Abstract
Mantle cell lymphoma (MCL) is a pre-germinal center neoplasm characterized by cyclin D1 overexpression resulting from t(11;14)(q13;q32). Since MCL is incurable with standard lymphoma therapies, new treatment approaches are needed that target specific biologic pathways. In the present study, we investigated a novel drug delivery nanovehicle enriched with the bioactive polyphenol, curcumin (curcumin nanodisks; curcumin-ND). Cells treated with curcumin-ND showed a dose-dependent increase in apoptosis. This was accompanied by enhanced generation of reactive oxygen species (ROS). The antioxidant, N-acetylcysteine, inhibited curcumin-ND induced apoptosis, suggesting that ROS generation plays a role in curcumin action on MCL cells. Curcumin-ND decreased cyclin D1, pAkt, pIκBα, and Bcl2 protein. In addition, enhanced FoxO3a and p27 expression as well as caspase-9, -3, and poly(ADP-ribose) polymerase (PARP) cleavage were observed. Curcumin-ND treatment led to enhanced G1 arrest in two cultured cell models of MCL.
Keywords: Nanodisks, mantle cell lymphoma, curcumin, cell cycle, apoptosis
Introduction
Mantle cell lymphoma (MCL) is an aggressive subtype of non-Hodgkin lymphoma characterized by the genetic translocation t(11;14)(q13;q32) and cyclin D1 overexpression [1]. The clinical course of MCL is aggressive, and is notable for failure to achieve long-term remission and poor response to conventional chemotherapy [2]. Other than allogeneic stem cell transplant, no curative therapy exists [3]. New therapeutic strategies are clearly needed.
Several investigations have reported the importance of the phytochemical, curcumin, in the treatment of hematological cancers [4–7], as well as other cancers [8,9]. Studies in cancer cell models suggest that curcumin suppresses cell proliferation and induces apoptosis [10,11] via effects on specific molecular targets, including transcription factors, growth factors, and cytokines [12]. However, drawbacks associated with curcumin as a potential therapeutic include insolubility in aqueous media and poor oral bioavailability.
Recently, we reported a novel formulation of curcumin that confers water solubility with retention of potent biological activity [13]. Curcumin was incorporated into nanodisks (ND), comprising a disk-shaped phospholipid bilayer whose edge is stabilized by a scaffold protein, recombinant human apoplipoprotein (apo) A-I [14–16]. We showed that formulation of curcumin in ND enhances its biological activity compared to free curcumin [13]. In the present study, mechanistic aspects of curcumin-ND induced apoptosis and cell cycle arrest at G1–S phase were investigated. The data obtained indicate that the exposure of MCL cells to curcumin-ND induces apoptosis via reactive oxygen species (ROS) generation as well as activation of the caspase-3 pathway. We also show that curcumin-ND increased growth arrest at G1, which correlated with a decrease in cyclin D1 levels.
Materials and methods
Cell lines and reagents
MCL cells (Granta) were cultured in RPMI-1640 containing 10% fetal bovine serum, 1% sodium pyruvate, penicillin, streptomycin, and glutamine at 37°C in a humidified atmosphere. NCEB and Jeko cells were cultured in the same medium, except sodium pyruvate was omitted. 2′,7′-Dichlorodihydro-fluorescein diacetate (H2DCFDA) was purchased from Molecular Probes, Inc. (Carlsbad, CA); propidium iodide (PI) was from Biosource (Camarillo, CA); curcumin was from Sigma Chemical Co. (St. Louis, MO); and a fluorescein isothiocyanate (FITC)–annexin-V Apoptosis Kit was from Invitrogen (Carlsbad, CA). The following antibodies were used: p27, poly (ADP-ribose) polymerase (PARP), caspase-9 and -3, pIκBα, IκBα, FoxO3a, Bcl2, Akt, pAkt (Cell Signaling), cyclin D1 (Santa Cruz), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Chemicon).
Curcumin-ND
Curcumin-ND were formulated as described by Ghosh et al. [13]. Briefly, dimyristoylphosphatidylcholine (Avanti Polar Lipids Inc., Alabaster, AL) was dissolved in chloroform/methanol (3:1 v/v) and dried under a stream of N2 gas. Following dispersal of the prepared lipids in phosphate buffered saline (PBS; 20 mM sodium phosphate, 150 mM sodium chloride, pH 7.0) by bath sonication, curcumin (4 mg/mL stock solution in dimethylsulfoxide [DMSO]) and recombinant apolipoprotein A-I [17] were added. The clarified solution was dialyzed to remove DMSO and sterile filtered. Empty-ND, lacking curcumin, were prepared in the same manner, except that curcumin was omitted from the formulation.
