Abstract
The particular importance of the vagus nerve for the pathophysiology of peritonitis becomes more and more apparent. In this work we provide evidence for the vagal modulation of inflammation in the murine model of colon ascendens stent peritonitis (CASP). Vagotomy significantly increases mortality in polymicrobial sepsis. This effect is not accounted for by the dilatation of gastric volume following vagotomy. As the stimulation of cholinergic receptors by nicotine has no therapeutic effect, the lack of nicotine is also not the reason for the reduced survival rate. In fact, increased septic mortality is a consequence of the absent modulating influence of the vagus nerve on the immune system: we detected significantly elevated serum corticosterone levels in vagotomised mice 24 h following CASP and a decreased ex vivo TNF-alpha secretion of Kupffer cells upon stimulation with LPS. In conclusion, the vagus nerve has a modulating influence in polymicrobial sepsis by attenuating the immune dysregulation.
1. Introduction
Peritonitis and subsequent sepsis remain a severe problem in the surgical field. The immune response of a septic organism is mediated by the adaptive and the innate immune system. Beside “classical” mechanisms like humoral and paracrine regulation or direct cell-to-cell interaction, the autonomic nervous system seems to be critically involved. Neural circuits that directly control certain immune reactions, also known as the “inflammatory reflex”, were identified [1–3]. Within these neural circuits, the vagus nerve plays an important role as it mediates afferent effects triggered by inflammatory mediators and also interacts with visceral organs by efferent activity.
During sepsis, the vagus nerve is essential for balancing anti- and pro-inflammation. Experimental vagotomy results in hyperinflammation and can lead to increased mortality [4–7]. Several studies showed that animals deficient in vagus nerve activity are more sensitive to inflammatory challenges like endotoxemia, sepsis, pancreatitis [8] and hypovolemic shock. This may be due to the uncontrolled hyperinflammation which is mirrored by a critically increased release of proinflammatory cytokines [1, 6, 7, 9].
An efferent vagal connection to adrenal glands is established [10–12]. However, there is still an incomplete understanding of the potential role of vagal nerve in context of adrenal gland derived glucocorticoids. It is known that adrenal gland function is essential for the outcome of patients with sepsis [13–15]. Nevertheless, glucocorticoid therapy in sepsis, based on their ability to induce IL-10 elevation und TNF-alpha decrease, was controversially discussed [16–18]. Additionally, vagal activity seems to be involved in fever regulation [19, 20], apoptosis [21, 22], and regeneration of hepatocytes [23]. The liver and its resident macrophages, the Kupffer cells (KCs), are considered to play a crucial role in the course of sepsis [24]. An anatomical link between the vagus nerve and the liver is well described: the hepatic branch of the vagus nerve [25, 26]. KCs produce many kinds of soluble mediators such as cyokines, especially IL-10 and TNF-alpha, prostanoids, proteases, and oxygen radicals [27, 28]. 80–90% of them are located in the liver [27]. A vagal modulation of the cytokine release by Kupffer cells in inflammation is presumed, but poorly understood [29].
The aim of this study was to further investigate the role of the vagus nerve in the course of peritonitis. Therefore, the colon ascendens stent peritonitis (CASP) was used. In contrast to models of LPS shock, CASP is a model of polymicrobial abdominal sepsis, that mirrors a common course of systemic infection in surgical intensive care patients [30–32]. The influence of subdiaphragmal vagotomy on corticosterone release by adrenal glands and KCs function after CASP were examined. Additionally, the potential therapeutic effect of nicotine as an unspecific agonist of nicotinic acetylcholine receptors (nACHRs) during peritonitis was analyzed.
2. Methods
2.1. Mice
For all experiments, 8- to 12-week-old female C57BL/6 mice purchased from Charles River (Sulzfeld, Germany) (weight 20–25 g) were used. Prior to surgery, mice were kept for at least 2 weeks in the animal facility to recover from transport. All experimental procedures were performed according to German animal safety regulations. For all surgical procedures, Avertin (Sigma-Aldrich Chemie, Taufkirchen) anaesthesia was used.
2.2. CASP Surgery
The surgical procedure for CASP was performed as previously described [30, 31]. After disinfection of the abdomen, the ascending colon was identified and a prepared catheter (16 gauge, Venflon; BOC, Ohmeda, Sweden) was implanted in the antimesenteric wall of the ascending colon. To ensure intraluminal positioning of the stent, stool was milked from the ascending colon into the stent. Afterwards, 0.5 mL of sterile saline solution was flushed into the peritoneal cavity before closure of the abdominal walls (single layer; 4/0 Polyester, Catgut, Markneukirchen, Germany).
2.3. Vagotomy
Upper abdominal wall was opened through a transverse incision. Esophagus was exposed by carefully keeping costal arc, liver, and stomach out of sight. Further preparation was done using a surgical microscope (40-times magnification, Leica M651, Bensheim, Germany). The ventral branch of the vagal nerve was exposed and about 3 mm were excised (see Figure 1). After its passage through the diaphragm, the esophagus was mobilized on its hepatic side and lifted. The dorsal branch of the vagal nerve was exposed and about 3–5 mm were resected. After fluid resuscitation (0.5 mL of sterile saline solution), the abdominal wall was closed (one layer; 4/0 Polyester, Catgut, Germany). For control purposes, sham operations without transsection of the vagal nerve were performed.
Figure 1.
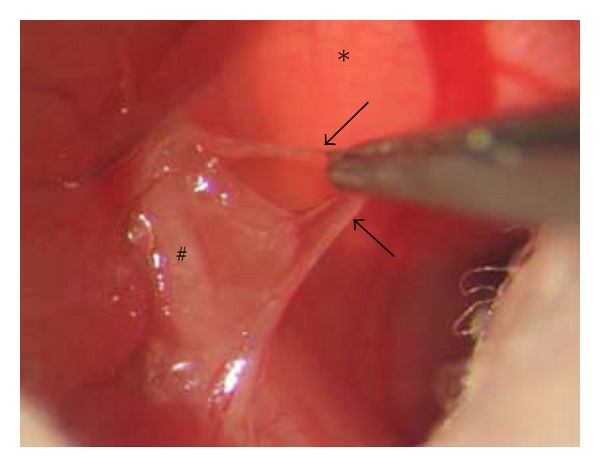
Surgical dissection of the vagus nerve using a microscope (40-fold magnification): the abdominal wall was opened through a transverse incision. Esophagus (#) and diaphragm (∗) were exposed and the ventral branch of the vagal nerve (arrows) was dissected. The dorsal branch of the vagus nerve was excised in the same way.
