Abstract
Since previous work using a nonreplicating adenovirus-expressing mouse interferon-β (Ad.mIFNβ) showed promising preclinical activity, we postulated that a vector-expressing IFNβ at high levels that could also replicate would be even more beneficial. Accordingly a replication competent, recombinant vaccinia viral vector-expressing mIFNβ (VV.mIFNβ) was tested. VV.mIFNβ-induced antitumor responses in two syngeneic mouse flank models of lung cancer. Although VV.mIFNβ had equivalent in vivo efficacy in both murine tumor models, the mechanisms of tumor killing were completely different. In LKRM2 tumors, viral replication was minimal and the tumor killing mechanism was due to activation of immune responses through induction of a local inflammatory response and production of antitumor CD8 T-cells. In contrast, in TC-1 tumors, the vector replicated well, induced an innate immune response, but antitumor activity was primarily due to a direct oncolytic effect. However, the VV.mIFNβ vector was able to augment the efficacy of an antitumor vaccine in the TC-1 tumor model in association with increased numbers of infiltrating CD8 T-cells. These data show the complex relationships between oncolytic viruses and the immune system which, if understood and harnessed correctly, could potentially be used to enhance the efficacy of immunotherapy.
Introduction
Lung cancer is the leading cause of cancer-related death throughout most of the world, with >160,000 cancer deaths per in the US alone. Nonsmall cell lung cancer is the most predominant subtype of lung cancer, which contributes >80% of lung cancer cases. Despite the use of surgery, chemotherapy and radiotherapy, a 5-year survival of about 15% has remained unchanged for decades. New therapeutic strategies are clearly needed.
The use of type I interferons (IFNs) (the IFNα family and IFNβ) as potential antitumor agents was proposed many years ago. IFNs have multiple anticancer mechanisms that include: direct inhibition on tumor cell proliferation and angiogenesis; induction of tumor-specific cytotoxic T-cells; plus other immunoregulatory effects on antibody production, natural killer (NK) cell activation, macrophage function, delayed-type hypersensitivity, and major histocompatibility complex antigen expression.1,2 Anticancer activity of type I IFNs has been demonstrated in patients with hematological malignancies (e.g., hairy cell leukemia) and solid tumors (e.g., renal cell carcinoma and malignant melanoma),1 however, the results and overall efficacy have been modest. This may be due to intrinsic resistance to IFN-induced cell death, to the short half-life (~30 minutes) of intravenously or subcutaneously dosed IFN, to dose-limiting systemic toxicities, and/or to the development of neutralizing antibodies against recombinant IFN protein.
One strategy to improve the specificity and efficacy of type I IFNs is to express them using viral vectors. Our group and others have shown that delivery of type I IFNs using replication-incompetent adenoviral vectors (Ad.IFN) in a variety of animal models induces high local levels of IFN and results in strong antitumor immune responses that effectively eliminate tumors, including lung cancer.3,4,5 Successful gene transfer using adenoviruses expressing IFNβ and IFNα2b to produce high levels and long-lasting IFN exposure to tumors has also been demonstrated in patients, and this has been associated with antitumor immune responses (such as activation of cytotoxic T-cells and NK cells, as well as generation of humoral responses) and promising clinical antitumor responses.6,7,8,9,10 However, there are some limitations to the use of Ad.IFN. First, because of almost complete “first pass” uptake in the liver after intravenous injection, Ad vectors can only be given locally, thus limiting the range of applicable tumors to diseases such as malignant mesothelioma, brain tumors, and bladder tumors. Second, the vector can only be administered once (or twice within a very short three day window) because rapid generation of anti-Ad neutralizing antibodies rapidly inactivate the vector and prevent generation of IFNβ after repeated dosing.6,7,8,9,10
One potential solution would be to develop alternative vectors. A vaccinia virus (VV) vector-expressing IFNβ was of particular interest for us, as the antitumor efficacy and safety of modified VV vectors have been demonstrated in other tumor models and recombinant VV vectors are being developed for clinical trials.11,12,13,14,15 The mouse IFNβ gene was originally inserted into VV to enhance safety since many tumor cells are resistant to the antiviral effects of type I IFNs, whereas viral replication in normal tissue is limited.11,12 Thus, production of IFNβ would theoretically not impede viral replication in the tumor, but would effectively prevent replication in normal tissues that have intact IFN-dependent antiviral defenses. However, to prevent VV-mediated neutralization of the IFNβ being produced, the vaccinia B18R gene (which binds and inactivates IFN) was deleted and the mouse IFNβ transgene was cloned into and replaced the thymidine kinase gene.11 The resulting virus displayed highly selective replication in the tumor with very little gene expression in any other organs or tissues, including the liver. Moreover, several strategies have been reported that can allow repeat delivery of vaccinia to the tumor even in the face of an antiviral immune response. These include cell-based delivery within cytokine-induced killer cell carrier vehicles16,17 and the incorporation of mutations that enhance production of the extracellular enveloped virus.18 The extracellular enveloped virus form of the virus is shrouded in a host cell-derived membrane containing host cell complement control proteins, and so is relatively protected from complement or antibody-mediated neutralization.12,18,19
We therefore hypothesized that this oncolytic VV mutant (TK−/B18R−/IFNβ+) would be a better therapeutic than Ad.IFNβ for treating lung cancer, due to its oncolytic effect plus IFNβ-mediated anticancer activity.
Results
Local and systemic administration of VV.mIFNβ induces therapeutic response in two mouse lung cancer models
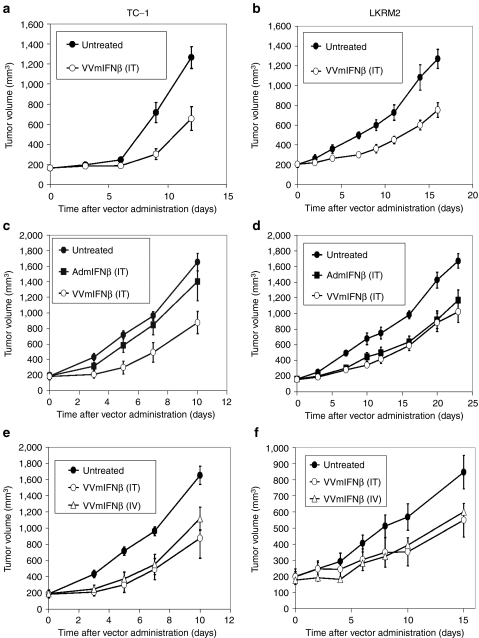
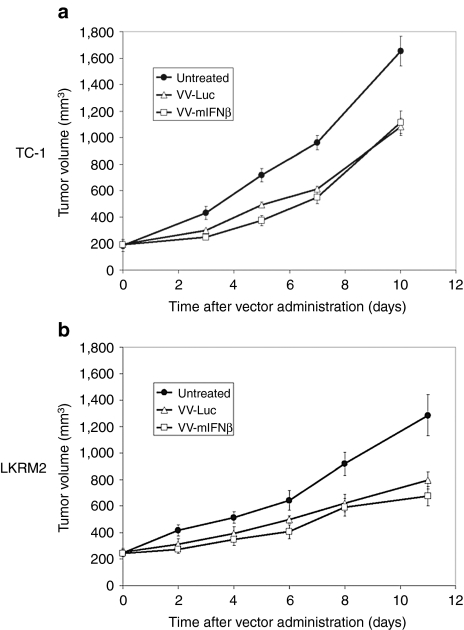
We first determined whether vaccinia viral vector-expressing mIFNβ (VV.mIFNβ) could induce therapeutic responses in two lung cancer models. TC-1 and LKRM2, two mouse lung cancer cell lines, were inoculated subcutaneously into the right flanks of syngeneic immunocompetent mice. When tumor sizes reached 200 mm3, a sublethal dose of VV.mIFNβ [108 plaque-forming units (pfu)]11 was given intratumorally and tumor measurements obtained. VV.mIFNβ was able to significantly (P < 0.05) slow tumor growth by ~40% in both mouse models (Figure 1a,b).
Figure 1.
Therapeutic responses induced by vaccinia viral vector-expressing mIFNβ (VV.mIFNβ) and adenovirus-expressing mouse interferon-β (Ad.mIFNβ) in two mouse lung cancer models, TC-1 (left panel) and LKRM2 (right panel). Tumor cells were inoculated subcutaneously into the right flanks of syngeneic mice. When tumor reached 200 mm3, a sublethal dose of VV.mIFNβ [108 plaque-forming units (pfu)] was given to mice either (a, b) intratumorally or (e, f) intravenously via tail vein. Antitumor efficacy of Ad.mIFNβ at the same dose, 108 pfu, was also compared with VV.mIFNβ in these (c, d) two mouse lung cancer models. The values are expressed as the mean + SEM (n = 6/group).
