunized with Ad-HPRT, or saline as a control (i.p.). Mice were euthanized at 30, 60, and 90 days post immunization. Brain sections were assessed by immunohistochemistry with an antibody specific for TK. The number of immunoreactive cells was quantified by stereology at each time point. (a) The dynamics of TK immunoreactivity in the brains of transgenic immune cell knockout mouse strains, i.e., CD4−/−, CD8−/−, Rag1−/−, which are displayed as the relationship of TK immunoreactive cells at each time point with respect to their levels at the day of immunization; *P < 0.05 compared to all other time points, one-way ANOVA followed by Tukey's test. (b) The dynamics of TK immunoreactivity in the brains of transgenic mouse strains with knockouts of specific effector molecules Prf−/−, Tnfrsf1α(−/−), or IFNγ−/−; *P < 0.05 compared to all other time points, two-way ANOVA followed by Tukey's test.
Stereological quantification of TK transgene expression in immunized mice lacking perforin and TNFα receptor expression revealed no transgene loss at 30 days after immunization; however, at 60 and 90 days after immunization, loss of transgene-expressing cells was seen in both knockout animal strains (Figure 4b). Stereological quantification of TK transgene expression in immunized IFNγ knockout mice revealed an inhibition of transgene loss at all time points studied. These data suggest that perforin and TNFα play a role in the early (30 days) phase of transgene elimination, while IFNγ is necessary at all time points examined (Figure b). All mice, except for Rag1(−/−) which lack T and B cells, showed increases in adenovirus-neutralizing titers (1:8 to 1:128; data not shown) following systemic immunization.
Loss of transgene expression is irreversible
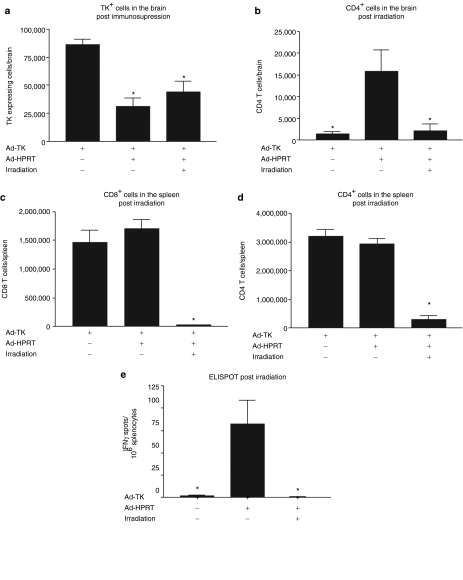
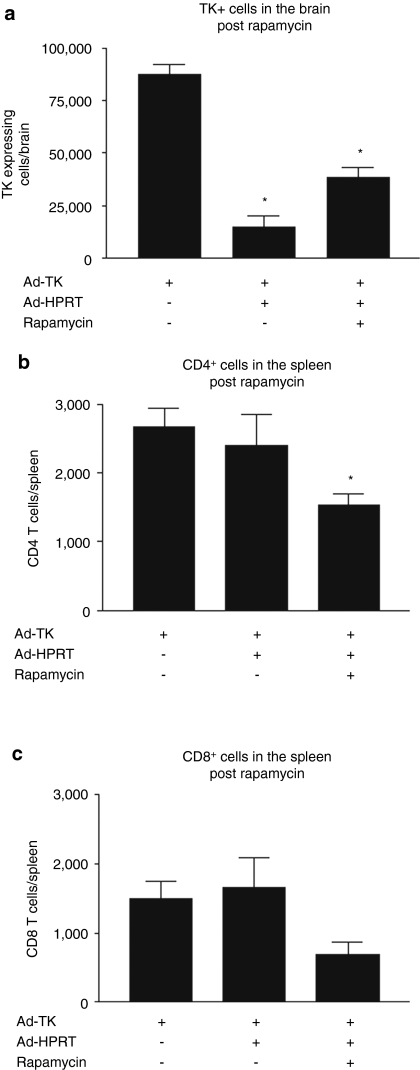
To assess whether the constant presence of immune cells is required to suppress Ad-mediated transgene expression in the mouse brain, we assessed transgene expression in the brains of immunized animals following immunosupression by either irradiation (Figure 5a–e) or treatment with rapamycin (Figure 6a–c). Following irradiation, TK expression did not increase (Figure 5a). Immunohistochemistry analysis of CD4+ T cells in the brains (Figure 5b) or flow cytometry analysis of CD4+ T cells and CD8+ T cells in the spleens (Figure 5c–d) confirms that irradiation dramatically reduces the levels of immune cells. Irradiation also caused a sharp reduction in the frequency of adenovirus-specific IFNγ-secreting T lymphocyte precursors in the mouse spleen (Figure 5e). The decrease in T-cell function was less following treatment with rapamycin, but TK expression did not recover back to control levels (Figure 6a–c). These results demonstrate that the constant presence of T cells is not required to suppress Ad-mediated transgene expression from the brain, thus suggesting that elimination of transgene expression is irreversible. The irreversibility of the loss of TK transgene expression following immune suppression strongly suggests the elimination of transduced cells from the brain.