Apoptosis assay
Cells were incubated with curcumin-ND or empty-ND or medium alone (untreated) for 48 h, washed, and resuspended in buffer containing 2.5 μL FITC–annexin-V and 5.0 μL PI for 15 min at 37°C in a CO2 incubator. Flow cytometry measurements were made on a Beckman Coulter EPICS XL-MCL cytometer [18].
Cell cycle progression assay
Cells were incubated with curcumin-ND, empty-ND, or medium alone (untreated) for 24 h [18]. Cells were fixed in 70% ethanol, washed, resuspended in 0.5 mL DNA extraction buffer (0.2 M Na2HPO4 in 0.1 M citric acid, pH 7.8), and centrifuged. The pellet was resuspended in 1 mL PBS containing PI (50 μg/mL) and ribonuclease A (200 μg/mL) and incubated at 37°C for 20 min in the dark. The percentage of cells in G1, S, and G2 was evaluated using ModFit LT for Win32 software (Verity Software House, Topsham, ME).
Reactive oxygen species
ROS production was measured by flow cytometry [18]. Granta and NCEB cells were seeded at a density of 0.5 × 106 cells/well in 24-well plates and treated with curcumin-ND (20 μM) or empty-ND for 1, 3, and 6 h. Cells were then stained with 5 μM H2DCFDA and 2 μg PI per well and incubated for 30 min at 37°C in a humidified CO2 incubator. Cells were washed with PBS, and ROS measured by flow cytometry. In some studies cells were treated with the ROS scavenger, N-acetylcysteine (NAC). All experiments were performed in triplicate.
Western blotting
Fifty micrograms of cell lysate was subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane, and probed with specified antibodies. Immune complexes were visualized by enhanced chemiluminescence.
Statistical analysis
Data from ROS measurements in live cells were analyzed and expressed as mean fluorescence, and data from apoptosis assays were expressed as percent of apoptotic cells (annexin-V-positive and PI-positive) over total cells. Statistical significance was performed by one-way analysis of variance (ANOVA) and Newman–Keuls multiple comparison test (GraphPad Software, Inc., San Diego, CA) to assess the effects of curcumin-ND on apoptosis or ROS production.
Results
Curcumin-ND induced apoptosis
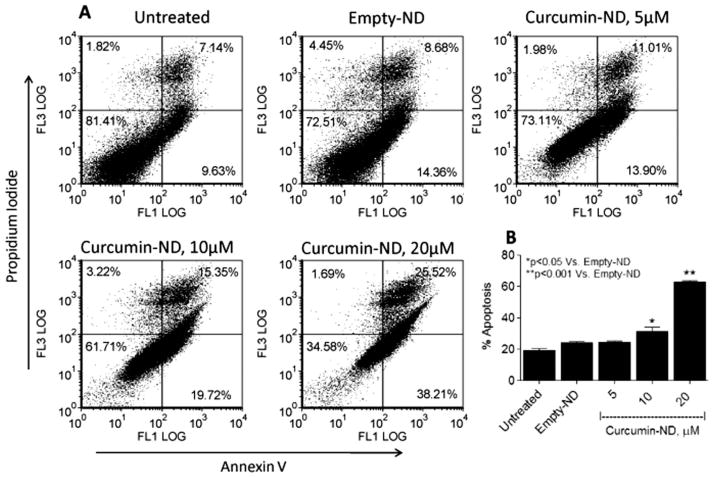
To verify and extend the pro-apoptotic effects of curcumin-ND on cultured MCL cells, Granta cells were incubated with curcumin-ND, empty-ND, or medium alone (untreated). Compared to medium alone, empty-ND had no effect. On the other hand, curcumin-ND produced a dose-dependent increase in apoptosis, achieving ~70% apoptosis at 20 μM curcumin [Figure 1(A)]. Early and late apoptotic percentages from multiple dot plots were combined to arrive at the apoptosis values in the histogram [Figure 1(B)].
Figure 1.
Curcumin-ND induced apoptosis in Granta cells. (A) Granta cells were exposed to medium alone (untreated), empty-ND, or curcumin-ND at 37°C in a 5% CO2 atmosphere for 48 h. (B) Early and late apoptotic percentages (annexin-V/PI-positive) from dot plots were combined to estimate total apoptosis. Values shown are the mean ± SD of three incubations.
