Abstract
Fractures are increased among men with prostate cancer, especially those on androgen deprivation therapy (ADT), but few data are available on men with localized prostate cancer. The purpose of this investigation was to estimate fracture risk among unselected community men with prostate cancer and systematically assess associations with ADT and other risk factors for fracture. In a population-based retrospective cohort study, 742 Olmsted County, MN men with prostate cancer first diagnosed in 1990–99 (mean age, 68.2 ± 8.9 years) were followed for 6821 person-years. We estimated cumulative fracture incidence; assessed relative risk by standardized incidence ratios; and evaluated risk factors in time-to-fracture regression models. Altogether, 482 fractures were observed in 258 men (71 per 1000 person-years). Overall fracture risk was elevated 1.9-fold, with an absolute increase in risk of 9%. Relative to rates among community men generally, fracture risk was increased even among men not on ADT but was elevated a further 1.7-fold among ADT-treated compared to untreated men with prostate cancer. The increased risk following various forms of ADT was mainly accounted for by associations with pathologic fractures (14% of all fractures). Among men not on ADT (62% of the cohort), more traditional osteoporosis risk factors were implicated. In both groups, underlying clinical characteristics prompting different treatments (indication bias) may have been partially responsible for the associations seen with specific therapies. To the extent that advanced stage disease and pathologic fractures account for the excess risk, the effectiveness of fracture prevention among men with prostate cancer may be limited.
Keywords: COHORT STUDY, EPIDEMIOLOGY, FRACTURES, POPULATION-BASED, PROSTATE CANCER
Introduction
Low serum estrogen levels adversely affect bone density in men, with a critical threshold comparable to that for postmenopausal women.(1) Orchiectomy lowers circulating estrogen levels into this postmenopausal range, and Olmsted County, MN men undergoing bilateral orchiectomy (98% for prostate cancer) were at greater risk of an osteoporotic fracture after excluding pathologic fractures and those found incidentally on skeletal surveys for metastasis.(2) However, orchiectomy is being superceded by “medical orchiectomy” with various androgen deprivation therapy (ADT) regimens, and the annual orchiectomy rate in Olmsted County fell from 73 per 100,000 men in 1986–90 to only 7 per 100,000 in 2000.(3) There is little doubt that pharmacologic ADT is associated with bone loss,(4) but steroidal antiandrogens (SAA), nonsteroidal antiandrogens (NSAA) and gonadotropin-releasing hormone (GnRH) agonists may have differing effects, and fracture is the most clinically relevant outcome in any case. Although bisphosphate treatment can slow bone loss in men undergoing ADT,(5) ADT is expensive(6) and bisphosphonates for any substantial portion of ~200,000 men who develop prostate cancer annually(7) would increase costs still further.(8)
Less is known about fracture risk among men with localized prostate cancer. Few studies have focused on this subset of patients,(9) who often serve as the referent group in assessing ADT risks.(10–15) Given the observation that men with the greatest bone mass have a higher risk of prostate cancer,(16,17) fractures might even be reduced among the men spared ADT. Conversely, fracture risk may be increased by chemotherapy or radiation treatment(18) whether or not ADT were prescribed. Moreover, in a study of fracture risk among patients with multiple myeloma, we showed that the problem related more to pathologic fractures than to osteoporotic fractures.(19) Since these possibilities have not been systematically addressed in a population-based study, we aimed to quantify the risk of different fractures among men with prostate cancer and to assess the influence of various types of treatment as well as other risk factors for fracture.
Materials and Methods
Olmsted County is well suited for disease association studies such as this because comprehensive medical records for the residents are available for review, and the pertinent records can be identified through a centralized index to diagnoses made by essentially all medical care providers used by the local population.(20) Following approval by the Institutional Review Boards of Mayo Clinic and the Olmsted Medical Center, we used this unique medical records linkage system (the Rochester Epidemiology Project) to identify all men who resided in Olmsted County when first diagnosed with tissue-confirmed prostate cancer in 1990–99, allowing for a decade or more of subsequent follow-up. Of 1538 potential cases screened, 278 did not have prostate cancer; 270 were not residents at diagnosis; 230 were initially diagnosed before 1990 or after 1999; four histories had been lost; one patient had been diagnosed postmortem; and 13 men declined to authorize the use of their medical records for research.(21) The remaining 742 patients were then followed forward in time through their community medical records (historical cohort study) until death or the most recent clinical contact. The original inpatient and outpatient medical records were reviewed by trained nurses to collect information about the prostate cancer and its treatment, as well as lifestyle factors and a diverse array of conditions predisposing to secondary osteoporosis or to falls.(22) Body mass index (BMI) was recorded at the time of diagnosis, and obesity was defined as BMI ≥ 30. Physical activity was assessed on a six-point scale, with subjects in the highest two categories classified as physically active. In addition, detailed data were collected from contemporary clinical notes regarding the use of various classes of drugs associated with bone loss or with osteoporosis treatment.
These records, including all community x-ray reports and emergency department records, as well as the original notes of all attending physicians, were also searched for the occurrence of any fracture. Mayo Clinic records, for example, contain the details of every inpatient hospitalization, every outpatient office or clinic visit, all emergency room and nursing home care, as well as all laboratory results, all radiographic and pathology reports, including autopsies, and all correspondence with each patient.(20) The records contained the clinical history and the radiologist’s report of each fracture, but original radiographs were not available for review. Thus, the diagnosis of vertebral fracture was accepted on the basis of a radiologist’s report of compression or collapse of one or more thoracic or lumbar vertebrae.(23) Ascertainment of clinically-evident fractures is believed to be complete.(24) By convention, fractures due to daily activities and falls from standing height or less were considered moderate trauma, while motor vehicle accidents and falls from a greater height were deemed severe trauma. Based on review of complete contemporary medical record documentation, we further distinguished fractures attributed by the attending physicians to a specific bone lesion, mainly metastatic malignancy (pathologic fractures), and such fractures were considered pathologic regardless of the apparent degree of trauma involved. We also identified fractures discovered only through monitoring of patients for bone metastases or found on caring for unrelated clinical problems (incidental fractures).