Figure 5.
Elimination of Ad-mediated transgene expression upon immunization is not reversed by irradiation. C57BL/6 mice were injected with Ad-TK in the brain and 30 days later immunized i.p. with either Ad-HPRT or saline as control. 30 days after immunization, mice were immunosuppressed using irradiation. Mice were euthanized 5 days post immunosupression for further analysis. (a) Stereological quantification of TK immunoreactive cells in the mouse brain following irradiation treatment. *P < 0.05 compared to nonimmunized mice, one-way ANOVA followed by Tukey's test. (b) Stereological quantification of CD4+ immunoreactive cells in the mouse brain following irradiation. *P < 0.05 compared to immunized mice, one-way ANOVA followed by Tukey's test. Flow cytometry analysis reveals that (c) CD8+ T cells and (d) CD4+ T cells are depleted from the spleens of irradiated mice; *P < 0.05 compared to nonimmunized mice, one-way ANOVA followed by Tukey's test. (e) ELISPOT analysis reveals that the frequency of adenovirus-specific IFNγ-secreting T lymphocyte precursors is dramatically reduced in the spleen of the irradiated mice; *P < 0.05 compared to immunized mice, one-way ANOVA followed by Tukey's test.
Figure 6.
Elimination of Ad-mediated transgene expression upon immunization is not reversed by rapamycin. C57BL/6 mice were injected with Ad-TK in the brain and 30 days later, immunized i.p. with either Ad-HPRT or saline as control. 30 days after immunization, mice were immunosuppressed by treatment with rapamycin. Mice were euthanized 5 days post immunosupression for further analysis. (a) Stereological quantification of TK immunoreactive cells in the mouse brain following rapamycin treatment. *P < 0.05 compared to nonimmunized mice, one-way ANOVA followed by Tukey's test. Flow cytometry analysis reveals that (b) CD4+ T cells and (c) CD8+ T cells are depleted from the spleens of rapamycin-treated mice; *P < 0.05 compared to nonimmunized mice, one-way ANOVA followed by Tukey's test.
Elimination of Ad-mediated transgene expression occurs mainly through the loss of transduced cells
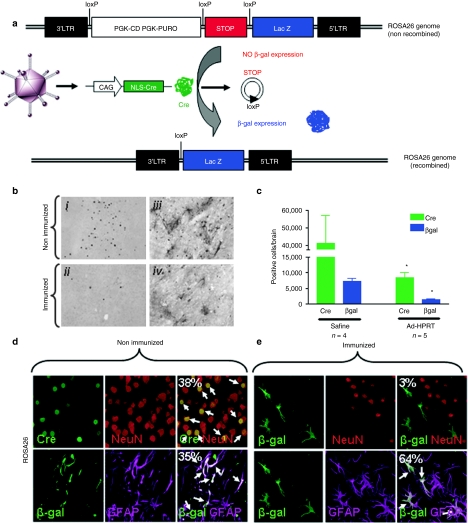
We next tested the hypothesis that elimination of transgene expression results from the elimination of transduced cells, rather than inhibition of vector-encoded transgene expression. To do so, we developed a novel method in ROSA26 mice. A first-generation Ad-vector expressing Cre recombinase (Ad-CAG-Cre) was injected into the brains of transgenic ROSA26 mice (B6;129Gt(ROSA) 26Sortm1/Sho/J). ROSA26 mice harbor a genomic lacZ gene with a STOP sequence flanked by loxP sites upstream of the lacZ start codon. This STOP sequence prevents translation of the lacZ gene. Cre recombinase expression (provided in trans by Ad-CAG-Cre) excises the STOP sequence, thus allowing constitutive and permanent β-galactosidase expression from the genome of transduced cells, while expression of Cre recombinase can be used to monitor transgene expression from the viral vector (Figure 7a). Upon immunization, changes in the expression of Cre recombinase (vector genome) will indicate changes in the expression from the viral vector; changes in the expression of β-galactosidase (ROSA26 genome) will indicate changes in the number of transduced cells. In this experimental paradigm, following antiadenoviral immunization, a purely functional inhibition of transgene expression will show a reduction of Cre recombinase, but no reduction in the level of β-galactosidase; a reduction in the expression of both Cre recombinase and β-galactosidase immunoreactive cells will indicate the loss of transduced cells, i.e., cell death.
Figure 7.