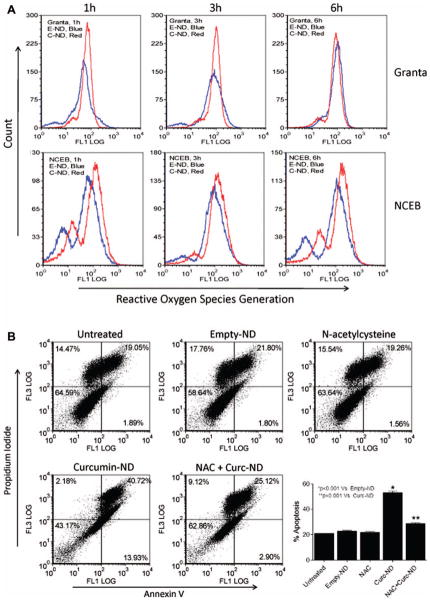
Curcumin-ND induced ROS generation
Several drugs used in chemotherapy induce cell death via ROS generation [19]. To examine whether curcumin-ND induce ROS generation in cultured MCL cells, Granta and NCEB cells were treated with curcumin-ND and intracellular ROS measured as a function of time. In Granta, ROS levels increased as early as 1 h, and declined after 3 h following incubation with curcumin-ND [Figure 2(A)]. In NCEB cells, however, ROS increased at 1 h and was sustained over a 6 h period. When Granta cells were co-incubated with the antioxidant and ROS scavenger, NAC, curcumin-ND induced apoptosis was prevented [Figure 2(B)].
Figure 2.
Curcumin-ND induced ROS generation in MCL cells. (A) Granta and NCEB cells were treated with 20 μM curcumin-ND (C-ND, red peak) and empty-ND (E-ND, blue peak) for 1, 3, and 6 h at 37°C. ROS production in live cells was measured by flow cytometry as described in ‘Materials and methods.’ In the two cell lines, there is an increase in ROS after curcumin-ND compared with empty-ND. (B) Granta cells were incubated with medium alone (untreated), empty-ND, NAC (10 mM), curcumin-ND (20 μM), and combination of curcumin-ND and NAC. Cells were pretreated with NAC for 4 h prior to incubation with curcumin-ND. After 48 h, cells were analyzed for apoptosis. Early and late apoptotic percentages (annexin-V/PI-positive) from each dot plot were combined to represent total apoptosis (summarized in the bar graph; n = 3).
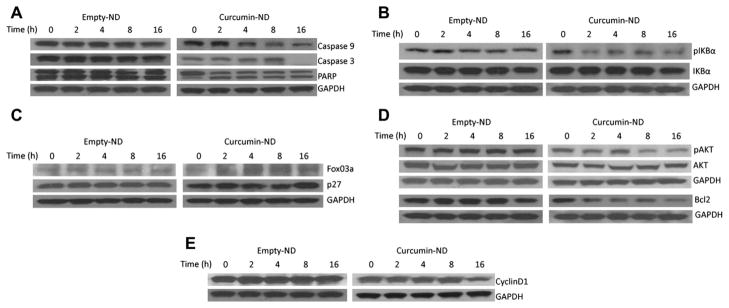
Curcumin-ND effects on apoptosis related proteins
To investigate the cellular pathways underlying curcumin-ND induced apoptosis, its effects on apoptosis-related proteins were studied. Following exposure to curcumin-ND, there was a time-dependent decrease in caspase-9 levels [Figure 3(A)]. Furthermore, 16 h after exposure to curcumin-ND, caspase-3 was undetectable. Loss of this band implies its activation, which was confirmed by PARP cleavage, wherein a decrease in the 116 kDa band was accompanied by an increase in its 85 kDa cleavage product. Curcumin-ND induced sustained PARP cleavage, even at 16 h incubation, suggesting continuous release of curcumin from the ND delivery vehicle. Constitutive activation of nuclear factor-κB (NF-κB) has been reported in a variety of cancers, including lymphoid malignancies [4,20]. In cultured Granta cells, within 2 h after exposure to curcumin-ND, IκBα phosphorylation was decreased, while IκBα protein levels were unchanged [Figure 3(B)].
Figure 3.
Expression of apoptosis related proteins in cells treated with curcumin-ND. Granta cells were incubated with empty-ND or curcumin-ND for specified times. Following incubation, cell homogenates were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies directed against caspase-9, caspase-3, PARP, and GAPDH (A), pIκBα, IκBα, and GAPDH (B), FoxO3a, p27, and GAPDH (C), pAkt, Akt, Bcl2, and GAPDH (D), and cyclin D1 and GAPDH (E).