The influence of prostate cancer on fracture risk was evaluated using three basic analyses, all carried out in SAS (SAS Institute Inc., Cary, NC). The primary analysis compared the fractures observed at each site (based on the first fracture of a given type per person) to the number expected in this cohort during their follow-up in the community, ie, standardized incidence ratios (SIR). As delineated elsewhere,(2) expected numbers were derived by applying local calendar year-, age- and sex-specific incidence rates for these fractures to the calendar year-and age-specific person-years of follow-up in the prostate cancer cohort and summing over the strata. Ninety-five percent confidence intervals (95% CI) for the SIRs were calculated assuming the expected rates are fixed and the observed fractures follow a Poisson distribution.(25)
In the second analysis, the cumulative incidence of fracture was estimated for up to 15 years following prostate cancer confirmation using the Kaplan-Meier method.(26) In the customary approach, patients who die are censored, although this may overestimate cumulative fracture incidence when the death rate is high; therefore, we treated death as a competing event in an alternative analysis.(27) Kaplan-Meier methods were also used to assess survival, with expected death rates from the Minnesota white population. Observed and expected cumulative incidence estimates, as well as observed and expected survival curves, were compared using the log-rank test.(28)
Finally, Andersen-Gill time-to-fracture regression models(29) assessed the impact of various covariates (eg, clinical stage, ADT) on relative fracture risk (hazard ratio [HR]). These models allow for multiple fractures per subject, while accounting for the correlation structure. Univariate relations between the risk of specific fractures and each clinical characteristic were first assessed, and stepwise methods with forward selection and backward elimination were then used to choose independent variables for the final models. The independent variables were age and the various clinical characteristics; drug exposures were handled as time-dependent variables. When fracture counts were low for a particular model, and coefficient estimates thereby unstable, Firth’s penalized maximum likelihood estimation was used.(30) Proportional hazards assumptions were checked for all models, but significant deviations from the assumption of constant coefficient estimates across follow-up time were seen with some (eg, models for pathologic fractures). Therefore, models were also fit allowing for coefficient estimates to vary by follow-up time after the prostate cancer diagnosis (up to 2 years, between 2 and 5 years, and greater than 5 years).
Results
All but 14 of the 742 Olmsted County men with prostate cancer first diagnosed in 1990–99 were white (by self-report), and mostly of Northern European extraction, reflecting this community (96% white in 1990). Their mean (±SD) age at diagnosis was 68.2±8.9 years (median, 67.9 years; range, 41 to 94 years). The clinical characteristics of these unselected community patients are delineated in Table 1. Thus, only 114 men (16%) had advanced disease (T3 or T4 or [T1 or T2 with N ≠ 0 or M ≠ 0]), 70 of whom had distant metastases at baseline, and only 9% of the total had a poorly differentiated grade (Gleason score > 7 or Mayo grade 4 if the Gleason score were missing). The mean PSA level at baseline was 42.5 ± 265 ng/mL (median, 7.5 ng/mL; range, 0.2 to 5930 ng/mL). On average, the men had been attended in the community for 38 years prior to recognition of their prostate cancer, and for 9.4 years afterward (median, 9.7 years; 42% followed until death). However, survival in this cohort was unimpaired (p = .119), as 48% remained alive after 15 years compared to 44% expected.
Table 1.
Clinical Characteristics of 742 Olmsted County, MN Men With Prostate Cancer First Diagnosed in 1990–99
| Advanced clinical stage (% yes)a | 114 (15.5%) | Glucocorticoid use (% ever) | 275 (37.1%) |
| Poorly differentiated grade (% yes)a | 66 (9.0%) | Bisphosphonate/osteoporosis drug use (% ever) | 54 (7.3%) |
| Baseline PSA (median)a | 7.5 ng/mL | ||
| Bilateral orchiectomy (% ever) | 102 (13.7%) | Estrogen or SERM use (% ever) | 9 (1.2%) |
| GnRH agonist (% ever) | 161 (21.7%) | Anticoagulant use (% ever) | 191 (25.7%) |
| Any primary androgen blockade (% ever) | 249 (33.6%) | Prior osteoporotic fracture (% yes)a | 69 (9.3%) |
| Non-steroidal anti-androgen use (% ever) | 155 (20.9%) | Risk factor for 2° osteoporosis (% ever)b | 373 (50.3%) |
| Steroidal anti-androgen use (% ever) | 54 (7.3%) | Risk factor for falling (% ever)c | 311 (41.9%) |
| Any combined androgen blockade (% ever) | 146 (19.7%) | Obese (% yes)a | 198 (26.7%) |
| Smoked cigarettes (% ever) | 466 (62.8%) | ||
| Pelvic radiation (% ever) | 294 (39.6%) | Used alcohol (% ever) | 650 (87.7%) |
| Chemotherapy (% ever) | 88 (11.9%) | Limited activity (% yes)a | 36 (4.9%) |
Baseline variable. Others could occur at any time point.
If YES to any of the following: thyroid adenoma, increased thyroid function, thyroidectomy, peptic ulcer disease, gastric resection, resection of large or small bowel, renal failure/uremia, rheumatoid arthritis, decreased or increased adrenal function, increased parathyroid function, pancreatitis, cirrhosis of liver, malabsorption syndrome, pernicious anemia, emphysema chronic bronchitis, or thyroid medication.
If YES to any of the following: stroke, hemiparesis, hemiplegia, transient ischemic attack, dementia, vertebral-basilar insufficiency, vertigo, cataracts, blindness, other vision problems, heart arrhythmia, and postural/orthostatic hypotension, sycopal attacks, parkinsonism, polio sequelae, multiple sclerosis or seizure.
During 6821 person-years (p-y) of observation (range, 14 days to 17.6 years per subject), 258 men experienced 482 fractures (crude incidence, 71 per 1000 p-y; 95% CI, 64–77). Altogether, 484 men (65%) had no fracture, while 149 (20%) had one fracture and 109 (15%) had two or more. Only 89 fractures (18%) resulted from severe trauma (eg, motor vehicle accident), while 311 (65%) were attributed to no more than moderate trauma (Table 2). Of these, 124 fractures were due to a fall from standing height or less, while 187 (mostly vertebral and rib fractures) occurred “spontaneously” during everyday activities. Sixty-six fractures (14%) resulted from a specific pathological lesion (almost all in the axial skeleton due to metastatic malignancy). The etiology of the remaining 16 fractures was uncertain.