We then compared similar doses of VV.mIFNβ with our adenovirus-expressing mouse IFNβ (Ad.mIFNβ) vector in which antitumor efficacy in mouse lung cancer has already been demonstrated by our group.3,4 A single dose of either IFNβ vector was given intratumorally to mice with large (200 mm3) TC-1 and LKRM2 tumors. Consistent with the previous study,4 Ad.mIFNβ significantly inhibited LKRM2 flank tumor growth (Figure 1d), however, at the dose used in this experiment (108 pfu), it had no antitumor activity against TC-1 flank tumor (Figure 1c). Since LKRM2 flank tumors spontaneously metastasize to the lung (Supplementary Figure S1),20 we also compared the ability of each vector to inhibit lung metastases and found that intratumoral VV.mIFNβ (versus Ad.mIFNβ) was superior (Supplementary Figure S2). Given that the viruses made similar amounts of IFNβ (see below), these data suggested that the additional oncolytic effect of VV.mIFNβ might be active in the TC-1 model.
Another possible advantage of VV.mIFNβ over an adenoviral vector is that it could potentially show activity when given intravenously.11 To test this, VV.mIFNβ was delivered either intratumorally or intravenously (via tail vein) to immunocompetent mice bearing large (200 mm3), subcutaneous TC-1 or LKRM2 tumors. The results showed that intravenous administration was equally effective as intratumoral injection on inhibition of subcutaneous tumor growth in both models (Figure 1e,f). Both routes of VV administration were equally effective in inhibiting growth of lung metastases in the LKRM2 model (Supplementary Figure S3).
To summarize, the recombinant VV-expressing mouse IFNβ was effective against both our mouse lung cancer models when given either locally or systemically. Moreover, VV.mIFNβ was as effective as, or better than Ad.mIFNβ at the same pfu in the TC-1 model.
Differential replication of VV.mIFNβ in the two mouse lung cancer models: the role of the oncolytic effect
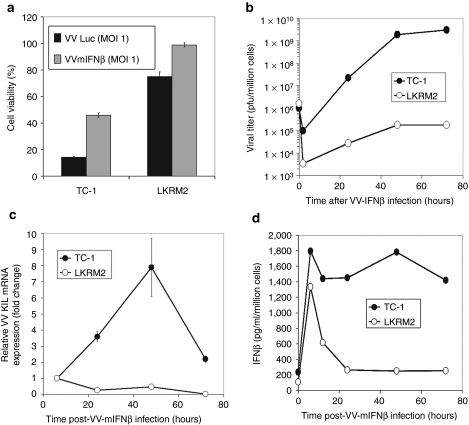
Given that VV.mIFNβ generated significant clinical responses in both mouse lung cancer models, we next investigated the antitumor mechanisms in each model. First, we examined how efficiently the virus replicated and killed tumor cells in culture, i.e., the oncolytic activity. TC-1 and LKRM2 cells were infected with either a recombinant VV-expressing luciferase (VV.Luc; control vector with the same TK−/B18R− background) or VV.mIFNβ (test vector) at an multiplicity of infection (MOI) 1, and the cell viability was determined 48 hours postinfection. Interestingly, despite the similar in vivo antitumor activity of VV.mIFNβ in the two mouse tumor models, TC-1 cells were killed much more efficiently by both control and test vectors compared with LKRM2 cells (Figure 2a). There was slightly less killing in both cell lines with the VV.mIFNβ vector compared to the VV.Luc vector, which likely reflects that these two tumor cell lines are partially “IFN-responsive,” as determined by their diminished susceptibilities to VSV-mediated killing in the presence of IFN (data not shown).
Figure 2.
The ability of our vaccinia virus (VV) vectors to (a) lyse tumor cells, (b, c) replicates and (d) induce interferon-β (IFNβ) in culture. TC-1 and LKRM2 tumor cells were infected with either VV.Luc or vaccinia viral vector-expressing mIFNβ (VV.mIFNβ) at multiplicity of infection (MOI) of 1. The cell viability was then determined with MTT assays 24 hours following infection (a). The viral replication was determined using (b) plaque assay and (c) real-time PCR. One million of TC-1 or LKRM2 cells were infected with either VV.Luc or VV.mIFNβ vector at MOI of 1, and cells were harvested at various timepoints to determine the amount of the infectious virus. At the same time, the culture supernatants were collected and analyzed with enzyme-linked immunosorbent assay (ELISA) to measure IFNβ protein level (d).
A likely explanation for the differences in VV-induced cell death is that VV's replicate more efficiently in TC-1 than in LKRM2 cells. Both lung cancer lines were thus infected with MOI 1 of VV.mIFNβ, and the infected cells were harvested over 72-hour period to check for viral titers with plaque assays, as well as viral gene expression by real-time PCR. As expected, VV.mIFNβ replicated efficiently in TC-1 cells, with the viral titer increasing by 4–5 logs over the first 48 hours (Figure 2b). Concurrently, the amount of VV K1L gene expression present in the infected cells following infection using real-time PCR increased linearly with time in TC-1 cells and reached the peak level 48 hours postinfection (Figure 2c). In contrast, the replication of VV.mIFNβ was limited in LKRM2 cells, with only approximately a one to two log increase over the first 48 hours (Figure 2b). Consistent with the viral titer data, VV K1L gene expression detected in LKRM2 cells remained relatively constant (Figure 2c).
Given that the VV vector expresses the IFNβ gene, one would hypothesize that the IFNβ secretion would increase while the virus was replicating. We infected both TC-1 and LKRM2 cells with VV.mIFNβ and measured IFNβ protein level over time using enzyme-linked immunosorbent assay. The result is consistent with the viral replication data (Figure 2d). IFNβ protein level was maintained at high level in TC-1 cell culture over 72 hours, whereas IFNβ levels reached their peak at 6 hour and dramatically decreased in LKRM2 within the first 24 hours. We also compared the amount of IFNβ (area under the curve) induced by either Ad.mIFNβ or VV.mIFNβ in an IFN-nonresponsive mesothelioma cell line, and found that they produced similar amounts of IFNβ protein in culture (Supplementary Figure S4).
Taken together, these results indicate that in the in vitro system, the differential susceptibility of tumor cells to VV-mediated oncolysis is dependant on how well the virus replicates in those cells. The amount of IFNβ transgene expression also correlates well with the amount of viral replication in culture.
Induction of IFNβ by VV.mIFNβ does not correlate with its ability to replicate in mice
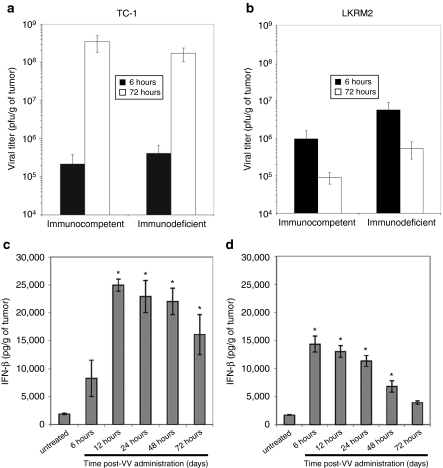
We next conducted studies to evaluate virus replication and IFNβ secretion in immunocompetent mice bearing established TC-1 or LKRM2 tumors. VV.mIFNβ was injected intratumorally into TC-1 or LKRM2 tumors when the tumor size reached ~300 mm3. Tumors were harvested 6 and 72 hours after injection, homogenized, and assayed for viral titer using plaque assays. Consistent with the in vitro data, the amount of virus recovered in TC-1 tumors increased by three logs within the first 72 hours (Figure 3a), while a log decrease in viral titer was seen in LKRM2 tumors (Figure 3b). We also conducted experiments to evaluate the role of the immune system in limiting viral replication. TC-1 and LKRM2 cells were inoculated into NOD/SCID/γ2 knockout (NSG) mice and VV.mIFNβ was injected into tumors when the size reached 250 mm3. Overall, we did not see any significant changes in the amount of viral replication in either tumor cell line when comparing immunocompetent to immunodeficient mice (Figure 3a,b).
Figure 3.