Subsequently, the effect of curcumin-ND on the expression of additional transcription factors and regulators involved in the apoptotic pathway were studied. MCL cells incubated with curcumin-ND exhibited up-regulation of FoxO3a expression at the 2 h time point, and this remained elevated over 16 h [Figure 3(C)]. Curcumin-ND also induced p27 expression 16 h after exposure. FoxO-dependent increases in p27 levels have been implicated in the induction of an apoptotic program [21].
Bcl2 is an anti-apoptotic protein that is overexpressed in MCL cells [22,23]. Consistent with its effects on apoptosis, curcumin-ND suppressed Bcl2 expression in Granta cells at 2 h [Figure 3(D)]. Whereas constitutive phosphorylation of Akt is implicated in survival and pathogenesis of MCL [24], the effect of curcumin-ND on pAkt levels is unknown, and hence was examined. As shown in Figure 3(D), between 2 and 8 h following treatment, curcumin-ND induced a decrease in pAkt levels. Since cyclin D1 is overexpressed in MCL cells, the levels of this protein were investigated as a function of time following exposure to curcumin-ND. Curcumin-ND induced a decrease in cyclin D1 expression [Figure 3(E)].
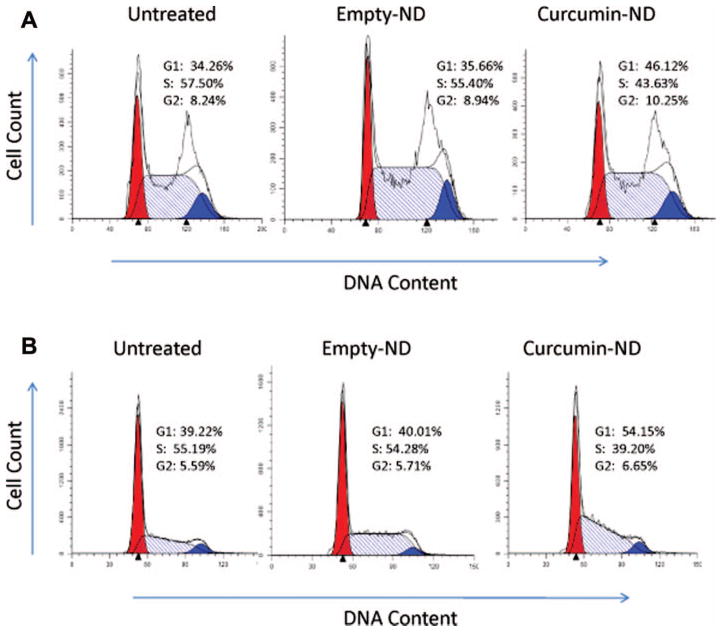
Cell cycle effects of curcumin-ND
Since curcumin-ND incubation led to a decrease in cyclin D1 levels, its effects on cell cycle progression in Granta and Jeko cells were examined. As seen in Figure 4, in both cell lines, curcumin-ND induced G1 cell cycle arrest.
Figure 4.
Curcumin-ND induced G1 growth arrest in MCL cells. (A) Granta and (B) Jeko cells were incubated for 24 h with medium alone (untreated), empty-ND, or curcumin-ND (20 μM curcumin). Following incubation, cells were analyzed for DNA content as described in ‘Materials and methods.’ G1 (red), G2 (blue), and S (hatched).
Discussion
An obstacle to the clinical application of curcumin as an antineoplastic agent relates to its inherent insolubility and poor bioavailability following oral administration. As such, the successful incorporation and solubilization of curcumin into ND [13] provides new opportunities to expand the use of this polyphenol. In the present study we investigated the response of cultured MCL cells to curcumin-ND, with a focus on the underlying mechanism of action. Curcumin-ND elicit pro-apoptotic effects in Jeko cells that are significantly greater than those seen with free curcumin [13]. In the present study, using another MCL cell line (Granta), curcumin-ND elicited similar effects. Furthermore, the observed MCL cellular response to curcumin in general is consistent with results reported using SP-53 cells [4], osteosarcoma [25], and lymphoma cells [26].