Table 2.
Distribution of Fractures Among 742 Olmsted County, MN Men Following a First Diagnosis of Prostate Cancer in 1990–99, by Fracture Site and Immediate Cause
| Fracture site | Fracture cause
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe trauma | Fall from standing | Spontaneous | Pathological | Uncertain | All causes | |||||||
| n | %a | n | %a | n | %a | n | %a | n | %a | n | %b | |
| Skull/face | 4 | 33.3 | 7 | 58.3 | 0 | 0.0 | 1 | 8.3 | 0 | 0.0 | 12 | 2.5 |
| Hands/fingers | 12 | 70.6 | 5 | 29.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 17 | 3.5 |
| Distal forearm | 7 | 33.3 | 12 | 57.1 | 2 | 9.5 | 0 | 0.0 | 0 | 0.0 | 21 | 4.4 |
| Other arm | 4 | 30.8 | 6 | 46.2 | 0 | 0.0 | 3 | 23.1 | 0 | 0.0 | 13 | 2.7 |
| Clavicle/scapula/sternum | 3 | 27.3 | 2 | 18.2 | 2 | 18.2 | 3 | 27.3 | 1 | 9.1 | 11 | 2.3 |
| Ribs | 18 | 21.2 | 23 | 27.1 | 17 | 20.0 | 14 | 16.5 | 13 | 15.3 | 85 | 17.6 |
| Thoracic/lumbar vertebrae | 11 | 5.3 | 13 | 6.2 | 156 | 74.6 | 29 | 13.9 | 0 | 0.0 | 209 | 43.4 |
| Cervical vertebrae | 3 | 27.3 | 4 | 36.4 | 1 | 9.1 | 2 | 18.2 | 1 | 9.1 | 11 | 2.3 |
| Pelvis | 4 | 23.5 | 6 | 35.3 | 1 | 5.9 | 6 | 35.3 | 0 | 0.0 | 17 | 3.5 |
| Proximal femur | 3 | 7.9 | 30 | 78.9 | 0 | 0.0 | 5 | 13.2 | 0 | 0.0 | 38 | 7.9 |
| Other leg | 11 | 33.3 | 13 | 39.4 | 5 | 15.2 | 3 | 9.1 | 1 | 3.0 | 33 | 6.8 |
| Feet/toes | 9 | 60.0 | 3 | 20.0 | 3 | 20.0 | 0 | 0.0 | 0 | 0.0 | 15 | 3.1 |
| All sites | 89 | 18.5 | 124 | 25.7 | 187 | 38.8 | 66 | 13.7 | 16 | 3.3 | 482 | 100 |
Percentage (%) of each type of fracture.
Percentage (%) of total.
After 15 years, an estimated 49% of these patients had at least one new fracture when follow-up was censored at death. With death treated as a competing risk, the cumulative incidence at 15 years was 41% compared to 32% expected (p<.001), for an absolute increase in fracture risk of 9%. Compared to expected rates, there was a 1.9-fold increase (95% CI, 1.6–2.1) in overall fracture risk following the prostate cancer diagnosis. The relative risk of fractures at specific skeletal sites is delineated in Table 3. Statistically significant increases were seen for most fractures of the axial skeleton, particularly the vertebrae (SIR, 8.0; 95% CI, 6.7–9.4). Overall, the relative risk of any axial fracture was 2.6 (95% CI, 2.2–3.0) compared to only 1.2 (95% CI, 0.95–1.4) for all limb fractures combined.
Table 3.
Fractures Observed (Obs)a Among 742 Olmsted County, MN Men Following Prostate Cancer First Diagnosed in 1990–99 Compared With the Numbers Expected (Exp) and Standardized Incidence Ratios (SIR), With 95% Confidence Intervals (CI)
| Fracture site | All fractures | Non-pathological, non-incidental fractures due to moderate trauma | ||||
|---|---|---|---|---|---|---|
| Obs | Exp | SIR (95% CI)b | Obs | Exp | SIR (95% CI) b | |
| Skull/face | 11 | 9.0 | 1.2 (0.6–2.2) | 7 | 4.3 | 1.6 (0.6–3.3) |
| Hands/fingers | 17 | 16.6 | 1.0 (0.6–1.6) | 5 | 7.4 | 0.7 (0.2–1.6) |
| Distal forearm | 21 | 7.6 | 2.8 (1.7–4.2) | 14 | 4.9 | 2.9 (1.6–4.8) |
| Other arm | 11 | 17.3 | 0.6 (0.3–1.1) | 5 | 11.9 | 0.4 (0.1–0.98) |
| Clavicle/scapula/sternum | 10 | 9.4 | 1.1 (0.5–1.9) | 4 | 5.2 | 0.8 (0.2–2.0) |
| Ribs | 67 | 38.5 | 1.7 (1.4–2.2) | 41 | 25.4 | 1.6 (1.2–2.2) |
| Thoracic/lumbar vertebrae | 145 | 18.2 | 8.0 (6.7–9.4) | 57 | 15.0 | 3.8 (2.9–4.9) |
| Cervical vertebrae | 10 | 3.5 | 2.9 (1.4–5.3) | 4 | 1.6 | 2.5 (0.7–6.5) |
| Pelvis | 15 | 8.0 | 1.9 (1.04–3.1) | 7 | 5.7 | 1.2 (0.5–2.5) |
| Proximal femur | 35 | 28.0 | 1.2 (0.9–1.7) | 27 | 24.3 | 1.1 (0.7–1.6) |
| Other leg | 28 | 22.2 | 1.3 (0.8–1.8) | 15 | 13.7 | 1.1 (0.6–1.8) |
| Feet/toes | 14 | 12.7 | 1.1 (0.6–1.8) | 5 | 2.7 | 1.8 (0.6–4.3) |
| Any site | 258 | 138 | 1.9 (1.6–2.1) | 139 | 102 | 1.4 (1.1–1.6) |
Note that the number of fractures observed at specific skeletal sites may differ from those reported in Table 2 because only the first fracture of each type per patient was counted in this analysis.