2.4. Implantation of Osmotic Pumps
Alzet osmotic pumps (Modell 1002, Alzet, Cupertino, USA) where filled with nicotine dissolved in 0.9% NaCl according to manufacturer instructions. With a liberation volume of 0.20–0.22 μL/h nicotine concentrations were adapted to the individual weight of each animal to ensure the expected liberation rate of 1, 1.5, and 3 mg/kg bodyweight (BW)/h. Control groups received osmotic pumps filled with 0.9% NaCl. After a short incision in the neck region of the mice, the primed pumps were placed in a subcutaneous pouch. Thereafter, the incision was closed (4/0 Polyester, Catgut, Germany).
2.5. Isolation of Kupffer Cells
The isolation procedure was modified from Valatas et al. [33]. All steps were performed with sterile solutions at pH = 7.4. The portal vein was identified, punctured with a 22-gauge cannula and irrigated by 50 mL pretempered HBSS. To allow the irrigation solution to evade the venous system, the inferior vena cava was incised right above its bifurcation. Afterwards, the liver was perfused with pronase E 0.4% for enzymatic digestion of hepatocytes and connective tissue and disintegration of cell junctions. A second enzyme mixture containing collagenase 0.0143% and DNAse 0.0014% was applied to dissolve the extracellular matrix and released DNA. The liver was transferred onto a petri dish, the capsule was cut and the parenchyma carefully fragmented before a third enzymatic digestion (using pronase E 0.1% and DNAse 0.01%) was performed under constant agitation. The cell suspension was pushed through a nylon mesh to remove larger cell aggregates. Subsequent steps were conducted with 4°C media to abate cell attachment on plastic surfaces.
A step of differential centrifugation followed (10 min, 380 g, 4°C) to wash out the residual enzymatic solution, DNA, and cell debris. The supernatant was discarded, the pellet resuspended and density gradient centrifugation was performed to separate parenchymal from nonparenchymal cells. Iodixanol (OptiPrep) was applied as seperation medium. HBSS and OptiPrep were added to the cell suspension to gain 4 mL of a 11.7% OptiPrep solution which was bedded on a density cushion of 4 mL 17.6% OptiPrep. An additional 4 mL of HBSS was used as a top finishing. Density centrifugation was carried out at 1400 g and 4°C for 17 min. The resulting layer of mainly non-parenchymal cells on top of the 11.7% OptiPrep cushion was carefully removed and transferred into RPMI+ medium followed by another centrifugation step (10 min, 380 g, 4°C). The pellet was resuspended with 1 mL RPMI+.
Total number of non-parenchymal cells was assed using the Neubauer chamber. Adding the appropriate amount of RPMI+ a final cell concentration of 5 × 104/mL was generated. For cell culture a 96-well culture plate was used and 200 μL of cell suspension was added. Kupffer cell function was shown by phagocytosis of fluorescing latex beads (3 μm Fluoresbrite). Kupffer cell purity was analysed by cell adherence to glass slides and subsequent immunofluorescence staining with FITC-conjugated anti-F4/80 antibody. We reached purity of 79 percent. Cells were kept at 37°C and 5% CO2-atmosphere. After 24 hours of incubation, cell media and all nonadherent cells were removed by thorough washing. All subsequent stimulation experiments were performed using FCS-free media.
2.6. Stimulation of Kupffer Cells
Kupffer cells were cultured in 96-well plates containing 1 × 104 cells per well in 200 μL cell culture medium (RPMI without FCS). After 24 hours, medium was changed and cells were stimulated with LPS (E. coli, Sigma-Aldrich Chemie, Taufkirchen) at concentrations of 0.1 μg/mL, 1 μg/mL and 10 μg/mL (same volume of medium in each well) dissolved in 1 × PBS. After 24 hours, the supernatant was transferred to 1.5 mL Eppendorf tubes and centrifuged (10 min; 16100 g; 4°C; Centrifuge 5415R, Eppendorf, Germany). Cytokines/chemokines were analysed using a commercial available kit (BD cytometric bead array mouse inflammation kit, BD bioscience, Heidelberg, Germany).
2.7. MRI Imaging and Analysis
MRI was modified from Partecke et al. [34]. For all MRI studies, anaesthesia had to be carried out using isoflurane (1%–1.5%). The depth of anaesthesia was monitored by the breathing rate (about 40 breaths per minute). MRI sequences were triggered by breathing rate. To reduce the influence of bowel motility in all MRI examinations, mice were kept nil per os (NPO) for at least 4 hours before starting MRI scans. For MRI scans, all mice were placed in a supine position. Mice were scanned in a high-field 7.0 Tesla MRI scanner for small animals (Bruker, ClinScan, 7.0 Tesla, 290 mTesla/m gradient strength, Bruker, Ettlingen, Germany). MRI scans were performed in a whole mouse body coil (Bruker, Ettlingen, Germany) using a T2-TSE (turbo spin echo) sequence. For size and volume assessment, we used high resolution coronary and axial T2-weighted images (coronary plane: TR (repetition time): ca. 1200 ms; TE (echo time): 41.0 ms; FA (flip angle): 180°; FoV (field of view): 42 mm × 42 mm; matrix: 240 × 320; 24 slices of 0,7 mm per slice, acquisition time: ca. 15 min; axial plane: TR: ca. 1250 ms; TE: 41.0 ms; FA: 180°; FoV: 40 mm × 40 mm; matrix: 240 × 320; 24 slices of 0,7 mm per slice, acquisition time: ca. 10 min). Generated images were analyzed using MIPAV (medical imaging processing and visualisation, National Institutes of Health, Bethesda, MD, USA) and Image J (Image Processing and Analysis in Java, National Institutes of Health). By defining regions of interest (ROI) on each slice, the software was able to calculate volumes and diameters. This was finally done by a complex algorithm using all image inherent information including thickness of slices, resolution as well as size of ROIs.