The ability of our vaccinia virus (VV) vectors to (a, b) replicate and induce (c, d) interferon-β (IFNβ) in flank tumors. (a, c) TC-1 and (b, d) LKRM2 tumor cells were inoculated into the right flanks of syngeneic immunocompetent C57Bl6 or hybrid mice, respectively. The NOD/SCID/γ2 knockout immunodeficient mice were used to study the role of immunological effect. When the tumors reached 300 mm3, 100 million plaque-forming units (pfu) of vaccinia viral vector-expressing mIFNβ (VV.mIFNβ) were injected intratumorally. At various timepoints, tumor tissues were then harvested and assayed for viral replication with plaque assay (a, b) or IFNβ production with enzyme-linked immunosorbent assay (ELISA) (c, d). The values are expressed as the mean + SEM. *Denotes for significant induction of IFNβ above the untreated control (P < 0.05), which was analyzed with one-way ANOVA.
We also measured the amount of IFNβ production induced by VV.mIFNβ within infected tumors. We found that, in general, the IFNβ levels correlated well with viral titers in the tumors (Figure 3c), with high and prolonged levels seen in the TC-1 tumors. VV.mIFNβ induced significant early levels of IFNβ in LKRM2 tumors (which peaked at 6 hours), then subsequent lower levels that decayed relatively quickly (Figure 3d).
Evaluation of oncolytic versus immunological effects in vivo
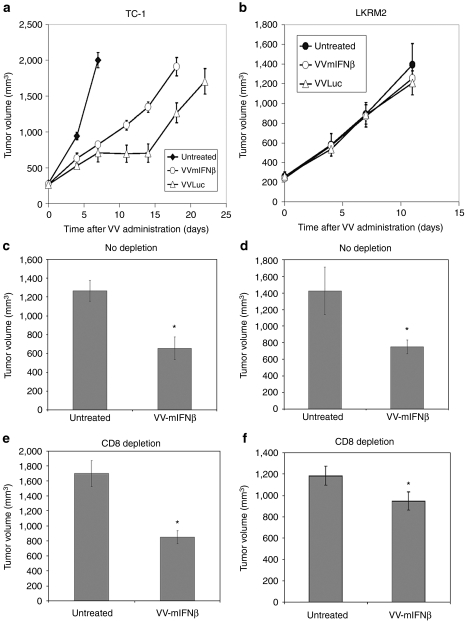
Since we found that the antitumor activity induced by VV.mIFNβ in the replication-resistant LKRM2 model was not due to its oncolytic effect, we tested if host immunity was required. TC-1 and LKRM2 tumor cells were inoculated subcutaneously to immunodeficient NSG mice. Once tumors reached 200 mm3, VV vectors expressing Luciferase or mIFNβ were injected intratumorally. Both VV.Luc and VV.mIFNβ vectors were still very effective in slowing TC-1 tumor growth in immunodeficient mice (Figure 4a). Consistent with its enhanced ability to replicate (see Figure 2a), the VV.Luc vector was even more effective than the VV.mIFNβ vector (Figure 4a). In contrast, in the LKRM2 model, both VV.Luc and VV.mIFNβ lost all of their antitumor efficacy in immunodeficient mice, indicating that intact host acquired immunity is required for antitumor efficacy in this model (Figure 4b).
Figure 4.
The role of host immune system on the antitumor efficacy of vaccinia virus (VV) vectors. (a) TC-1 and (b) LKRM2 tumors were inoculated into NSG immunodeficient mice. When tumor reached ~200 mm3, VV.Luc and vaccinia viral vector-expressing mIFNβ (VV.mIFNβ) were then injected into mice intratumorally and tumor size was monitored twice a week. The role of CD8+ cells on VV-mediated antitumor effect was also investigated by inoculating (c, e) TC-1 and (d, f) LKRM2 cells into syngeneic immunocompetent mice. Intraperitoneal injection anti-CD8 antibody was used to deplete CD8+ cells. The timepoint chosen for the comparison was 10 days post-VV administration. The values on the bar charts are expressed as the mean + SEM (n = 6/group). *Denotes for significant induction of interferon-β (IFNβ) above the untreated control (P < 0.05), which was analyzed with Student's t-test.
To more precisely define the immunologic effector cells in VV.mIFNβ-mediated therapeutic activity, we selectively depleted CD8 T-cells by administering an anti-CD8 antibody to immunocompetent mice-bearing TC-1 or LKRM2 tumors before and after vector administration. FACS analysis confirmed the successful depletion of CD8 T-cells in these mice prior to virus administration (Supplementary Figure S6a). Similar to the data with immunodeficient mice, depletion of CD8 had no effect on the overall VV-induced antitumor activity in TC-1 model; we saw 48% versus 50% decreases in tumor growth in undepleted and CD8-depleted mice, respectively (Figure 4c,e). VV-induced antitumor activity in LKRM2 was dampened, but still significant, when CD8 T-cells were depleted: we saw a 48% decrease in tumor size in intact animals, but only a 20% decrease in tumor size in the CD8-depleted mice (Figure 4d,f). However, VV.mIFNβ completely failed to inhibit lung metastases in CD8-depleted mice (Supplementary Figure S5).
To further explore the immunologic mechanisms in the LKRM2 model, we also depleted other immune cell types after administration of VV.mIFNβ and assessed the influence the antitumor response. Anti-CD4, anti-asialo-GM, and anti-Ly6G antibodies were given to mice to deplete CD4 cells, NK cells, and neutrophils, respectively. Successful depletion was demonstrated with FACS analyses (Supplementary Figure S6). However, no effect on therapeutic efficacy was seen in these immune subset-depleted mice (Supplementary Figure S7). These data thus indicate that induction of antitumor CD8 T-cells by VV.mIFNβ in the LKRM2 model, but not CD4 cells, NK cells, or neutrophils, play an important (but not exclusive role) in the antitumor activity.
The role of IFNβ transgene in the overall antitumor activity of VV
Given our previous data with Ad.mIFNβ showing that almost all of the antitumor activity was due to expression of the transgene,3,4 we had originally assumed that VV.mIFNβ would show more therapeutic benefits than the control VV.Luc vector. To test this hypothesis, immunocompetent mice bearing established TC-1 or LKRM2 tumors were given either VV.Luc or VV.mIFNβ intravenously. In contrast to our experience with adenovirus, we saw the antitumor effects were quite similar between the VV.Luc “control” vector versus the VV.mIFNβ vector in both TC-1 and LKRM2 tumor models (Figure 5). In addition, no difference was observed in inhibition of LKRM2 spontaneous lung metastases (Supplementary Figure S8).
Figure 5.
The role of interferon-β (IFNβ) transgene in the antitumor efficacy of our vaccinia virus (VV) vector. (a) TC-1 and (b) LKRM2 tumors were inoculated into the right flanks of syngeneic mice. When tumor reached ~200 mm3, VV.Luc and VV.mIFNβ were then injected into mice intravenously and tumor size was monitored twice a week. The values are expressed as the mean + SEM (n = 6/group).
To determine whether this observation was also true in another lung cancer model, we examined the anticancer efficacy of intravenous VV.Luc and VV.mIFNβ in an orthotopic lung cancer model driven by the activation of a k-ras mutation.21 When examined 70 days after administration, animals given either VV.Luc or VV.mIFNβ had 100% survival when compared to 70% survival in control mice (Supplementary Figure S9). Neither treatment was curative, however, as all of VV-treated mice died by 100 days after vector administration, compared with the untreated mice who died within 80 days (data not shown).
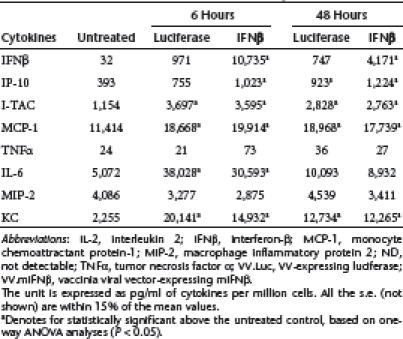
Given the observation that VV can be immunogenic through its ability to induce inflammatory responses via type I IFNs and other mediators,12 we measured the levels of eight different cytokines/chemokines, including IFNβ, IP-10 (CXCL10), I-TAC (CXCL11), monocyte chemoattractant protein-1 (MCP-1) (CCL2), tumor necrosis factor-α, interleukin-6, macrophage inflammatory protein 2 (MIP-2) (CXCL2), and KC (CXCL1), in both cell culture and in mice after infecting with VV.Luc or VV.mIFNβ at an MOI of 1. We saw significant increases in levels of IFNβ, interferon gamma-inducible protein-10 (IP-10), and MCP-1 in both cell lines with both vectors (Table 1 and 2), although the levels of IFNβ, IP-10, and I-TAC were the highest in the supernatants harvested from TC-1 cells treated with VV.mIFNβ.
Table 1. Cytokine induction by VV.Luc or VV.mIFNβ in TC-1 cell culture.