Statistically significant (p<.05) associations are bolded.
When pathologic fractures were excluded, the overall risk of a subsequent fracture remained elevated (SIR, 1.7; 95% CI, 1.5–2.0). Further excluding 102 non-pathologic fractures discovered incidentally (17 in the course of cancer monitoring and 85 found on radiographs taken for some other purpose), to allow for possible ascertainment bias, the overall risk of fracture was increased to a lesser degree (SIR, 1.2; 95% CI, 1.1–1.4). However, the fractures typically ascribed to bone loss include only those due to minimal or moderate trauma. As also shown in Table 3, the risk of any subsequent moderate trauma vertebral fracture was elevated 3.8-fold (95% CI, 2.9–4.9) compared to the 8-fold increase when all thoracic and lumbar spine fractures were included. The only other statistically increased risks were for distal forearm (SIR, 2.9; 95% CI, 1.6–4.8) and rib fractures (SIR, 1.6; 95% CI, 1.2–2.2). The risk of any osteoporotic fracture (hip, spine or wrist fracture due to moderate trauma but not pathologic nor incidental) was somewhat elevated (SIR, 1.8; 95% CI, 1.4–2.2). After 15 years, an estimated 19% of the prostate cancer patients had experienced at least one new osteoporotic fracture compared to an expected 12% (p<.001).
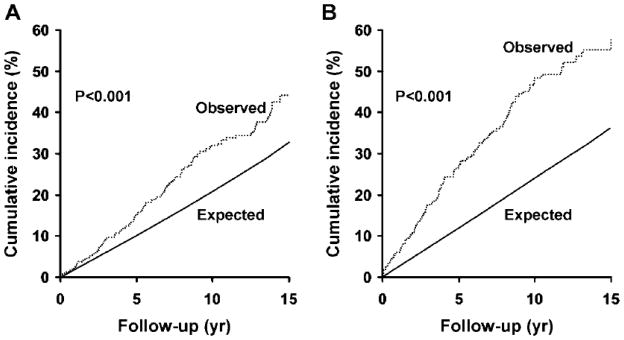
After 15 years, 44% the 463 men (62% of the entire cohort) not treated with ADT had experienced at least one fracture compared to an expected cumulative incidence of 33% (Fig. 1A). Similarly, it was expected that 36% of ADT-treated men would have a fracture over the same interval, but fractures were actually observed in an estimated 58% of them (Fig. 1B), who were exposed to a diverse array of regimens: 167 men (23%) were started on primary ADT (85 bilateral orchiectomy, 82 GnRH), 64 of whom went on to combined androgen blockade (CAB) with an anti-androgen after a median 1.2 years; only 82 men (11%) had CAB as the initial therapy. Of 146 men ultimately treated with CAB, the anti-androgens used included SAA in 54 (mostly megace or nilutamide) and NSAA in 155 (mostly flutamide in the early 1990s and bicalutamide since 1995), with some exposed to multiple agents. Only 9 men were exposed to estrogens or selective estrogen receptor modulators, which were not evaluated further. The cumulative initiation of various treatments is illustrated in Fig. 2.
Fig. 1.
Observed versus expected cumulative incidence of any fracture among Olmsted County, MN men following prostate cancer first diagnosed in 1990–99, comparing 463 men not treated with androgen deprivation therapy (panel A, p<.001) and 279 who received any one of several such treatment regimens (Panel B; p<.001).
Fig. 2.
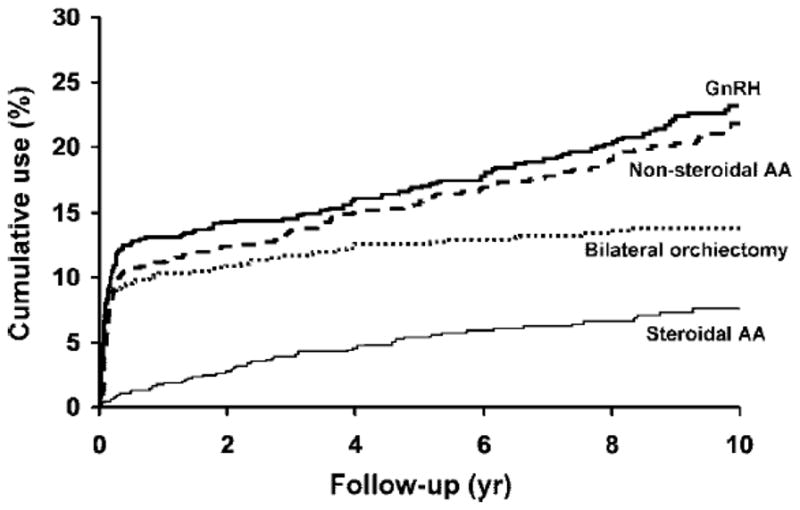
Cumulative initiation of various components of androgen deprivation therapy (bilateral orchiectomy, gonadotropin releasing hormone [GnRH] agonists and steroidal and non-steroidal anti-androgens [AA]) among Olmsted County, MN men, by time after first diagnosis of prostate cancer in 1990–99.
After adjusting for age (HR per 10-year increase, 1.8; 95% CI, 1.5–2.1), associations with most variables related to prostate cancer and its treatment were accounted for by pathological fractures among ADT-treated men (Table 4). These included significantly increased risks with advanced stage, PSA level, CAB, NSAA use, pelvic irradiation (9 interstitial, 287 external beam), chemotherapy and use of glucocorticoids or osteoporosis drugs (18 on oral bisphosphonates [alendronate, risedronate], 9 on intravenous bisphosphonates [pamidronate, zoledronic acid], 3 on calcitonin). By contrast, pathologic fractures were uncommon among the men not given ADT, where risk factors traditionally linked to osteoporosis were more prominent (Table 4). Thus, osteoporotic fractures were significantly associated with the use of osteoporosis drugs (21 on oral and 2 on intravenous bisphosphonates, 6 on calcitonin), risk factors for secondary osteoporosis (eg, hyperthyroidism, thyroid replacement, chronic obstructive lung disease) or for falling (eg, stroke, dementia, vertigo), and use of anticoagulants.