2.8. Serum Corticosterone Levels
For the detection of serum corticosterone levels, we used a commercially available ELISA-kit (Corticosterone (Rat/Mouse) Elisa, DRG Instruments GmbH, Marburg, Germany) following customers instructions. Serum was separated by centrifugation of whole blood (10 min; 16100 g; Centrifuge 5415R, Eppendorf, Germany).
2.9. Survival Analysis
Survival of animals was observed for 240 hours after CASP induction.
2.10. Statistical Methods
Statistical analysis was performed using GraphPad Prism for Windows software (GraphPad Software, San Diego, CA, USA). Statistical differences in survival rates were assessed using log-rank test. Results from cytokine levels were analyzed using the two-tailed Mann-Whitney U test for nonparametric probes. A significance level of 0.05 was applied for all calculations.
3. Results
3.1. Vagotomy Increases the Mortality in Polymicrobial Sepsis
To analyse the influence of the vagus nerve on the mortality in polymicrobial sepsis, we compared the survival rate of mice in the following surgical procedures: CASP, vagotomy (VGX), CASP in combination with vagotomy. Sham-operation was performed in the control group. Sham-surgery as well as vagotomy did not change the survival rate of mice as both were 100% (Figure 2, n = 10 per group). Comparable to our recent data, the induction of a septic peritonitis by CASP significantly decreased the survival rate to 63.6% (P = 0.025, n = 33). The survival of the vagotomised CASP group (VGX + CASP, n = 33) was significantly decreased further to 35.3% (P = 0.048). Thus, the intact vagus function is essential for the survival in polymicrobial sepsis, whereas vagotomy in absence of a septic focus does not affect the survival rate.
Figure 2.
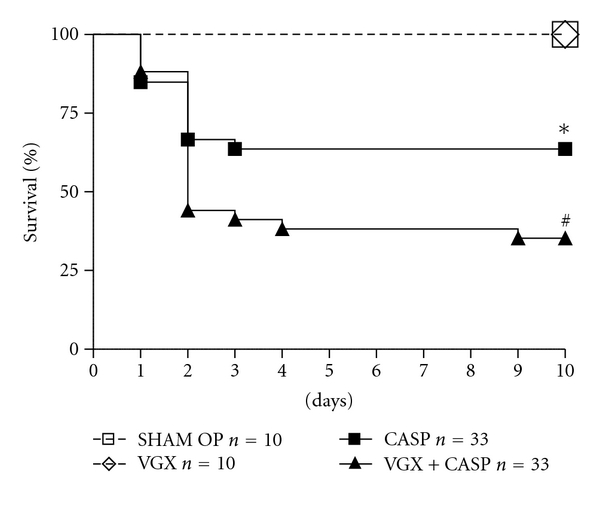
The mortality in polymicrobial sepsis (CASP) is significantly increased compared to sham-operation (laparotomy without CASP or vagotomy) by 63.6% versus 100% (*P = 0.025, n = 10 and 33, resp.). The survival of the vagotomised CASP group (VGX + CASP, n = 33) is significantly decreased further to 35.3% (CASP versus VGX + CASP: # P = 0.048). Vagotomy itself (n = 10) does not affect the survival rate (100%).
3.2. The Effect of Enlarged Gastric Volume by Vagotomy Is Independent of the Presence of Sepsis
By visualization of the stomach in small animal 7-Tesla-MRI, we confirmed that vagotomy results in an increased gastric volume. Figure 3(a) displays the regular empty gastric volume (193 ± 9 mm3) in untreated animals. Seven days following vagotomy, the volume was extended to 1064 ± 71.7 mm3. This difference is significant as shown in panel c (n = 6, P < 0.0001). In polymicrobial sepsis, the gastric volume was detected with 320.8 ± 41.64 mm3, which is a significant increase to the control group (P < 0.05) but a decrease as compared with the volume following the vagotomy procedure (P < 0.0001). Induction of peritonitis in vagotomised mice results in an enlarged gastric volume of 836.7 ± 151 mm3 at 24 hours postoperative. Vagotomy increases the gastric volume in the presence as well as in the absence of sepsis. These data suggest that the dilated intestine and the resulting ileus may not be the reason for the increased mortality in vagotomised septic mice, as these findings are also observed in septic mice (100% survival).
Figure 3.