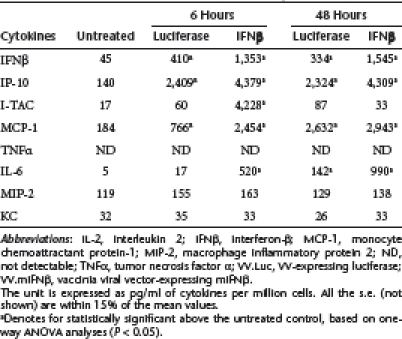
Table 2. Cytokine induction by VV.Luc or VV.mIFNβ in LKRM2 cell culture.
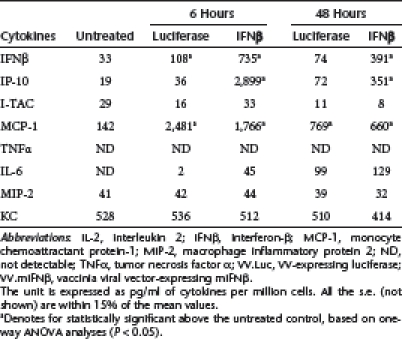
Interestingly, in the mouse tumors, VV.Luc and VV.mIFNβ induced similar amount of many cytokines, including I-TAC, MCP-1, interleukin-6, MIP-2, and KC in tumors derived from both cell lines (Table 3 and 4). The main differences were in the levels of IFNβ and IP-10, especially in the TC-1 tumors. These results indicate that the VV.TK−/B18R−/Luc vector itself, appears to induce a significant amount of inflammatory responses, including an IFN response, within our lung cancer models. This may help to explain the lack of difference in therapeutic responses seen between VV.Luc- and VV.mIFNβ-treated groups.
Table 3. Cytokine induction by VV.Luc or VV.mIFNβ in TC-1 tumors.
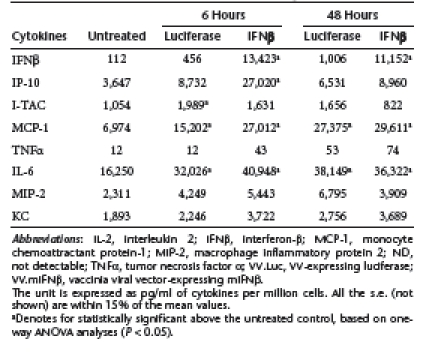
Table 4. Cytokine induction by VV.Luc or VV.mIFNβ in LKRM2 tumors.
We have preliminary data suggesting that it is the lack of the B18R gene that may be important in inducing inflammation in the VV.luc vector. Although not definitive, since the vector backbones are slightly different, we did note that another luciferase producing VV vector that retained the B18R gene, but had deletions of the VV growth factor (VGF) and TK genes (VV.(VGF−/TK−)-Luc), completely lost its antitumor effect in LKRM2 tumor model, although some efficacy was still seen in TC-1 model (Supplementary Figure S10). While this vector was able to replicate in both TC-1 and LKRM2 cells both in vitro and in vivo (Supplementary Figure S11), it did not induce much chemokine response in the infected tumors (Supplementary Table S1); in fact, we actually observed a reduction in most of chemokines tested within the infected tumor tissues. We are currently conducting more careful comparisons of VV's with and without the B18R gene to study the importance of virus-induced inflammation as part of the overall antitumor efficacy mediated by VV.
VV.mIFNβ, but not VV.Luc, augments the efficacy of immunotherapy
Given the ability of both vectors to strongly induce intratumoral production of inflammatory cytokines (like IFNβ) and T-cell attracting chemokines (such as IP-10), we hypothesized that if antitumor T-cells were first generated by a vaccine, subsequent administration of the VV vectors might serve to attract these cytotoxic T-cells to the tumors and enhance antitumor efficacy. To test this hypothesis, we combined a cancer vaccine for TC-1 tumors (which express HPV-E7 antigen), specifically an adenovirus-expressing HPV- E7 antigen (Ad.E7),22 with our two VV vectors. A “prime” dose of Ad.E7 was injected to mice subcutaneously when tumor sizes were around 100–150 mm3, and a “boost” dose was given a week later to generate activated T-cell response against TC-1 tumor antigen. A single intravenous injection of VV vector was administered 2 days after the second dose of Ad.E7, in an attempt to generate a chemokine gradient to attract the activated T-cells to the tumor site. Controls were also done with administration of just VV.luc or VV.IFNβ alone.
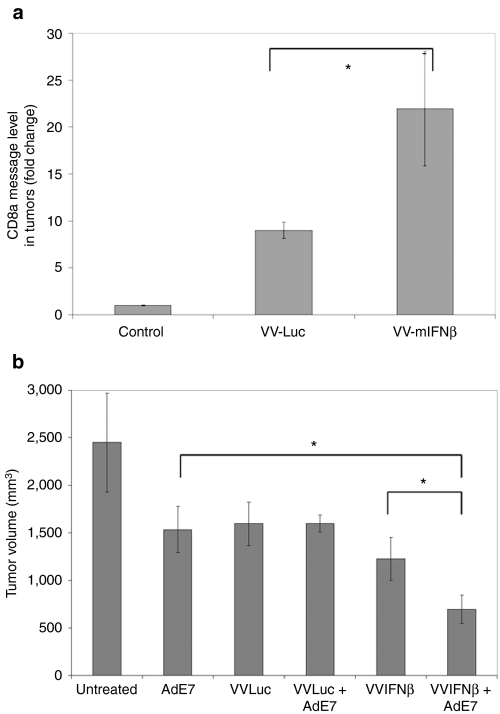
Two days later, we performed real-time PCR in a subgroup of tumors to look for CD8 mRNA message. We found that both VVs significantly increased the amount of CD8 mRNA, however, VV.IFNβ induced a much higher amount of CD8 mRNA in TC-1 tumors (22-fold compared to Ad.E7 tumors) than did the VV.Luc vector (a ninefold increase compared to Ad.E7 tumors (Figure 6a). The remainder of the tumors were harvested 2 weeks after VV administration (Figure 6b). Although all the treatments led to significantly smaller tumors than untreated controls, addition of VV.mIFNβ, but not VV.Luc, was able to significantly enhance the effect of this immunotherapy.
Figure 6.
Vaccinia viral vector-expressing mIFNβ (VV.mIFNβ) enhances the efficacy of immunotherapy by recruiting more CD8 T-cells into tumors than VV.Luc. TC-1 tumor cells were inoculated into the right flanks of C57Bl6 mice. When tumors reached 150 mm3, the first dose of Ad.E7 [109 plaque-forming units (pfu)] was given to the mice contralaterally to their flank tumors. A second boost dose of Ad.E7 vaccine was given 5 days later to induce antitumor immune response, and a single intravenous injection of VV.mIFNβ or VV.Luc was given to mice. Two days after vaccinia virus (VV) injection, some tumors were harvested to check for CD8 expression by (a) real-time PCR. The remainder of the tumors were measured over time, and the time point chosen was 2 weeks after VV administration for comparison (b). The values are expressed as the mean + SEM (n = 6/group). *Denotes significant difference between two groups, analyzed with Student's t-test (P < 0.05).
In summary, our data showed that the IFNβ transgene was associated with increased trafficking of CD8 T-cells into animals that had been vaccinated and was able to enhance the efficacy of this form of immunotherapy.
Discussion
We report here that an attenuated VV-expressing IFNβ may be a promising therapeutic agent to treat lung cancer due to its ability to replicate and lyse tumors cells, to make high levels of IFNβ through either viral replication or induction of host immune responses, and its ability to induce other types of acute inflammatory responses within tumors. Moreover, this viral vector, unlike adenovirus, had efficacy after intravenous injection, suggesting that it can potentially be given systemically to target diffuse disease. However, this study also illustrates a number of interesting issues relating to the complexity of how these vectors exert their antitumor effects.