Table 4.
Predictorsa of Fracture (Fx) Risk Among 742 Olmsted County, MN Men With Prostate Cancer First Diagnosed in 1990–99, After Adjustment for age at Diagnosis, by Treatment Group and Fracture Type
| Entire cohort | ADTb | No ADTc | |||
|---|---|---|---|---|---|
| Risk factor | Any Fx N = 482 | Any Fx (N=201) | Pathologic Fx (N=53) | Any Fx (N=281) | Osteoporotic Fxd(N=73) |
| HR (95% CI)e | HR (95% CI)e | HR (95% CI)e | HR (95% CI)e | HR (95% CI)e | |
| Advanced stage | 2.1 (1.4–3.1) | 1.7 (1.1–2.6) | 3.7 (1.4–10) | 1.5 (0.6–4.0) | 0.9 (0.3–2.7) |
| Poorly differentiated grade | 1.6 (0.96–2.7) | 1.3 (0.8–2.3) | 1.6 (0.5–5.4) | 0.6 (0.2–1.7) | 0.4 (0.0–6.0) |
| PSA at baseline (per 500 units) | 1.3 (1.1–1.6) | 1.3 (1.04–1.6) | 1.5 (1.1–1.9) | 0.0 (0.0–10) | 0.7 (0.02–36) |
| Bilateral orchiectomy | 1.8 (1.2–2.6) | 1.3 (0.9–2.1) | 2.3 (0.9–5.8) | - | - |
| Use of GnRH | 1.4 (0.98–2.0) | 0.9 (0.6–1.3) | 0.5 (0.2–1.2) | - | - |
| Primary androgen blockade | 1.7 (1.3–2.3) | 1.3 (0.6–2.7) | 1.4 (0.2–11) | - | - |
| Use of nonsteroidal anti-androgens | 1.9 (1.3–2.7) | 1.4 (0.9–2.1) | 4.7 (1.8–12) | - | - |
| Use of steroidal anti-androgens | 2.1 (1.2–3.7) | 1.5 (0.9–2.7) | 1.4 (0.5–4.4) | - | - |
| Any combined androgen blockade | 2.0 (1.3–2.8) | 1.5 (0.99–2.2) | 3.2 (1.2–8.1) | - | - |
| Any pelvic radiation therapy | 1.4 (1.1–1.9) | 1.5 (0.99–2.2) | 6.0 (1.4–26) | 1.3 (0.8–2.0) | 1.5 (0.8–2.8) |
| Any chemotherapy | 2.0 (1.3–3.1) | 2.0 (1.2–3.3) | 6.0 (2.4–15) | 1.8 (0.9–3.7) | 1.0 (0.2–3.6) |
| Use of glucocorticoids | 2.0 (1.4–2.7) | 2.4 (1.5–3.7) | 5.8 (2.7–12) | 1.6 (1.04–2.5) | 1.5 (0.8–2.8) |
| Prior osteoporotic fracture | 1.7 (1.1–2.7) | 1.5 (0.9–2.5) | 0.2 (0.02–1.2) | 1.8 (0.9–3.5) | 1.4 (0.5–4.0) |
| Use of osteoporosis drugs | 3.5 (2.3–5.5) | 2.5 (1.3–4.8) | 3.8 (1.2–12) | 4.6 (2.7–7.8) | 9.7 (5.0–19) |
| Risk factors for 2° osteoporosis | 1.3 (0.95–1.8) | 1.0 (0.7–1.6) | 0.8 (0.3–2.2) | 1.6 (1.02–2.4) | 2.0 (1.1–3.8) |
| Risk factors for falling | 1.4 (1.04–1.8) | 1.2 (0.8–1.8) | 0.7 (0.2–2.4) | 1.6 (1.1–2.4) | 2.3 (1.2–4.3) |
| Use of anticoagulants | 1.7 (1.3–2.3) | 1.7 (1.1–2.5) | 1.8 (0.7–4.7) | 1.8 (1.2–2.8) | 2.1 (1.2–3.7) |
| Obesity | 1.1 (0.8–1.5) | 0.7 (0.4–1.1) | 0.4 (0.1–1.2) | 1.4 (0.9–2.1) | 0.9 (0.4–1.9) |
| Inactivity | 1.1 (0.5–2.3) | 0.8 (0.4–2.0) | 0.2 (0–3.5) | 1.1 (0.3–3.7) | 0.2 (0–3.9) |
Age-adjusted univariable analyses; statistically significant (P < 0.05) associations are bolded. The numbers of affected men in each instance are shown in Table 1.
Ever had any androgen deprivation therapy (ADT); time in model begins at first ADT (N = 279).
Never had ADT (N=463) or time in model prior to ADT in those who had it (N=247).
Fractures of the proximal femur, distal radius or thoracic/lumbar vertebrae due to minimal or moderate trauma, excluding pathologic fractures and those diagnosed incidentally on follow-up x-rays.
Hazard ratio (HR) and 95% confidence interval (CI).
In a multivariable analysis, the independent predictors of pathologic fractures included age (HR, 1.6; 95% CI, 1.01–2.6), orchiectomy (HR, 5.1; 2.1–12), pelvic radiation (HR, 4.0; 95% CI, 1.4–11) or chemotherapy (HR, 4.0; 95% CI, 1.8–8.7), and use of NSAA (HR, 7.0; 95% CI, 3.2–15), glucocorticoids (HR, 4.0; 95% CI, 2.0–8.2) or anticoagulants (HR, 2.3; 95% CI, 1.05–4.8); obesity at baseline (HR, 0.3; 95% CI, 0.1–0.6) and prior osteoporotic fracture (HR, 0.1; 95% CI, 0.01–0.96) were protective after adjusting for the other factors. Similarly, the independent predictors of an osteoporotic fracture were age (HR, 1.7; 95% CI, 1.2–2.3) and the presence of conditions associated with secondary osteoporosis (HR, 1.8; 95% CI, 1.1–2.9) or falling (HR, 1.8; 95% CI, 1.1–2.9), as well as exposure to osteoporosis drugs (HR, 3.8; 95% CI, 2.1–6.9), primary ADT (HR, 1.7; 95% CI, 1.1–2.6) or anticoagulants (HR, 2.2; 95% CI, 1.4–3.5).