Elimination of Ad-mediated transgene expression occurs primarily through loss of transduced cells. (a) Illustration of novel reporter system to discriminate between loss of transduced cells and downregulation of transgene expression without loss of Ad-transduced brain cells. The ROSA26 transgenic mouse strain contains a STOP sequence flanked with loxP sites located upstream of the transcriptional start site of the lacZ gene. In these animals, genomic β-galactosidase is only expressed after Cre-recombinase-mediated excision of loxP-flanked STOP sequence (Cre-recombinase is provided in trans from a first-generation adenoviral vector). Downregulation of Ad-mediated transgene expression should result in loss of Cre-recombinase expression without loss of genomic β-galactosidase expression, whereas loss of both Cre-recombinase and β-galactosidase expression would be the result of loss of Ad-infected brain cells. (b) ROSA26 mice were injected in the brain with Ad-Cre and immunized systemically with Ad-HPRT, or saline as a control, 7 days later. ROSA26 mice were euthanized 35 days later and brain sections were analyzed by immunohistochemistry with antibodies specific for Cre-recombinase and β-galactosidase. Representative images illustrate nuclear Cre-recombinase expression or β-galactosidase expression. (c) Quantitative stereological analysis of Cre-recombinase and β-galactosidase immunoreactive cells in the brains of nonimmunized and immunized ROSA26 transgenic mice is shown; *P < 0.05 compared to nonimmunized mice, two-way ANOVA followed by Tukey's test. Brain sections from (d) nonimmunized and (e) immunized mice were double labeled with antibodies specific for Cre-recombinase (transgene expression, green) and neurons (red, NeuN), or β-galactosidase (transgene-mediated genomic expression, green) and astrocytes (magenta, GFAP). Immunofluorescence was analyzed by confocal microscopy colocalization of transgene expression and neurons or astrocytes. The percentage of double labeled cells is shown.
Figure 7b displays representative immunohistochemistry images of Cre recombinase and β-galactosidase from either immunized or nonimmunized ROSA26 mice injected with Ad-CAG-Cre in the brain. Stereological quantification of Cre recombinase and β-galactosidase immunoreactive cells in the brains of ROSA26 mice reveal a significant reduction in the number of both Cre recombinase and β-galactosidase immunoreactive cells. These data demonstrate that immunization eliminates adenovirally transduced cells from the brain (Figure 7c). To assess whether Ad-transduced astrocytes and/or neurons are killed following immunization, we performed double labeling confocal microscopy with a neuronal marker (NeuN) or an astrocyte marker (GFAP) and Cre recombinase or β-galactosidase expression in nonimmunized (Figure 7d) or immunized ROSA26 mice (Figure 7e). Even though both populations of transduced cells are significantly reduced, in the immunized animals, a higher percentage of cells that survived the immune attack are astrocytes.
Discussion
The elucidation of the cellular and molecular mechanisms by which the immune response clears viral gene expression from the brain is crucial to the safety and efficacy of clinical trials in neurological gene therapy. If host defense mechanisms simply abolish transgene expression, the effectiveness of gene therapy will be reduced. However, if the immune response both suppresses transgene expression and eliminates transduced brain cells, the symptomatology of patients suffering from chronic neurodegenerative disorders that involve neuronal loss would worsen. Elucidating how the immune system regulates transgene expression in the CNS is therefore of central importance to clinical neurological gene therapy,1,13,15,30,35,36 especially in view of the continuing use of first-generation adenoviral vectors for the treatment of brain diseases, specifically brain tumors.4,5,6,7,8,9,11,12,37
Utilizing a well established mouse model of brain immune responses to adenoviruses, we demonstrate that T cells mediate elimination of transgene expression from the brain through what both cytotoxic and noncytotoxic mechanisms.2,3,16,17,18 Kinetics of infiltration of immune cells into the brain produced a number of interesting and unexpected observations. The first cells to infiltrate the brain were CD4+ cells, which peaked at 7 days after immunization, remained high for the first month, and then slowly decreased to basal levels during more than 4 months. To our surprise, CD8+ cells entered the brain much later, with very low levels of CD8+ T cells found in the brain at 30 days after immunization, peaking only much later, at 2–3 months after immunization, and then returning to basal levels by 4 months. Macrophages/monocytes increased more slowly than CD4+ T cells and achieved a clear peak in the CNS at 2 months after immunization. This indicates that at the peak of the reduction seen in transduced cells, i.e., at 30 days after immunization, CD4+ T cells are the most abundant in the brain, followed by macrophages, and CD8+ T cells, a distant third. If numbers of T cells in the brain were to determine function, then CD4+ T cells would be the ones mostly responsible for the decrease of transduced cells. However, when we carefully examined the capacity of T cells to establish morphological contacts with transduced target cells in the brain, we found that both CD4+ and CD8+ T cells did indeed establish contacts with transduced cells, but that, overall, these contacts were very few. Specifically, less than 10% of all target cells are contacted by either CD4+ or CD8+ T cells at the peak of reduction in the number of transgene expression, though up to 75% of transduced cells are contacted by macrophages. Thus, in spite of the known importance of immunological synapses and their role in mediating communication between effector T cells and target cells in the brain,3,16,18,38,39,40 the low number of contacts detected between T cells and target cells would appear to argue that most of the elimination of transduced cells would indeed be exerted by T cells in an indirect manner likely through cytokine secretion. Furthermore, the contacts detected between macrophages and transduced cells indicated clearly that macrophages were able to phagocytose transduced target cells. Although our experiments do not allow us to determine whether macrophages do so after the fact that the target cell has been damaged, or do the damage themselves, we demonstrate that macrophages can phagocytose transduced cells, a fact we show for cells transduced with two different viral vectors and two different transgenes.