The first issue relates to the differential ability of VV to replicate within different tumor cell lines. The factors that determine whether an oncolytic vector will replicate well within tumors are still not known for certain. For some viruses, the presence or absence of an intact IFN-response pathway is highly predictive. Viral nucleic acids are often recognized by innate pattern recognition receptors such as Toll-like receptors, RIG-I-like receptors, double-stranded RNA-activated protein kinase, and melanoma differentiation-associated protein 5.23 Binding of viral nucleic acids to these receptors result in type I IFN secretion with subsequent binding to the IFN receptor in an autocrine and paracrine fashion, that initiates the expression of a cohort of IFN-stimulated genes to inhibit virus replication through multiple mechanisms.24 Numerous studies have demonstrated that many tumor cells have increased susceptibility to oncolytic viruses, including recombinant VV, if they have an impaired ability to respond to IFNs.11,25,26 In our previous study with vesicular stomatitis virus (VSV), we were able to predict with a high degree of certainty whether the tumors would be susceptible to VSV-mediated oncolysis by the presence or absence of an intact IFN-response pathway. Although we did observe a small decrease in VV replication when our tumor cells were infected with VV.mIFNβ vector compared with the VV.Luc vector (Figure 2a), in contrast to our findings with VSV, IFN-responsiveness did not seem to be the major factor predicting whether or not the VV would replicate. This is consistent with considerable literature showing that VV is fairly resistant to inhibition by IFN, likely due to an extremely robust set of viral genes that inhibit all aspects of the IFN-response pathway (reviewed in ref. 27). Both TC-1 and LKRM2 are both somewhat IFN-responsive (with regard to supporting VSV growth), but were very different in their VV permissiveness. We also tested a mouse mesothelioma cell line, AB12, in which the IFN-response pathway was completely inactivated. To our surprise, the replication of VV in AB12 cells or tumors was limited, very similar to the pattern we saw in LKRM2 (data not shown).
Ras activation has been demonstrated by many groups to enhance viral infection and replication of many viruses, including VV, VSV, HIV, and reovirus.28,29,30 However, both cell lines used in this study have a mutated ras gene.21,31 We are thus still unclear why we see the differential ability of VV to replication in our tumor cell lines. A better understanding of this question may help us to predict which patients might have the best antitumor efficacy with this virus.
An issue of even more interest relates to the observation that even though the VV.mIFNβ vector showed equivalent antitumor efficacy in two different lung cancer animal models (Figure 1), the mechanisms responsible for the effects seemed to be completely different. In the TC-1 model, VV.mIFNβ was able to efficiently replicate and produce high and prolonged levels of IFNβ (Figures 2b–d and 3a,c). However, the antitumor efficacy seemed to be almost entirely due to the oncolytic effects, as there was no loss of efficacy in profoundly immunodeficient mice (Figure 4a). In contrast, in the LKRM2 model, VV was not able to replicate well, but produced moderately high levels of IFNβ in tumors (Figures 2b–d and 3b,d). In this model, the antitumor effects seen appeared to be primarily CD8 T-cell-mediated, because all the efficacy was lost in immunodeficient mice and was significantly dampened in CD8-depleted mice (Figure 4b,e,f; Supplementary Figure S8), while other immune cells, such as CD4 T-cells, NK cells, and neutrophils, seemed to play little role in the VV-mediated antitumor activity.
A previous study using the same vector showed that the addition of IFNβ to the B18R-deleted virus increased efficacy to a small extent, but had the greatest effect on the clearance of the virus from normal tissues, rather than on increased antitumor effects.11 In our study, it was of interest that we saw virtually identical antitumor effect when comparing the VV.mIFNβ vector with the control VV.Luc vector lacking the B18R gene (Figure 5). An explanation for this might be expected in the TC-1 model which was primarily, but not solely, dependent on viral replication. In this case, it might even be expected that the IFNβ produced by the VV.mIFNβ would actually decrease replication (and thus efficacy). As discussed above, however, the presence of IFNβ seemed to have only minimal antiviral effects. Less expected was the observation that the VV.Luc vector seemed just as efficacious as the VV.mIFNβ vector in the LKRM2 model (Figure 5a), where antitumor immune responses were responsible for efficacy. This observation is similar to that recently reported in a study of VSV encoding CD40L32 where no difference was observed between VSV.GFP and VSV.CD40L. We believe the data in Tables 1–4 provide a possible explanation for this finding of equivalent efficacy. In the LKRM2 model, both vectors induced initial innate immune responses marked by upregulation of number of proinflammatory cytokines and chemokines. Although the VV.mIFNβ vector induces more IFNβ and IP-10 at an early time point, a threefold increase in IFNβ was stimulated by the VV.Luc vector at 6 hours. Similar levels of strong induction of MCP-1, KC, and interleukin-6 were seen in both vectors at early and late time points. In contrast, when we studied a different VV control vector with a different backbone, deleted in the VGF and TK genes, we saw relatively little or no antitumor effect (Figure 6b) and no induction of proinflammatory cytokines (Supplementary Table S1).
These data suggest that this ability to induce intratumoral inflammation with subsequent generation of a CD8 T-cell-mediated antitumor response may be dependent on the viral backbone. In this study, we speculate that the lack of the B18R gene may have played just as important a role as the expression of the IFNβ transgene. Like all other viruses, VV encodes proteins that block the innate immune response to viral infection.27 B18R, a soluble type I IFN-binding protein, can sequester the type I IFN produced in response to viral infection.33,34 Our vector was purposely engineered to delete this key protein to prevent inactivation of the IFNβ transgene.11 However, it appears that just the loss of this protein in a VV not carrying other transgenes enhances antitumor immune responses. Although there are very few studies using the B18R-deleted VV variant, a recent paper by Waibler and the colleagues35 showed that the wild-type VV could inhibit systemic type I IFN responses, whereas the B18R-deleted variant actually induced IFN. We show here, for the first time, that this B18R-deleted VV not only induced IFNβ, but also stimulated production of IFN-inducible chemokines (i.e., IP-10 and I-TAC) and other chemokines and cytokines that might aid in its antitumor efficacy. This is consistent with predictions that “more new functions will be found for already known genes” involved in blocking the IFN pathway.27 Future investigations to examine the role of B18R gene expression or to regulate the VV-induced IFN response will be helpful for vector development, since our observations are limited by the fact that we did not directly compare the VV.(B18R+/TK−) and VV.(B18R−/TK−) vectors.
Although we did not see major differences in antitumor efficacy between the VV.Luc and the VV.mIFNβ vectors when given by themselves, we did note that the VV.mIFNβ vector was superior in attracting CD8 T-cells and augmenting antitumor efficacy when combined with a tumor vaccine in the TC-1 model (Figure 6). Although, the reason for this is not known for certain, the vectors did differ in the degree of induction of intratumoral IFNβ and in the IFN-dependent chemokine, IP-10, in the TC-1 model. Given the importance of type 1 IFNs and IP-10 in activating and attracting CD8 T-cells,1,36,37 we speculate that these activities are responsible for the observed augmentation of efficacy of immunotherapy.
Vaccinia is know to be highly immunogenic, as seen during its use in the smallpox eradication program, and with its subsequent use in antigen-expressing vaccines for the treatment or prevention of a variety of diseases. However, it is also known to express multiple immune modulating virulence genes, many of which act as secreted decoy receptors for binding of Th1-associated cytokines.27,35,38,39 It is therefore possible that the expression of these genes (including B18R) may limit the CTL response raised by the virus, and so could at least partially explain the lack of clinical success with vaccinia-based therapeutic cancer vaccines to date.40 In order to enhance virally generated antitumor immune responses, there are a number of approaches being explored. One approach is to delete viral genes that encode for specific proteins to suppress host immunity.33,34,35,39 The B18R gene, as discussed above, appears to be a good example.33,34 A second approach involves addition of transgenes that can enhance the host immunity;13,14,41 for example, the current promising VV vector-expressing GM-CSF in the phase II trials. Another strategy involves engineering tumor-associated antigens into the viral vector.42 A final approach is illustrated by our study (Figure 6), where antitumor CD8 T-cells were generated with active immunotherapy (i.e., a vaccine) and then the viral vector was used to induce cytokine or chemokines that would produce local inflammation (such as IFNβ and IP-10) leading to chemoattraction and potential activation or expansion of these vaccine-generated antitumor T-cells.
Our findings support the growing recognition of the complex interactions between oncolytic vectors and the immune system.43 It is becoming increasingly clear that none of the available oncolytic viruses can effectively destroy large, established tumors through viral replication alone, but also need an accompanying antitumor immune response. If the immune reaction induced by the virus is too strong, replication can be totally inhibited and the immune response induced will be dominated by antiviral rather than antitumor responses. If the immune response to the virus is too weak, viral replication may be enhanced, but an effective antitumor immune responses may not be produced. As Prestwich and colleagues44 have recently summarized, “the mechanisms of activity of oncolytic viruses involve direct oncolysis, vascular effects, and innate adaptive immunity. The extent of viral replication does not necessarily correlate with therapeutic efficacy. Immune interactions may be either beneficial or detrimental.” Our data point out that differences in mechanisms can exist with the same virus, depending on the tumor cell line treated. This observation is also supported by a recent study showing that the same oncolytic VV can destroy the same tumor by different mechanisms depending on whether the mouse has been preimmunized or not.16
It should be noted that human cancer cells are, in general, more sensitive to direct oncolytic effect of VV than mouse cells in culture (data not shown). Whether or not VV-mediated antitumor activity in patients will be primarily due to oncolytic activity or immune effect still remains to be elucidated.