However, compared to the others, men with advanced stage cancer were more likely to undergo ADT (84% versus 29%), pelvic radiation (63% versus 35%), chemotherapy (22% versus 10%) or use of glucocorticoids (50% versus 34%). Likewise, 87% of the men on bisphosphonate therapy had metastatic disease, prior osteoporotic fracture, glucocorticoid use or another risk factor for secondary osteoporosis, while 75% of the men ever given NSAAs had metastatic prostate cancer, prior fracture, glucocorticoid use or another risk factor for secondary osteoporosis. Moreover, changes in case mix may have occurred over time: with follow-up broken into three epochs (≤2 years, 2–5 years, > 5 years), HRs for ADT, CAB and use of GNHA, NSAA, SAA or chemotherapy were greater between 2 and 5 years than for either shorter or longer follow-up. For example, NSAA use was not associated with a significant increase in fracture risk within the first two years (HR, 1.6; 95% CI, 0.7–3.5), but there was a 5.3-fold increase (95% CI, 3.1–8.9) in fracture risk overall (and a 10-fold increased risk of pathologic fractures) with NSAA therapy in years 2–5; this declined again to non-significance in follow-up beyond 5 years (HR, 1.0; 95% CI, 0.6–1.7). However, 90% of the men with less than 5 years of follow-up had died, and they were more likely to have had advanced disease at baseline. Among those surviving beyond 5 years, only 10% had advanced stage prostate cancer.
Discussion
In this inception cohort of unselected community men with prostate cancer, the overall risk of any subsequent fracture was elevated 1.9-fold, consistent with results from a large case-control study in Denmark, where overall fracture risk was elevated 1.8-fold in men with prostate cancer.(14) By contrast, a case-control study in Canada found no association between prostate cancer and fractures of the hip, spine and forearm combined.(31) Although fractures in men with prostate cancer are often attributed to treatment-related bone loss,(4) pathologic fractures accounted for over half of the difference between observed and expected fractures. Moreover, 14% of all fractures in this investigation were pathologic, whereas only 2% of fractures among adults in the general population are due to a specific skeletal lesion.(24) The elevated fracture risk was mainly confined to the axial skeleton, but many vertebral fractures were only discovered incidentally, raising the additional possibility of ascertainment bias. When the analysis was confined to fractures resulting from moderate trauma (the sort conventionally attributed to osteoporosis), excluding the pathological fractures and those found incidentally, the overall relative risk was reduced to 1.4.
Nevertheless, fracture risk was clearly greater among the men undergoing ADT. The overall 1.7-fold relative risk of fracture associated with ADT in the present historical cohort was identical to the odds ratios of 1.7 obtained from the case-control studies in Denmark and Canada.(14,31) The Danish authors also documented a 1.7-fold increase in overall fracture risk following orchiectomy;(14) this is again identical to our estimated relative risk of 1.7 with orchiectomy in the multivariable analysis, although slightly less than the 2.0-fold increase in overall fracture risk we observed in a somewhat older cohort of men who had a bilateral orchiectomy (almost all for prostate cancer) in 1956–2000(2) or the 2.1-fold increase in hip fractures seen among Swedish men following orchiectomy.(10) Among men with prostate cancer in the Surveillance, Epidemiology and End Results (SEER) program, orchiectomy was associated with a 1.5-fold increase in fracture risk.(13) However, the use of bilateral orchiectomy has been declining,(3) and only 14% of men in the present cohort had an orchiectomy. Instead, primary ADT was induced by GnRH therapy over half of the time. Two recent studies based on administrative claims data both found a 1.2-fold increase in fracture risk among prostate cancer patients treated with a GnRH agonist,(11,12) and similar results were seen in the SEER study.(13) We saw no overall increase in fractures with GnRH, but our post hoc power to detect a relative risk of 1.2 was only 38%. CAB was associated with a further 2.0-fold increase in fractures. Any form of ADT was associated with a 1.3-fold increase in fractures in a study based on claims data,(15) which reported that 64% of the men with prostate cancer had undergone ADT at some point compared to 62% in our cohort.
Less obvious is the extent to which elevated fracture risk was due to the various treatments per se or, instead, to clinical characteristics that may have dictated such treatment (indication bias), which is a particular concern in observational studies of treatment outcomes.(32) Thus, compared to the men not on ADT, treated men were more likely to have advanced disease, glucocorticoid use, pelvic radiation and chemotherapy, all of which were themselves risk factors for fracture. Indeed, fracture risk may be greater even before treatment in men destined for subsequent ADT.(13) Conversely, NSAA treatment has been associated with relatively less bone loss compared to alternatives,(33,34) but it was an independent predictor of increased fracture risk in our study. However, the association of NSAA with fracture risk varied over follow-up, and such apparent changes in HRs over time have been attributed to selection bias, eg, time-dependent changes in case mix resulting from death of the highest risk patients.(35) Likewise, an increased risk of fracture was seen following bisphosphonate therapy, despite data from large randomized clinical trials documenting the antifracture efficacy of these agents.(36) Thus, to the extent that men at high risk of fracture are disproportionately selected to receive NSAA or bisphosphonate therapy, this can lead to “implausibly worse” outcomes.(37) By contrast, glucocorticoids, used in some chemotherapy regiments, are well known to cause bone loss and fractures,(38) as seen here. However, the increased fracture risk associated with chemotherapy and pelvic radiation, reported by others and observed in this study as well, could also be due to confounding by treatment indication as reviewed elsewhere.(39)
The majority of the men in this community had localized prostate cancer and were never treated with ADT. In this subgroup, pathologic fractures were uncommon, and traditional osteoporosis risk factors more prominent. These included numerous conditions associated with secondary osteoporosis or an increased risk of falling,(22) as well as a previously reported association of anticoagulant use with fractures of the axial skeleton.(40) In addition, fracture risk was greater among men who had already experienced an osteoporotic fracture, as would be expected.(41) Greater body mass is generally protective of fractures,(42) and other investigators have found fractures to be reduced in obese men with prostate cancer;(43) however, we found no reduction in overall fracture risk among the 27% of men in this study who were obese at baseline.