Our data conclusively show that CD4+ and CD8+ T cells are required to eliminate transgene expression and that expression of both IFNγ and the early expression of perforin and TNFα are also necessary for transgene elimination. Our results, taken from studies in several lines of transgenic animals, devoid of specific immune cell populations and effector molecules, show that there are several specific pathways at work in the elimination of adenoviral-mediated transgene expression from the CNS. In contrast, the need for the adaptive immune system and specific CD4+ and CD8+ T cells was demonstrated by persistent transgene expression in immunodeficient Rag1(−/−) mice as well as in CD4(−/−), and CD8(−/−) mice. Elimination of expression of the key T cell cytokine effectors using IFNγ(−/−) Prf(−/−) and Tnfrsf1α(−/−) mice clearly implicates these cytokines in transgene expression elimination.30,35,41 Importantly, we demonstrate that neurons represent ~80% of transduced cells, and astrocytes ~10%. As the number of transduced cells falls to below 10% of nonimmunized levels, these data strongly indicate that the majority of transduced neurons is indeed being lost.
The largest decrease in transgene expression is seen between 14 and 30 days following immunization, at which time mainly CD4+ lymphocytes have infiltrated the brain. These data suggest that CD4+ T cells initiate the process of transgene elimination from the brain, a conclusion supported by results from animals deficient in CD4+ T cells. The influx of CD4+ T cells occurs simultaneously with an increase in F4/80+ macrophages/microglia. CD8+ T cells infiltrated the brain at later time points, suggesting that they are responsible for the late phase of transgene elimination.
Immunosuppression experiments demonstrate that persistence of immune cells in the CNS is not required to inhibit viral gene expression, as the reduction of transgene expression was irreversible following either irradiation or rapamycin treatment.
The challenge of determining whether brain cells are being killed or whether transgene expression from vectors is being inhibited led us to develop a novel method to detect death of brain cells transduced by viral vectors. This method demonstrates that a majority of transduced cells (>70%) is effectively eliminated by the immune system (i.e., loss of expression of the transgene encoded by the vector and the gene encoded within the host cell's genome that marks the cell as having been infected by a recombinant adenoviral vector expressing Cre recombinase). A small population of cells remained unaffected by the immune response.
Further evidence of cytotoxicity comes from phagocytosis of transduced cells by F4/80+ labeled macrophages/microglia. Phagocytosis represents a late stage in the process of transduced cell death. The exact mechanism by which brain cells actually die remains to be determined. Detection of apoptosis using either immunostaining to detect activated caspase 3 or staining for apoptosis via Terminal dUTP nick end labeling failed to label transduced cells (results not shown).
In summary, our results provide strong evidence that elimination of virally transduced brain cells occurs as a result of the systemic immunization against adenoviral vectors. Our experiments demonstrate that CD4+ and CD8+ T cells are necessary for transgene elimination and that their effects are mediated, at least in part, by IFNγ, perforin, and TNFα. Our data demonstrating that the immune system can eliminate adenovirally transduced brain cells indicates that this phenomenon will have to be carefully studied and monitored during future clinical trial using adenoviral vectors, or potentially other viral vectors.
Materials and Methods
Adenoviral vectors. Adenoviruses used in this study were first-generation E1/E3-deleted recombinant adenovirus vectors based on adenovirus type 5. The construction of Ad-TK (expressing HSV1-TK), Ad-β-gal (expressing β-galactosidase), and Ad-HPRT (expressing hypoxanthine-guanine phosphoribosyl-transferase) has been described in detail elsewhere.42,43,44 In both vectors, the transgenes are under the major immediate early human cytomegalovirus promoter (hCMV). All viruses tested negative for the presence of replication competent adenoviral vectors (RCA) and lipopolisacharide (LPS) as described before.45 Ad-CAG-Cre (Ad-Cre) previously described was a generous gift from Dr Saito.31
Animals, surgical procedures, viruses. C57BL/6, B6;129Gt(ROSA)26Sortm1Sho/J (ROSA26 mice) and transgenic knockout mice Rag1(−/−), CD4(−/−), CD8(−/−), IFNγ(−/−), Tnfrsf1α(−/−), and Prf1(−/−), all on C57BL/6 background, were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in specific pathogen-free conditions in the Department of Comparative Medicine of Cedars-Sinai Medical Center. All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by Cedars-Sinai Medical Center Institutional Animal Care and Use Committee (CSMC IACUC). Mice were anesthetized using ketamine (75 mg/kg) and medetomidine (0.5 mg/kg) and placed in a stereotactic apparatus modified for mice. Animals were injected into the right striatum (stereotactic coordinates: 0.05 mm anterior, 0.22 mm lateral from bregma and 0.32 mm ventral from the brain's surface) with 1 × 107 infectious units of adenoviral vector within 0.5 µl of volume, using a 5 µl Hamilton syringe. Each injection was performed over a period of 3 minutes, with the needle being left in place for an additional 5 minutes before withdrawal. Thirty days after viral vector injection into the brain, animals were immunized systemically (i.p. injection) with 3.28 × 108 infectious units (iu) of Ad-HPRT in 100 µl of saline solution. At experimental endpoints, mice were anesthetized via i.p. injection of an overdose of ketamine (50 mg/kg) and xylazine (50 mg/kg) and transcardially perfused with oxygenated Tyrode's solution alone (for brains to be used for molecular studies) or perfused-fixed with oxygenated Tyrode's solution followed by 4% paraformaldehyde in phosphate buffered saline (PBS). Brain tissue was removed and postfixed for 48 hours before immunohistochemistry and further analysis. Unless indicated, experiments were performed on groups of 3–5 animals per group.