In conclusion, VV.mIFNβ was demonstrated here to be a promising therapeutic agent in two different lung cancer models when delivered intratumorally or intravenously. In one model, it has better effects than an adenovirus-expressing IFNβ. However, the mechanisms of antitumor effect were different. In one model, tumor killing was mediated by viral replication and oncolysis, whereas in the other model, efficacy was dependent on acquired antitumor immune response. We also found that a VV vector lacking the B18R gene was effective in inducing local tumor inflammatory response. The factors that enable VV to replicate in different lung cancer models is still not clear, but it was found not to be limited by the IFN-response status or ras mutation of the infected cells. An understanding of the complexity of virus-tumor-immune system interactions will be important to define as these vectors are moved into clinical trials in human cancer patients.
Materials and Methods
Viruses. The VV.(B18R−/TK−) vectors encoding firefly luciferase (VV.Luc) or the firefly luciferase plus murine IFNβ (VV.mIFNβ) genes were generated as previously described.11 In brief, the cloning plasmid pSC65 was modified so that the firefly luciferase gene was expressed from the strong synthetic early/late promoter (pSE/L) and the murine IFNβ (IFNβ) gene was expressed from the weak p7.5 early/late promoter. The plasmid was then used to insert the cDNA into the vaccinia TK gene of B18R-deleted strain of Western Reserve VV by homologous recombination. Successful recombination events were selected for by luciferase expression, and correct insertion of plaque-purified clones was verified by PCR. Viral stocks were then amplified in human HeLa cervical cancer cells, purified with 0.05 µm MicroKros filter (Cat no. X10S-100-04N; Spectrum Laboratories, Rancho Dominguez, CA) in addition to traditional sucrose cushion, and were titered using standard plaque assay in green monkey BSC-1 kidney cells. The VV (VGF−/TK−) vector encoding firefly luciferase was also generated in similar manner as previously described.45 The E1/E3-deleted type 5 adenoviral vector-encoding murine IFNβ (Ad.mIFNβ was previously generated by the Vector Core of the Institute for Human Gene Therapy (University of Pennsylvania Medical Center).46
Cell lines. Mouse TC-1 lung cancer cells were derived from mouse lung epithelial cells immortalized with human papillomavirus-16 E6 and E7 and transformed with the c-Ha-ras oncogene,31 and maintained in RPMI1640 medium (Gibco; Invitrogen, Carlsbad, CA) supplemented with 10 % fetal bovine serum (Georgia Biotechnology, Atlanta, GA), 2 mmol/l -glutamine and 1% penicillin/streptomycin. The original mouse “LKR” cell line was obtained as a gift from Dr Friedberg (University of Pennsylvania School of Medicine). These cells were derived from an explant of a pulmonary tumor from an activated KrasG12D mutant mouse grown in Dr Tyler Jacks' lab at M.I.T. (Boston, MA).21 The LKRM2 cells were derived in our lab from spontaneous lung metastases in a mouse bearing subcutaneous LKR tumor. LKRM2 cells are more aggressive than its parental LKR cells in terms of forming lung metastases in mice, and they were maintained in high glucose DMEM (Gibco; Invitrogen) supplemented with 10% fetal bovine serum, -glutamine, and penicillin/streptomycin. Green monkey kidney BSC-1 epithelial cells and human HeLa cervical carcinoma cells were purchased from ATCC (Manassas, VA), and they were also maintained in DMEM with 10% fetal bovine serum, -glutamine, and penicillin/streptomycin.
Animals. Pathogen-free C57BL/6 mice, hybrid (C57BL/6J female × 129P3/J male F1) mice, and NOD/SCID/IL2 receptor γ2 chain knockout (NSG) mice were purchased from Charles River Laboratories (Wilmington, MA), Jackson Labs (Bar Harbor, ME) and Wistar Institute (Philadelphia, PA) respectively. Animals used for all experiments were female mice between 6 and 10 weeks old, and were housed in a pathogen-free animal facility at Wistar Institute. All protocols were approved by the Animal Use Committees of the Wistar Institute and University of Pennsylvania, and were in compliance with the guide and care and use of animals.
Animal studies. To create flank tumors, mice were injected with 1.2 × 106 of TC-1 or 2 × 106 of LKRM2 cells subcutaneously on the right hind flank. Unless otherwise stated, a single intratumoral dose (108 pfu) in 100 µl of saline) of viral vectors (Ad.mIFNβ, VV.Luc, or VV.mIFNβ) was administered to mice when tumors reached ~200 mm3 in size. For some experiments, VV vectors were injected through tail vein. Tumors were measured two to three times per week with calipers, and mice were monitored for toxicity. Mice were euthanized if toxicity was evident or tumor burden exceeded 2,500 mm3. All experiments had at least five mice per group and were repeated at least two times.
The LKRM2 cell line was also used to study the effect of VV vectors on distant diseases, which has been previously described.20 This cell line was produced from a spontaneous lung metastasis formed in a LKR tumor-bearing mouse, and after two in vivo passage cycles, a stable line was established with a reproducible pattern of spontaneous lung metastatic induction (>40% of untreated mice in 5 weeks). Hybrid (B6X129/J1) mice were injected on the right flank with 2 × 106 LKR-M cells. After flank tumors reached an average size of 200 mm3, mice were randomized to the untreated group or VV-treated group. Forty days after tumor inoculation, the mice were killed and their lungs were excised, separated into discrete lobes, and weighed. Sections were cut from each of the five lobes and stained with hematoxylin and eosin. In each lung, the total number of nodules was counted, and the total tumor area measured and divided by the total area of the lung section, using Image J (National Institutes of Health, Bethesda, MD) software. These evaluations were performed by a technician blinded to the group origin of the lung.
To deplete CD8+ T-cells systemically prior to administration of VV vectors, mice received intraperitoneal injections of 200 µg of purified monoclonal antibodies from the anti-CD8 hybridoma 53-6.72 (Harlan Bioproducts, Indianapolis, IN). Injections were administered 2 days before virus injection. Thereafter, a maintenance dose of antibody was injected intraperitoneally twice a week throughout the entire experimental period to ensure depletion. Cell depletion was confirmed by flow cytometry of splenic suspensions at different time points. We used the similar approach to deplete other immune subsets, including CD4, NK, and neutrophils. The anti-CD4 antibody was purified from anti-CD4 hybridoma GK1.5 (ATCC), and anti-asialo (GM1; NK) and anti-Ly6G (1A8; neutrophils) antibodies were purchased from Wako Pure Chemical Industries (Osaka, Japan) and BioXcell (West Lebanon, NH), respectively. Successful depletion was confirmed with flow cytometry from splenic population.
The orthotopic lung cancer model using intranasal Ad.Cre administration in transgenic K-ras mice has been previously described in detail.4 Briefly, to activate the conditional oncogene and induce tumors, 100 µl of saline with 3 × 1010 particles of adenovirus containing Cre recombinase (Ad.Cre) were administered to LSL KrasG12D mice intranasally. Four weeks after instillation of Ad.Cre, mice were treated with 108 pfu of VV.Luc or VV.mIFNβ intravenously. Mice were checked daily to monitor their health status. Animals were sacrificed when they exhibited any sort of distress or shortness of breath.
For the combination studies with the Ad.E7 cancer vaccine, mice-bearing TC-1 tumors (~200 mm3 in size) were vaccinated subcutaneously in the left flank (contralateral to the tumor) with 1 × 109 pfu of Ad.E7 vector.22 Five days following the initial vaccination, mice received a booster vaccine of 1 × 109 pfu of Ad.E7 in the left flank. Two days later, VV-Luc and VV-mIFNβ were injected into corresponding mice intravenously via tail vein. Tumors were monitored three times weekly until they reached large size.
MTT assays. To perform MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assays, TC-1 and LKRM2 cells were plated in quadruplicate on 96-well plates (5,000 cells/well in RPMI or DMEM medium with 10% fetal bovine serum) and were infected with MOIs of 1 of the VV-Luc and VV-mIFNβ viruses. Viability was assessed 72 hours postinfection by developing the reaction assay per manufacturer's instruction (Promega, Madison, WI). Optical density was read at 570 nm and corrected using a background control value.