The present investigation has a number of strengths. The study was population-based, comprised of unselected community men followed from the time their prostate cancer was first diagnosed (inception cohort), and it represents the current clinical spectrum of the disease. During extensive follow-up, a large number of fractures were documented in medical records that spanned each subject’s entire period of residency in the community. Since the vast majority of fractures come to medical attention,(24) ascertainment should be nearly complete with the possible exception of some vertebral and rib fractures. Indeed, the observed incidence of fractures in this cohort (71 per 1000 p-y) was over twice that reported (32 per 1000 p-y) in a smaller population-based study from Australia.(9) In addition, access to complete inpatient and outpatient records allowed us to classify pathologic fractures on the basis of contemporary documentation by attending physicians, which is preferable to using “pathologic” fracture diagnoses in administrative databases that often refer to osteoporosis rather than metastatic malignancy.(44) We were also able to identify clinical characteristics of the men with prostate cancer that may have influenced treatment choices. Moreover, these potentially confounding clinical characteristics had been recorded in the records prior to knowledge of subsequent fractures.
There are also corresponding limitations of a study based on medical records. One may be the generalizability of these data from a small Midwestern community that is predominantly white and better educated than the white population of the country as a whole,(20) although the annual incidence of hip fractures in this community for those age 50 years and over is quite comparable to national figures for United States whites generally (386 vs. 391 per 100,000), and our secular trends in hip fracture incidence mirror those seen nationally.(45) In addition, measurements of bone density or biochemical markers of bone turnover were not routinely performed so the role of bone loss in fracture risk could not be assessed directly. Finally, observational studies such as this do not represent a strong design for determining causality due to potential confounding by treatment indication.(37)
Conclusions
While randomized controlled clinical trials are required to determine the efficacy of specific therapies on improving prostate cancer survival, and indeed to establish causality with respect to adverse skeletal outcomes of such treatment, observational studies like this one are needed to estimate the positive and negative outcomes among unselected patients in routine clinical practice. Both prostate cancer and osteoporosis are common conditions in older men, but our data confirm other reports that age-adjusted fracture risk is increased among men with prostate cancer, with an absolute increase in fracture risk of 9%. However, putative associations of these fractures with disease- or treatment-related bone loss may have been overestimated in the past due to the fact that pathologic fractures are very common in men with prostate cancer, accounting for 14% of the total in this study, while another 21% of the fractures observed here were only found incidentally (ascertainment bias). Nonetheless, our results are generally consistent with earlier reports of elevated fracture risk associated with various treatments for prostate cancer, although the treatment patterns observed were complex and difficult to partition; moreover, fracture risk was elevated even among men not on ADT. Such associations in observational studies may be partly explained by factors that themselves enhance fracture risk while also prompting ADT, radiation or chemotherapy for prostate cancer (indication bias). To the extent that advanced stage disease and pathologic fractures are responsible for the excess risk, the effectiveness of fracture prophylaxis may be limited.
Acknowledgments
This project was supported by grants AG-04875 and AG-034676 from the National Institute on Aging, U.S. Public Health Service.
The authors would like to thank Leona Bellrichard, R.N., Marcia Erickson, R.N., Wendy Gay, R.N., Joan LaPlante, R.N. and Barbara Nolte, R.N. for assistance with data collection and Mary Roberts for help in preparing the manuscript.
Footnotes
Conflict of interest All authors have no conflicts of interest
Contributor Information
L. Joseph Melton, III, Email: melton.j@mayo.edu.
Michael M. Lieber, Email: lieber.michael@mayo.edu.
Elizabeth J. Atkinson, Email: atkinson@mayo.edu.
Sara J. Achenbach, Email: achenbach.sara@mayo.edu.
Horst Zincke, Email: zincke.horst@mayo.edu.
Terry M. Therneau, Email: therneau@mayo.edu.
Sundeep Khosla, Email: khosla.sundeep@mayo.edu.