Immunohistochemistry and immunofluorescence. Sections of the striatum (50 µm) were cut into six series with a vibratome and analyzed by immunohistochemistry with antibodies specific for either transgene expression (TK) or specific immune cells as described previously.43 Sections were then incubated for 4 hours with biotin-conjugated secondary antibodies, followed by 4-hour additional incubation with avidin-biotin complex (Vector Laboratories, Ontario, Canada). Nickel-enhanced 0.02% 3,3′-diaminobenzidine in sodium acetate was used as the chromogen. Finally, the sections were mounted onto gelatin-coated slides, dehydrated, and cover slipped using Di-n-butylPhathalate in Xylene mounting media for histology (Sigma-Aldrich, St. Louis, MO). For immunofluorescence, 50-µm sections were treated with 0.5% citrate buffer (70 °C, with constant shaking) for 30 minutes to increase antigen retrieval and penetration of the antibodies into the tissues. Nonspecific Fc binding sites were blocked with 10% horse serum, and sections were incubated for 48 hours (room temperature, constant shaking) with primary antibody diluted in PBS containing 1% horse serum, 0.5% Triton X-100, and 0.1% sodium azide. Sections were incubated for 4 hours in labeled secondary antibody and after PBS washes, sections were incubated with 4',6-diamidino-2-phenylindole (DAPI) solution (1:1,000) in 1× PBS for 30 minutes. After washing, sections were incubated with DAPI solution for 30 minutes to label the nuclei. Sections were washed, mounted using Prolong antifade reagent (Invitrogen; Carlsbad, California), and examined using confocal microscopy (Leica DMIRE2, Wetzlar, Germany). Primary antibodies included custom-made rabbit polyclonal anti-TK (1:10,000)46 and anti-β-gal (1:1,000)47, rabbit anti-Cre recombinase (1:10,000; Novagen-EMD, Gibbstown, NJ), rat anti-mouse CD8α (1:750; clone YTS169.4, Serotec, Kidlington, UK), rat anti-mouse CD4, (1:750; clone kt174, Serotec), rat anti-mouse CD45, (1:1,000; clone YW62.3, Serotec), and rat anti-mouse F4/80 (1:100, clone Cl:A3-1; Serotec). Secondary antibodies included biotin-conjugated goat anti-rabbit IgG (1:800; DAKO, Carpinteria, CA), Texas Red–conjugated goat anti-rabbit (1:1,000) and fluorescein (FITC)-conjugated goat anti-rat IgG (1:1,000), both from Jackson ImmunoResearch Laboratories (West Grove, PA), and Alexa 488-conjugated goat anti-rabbit (1:1,000; Molecular Probes, Carlsbad, CA).
Quantification and stereological analysis. The optical fractionator protocol used for unbiased stereological cell estimation in the striatum of mice injected with Ad-TK was as described earlier. Striatum and external capsule were defined according to the Mouse Brain Atlas.48 Quantification of DAB or fluorescent-labeled cells in the striatum was performed by the examination of five coronal sections in series from each animal. Analysis was done by stereological methods using a computer-assisted image analysis system (Stereoinvestigator software version 5.0, Microbrightfield, Vermont) with a Zeiss Axioplan 2 microscope controlled by a Ludl electronic MAC 5000 XY stage control (Ludl Electronics Products, Hawthorne, NY) and Axioplan Z-axis control (Carl Zeiss, Thornwood, NY) connected to a digital camera. The region of interest was traced using the 1.25× objective. The number of cells was measured in 200 × 200 µm fields that covered the surface of the analyzed region. Labeled cells were counted using the 20× objective with 55 counting frames throughout the delineated area of the striatum in each section via the Optical Fractionator. The thickness of each counting frame was 50 µm and positive cells were counted only when found to be in the limit of the square. Data were expressed as an absolute number of positive cells in each anatomical region analyzed, as described previously.
In vivo cytotoxic T lymphocyte assay. Fourteen and seven days prior to receiving transfer of splenocytes, recipient C57BL/6 mice were immunized with either saline or 3 × 108 iu of Ad-HPRT. Splenocyte donor mice were perfused with oxygenated Tyrode's solution, splenocytes were isolated and cultured in Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum. Splenocytes were pulsed overnight with 4 µg of fiber peptide (VGNKNNLGL) and 1 × 109 iu of heat-inactivated Ad-HPRT (multiplicity of infection = 10). Pulsed splenocytes were labeled with 2 µM carboxyfluorescein diacetate, succinimidyl ester (CFSE) (CFSEhi) and control nonpulsed splenocytes were incubated with 0.2 µM carboxyfluorescenin diacetate, succinimidyl ester (CSFE) (CFSElo). CFSEhi and CFSElo cells were mixed at a 1:1 ratio and 2 × 108 of total splenocytes was injected into immunized or nonimmunized mice by tail vein injection. Eighteen hours after transfer, recipient mice were perfused with oxygenated Tyrode's solution and splenocytes were isolated and analyzed for the presence of CFSEhi and CFSElo populations by flow cytometry.49 Animals that exhibit cytolytic T cells specific for adenovirus will display a reduction in the population of CFSEhi target cells, which had been pulsed with adenovirus epitopes.