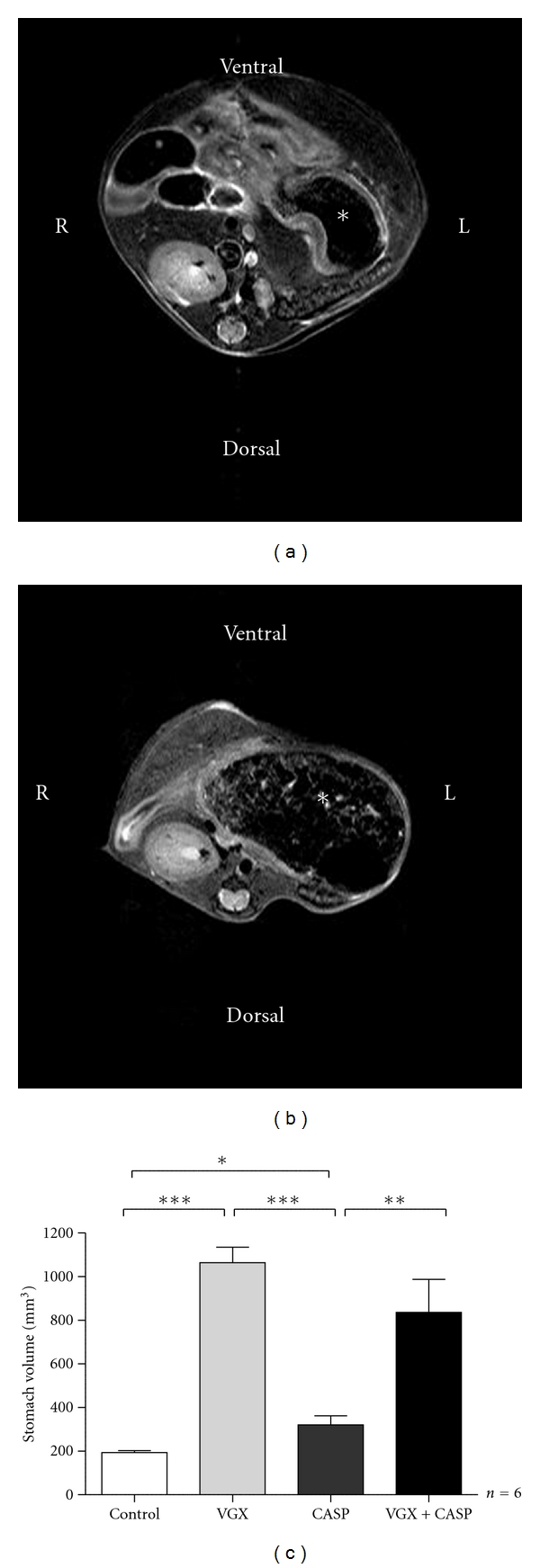
Images of MRI of the upper abdomen of mice. The asterisk identifies the stomach. Panel a shows the sectional image of an untreated mouse with normal gastric volume (a). Panel b displays the representative scan of a mouse with increased gastric volume after CASP and vagotomy (b). Values of gastric volume are shown in panel c. CASP alone led to a slight but significant increase of gastric volume. In both vagotomy groups, a strong dilatation of the stomach was measured that was independent from the presence of sepsis (c). (n = 6 per group, *P < 0.05, **P < 0.01, and ***P < 0.0001).
3.3. The Stimulation of Nicotinic Acetylcholine Receptors Has No Effect on the Survival in Polymicrobial Sepsis
The influence of a continuous application of nicotine on the survival in sepsis was analysed following implantation of osmotic pumps subcutaneously before induction of CASP. By this method, a detectable and dose-dependent serum level of cotinine, a metabolite of nicotine, can be reached (Figure 4(a)). The survival rate in septic peritonitis during continuous administration of nicotine in different body-weight (BW)-adapted dosages was compared: 1 mg/kgBW/h, 1.5 mg/kgBW/h, and 3 mg/kgBW/h. In the control group the osmotic pumps released NaCl 0.9%, and the survival rate was detected by 9.67% (Figure 4(b), n = 31). A dosage of 1 mg/kgBW/h correlates with a survival of 20% (n = 10), 1.5 mg/kgBW/h (n = 32) leads to a survival of 21.85% and 3 mg/kgBW/h to a survival of 12.5% (n = 8). These data suggest that a systemic application of the unspecific nicotinic acetylcholine receptor agonist nicotine has no protective effect on the outcome of septic peritonitis.
Figure 4.
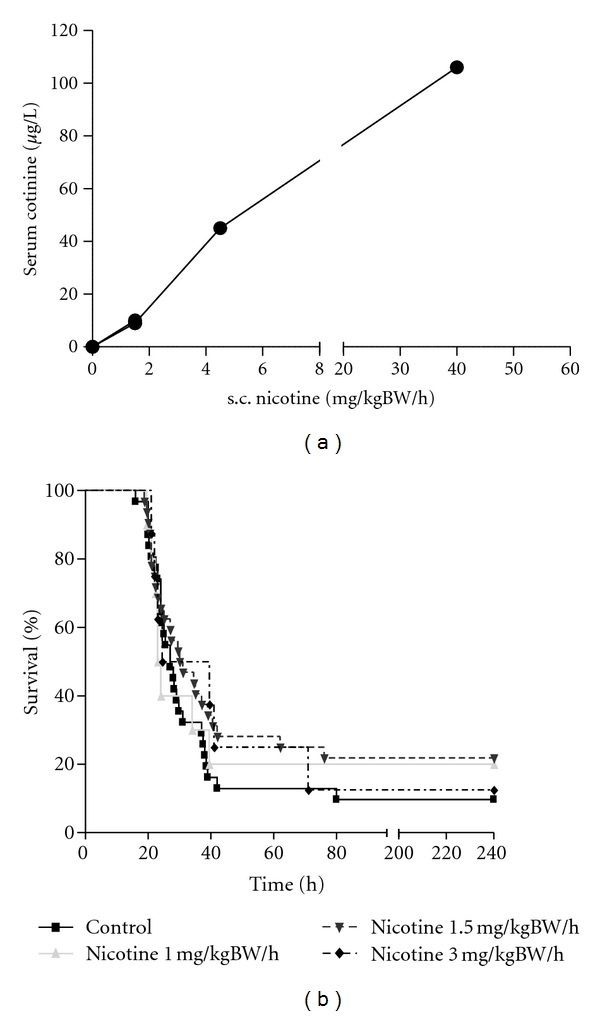
(a) To verify the therapeutic dose of nicotine in the murine serum, we analysed the serum cotinine level as this correlates with the amount of nicotine dose applicated by osmotic pumps. Application of nicotine in a dose of 1.5 mg/kgBW/h results in a serum cotinine level of 9.24 μg/l (n = 4). 4.5 mg/kgBW/h nicotine leads to a cotinine serum concentration of 45 μg/l (n = 1) and 40 mg/kgBW/h nicotine induces a cotinine serum concentration of 106 μg/l (n = 1). Serum samples were analysed 18 hours following CASP. (b) The survival in CASP is not altered by continuous nicotine administration through subcutaneously implanted osmotic pumps. A nicotine exposition of 1 mg/kg bw per hour correlates with a survival rate of 20% (n = 10), 1.5 mg/kgBW/h (n = 32) nicotine leads to a survival rate of 21.85% and 3 mg/kgBW/h nicotine correlates with a survival rate of 12,5% (n = 8). In the control group, NaCl was applied where the survival rate was detected with 9.76% (n = 31).