Plaque assays. To evaluate the ability of VV-mIFNβ to replicate in lung cancer cells, TC-1 and LKRM2 cells were infected at an MOI of 1, and after a 2-hour absorption period, the inoculum was removed, and the monolayer washed with phosphate-buffered saline (PBS). The cell lysates and supernatants were collected at various time points (6, 24, 48 and 72 hours; unless stated otherwise), and titered with standard plaque protocol on BSC-1 cells and visualized with 0.05% crystal violet (wt/vol). For tumor samples from mice, TC-1 and LKRM2 tumors were harvested at various timepoints following intratumoral injection of VV-mIFNβ. Tumors were then homogenized in Tris–HCl buffer (pH 9) with tissue grinder (Tissuemiser; Fisher Scientific, Waltham, MA) till all material was in suspension. Tumor lysates were subjected to three freeze-thaw cycles and centrifuged to pellet debris. The resulting supernatants were then tittered on BSC-1 cells.
RNA extraction and real time-PCR. To collect total RNA for reverse transcription-PCR after VV.mIFNβ infection, TC-1, and LKRM2 cells (106 cells/3 ml/well) were seeded in 6-well plate, incubated overnight, and then infected with VV.mIFNβ at an MOI of 1. Two hour postinfection, the viral inoculum was removed, cells were washed twice with PBS, and 2.5 ml of supplemented RPMI medium was applied in each of the flasks. At various timepoints the culture medium was removed, cells were washed twice with PBS, and one ml of TRIzol Reagent (Invitrogen) was added to each well to lyse cells. Total RNA were isolated using the protocol provided by the manufacturer, followed by removal of contaminating genomic DNA by DNAse I treatment (Roche Molecular Biochemicals, Indianapolis, IN). High-quality RNA was confirmed by running an aliquot of each sample on a denaturing formaldehyde/agarose/EtBr gel.
Quantitative analysis of mRNA expression was performed using real-time reverse transcriptase-PCR. Three micrograms of total RNA were reverse transcribed to cDNA, using Oligo(dT)15 primer (Promega) and SuperScript III reverse transcriptase (Invitrogen), following the protocol provided by the manufacturer. Synthesized cDNA was normalized to β-actin or GAPDH mRNA levels. Relative VV K1L gene expression (fold-change in VV.mIFNβ-treated samples versus control) was determined, which gives a 131-base pair PCR amplified product. The sequence for forward primer of K1L gene is: GGACACGTGGATATGATGATTCTC, and the one for the reverse primer is: TGAACAGAGCCTGTAACATCTCAAT. The annealing temperature for this primer pairs is 60 ºC. Each sample was run in quadruplicate and the experiment was repeated at least once using the using Smart Cycler System (Cepheid, Sunnyvale, CA).
Cytokine production measured with enzyme-linked immunosorbent assays. For culture supernatants, TC-1 and LKRM2 cells (106) were plated in 6-well plates in triplicate and were incubated in 3 ml of supplemented RPMI or DMEM medium overnight. On the next day, the cells were infected with different VV vectors at MOI 1 in a total volume of 1 ml. After 2 hours, the inoculum was aspirated, cells were washed with PBS twice and 3 ml of culture medium were applied. The culture supernatants were collected, spun for 10 minutes at 13,000 r.p.m. to remove detached cells and kept at −80 °C till cytokine analyses. For tumor samples from mice, TC-1 and LKRM2 tumors were harvested at various timepoints. Tumors were weighed, and lysis buffer (PBS with 1% Triton-X (FisherBiotech, Fair Lawn, NJ), 1 mmol/l phenylmethanesulfonyl fluoride (cat no. P7626-5G; Sigma-Aldrich, St Louis, MO) plus other protease inhibitors (Complete Mini Protease inhibitors; cat no: 13999600; Roche Diagnostic, Mannheim, Germany) were added according to the tumor weight (100 mg/ml of lysis buffer). All the enzyme-linked immunosorbent assay protocols provided by the manufacturers were followed: including IFNβ (cat no: 42400-1; PBL Interferonsource, Piscataway, NJ), IP-10 (cat no: Duoset DY466; R&D Systems; Minneapolis, MN), CXCL11 (cat no: Duoset DY572; R&D Systems), MCP-1 (cat no: 555260; BD Biosciences, Franklin Lakes, NJ), KC (cat no: Duoset DY453; R&D Systems), tumor necrosis factor-α (cat no: 555268; BD Biosciences), interleukin-6 (cat no: 555240; BD Biosciences) and MIP-2 (Duoset DY452; R&D Systems).
Statistical analyses. For the reverse transcriptase-PCR and flank tumor studies comparing differences between two groups, we used unpaired Student t-tests. For flank tumor studies comparing more than two groups, we used one-way ANOVA with appropriate post hoc testing. Kaplan–Meier data were analyzed using Epi Info software developed by Centers for Disease Control and Prevention (Atlanta, GA). Differences were considered significant when P < 0.05. Data are presented as mean ± SEM.
SUPPLEMENTARY MATERIAL Figure S1. Spontaneous lung metastases formed in LKRM2 flank tumor model. Figure S2. Effect of Ad.mIFNβ and VV.mIFNβ on spontaneous lung metastases in LKRM2 model. Figure S3. Effect of different routes of VV.mIFNβ administration on spontaneous lung metastases in LKRM2 model. Figure S4. IFNβ production by Ad.mIFNβ and VV.mIFNβ in AB12 mouse mesothelioma cells. Figure S5. Effect of CD8 depletion on VV.mIFNβ-induced lung metastases growth inhibition. Figure S6. Flow cytometry analyses of splenic population upon depletion of various immune subsets. Figure S7. Effect of CD4, NK, and neutrophil depletion on VV.mIFNβ-mediated antitumor response. Figure S8. Comparison of VV.Luc- and VV.mIFNb-induced growth inhibition on spontaneous lung metastases. Figure S9. VV.Luc and VV.mIFNβ induced moderate antitumor response in a mutated k-ras orthotopic model. Figure S10. Loss of antitumor efficacy of VV vector with VGF/TK deleted backbone. Figure S11. Replication of VV.(VGF−/TK−).Luc in vitro and in vivo. Table S1. Chemokine induction by VV.(VGF−/TK−).Luc in TC-1 and LKRM2 tumors.
Acknowledgments
This work was supported by NCI (National Cancer Institute) grant P01 CA66726 (to S.M.A.), and an NRSA (National Research Service Award) training grant 5-T32 HL07748 (to L.-C.S.W.). S.N.I. was supported by U01 AI066333, and S.H.T. was supported by NCI grant R01 CA140215. The authors thank Jennerex Biotherapeutics for providing the vaccinia viral vectors. The authors declared no conflict of interest.
Supplementary Material
Spontaneous lung metastases formed in LKRM2 flank tumor model.
Effect of Ad.mIFNβ and VV.mIFNβ on spontaneous lung metastases in LKRM2 model.
Effect of different routes of VV.mIFNβ administration on spontaneous lung metastases in LKRM2 model.
IFNβ production by Ad.mIFNβ and VV.mIFNβ in AB12 mouse mesothelioma cells.
Effect of CD8 depletion on VV.mIFNβ-induced lung metastases growth inhibition.
Flow cytometry analyses of splenic population upon depletion of various immune subsets.
Effect of CD4, NK, and neutrophil depletion on VV.mIFNβ-mediated antitumor response.
Comparison of VV.Luc- and VV.mIFNb-induced growth inhibition on spontaneous lung metastases.
VV.Luc and VV.mIFNβ induced moderate antitumor response in a mutated k-ras orthotopic model.
Loss of antitumor efficacy of VV vector with VGF/TK deleted backbone.
Replication of VV.(VGF−/TK−).Luc in vitro and in vivo.
Chemokine induction by VV.(VGF−/TK−).Luc in TC-1 and LKRM2 tumors.