Quantification of cell contacts. Contacts between immune cells (CD4+, CD8+, or F4/80-immunoreactive cells) and TK-immunoreactive cells were quantified in mouse brains by confocal microscopy. The number of contacts was defined using a Leica DMIRE2 microscope with the 63× oil objective and Leica Confocal Software (Solms, Germany). A series range for each section was determined by setting an upper and lower threshold using the Z/Y Position for Spatial Image Series setting, and confocal microscope settings were established and maintained by Leica and local technicians for optimal resolution. 0.5-µm thick confocal layers of each section were made by choosing a number of sections through each layer. In each of the sections analyzed, regions for the quantification of cell contacts were selected based on areas where immune cells and TK-expressing cells overlapped anatomically. Contacts were defined as areas where colocalization of both markers occurs between two cells in single 0.5-µm thick optical sections; most contacts were present over at least two to three 0.5-µm optical sections in the z-axis. Contacts are also illustrated as they appear throughout the stack of sections, e.g., side-views are shown in figures illustrating the cell to cell contacts. In each single 0.5-µm layer, the total number of immune cells (CD4+ or CD8+), TK-expressing cells, and contacts were counted with Leica Confocal Software (Solms, Germany). The results were expressed as (i) the percentage of immune cells contacting TK-immunoreactive cells, (ii) the percentage of TK-immunoreactive cells that had contacts, and (iii) the mean number of immune cells that contact each TK cell.
Immunosuppression. C57BL/6 mice were injected stereotactically with 1 × 107 iu of Ad-TK. Mice were immunized with 3 × 108 iu of Ad-HPRT (i.p.) 30 days after CNS injection. Mice were then immunosuppressed using either irradiation or by treatment with rapamycin. For irradiation treatment, mice were placed in an irradiation chamber and exposed to 800 rads over the course of 8 minutes (100 rads/min; lethal irradiation). Mice were euthanized 5 days after irradiation, as they cannot survive longer following lethal irradiation; spleens were kept to quantitate the levels of CD4+ and CD8+ T cells by flow cytometry and to assess the frequency of adenovirus-specific IFNγ-secreting T lymphocyte precursors by ELISPOT. Brains were perfused-fixed using Tyrode's and 4% paraformaldehyde and immunohistochemistry was performed using either rabbit polyclonal TK antibody for determining transgene expression or rat anti-CD4+ antibody to determine the levels of CD4+ T-cell infiltration into the brain. For immunosupression by rapamycin, mice were treated with 3 mg/kg rapamycin (Sigma-Aldrich) dissolved in 2% carboxymethylcellulose (Sigma-Aldrich) every other day for 25 days. All animals were perfused-fixed with 4% paraformaldehyde 90 days after CNS injection and processed for immunohistochemistry as previously described.2
Flow cytometry analysis. Mice were perfused with Tyrode's solution and brain tissue was removed. The area around the injection site was dissected, and tissue was then diced with a razor blade before homogenizing in Roswell Park Memorial Institute medium (Gibco; Carlsbad, CA) using a glass Tenbroeck homogenizer. CNS mononuclear cells were purified from brain tissue by centrifugation through a Percoll gradient (Sigma-Aldrich). Cells were counted and labeled with antibodies for analysis by flow cytometry. Briefly, cells were resuspended at 5 × 105 cells/ml in 1 ml of staining buffer (0.1 mol/l PBS with 1% FBS, 0.1% sodium azide). Cells were centrifuged and the supernatant was discarded. The cells were resuspended in 100 µl staining buffer containing the antibodies described below and incubated for 30 minutes at 4 °C. After this incubation, the samples were washed in 1 ml staining buffer and analyzed by flow cytometry. Cells were stained with CD3-PE, CD4-PerCP, and CD8a-FITC (BD Pharmingen; San Jose, CA) to identify CD4+ and CD8+ T cells. Analysis of cell population was performed using Summit software (Cytomation; Fort Collins, CO).
ELISPOT. The frequency of IFNγ-secreting T lymphocyte precursors specific for adenovirus was assessed as described previously.50 Heat-inactivated adenovirus was used as a stimuli.
Statistical analysis. Data were analyzed using one-way analysis of variance followed by Tukey's test. Transduction efficiency and recombination in ROSA26 cells was analyzed using T-test. The results were expressed as mean values ± SEM. For all tests used, P value <0.05 was considered the cutoff for significance.