3.4. Serum Corticosterone Levels in Sepsis Are Significantly Elevated in Vagotomised Mice
Due to the central role assigned to adrenal glands in peritonitis [13–15, 35], we focused on the influence of the vagus nerve on the adrenal gland function in polymicrobial sepsis (Figure 5). Vagotomy in the nonseptic organism did not influence the corticosterone level (205.9 ± 56.75 ng/mL) as compared to a control group. In polymicrobial sepsis, the serum corticosterone was detected with 159.4 ± 53.24 ng/mL. In contrast, 24 h following CASP in vagotomised mice the corticosterone level is significantly increased up to 975.4 ± 261.9 ng/mL (*P = 0.031, n = 5). This indicates that the serum levels of corticosterone in sepsis are modulated by the vagus nerve.
Figure 5.
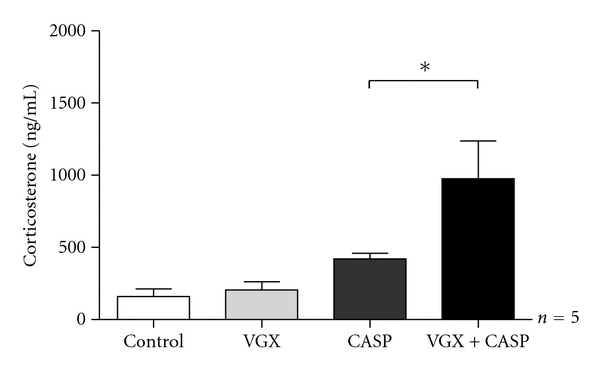
The serum levels of corticosterone in sepsis are modulated by the vagus nerve. Vagotomy in the nonseptic organism does not influence the corticosterone level (205.9 ± 56.75 ng/mL). In polymicrobial sepsis, serum corticosterone is detected with 159.4 ± 53.24 ng/mL. In contrast, 24 h following CASP in vagotomised mice the corticosterone level is significantly increased up to 975.4 ± 261.9 ng/mL (*P = 0.031, Mann-Whitney, n = 5).
3.5. The Vagus Nerve Has a Stimulating Effect on the EX Vivo Cytokine Release of Kupffer Cells
Kupffer cells were isolated seven days following vagotomy. The levels of TNF-α were detected in the cell culture supernatant. Kupffer cells isolated from untreated animals served as control. The basal TNF-α release of Kupffer cells ex vivo was significantly decreased as compared with the control group (164.7 ± 40.9 pg/mL versus 61.1 ± 4.4 pg/mL, *P = 0.04). Furthermore, we stimulated Kupffer cells ex vivo with 1 μg/mL lipopolysaccharide from E. coli (LPS). In the septic organism, there was a significantly decreased TNF-α release by stimulated Kupffer cells from vagotomised mice (2960 ± 513.1 pg/mL) when compared to mice with an intact vagus nerve (5746 ± 292.5 pg/mL, ***P = 0.0002, n = 10 per group). These data substantiate the hypothesis that the vagus nerve has an immunological influence on Kupffer cell function.
4. Discussion
The present study underlines the crucial role of the vagus nerve for the survival in septic peritonitis. Impaired vagal function results in increased mortality rates in inflammatory animal models (CASP) [7]. Stimulation of nervus vagus function ameliorates survival as described for different animal models like CLP, LPS application, or i.p. E. coli injection [1, 6, 36]. Additionally, the vagus nerve has strong influence on the intestinal tonus: increased ileus incidence is described following vagotomy [37], whereas vagus stimulation attenuates the postoperative ileus [38].
Surgical trauma or peritoneal inflammation can result in paralysis and consecutive ileus, too. It could therefore be possible that the ileus triggered by vagotomy is the critical factor of decreased survival rate in CASP following vagotomy. Vagotomy itself causes an increased pylorus tone with delayed gastric emptying (DGE) as we could describe by measuring gastric volumes in MRI scans (Figure 3(a)). Vagotomy without induction of sepsis significantly enlarged gastric volume when compared to CASP mice. In combination of both, Vagotomy and CASP, the vagotomy effect seems to stimulate ileus by sepsis. Our data on postoperative mortality suggest an unchanged survival rate in vagus dissected animals (Figure 2). Surgical sectioning of the vagus nerve per se is performed in procedures like gastrectomy or oesophagectomy and itself does not result in elevated mortality rates [39–42]. Therefore, vagal impairment is responsible for marked DGE and ileus but not for higher mortality.
Vagotomy results in an attenuated release of nicotine in efferent signaling. The role of nicotine in sepsis, especially its possible therapeutic effect, and stimulation of the vagus nerve were subject of several studies: The et al. described reduced experimental postoperative Ileus [43] using a central stimulus for the cholinergic pathway. Other studies define better survival in CLP and LPS models using nicotine as an unspecific stimulator of nACHR [1, 36]. In our experiments, we decided for continuous nicotine administration by subcutaneously implanted osmotic pumps, since in our opinion permanent administration ensures sufficient serum levels, especially due to the short half life of nicotine in C57Bl/6 mice (about 9 minutes) [44]. We reached adequate doses as demonstrated by the serum level of the nicotine metabolite cotinine (Figure 4(a)). We observed no change in survival rates by administration of nicotine (Figure 4(b)). This observation may attribute to our CASP model which in contrast to CLP or LPS models is associated with a very high bacterial load [30, 32]. Our finding of worse survival correlates with Westerloo et al. who administered living E. coli i.p. in mice [6]. They also detected even decreased survival after oral nicotine substitution. Action potentials transmitted in the vagus nerve lead to release of acetylcholine that blocks cytokine production by cells-expressing acetylcholine receptors. In particular, the efferent vagus can inhibit inflammation via interaction between acetylcholine and α7 subunit of cholinergic receptors [45]. Signal transduction by the nicotinic α7 cholinergic receptor subunit is the regulator of intracellular signals that control cytokine transcription and translation. Neutrophils expressing several nicotinic receptors, including the α7 cholinergic receptor [23], and stimulation of these receptors have been shown to inhibit neutrophil migration by a mechanism that involves inhibition of adhesion molecule expression on both the endothelial surface and neutrophils [23]. Mice deficient in α7 cholinergic subunit have an optimized bacterial clearance caused by a faster recruitment of neutrophils [46]. As the early recruitment of neutrophils to the site of an infection is considered important for an adequate antibacterial defense our present results are consistent with reports showing that nicotine (which stimulates α7 receptors) facilitates the growth and dissemination of E. coli after intraperitoneal infection [6]–the protective effect of nicotine administration seems to be not potent enough in case of massive living pathogen load.