REFERENCES
- Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A., and, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- Vannucchi S, Chiantore MV, Mangino G, Percario ZA, Affabris E, Fiorucci G.et al. (2007Perspectives in biomolecular therapeutic intervention in cancer: from the early to the new strategies with type I interferons Curr Med Chem 14667–679. [DOI] [PubMed] [Google Scholar]
- Odaka M, Wiewrodt R, DeLong P, Tanaka T, Zhang Y, Kaiser L.et al. (2002Analysis of the immunologic response generated by Ad.IFN-β during successful intraperitoneal tumor gene therapy Mol Ther 6210–218. [DOI] [PubMed] [Google Scholar]
- Wilderman MJ, Sun J, Jassar AS, Kapoor V, Khan M, Vachani A.et al. (2005Intrapulmonary IFN-β gene therapy using an adenoviral vector is highly effective in a murine orthotopic model of bronchogenic adenocarcinoma of the lung Cancer Res 658379–8387. [DOI] [PubMed] [Google Scholar]
- Brin E, Atencio I, Helmich BK, Maneval D., and, Laface D. Adenovirus delivery provides extended interferon-α exposure and augments treatment of metastatic carcinoma. Cancer Gene Ther. 2006;13:664–675. doi: 10.1038/sj.cgt.7700942. [DOI] [PubMed] [Google Scholar]
- Sterman DH, Gillespie CT, Carroll RG, Coughlin CM, Lord EM, Sun J.et al. (2006Interferon β adenoviral gene therapy in a patient with ovarian cancer Nat Clin Pract Oncol 3633–639. [DOI] [PubMed] [Google Scholar]
- Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A.et al. (2007A phase I clinical trial of single-dose intrapleural IFN-β gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses Clin Cancer Res 1315 Pt 14456–4466. [DOI] [PubMed] [Google Scholar]
- Sterman DH, Recio A, Haas AR, Vachani A, Katz SI, Gillespie CT.et al. (2010A phase I trial of repeated intrapleural adenoviral-mediated interferon-β gene transfer for mesothelioma and metastatic pleural effusions Mol Ther 18852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca EA, Smith KM, McKinney B, Palmer CA, Rosenfeld S, Lillehei K.et al. (2008A phase I trial of Ad.hIFN-β gene therapy for glioma Mol Ther 16618–626. [DOI] [PubMed] [Google Scholar]
- Sterman DH, Haas A, Moon E, Recio A, Schwed D, Vachani A.et al. (2011A trial of intrapleural adenoviral-mediated interferon-{α}2b gene transfer for malignant pleural mesothelioma Am J Respir Crit Care Medepub ahead of print). [DOI] [PMC free article] [PubMed]
- Kirn DH, Wang Y, Le Boeuf F, Bell J., and, Thorne SH. Targeting of interferon-β to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH., and, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC.et al. (2008Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial Lancet Oncol 9533–542. [DOI] [PubMed] [Google Scholar]
- Liu TC, Hwang T, Park BH, Bell J., and, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- Adamina M, Weber WP, Rosenthal R, Schumacher R, Zajac P, Guller U.et al. (2008Heterologous prime-boost immunotherapy of melanoma patients with Influenza virosomes, and recombinant Vaccinia virus encoding 5 melanoma epitopes and 3 co-stimulatory molecules. A multi-centre phase I/II open labeled clinical trial Contemp Clin Trials 29165–181. [DOI] [PubMed] [Google Scholar]
- Thorne SH, Liang W, Sampath P, Schmidt T, Sikorski R, Beilhack A.et al. (2010Targeting localized immune suppression within the tumor through repeat cycles of immune cell-oncolytic virus combination therapy Mol Ther 181698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH, Negrin RS., and, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Liang W, Contag CH., and, Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68:2071–2075. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A., and, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83 Pt 12:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Kapoor V, Buchlis G, Cheng G, Sun J, Wang LC.et al. (2011Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells Am J Respir Cell Mol Biol 44230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA.et al. (2001Somatic activation of the K-ras oncogene causes early onset lung cancer in mice Nature 4101111–1116. [DOI] [PubMed] [Google Scholar]
- Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V.et al. (2006Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine Clin Cancer Res 12214–222. [DOI] [PubMed] [Google Scholar]
- Diebold S. Innate recognition of viruses. Immunol Lett. 2010;128:17–20. doi: 10.1016/j.imlet.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Samuel CE.2001Antiviral actions of interferons Clin Microbiol Rev 14778–809.table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd P., and, Lemay G. Correlation between interferon sensitivity of reovirus isolates and ability to discriminate between normal and Ras-transformed cells. J Gen Virol. 2005;86 Pt 5:1489–1497. doi: 10.1099/vir.0.80628-0. [DOI] [PubMed] [Google Scholar]
- Saloura V, Wang LC, Fridlender ZG, Sun J, Cheng G, Kapoor V.et al. (2010Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-β for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy Hum Gene Ther 2151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero B., and, Esteban M. The interferon system and vaccinia virus evasion mechanisms. J Interferon Cytokine Res. 2009;29:581–598. doi: 10.1089/jir.2009.0073. [DOI] [PubMed] [Google Scholar]
- Battcock SM, Collier TW, Zu D., and, Hirasawa K. Negative regulation of the α interferon-induced antiviral response by the Ras/Raf/MEK pathway. J Virol. 2006;80:4422–4430. doi: 10.1128/JVI.80.9.4422-4430.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmulevitz M, Pan LZ, Garant K, Pan D., and, Lee PW. Oncogenic Ras promotes reovirus spread by suppressing IFN-β production through negative regulation of RIG-I signaling. Cancer Res. 2010;70:4912–4921. doi: 10.1158/0008-5472.CAN-09-4676. [DOI] [PubMed] [Google Scholar]
- Katsafanas GC., and, Moss B. Vaccinia virus intermediate stage transcription is complemented by Ras-GTPase-activating protein SH3 domain-binding protein (G3BP) and cytoplasmic activation/proliferation-associated protein (p137) individually or as a heterodimer. J Biol Chem. 2004;279:52210–52217. doi: 10.1074/jbc.M411033200. [DOI] [PubMed] [Google Scholar]
- Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM.et al. (1996Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen Cancer Res 5621–26. [PubMed] [Google Scholar]
- Galivo F, Diaz RM, Thanarajasingam U, Jevremovic D, Wongthida P, Thompson J.et al. (2010Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus Hum Gene Ther 21439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamonici OR, Domanski P, Sweitzer SM, Larner A., and, Buller RM. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon α transmembrane signaling. J Biol Chem. 1995;270:15974–15978. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- Alcamí A, Symons JA., and, Smith GL. The vaccinia virus soluble α/β interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J Virol. 2000;74:11230–11239. doi: 10.1128/jvi.74.23.11230-11239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waibler Z, Anzaghe M, Frenz T, Schwantes A, Pöhlmann C, Ludwig H.et al. (2009Vaccinia virus-mediated inhibition of type I interferon responses is a multifactorial process involving the soluble type I interferon receptor B18 and intracellular components J Virol 831563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J., and, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri E., and, Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:109–118. doi: 10.2174/1568008053174723. [DOI] [PubMed] [Google Scholar]
- Jackson SS, Ilyinskii P, Philippon V, Gritz L, Yafal AG, Zinnack K.et al. (2005Role of genes that modulate host immune responses in the immunogenicity and pathogenicity of vaccinia virus J Virol 796554–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamí A., and, Smith GL. A soluble receptor for interleukin-1 β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Buller RM., and, Palumbo GJ. Poxvirus pathogenesis. Microbiol Rev. 1991;55:80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkova K, Babiarova K, Hainz P, Krystofova J, Kutinova L, Otahal P.et al. (2009The expression of the soluble isoform of hFlt3 ligand by recombinant vaccinia virus enhances immunogenicity of the vector Oncol Rep 211335–1343. [DOI] [PubMed] [Google Scholar]
- Jäger E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D.et al. (2006Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients Proc Natl Acad Sci USA 10314453–14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik, JD, Twelves, CJ, Selby, PJ, Vile, RG and Chester, JD (2011). Immune recruitment and therapeutic synergy: keys to optimizing oncolytic viral therapy? Clin Cancer Res 17: 4214–4224. pp. [DOI] [PMC free article] [PubMed]
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA.et al. (2009The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon Hum Gene Ther 201119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK.et al. (2001Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes Cancer Res 618751–8757. [PubMed] [Google Scholar]
- Odaka M, Sterman DH, Wiewrodt R, Zhang Y, Kiefer M, Amin KM.et al. (2001Eradication of intraperitoneal and distant tumor by adenovirus-mediated interferon-β gene therapy is attributable to induction of systemic immunity Cancer Res 616201–6212. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spontaneous lung metastases formed in LKRM2 flank tumor model.
Effect of Ad.mIFNβ and VV.mIFNβ on spontaneous lung metastases in LKRM2 model.
Effect of different routes of VV.mIFNβ administration on spontaneous lung metastases in LKRM2 model.
IFNβ production by Ad.mIFNβ and VV.mIFNβ in AB12 mouse mesothelioma cells.
Effect of CD8 depletion on VV.mIFNβ-induced lung metastases growth inhibition.
Flow cytometry analyses of splenic population upon depletion of various immune subsets.
Effect of CD4, NK, and neutrophil depletion on VV.mIFNβ-mediated antitumor response.
Comparison of VV.Luc- and VV.mIFNb-induced growth inhibition on spontaneous lung metastases.
VV.Luc and VV.mIFNβ induced moderate antitumor response in a mutated k-ras orthotopic model.
Loss of antitumor efficacy of VV vector with VGF/TK deleted backbone.
Replication of VV.(VGF−/TK−).Luc in vitro and in vivo.
Chemokine induction by VV.(VGF−/TK−).Luc in TC-1 and LKRM2 tumors.