Acknowledgments
Work in the GTRI is funded by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant 1UO1 NS052465.01. The brain tumor program in our institute is funded by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants 1RO1-NS 057711 and 1R21-NSO54143 (to M.G.C.) and National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants 1 RO1 NS 054193 and RO1 NS 42893 (to P.R.L). The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics (to P.R.L. and M.G.C., respectively). The Drown Foundation, the Linda Tallen & David Paul Kane Foundation Annual Fellowship, and the Board of Governors at CSMC also provided support. We wish to thank S. Stohlman (Department of Neurosciences, Cleveland Clinic) for providing the IFNγ (−/−) mice and Izumu Saito (Institute of Medical Science, University of Tokyo) for the kind donation of Ad-CAG-Cre. We are very grateful to the Board of Governors at Cedars-Sinai Medical Center for their creation and support of the GTRI.
REFERENCES
- Thomas CE, Schiedner G, Kochanek S, Castro MG., and, Löwenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Jimenez-Dalmaroni M, Kroeger KM, Puntel M, Rapaport AJ, Larocque D.et al. (2007One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications Mol Ther 152154–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M.et al. (2006In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain J Exp Med 2032095–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Määttä AM, Samaranayake H, Pikkarainen J, Wirth T., and, Ylä-Herttuala S. Adenovirus mediated herpes simplex virus-thymidine kinase/ganciclovir gene therapy for resectable malignant glioma. Curr Gene Ther. 2009;9:356–367. doi: 10.2174/156652309789753365. [DOI] [PubMed] [Google Scholar]
- Wirth T, Samaranayake H, Pikkarainen J, Määttä AM., and, Ylä-Herttuala S. Clinical trials for glioblastoma multiforme using adenoviral vectors. Curr Opin Mol Ther. 2009;11:485–492. [PubMed] [Google Scholar]
- Pulkkanen KJ., and, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- Asadi-Moghaddam K., and, Chiocca EA. Gene- and viral-based therapies for brain tumors. Neurotherapeutics. 2009;6:547–557. doi: 10.1016/j.nurt.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F.et al. (2004A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting Mol Ther 10958–966. [DOI] [PubMed] [Google Scholar]
- Jiang H, Gomez-Manzano C, Lang FF, Alemany R., and, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–427. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA.et al. (2006Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy Blood 1083321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S.et al. (2003Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results J Clin Oncol 212508–2518. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR., and, Castro MG. Uncertainty in the translation of preclinical experiments to clinical trials. Why do most phase III clinical trials fail. Curr Gene Ther. 2009;9:368–374. doi: 10.2174/156652309789753392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, MacLaren RE., and, Charlton HM. Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. J Neurosci. 1996;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Abordo-Adesida E, Maleniak TC,, Stone D, Gerdes G., and, Lowenstein PR.2000Gene transfer into rat brain using adenoviral vectors Gerfen JN, McKay R, Rogawski MA,, Sibley DR., and, Skolnick P.ed). Current Protocols in Neuroscience vol. 4.23.1–4.23.40Wiley: New York; pp. 24.23.21–24.23.40. [Google Scholar]
- Byrnes AP, Rusby JE, Wood MJ., and, Charlton HM. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66:1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Barcia C, Sanderson NS, Barrett RJ, Wawrowsky K, Kroeger KM, Puntel M.et al. (2008T cells' immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury PLoS ONE 3e2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C.et al. (2005CD8 T cells are directly involved in silencing adenoviral vector-encoded transgene expression in the rat brain activated by systemic immune response. Society for Neuroscience Annual Meeting - Abstract Mol Ther 11S366–S366. [Google Scholar]
- Barcia C, Gerdes C, Xiong WD, Thomas CE, Liu C, Kroeger KM.et al. (2006Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells Neuron Glia Biol 2309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Lillemeier BF, Kuhns MS, Chen DS., and, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- O'Keefe JP., and, Gajewski TF. Cutting edge: cytotoxic granule polarization and cytolysis can occur without central supramolecular activation cluster formation in CD8+ effector T cells. J Immunol. 2005;175:5581–5585. doi: 10.4049/jimmunol.175.9.5581. [DOI] [PubMed] [Google Scholar]
- Shi L, Keefe D, Durand E, Feng H, Zhang D., and, Lieberman J. Granzyme B binds to target cells mostly by charge and must be added at the same time as perforin to trigger apoptosis. J Immunol. 2005;174:5456–5461. doi: 10.4049/jimmunol.174.9.5456. [DOI] [PubMed] [Google Scholar]
- McGavern DB, Homann D., and, Oldstone MB. T cells in the central nervous system: the delicate balance between viral clearance and disease. J Infect Dis. 2002;186 Suppl 2:S145–S151. doi: 10.1086/344264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol Res. 2010;47:123–133. doi: 10.1007/s12026-009-8143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Mehlhop E, Oliphant T., and, Samuel MA. The host immunologic response to West Nile encephalitis virus. Front Biosci. 2009;14:3024–3034. doi: 10.2741/3432. [DOI] [PubMed] [Google Scholar]