Another advice for higher mortality in polymicrobial sepsis are the significantly increased corticosterone levels in vagotomized mice (Figure 5). Especially human cortisol has several anti-inflammatory and immunosuppressive effects, that is, reduced TNF-alpha, increased IL-10-concentrations and apoptosis of mature T-lymphocytes [16, 47]. Adrenal insufficiency is frequently diagnosed in critical ill patients with sepsis [48] and it is associated with a high mortality rate [13–15]. A glucocorticoide administration during human sepsis was a discussed controversial [17, 18]. In rodents cortisol is replaced by corticosterone because of lack of C17-hydroxylase function [49, 50], in many ways [51]. We found increased corticosterone levels in septic animals that had undergone subdiaphragmal vagotomy seven days before CASP procedure (Figure 5). CASP mice with intact vagus nerve had a moderate but not significant elevation of serum corticosterone, whereas vagotomy alone had no effect on serum corticosterone levels.
To compensate for vagotomy, the endocrine axis not only seems to develop a stronger countereffect, as reflected by the corticosterone response [52]: Our data support the hypothesis of a relevant connection between vagus nerve and adrenal glands [10–12, 53] and suggest a vagal regulative function concerning hypoinflammation during sepsis via corticosterone.
In the course of sepsis immune system alternates between hyper- and hypo-inflammation. Increased proinflammatory TNF-levels and compensatory anti-inflammation with increased IL-10 levels contribute to immune paralysis status [54–56]. This effect seems to be dependent of vital pathogens, corticosterone responses were not affected in LPS models evaluating effects of vagotomy by Hansen et al. [57] or Gaykema et al. [58].
Furthermore, our data from in vitro experiments with Kupffer cells (KCs) indicate in the same line. KCs taken from vagotomized mice secreted reduced TNF-alpha amount after LPS stimulus (Figure 6).
Figure 6.
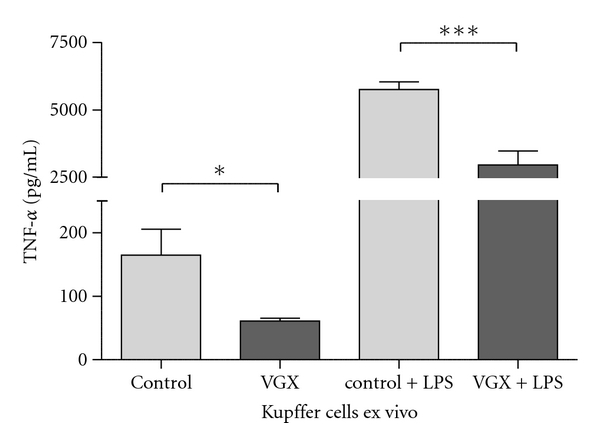
The vagus nerve has a stimulating effect on kupffer cells. Kupffer cells were isolated from control mice (column “control”) and from vagotomised mice without sepsis (column “VGX”). The basal TNF release of kupffer cells ex vivo is significantly decreased if mice were vagotomised 7 days before cell isolation (164.7 ± 40.9 pg/mL versus 61.1 ± 4.4 pg/mL, *P = 0.04). In addition, we stimulated the cells ex vivo with 1 μg/mL lipopolysaccharide from E.coli (LPS). In the septic organism, there is a significantly decreased TNF-α release by stimulated kupffer cells from vagotomised mice (2960 ± 513.1 pg/mL) when compared to mice with an intact nervus vagus (5746 ± 292.5 pg/mL, ***P = 0.0002, n = 10 per group).
Kupffer-cells are tissue macrophages located in the liver [27]. They liberate cytokines like TNF-alpha, IL-1, IL-6, and IL-10 and chemokines such as MCP-1 during inflammation [28]. Ikeda et al. stimulated Kupffer cells with acetylcholine and detected increased IL-6 secretion rates [23]. In our model, we performed subdiaphragmal vagotomy to dissect the established anatomical link by the hepatic branch of the vagus nerve [25, 26]. Our data correlate with Ikedas studies on cytokine release: ex vivo LPS stimulation induces a high level of TNF-alpha secretion. This LPS effect is attenuated in KCs isolated from animals that had undergone vagotomy seven days before (Figure 6). Basal rate (without LPS) of TNF-alpha secretion is significantly lower in vagotomy group. This underlines that the vagus nerve has a stimulating effect on the Kupffer cell activity. Following vagotomy, these macrophages are inhibited in their immunologic function, so the organism is impaired in its ability to cope with the septic situation. Their sensitivity seems to be downregulated resulting in less release of proinflammatory cytokines.
In summary, past studies have shown that the vagus nerve controls the immune response of hyperinflammation. Yet, our studies also suggest that the vagus nerve controls both stimulation as well as inhibition of inflammatory responses to severe bacterial threats. A high load of pathogens has unmasked the effects of the vagus nerve on states of hypoinflammation in our CASP model. Future studies will show the impact of further therapeutic modulation.
5. Conclusion
The vagal nerve plays an important role during peritonitis and leads to increased sepsis mortality after vagotomy. A stimulation of cholinergic receptors by nicotine has no therapeutic effect. Increased septic mortality seems to be a consequence of the absent modulating influence of the vagus nerve on the immune system. To underline this hypothesis we detect significantly elevated serum corticosterone levels in vagotomised mice 24 h following CASP and a decreased ex vivo TNF-alpha secretion of Kupffer cells upon stimulation with LPS. The recent study suggests that the vagus nerve controls both stimulation as well as inhibition of inflammatory responses to severe bacterial threats.