References
- 1.Khosla S, Melton LJ, 3rd, Riggs BL. Clinical review 144: Estrogen and the male skeleton. J Clin Endocrinol Metab. 2002;87:1443–1450. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ, 3rd, Alothman KI, Khosla S, Achenbach SJ, Oberg AL, Zincke H. Fracture risk following bilateral orchiectomy. J Urol. 2003;169:1747–1750. doi: 10.1097/01.ju.0000059281.67667.97. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, 3rd, Alothman KI, Achenbach SJ, O’Fallon WM, Zincke H. Decline in bilateral orchiectomy for prostate cancer in Olmsted county, Minnesota, 1956–2000. Mayo Clin Proc. 2001;76:1199–1203. doi: 10.4065/76.12.1199. [DOI] [PubMed] [Google Scholar]
- 4.Higano CS. Androgen-deprivation-therapy-induced fractures in men with nonmetastatic prostate cancer: what do we really know? Nat Clin Pract Urol. 2008;5:24–34. doi: 10.1038/ncpuro0995. [DOI] [PubMed] [Google Scholar]
- 5.Polascik TJ. Bone health in prostate cancer patients receiving androgen-deprivation therapy: the role of bisphosphonates. Prostate Cancer Prostatic Dis. 2008;11:13–19. doi: 10.1038/sj.pcan.4501019. [DOI] [PubMed] [Google Scholar]
- 6.Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst. 2000;92:1731–1739. doi: 10.1093/jnci/92.21.1731. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Elkin EB, Girotra M, Morris MJ. Cost-effectiveness of fracture prevention in men who receive androgen deprivation therapy for localized prostate cancer. Ann Intern Med. 2010;152:621–629. doi: 10.7326/0003-4819-152-10-201005180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahlborg HG, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Incidence and risk factors for low trauma fractures in men with prostate cancer. Bone. 2008;43:556–560. doi: 10.1016/j.bone.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Dickman PW, Adolfsson J, Astrom K, Steineck G. Hip fractures in men with prostate cancer treated with orchiectomy. J Urol. 2004;172:2208–2212. doi: 10.1097/01.ju.0000143930.73016.c6. [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–139. doi: 10.1016/S0022-5347(05)00033-9. discussion 139. [DOI] [PubMed] [Google Scholar]
- 13.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsen B, Nielsen MF, Eskildsen P, Andersen JT, Walter S, Brixen K. Fracture risk in Danish men with prostate cancer: a nationwide register study. BJU Int. 2007;100:749–754. doi: 10.1111/j.1464-410X.2007.07163.x. [DOI] [PubMed] [Google Scholar]
- 15.Krupski TL, Foley KA, Baser O, Long S, Macarios D, Litwin MS. Health care cost associated with prostate cancer, androgen deprivation therapy and bone complications. J Urol. 2007;178:1423–1428. doi: 10.1016/j.juro.2007.05.135. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Kiel DP, Ellison RC, Schatzkin A, Dorgan JF, Kreger BE, Cupples LA, Felson DT. Bone mass and the risk of prostate cancer: the Framingham Study. Am J Med. 2002;113:734–739. doi: 10.1016/s0002-9343(02)01382-7. [DOI] [PubMed] [Google Scholar]
- 17.Farhat GN, Taioli E, Cauley JA, Zmuda JM, Orwoll E, Bauer DC, Wilt TJ, Hoffman AR, Beer TM, Shikany JM, Daniels N, Chan J, Fink HA, Barrett-Connor E, Parsons JK, Bunker CH Osteoporotic Fractures in Men Study Group. The association of bone mineral density with prostate cancer risk in the Osteoporotic Fractures in Men (MrOS) Study. Cancer Epidemiol Biomarkers Prev. 2009;18:148–154. doi: 10.1158/1055-9965.EPI-08-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: pathogenesis and management. J Clin Oncol. 2000;18:1570–1593. doi: 10.1200/JCO.2000.18.7.1570. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ, 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005;20:487–493. doi: 10.1359/JBMR.041131. [DOI] [PubMed] [Google Scholar]
- 20.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ., 3rd The threat to medical-records research. N Engl J Med. 1997;337:1466–1470. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 22.USDHHS. Bone Health and Osteoporosis: A Report of the Surgeon General. U.S. Department of Health and Human Services; Rockville, MD: 2004. [Google Scholar]
- 23.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 24.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 25.Cox DR. Some simple approximate tests for Poisson variates. Biometrika. 1953;40:354–360. [Google Scholar]
- 26.Kaplan EL, Meier P. Non-parametic estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley and Sons; New York, NY: 1980. [Google Scholar]
- 29.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer-Verlag; New York, NY: 2000. [Google Scholar]
- 30.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 31.Lau YK, Lee E, Prior HJ, Lix LM, Metge CJ, Leslie WD. Fracture risk in androgen deprivation therapy: a Canadian population based analysis. Can J Urol. 2009;16:4908–4914. [PubMed] [Google Scholar]
- 32.Psaty BM, Siscovick DS. Minimizing bias due to confounding by indication in comparative effectiveness research: the importance of restriction. JAMA. 2010;304:897–898. doi: 10.1001/jama.2010.1205. [DOI] [PubMed] [Google Scholar]
- 33.Sieber PR, Keiller DL, Kahnoski RJ, Gallo J, McFadden S. Bicalutamide 150 mg maintains bone mineral density during monotherapy for localized or locally advanced prostate cancer. J Urol. 2004;171:2272–2276. doi: 10.1097/01.ju.0000127738.94221.da. quiz 2435. [DOI] [PubMed] [Google Scholar]
- 34.Smith MR, Goode M, Zietman AL, McGovern FJ, Lee H, Finkelstein JS. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004;22:2546–2553. doi: 10.1200/JCO.2004.01.174. [DOI] [PubMed] [Google Scholar]
- 35.Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 37.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ, 3rd, Tenenhouse A, Reeve J, Silman AJ, Pols HA, Eisman JA, McCloskey EV, Mellstrom D. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–899. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 39.Vestergaard P, Rejnmark L, Mosekilde L. Methotrexate, azathioprine, cyclosporine, and risk of fracture. Calcif Tissue Int. 2006;79:69–75. doi: 10.1007/s00223-006-0060-0. [DOI] [PubMed] [Google Scholar]
- 40.Caraballo PJ, Heit JA, Atkinson EJ, Silverstein MD, O’Fallon WM, Castro MR, Melton LJ., 3rd Long-term use of oral anticoagulants and the risk of fracture. Arch Intern Med. 1999;159:1750–1756. doi: 10.1001/archinte.159.15.1750. [DOI] [PubMed] [Google Scholar]
- 41.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 42.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 43.Oefelein MG, Ricchuiti V, Conrad W, Seftel A, Bodner D, Goldman H, Resnick M. Skeletal fracture associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patients with prostate cancer. J Urol. 2001;166:1724–1728. doi: 10.1016/s0022-5347(05)65661-3. [DOI] [PubMed] [Google Scholar]
- 44.Curtis JR, Taylor AJ, Matthews RS, Ray MN, Becker DJ, Gary LC, Kilgore ML, Morrisey MA, Saag KG, Warriner A, Delzell E. “Pathologic” fractures: should these be included in epidemiologic studies of osteoporotic fractures? Osteoporos Int. 2009;20:1969–1972. doi: 10.1007/s00198-009-0840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melton LJ, 3rd, Kearns AE, Atkinson EJ, Bolander ME, Achenbach SJ, Huddleston JM, Therneau TM, Leibson CL. Secular trends in hip fracture incidence and recurrence. Osteoporos Int. 2009;20:687–694. doi: 10.1007/s00198-008-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]