- Hausmann J, Pagenstecher A, Baur K, Richter K, Rziha HJ., and, Staeheli P. CD8 T cells require gamma interferon to clear borna disease virus from the brain and prevent immune system-mediated neuronal damage. J Virol. 2005;79:13509–13518. doi: 10.1128/JVI.79.21.13509-13518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantug GR, Cekinovic D, Bradford R, Koontz T, Jonjic S., and, Britt WJ. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J Immunol. 2008;181:2111–2123. doi: 10.4049/jimmunol.181.3.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Chen BP, Liu MT., and, Lane TE. Mouse hepatitis virus infection of the central nervous system: chemokine-mediated regulation of host defense and disease. Viral Immunol. 2002;15:261–272. doi: 10.1089/08828240260066215. [DOI] [PubMed] [Google Scholar]
- Conrady CD, Drevets DA., and, Carr DJ. Herpes simplex type I (HSV-1) infection of the nervous system: is an immune response a good thing. J Neuroimmunol. 2010;220:1–9. doi: 10.1016/j.jneuroim.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall GF, Mucke L., and, Oldstone MB. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol. 2003;3:493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegae Y.et al. (1995Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase Nucleic Acids Res 233816–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG., and, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed BY, Chakravarthy S, Eggers R, Hermens WT, Zhang JY, Niclou SP.et al. (2004Efficient delivery of Cre-recombinase to neurons in vivo and stable transduction of neurons using adeno-associated and lentiviral vectors BMC Neurosci 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie J, Lu H, Chen L, Hauck B, Samulski RJ.et al. (2007Existence of transient functional double-stranded DNA intermediates during recombinant AAV transduction Proc Natl Acad Sci USA 10413104–13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Parra B, Hinton DR, Ramakrishna C, Dowdell KC., and, Stohlman SA. Perforin and gamma interferon-mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J Virol. 2004;78:1739–1750. doi: 10.1128/JVI.78.4.1739-1750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JS., and, Stohlman SA. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990;64:4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, McCormick F, Lang FF, Gomez-Manzano C., and, Fueyo J. Oncolytic adenoviruses as antiglioma agents. Expert Rev Anticancer Ther. 2006;6:697–708. doi: 10.1586/14737140.6.5.697. [DOI] [PubMed] [Google Scholar]
- Barcia C, Wawrowsky K, Barrett RJ, Liu C, Castro MG., and, Lowenstein PR. In vivo polarization of IFN-gamma at Kupfer and non-Kupfer immunological synapses during the clearance of virally infected brain cells. J Immunol. 2008;180:1344–1352. doi: 10.4049/jimmunol.180.3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sanderson NS, Wawrowsky K, Puntel M, Castro MG., and, Lowenstein PR. Kupfer-type immunological synapse characteristics do not predict anti-brain tumor cytolytic T-cell function in vivo. Proc Natl Acad Sci USA. 2010;107:4716–4721. doi: 10.1073/pnas.0911587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K., and, Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7:347–360. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra B, Hinton DR, Marten NW, Bergmann CC, Lin MT, Yang CS.et al. (1999IFN-gamma is required for viral clearance from central nervous system oligodendroglia J Immunol 1621641–1647. [PubMed] [Google Scholar]
- Southgate TD, Bain D, Fairbanks LD, Morelli AE, Larregina AT, Simmonds HA.et al. (1999Adenoviruses encoding HPRT correct biochemical abnormalities of HPRT-deficient cells and allow their survival in negative selection medium Metab Brain Dis 14205–221. [DOI] [PubMed] [Google Scholar]
- Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ.et al. (1999Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials Nat Med 51256–1263. [DOI] [PubMed] [Google Scholar]
- Wilkinson GW., and, Akrigg A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate T, Kroeger KM, Liu C, Lowenstein PR, Castro MG.2008Gene transfer into neural cells in vitro using adenoviral vectors Jacqueline NC.et al. (eds). Current protocols in neuroscience Wiley: Chichester, New York; ch. 4. pp. 24.23.21–24.23.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S.et al. (2005Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model Cancer Res 657194–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Arica JR, Morelli AE, Larregina AT, Smith J, Lowenstein PR., and, Castro MG. Cell-type-specific and regulatable transgenesis in the adult brain: adenovirus-encoded combined transcriptional targeting and inducible transgene expression. Mol Ther. 2000;2:579–587. doi: 10.1006/mthe.2000.0215. [DOI] [PubMed] [Google Scholar]
- Paxinos G., and, Watson C. Academic press.; 1986. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Chen J, Hsu HC, Zajac AJ, Wu Q, Yang P, Xu X.et al. (2006In vivo analysis of adenovirus-specific cytotoxic T lymphocyte response in mice deficient in CD28, fas ligand, and perforin Hum Gene Ther 17669–682. [DOI] [PubMed] [Google Scholar]
- Puntel M, Kroeger KM, Sanderson NS, Thomas CE, Castro MG., and, Lowenstein PR.2010Gene transfer into rat brain using adenoviral vectors Jacqueline NC.et al. (eds). Current Protocols in Neurosciencech. 4, pp. 24.1–24.49. [DOI] [PMC free article] [PubMed]