Author's Contribution
W. Kessler and S. Diedrich contributed equally to this paper.
Acknowledgments
The expert technical assistance of Antje Janetzko, Kathrin Mülling and Doreen Biedenweg is gratefully, acknowledged. This work was supported by DFG - GK 840 “Host pathogen interactions” and by the Bundesministerium für Forschung und Technolgie (BMBF no. 01ZZ0403 and no. 0314107).
References
- 1.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 2.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ. Reflex control of immunity. Nature Reviews Immunology. 2009;9(6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. Journal of Internal Medicine. 2009;265(6):663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlov VA, Tracey KJ. Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochemical Society Transactions. 2006;34(6):1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- 6.van Westerloo DJ, Giebelen IAJ, Florquin S, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. Journal of Infectious Diseases. 2005;191(12):2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 7.Kessler W, Traeger T, Westerholt A, et al. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbeck’s Archives of Surgery. 2006;391(2):83–87. doi: 10.1007/s00423-006-0031-y. [DOI] [PubMed] [Google Scholar]
- 8.van Westerloo DJ, Giebelen IA, Florquin S, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130(6):1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 10.Niijima A. Electrophysiological study on the vagal innervation of the adrenal gland in the rat. Journal of the Autonomic Nervous System. 1992;41(1-2):87–92. doi: 10.1016/0165-1838(92)90130-9. [DOI] [PubMed] [Google Scholar]
- 11.Coupland RE, Parker TL, Kesse WK, Mohamed AA. The innervation of the adrenal gland. III. Vagal innervation. Journal of Anatomy. 1989;163:173–181. [PMC free article] [PubMed] [Google Scholar]
- 12.González-Fernández I, Gonzalo-Sanz LM. Vagal influence on the adrenocortical function of the rat. Revista Espanola de Fisiologia. 1987;43(2):203–207. [PubMed] [Google Scholar]
- 13.Lipiner-Friedman D, Sprung CL, Laterre PF, et al. Adrenal function in sepsis: the retrospective Corticus cohort study. Critical Care Medicine. 2007;35(4):1012–1018. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 14.Annane D. Adrenal insufficiency in sepsis. Current Pharmaceutical Design. 2008;14(19):1882–1886. doi: 10.2174/138161208784980626. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Liu L, Zhao B, et al. Relationship between adrenal function and prognosis in patients with severe sepsis. Chinese Medical Journal. 2007;120(18):1578–1582. [PubMed] [Google Scholar]
- 16.Prigent H, Maxime V, Annane D. Clinical review: corticotherapy in sepsis. Critical Care. 2004;8(2):122–129. doi: 10.1186/cc2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briegel J, Sprung CL, Annane D, et al. Multicenter comparison of cortisol as measured by different methods in samples of patients with septic shock. Intensive Care Medicine. 2009;35(12):2151–2156. doi: 10.1007/s00134-009-1627-9. [DOI] [PubMed] [Google Scholar]
- 18.Kalil AC, Sun J. Low-dose steroids for septic shock and severe sepsis: the use of Bayesian statistics to resolve clinical trial controversies. Intensive Care Medicine. 2011;37(3):420–429. doi: 10.1007/s00134-010-2121-0. [DOI] [PubMed] [Google Scholar]
- 19.Roth J, De Souza GEP. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Brazilian Journal of Medical and Biological Research. 2001;34(3):301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- 20.Blatteis CM. The onset of fever: new insights into its mechanism. Progress in Brain Research. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- 21.Kiba T, Saito S, Numata K, Kon Y, Mizutani T, Sekihara H. Expression of apoptosis on rat liver by hepatic vagus hyperactivity after ventromedial hypothalamic lesioning. American Journal of Physiology. 2001;280(5):G958–G967. doi: 10.1152/ajpgi.2001.280.5.G958. [DOI] [PubMed] [Google Scholar]
- 22.Hiramoto T, Chida Y, Sonoda J, Yoshihara K, Sudo N, Kubo C. The hepatic vagus nerve attenuates Fas-induced apoptosis in the mouse liver via alpha7 nicotinic acetylcholine receptor. Gastroenterology. 2008;134(7):2122–2131. doi: 10.1053/j.gastro.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda O, Ozaki M, Murata S, et al. Autonomic regulation of liver regeneration after partial hepatectomy in mice. Journal of Surgical Research. 2009;152(2):218–223. doi: 10.1016/j.jss.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 24.Traeger T, Mikulcak M, Eipel C, et al. Kupffer cell depletion reduces hepatic inflammation and apoptosis but decreases survival in abdominal sepsis. European Journal of Gastroenterology and Hepatology. 2010;22(9):1039–1049. doi: 10.1097/MEG.0b013e32833847db. [DOI] [PubMed] [Google Scholar]
- 25.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Autonomic Neuroscience. 2000;85(1–3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 26.Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anatomy and Embryology. 1992;186(5):431–442. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- 27.Arii S, Imamura M. Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. Journal of Hepato-Biliary-Pancreatic Surgery. 2000;7(1):40–48. doi: 10.1007/s005340050152. [DOI] [PubMed] [Google Scholar]
- 28.Bone-Larson CL, Simpson KJ, Colletti LM, et al. The role of chemokines in the immunopathology of the liver. Immunological Reviews. 2000;177:8–20. doi: 10.1034/j.1600-065x.2000.17703.x. [DOI] [PubMed] [Google Scholar]
- 29.Wu R, Dong W, Cui X, et al. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Annals of Surgery. 2007;245(3):480–486. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traeger T, Koerner P, Kessler W, et al. Colon Ascendens Stent Peritonitis (CASP)—a standardized model for polymicrobial abdominal sepsis. Journal of Visualized Experiments. 2010;(46) doi: 10.3791/2299. Article ID e2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maier S, Traeger T, Entleutner M, et al. Cecal ligation and puncture versus colon ascendens stent peritonitis: two distinct animal models for polymicrobial sepsis. Shock. 2004;21(6):505–511. doi: 10.1097/01.shk.0000126906.52367.dd. [DOI] [PubMed] [Google Scholar]
- 32.Buras JA, Holzmann B, Sitkovsky M. Model organisms: animal models of sepsis: setting the stage. Nature Reviews Drug Discovery. 2005;4(10):854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 33.Valatas V, Xidakis C, Roumpaki H, Kolios G, Kouroumalis EA. Isolation of rat Kupffer cells: a combined methodology for highly purified primary cultures. Cell Biology International. 2003;27(1):67–73. doi: 10.1016/s1065-6995(02)00249-4. [DOI] [PubMed] [Google Scholar]
- 34.Partecke IL, Kaeding A, Sendler M, et al. In vivo imaging of pancreatic tumours and liver metastases using 7 Tesla MRI in a murine orthotopic pancreatic cancer model and a liver metastases model. BMC Cancer. 2011;11, article 40 doi: 10.1186/1471-2407-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nougaret S, Jung B, Aufort S, Chanques G, Jaber S, Gallix B. Adrenal gland volume measurement in septic shock and control patients: a pilot study. European Radiology. 2010;20(10):2348–2357. doi: 10.1007/s00330-010-1804-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature Medicine. 2004;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 37.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nature Immunology. 2005;6(8):844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 38.The FO, Boeckxstaens GE, Snoek SA, et al. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133(4):1219–1228. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Woodward A, Schu W, Wojtowycz AR, Sillin LF. Gastric emptying of solids after Roux-en-Y gastrectomy: is extragastric vagal innervation important? Surgery. 1991;110(4):793–798. [PubMed] [Google Scholar]
- 40.Tomita R. Gastric emptying function in patients 5 years after pylorus-preserving distal gastrectomy with or without preserving pyloric and hepatic branches of the vagal nerve for early gastric cancer. World Journal of Surgery. 2009;33(10):2119–2126. doi: 10.1007/s00268-009-0147-7. [DOI] [PubMed] [Google Scholar]
- 41.Ando H, Mochiki E, Ohno T, et al. Effect of distal subtotal gastrectomy with preservation of the celiac branch of the vagus nerve to gastrointestinal function: an experimental study in conscious dogs. Annals of Surgery. 2008;247(6):976–986. doi: 10.1097/SLA.0b013e31816ffb1c. [DOI] [PubMed] [Google Scholar]
- 42.Banki F, Mason RJ, DeMeester SR, et al. Vagal-sparing esophagectomy: a more physiologic alternative. Annals of Surgery. 2002;236(3):324–336. doi: 10.1097/00000658-200209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The FO, Cailotto C, van der Vliet J, et al. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus. British Journal of Pharmacology. 2011;163(5):1007–1016. doi: 10.1111/j.1476-5381.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siu ECK, Tyndale RF. Characterization and comparison of nicotine and cotinine metabolism in vitro and in vivo in DBA/2 and C57BL/6 mice. Molecular Pharmacology. 2007;71(3):826–834. doi: 10.1124/mol.106.032086. [DOI] [PubMed] [Google Scholar]
- 45.Huston JM, Ochani M, Rosas-Ballina M, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. Journal of Experimental Medicine. 2006;203(7):1623–1629. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giebelen IAJ, Le Moine A, van den Pangaart PS, et al. Deficiency of α7 cholinergic receptors facilitates bacterial clearance in Escherichia coli peritonitis. Journal of Infectious Diseases. 2008;198(5):750–757. doi: 10.1086/590432. [DOI] [PubMed] [Google Scholar]
- 47.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular and Cellular Endocrinology. 2010 doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernández J, Escorsell A, Zabalza M, et al. Adrenal insufficiency in patients with cirrhosis and septic shock: effect of treatment with hydrocortisone on survival. Hepatology. 2006;44(5):1288–1295. doi: 10.1002/hep.21352. [DOI] [PubMed] [Google Scholar]
- 49.Touitou Y, Auzeby A, Bogdan A. Cortisol and cortisone production in rat and mouse adrenal incubations. Journal of Steroid Biochemistry and Molecular Biology. 1990;37(2):279–284. doi: 10.1016/0960-0760(90)90339-m. [DOI] [PubMed] [Google Scholar]
- 50.Missaghian E, Kempná P, Dick B, et al. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. Journal of Endocrinology. 2009;202(1):99–109. doi: 10.1677/JOE-08-0353. [DOI] [PubMed] [Google Scholar]
- 51.Al-Dujaili EAS, Mullins LJ, Bailey MA, Andrew R, Kenyon CJ. Physiological and pathophysiological applications of sensitive ELISA methods for urinary deoxycorticosterone and corticosterone in rodents. Steroids. 2009;74(12):938–944. doi: 10.1016/j.steroids.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. American Journal of Physiology. 2007;293(3):G560–G567. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]
- 53.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. Journal of the Autonomic Nervous System. 1993;42(2):153–170. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 54.Maier S, Traeger T, Westerholt A, Heidecke CD. Special aspects of abdominal sepsis. Chirurg. 2005;76(9):829–836. doi: 10.1007/s00104-005-1066-2. [DOI] [PubMed] [Google Scholar]
- 55.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. The New England Journal of Medicine. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 56.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thrombosis and Haemostasis. 2009;101(1):36–47. [PubMed] [Google Scholar]
- 57.Hansen MK, Nguyen KT, Fleshner M, et al. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. American Journal of Physiology. 2000;278(2):R331–R336. doi: 10.1152/ajpregu.2000.278.2.R331. [DOI] [PubMed] [Google Scholar]
- 58.Gaykema RPA, Dijkstra I, Tilders FJH. Subdiaphragmatic vagotomy suppresses endotoxin-induced activation of hypothalamic corticotropin-releasing hormone neurons and ACTH secretion. Endocrinology. 1995;136(10):4717–4720. doi: 10.1210/endo.136.10.7664696. [DOI] [PubMed] [Google Scholar]