Apoptosis, cell cycle, and immune responses are regulated in large part by NF-κB. Constitutive activation of NF-κB has been reported in a variety of cancers, including lymphoid malignancies [4,20]. In unstimulated cells, NF-κB is sequestered in the cytoplasm as an inactive heterotrimer consisting of p50, p65, and IκBα subunits. Upon activation, IκBα becomes phosphorylated, ubiquitinylated, and ultimately degraded [27]. Upon activation, NF-κB translocates to the nucleus where it serves to activate target genes regulated by κB sites. In Granta cells, we examined the effect of curcumin-ND on the phosphorylation status of IκBα and found that, within 2 h after exposure to curcumin-ND, IκBα phosphorylation was inhibited. This result is consistent with a previous study of the effects of curcumin on IκBα phosphorylation in cultured MCL cells [4]. Importantly, however, when presented to MCL cells as curcumin-ND, the onset of inhibition was earlier and the down-regulation was sustained. In a similar manner, compared to free curcumin [4], curcumin-ND led to an earlier onset of suppression of Bcl2 expression in Granta cells.
ROS are mediators of apoptosis in MCL cells [18,28], and this feature can be exploited therapeutically. It is suggested that mitochondria play a role in curcumin-induced apoptosis [29], suggesting that curcumin may activate mitochondrial enzymes leading to ROS generation. The generation of ROS by curcumin could occur through thioredoxin reductase [30]. In this study, it was observed that incubation of curcumin-ND with cultured MCL cells induced significant ROS generation. Moreover, NAC prevented curcumin-ND effects on MCL cells. Given that the antioxidant, NAC, inhibits curcumin-ND induced effects on apoptosis, it may be concluded that ROS generation in response to curcumin is involved in the cell death pathway. It follows that exposure to curcumin-ND would cause more ROS mediated damage and sensitize mantle cells to trigger apoptosis. Consistent with a classical apoptotic response, curcumin-ND also induced down-regulation of cyclin D1, decreased phosphorylation of Akt, and enhanced levels of FoxO3a and p27 as well as cleavage of caspases-9 and -3, and PARP. Furthermore, curcumin-ND induced G1 cell cycle arrest in two cultured cell models of MCL.
Taken together, the data indicate that formulation of curcumin into ND enhances and prolongs its biological effects. Unlike free curcumin, which is virtually insoluble in water, curcumin in ND is water soluble, allowing intravenous administration. Thus, a therapeutic advantage of curcumin-ND relates to the potential for enhanced payload delivery compared to free drug. Indeed, when curcumin was formulated into a nanoemulsion [31] or a micelle nanocarrier [32] delivery particle, studies in a prostate cancer cell model [31] or B6-F10 mouse melanoma cells [32] showed enhanced cell death. Curcumin-ND possess several distinct advantages including formulation via self assembly, nanoscale size, high curcumin binding capacity, and targeting potential. Thus, curcumin-ND represent a potentially effective strategy for the treatment of MCL or other cancers.
Acknowledgments
This study is supported by grants from The Northwestern University Feinberg School of Medicine Department of Medicine Lymphoma Research Fund (L.I.G.), the Meyer Research Fund (L.I.G.), and the Philip Bligh Research Fund (L.I.G.). The work was also supported by National Institutes of Health (NIH) grant HL-64159 (R.O.R.) from the National Heart, Lung and Blood Institute and the NIH Small Business Initiative Research award 1R43CA141904 (T.M.F.) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. M.G. was supported by an American Heart Association Western States Affiliate Predoctoral Fellowship (#10PRE3600031).
The authors gratefully thank Dr. Steven H. Bernstein (University of Rochester, NY) for Granta and NCEB cells; and Dr. Steven Rosen (Northwestern University, Chicago, IL) for Jeko cells.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Bosch F, Jares P, Campo E, et al. PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood. 1994;84:2726–2732. [PubMed] [Google Scholar]
- 2.Hartmann EM, Ott G, Rosenwald A. Molecular biology and genetics of lymphomas. Hematol Oncol Clin North Am. 2008;22:807–823. doi: 10.1016/j.hoc.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni F, Ponzoni M. The cellular origin of mantle cell lymphoma. Int J Biochem Cell Biol. 2007;39:1747–1753. doi: 10.1016/j.biocel.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Hussain AR, Ahmed M, Al-Jomah NA, et al. Curcumin suppresses constitutive activation of nuclear factor-KB and requires functional Bax to induce apoptosis in Burkitt’s lymphoma cell lines. Mol Cancer Ther. 2008;7:3318–3329. doi: 10.1158/1535-7163.MCT-08-0541. [DOI] [PubMed] [Google Scholar]
- 6.Tan TW, Tsai HR, Lu HF, et al. Curcumin-induced cell cycle arrest and apoptosis in human acute promyelocytic leukemia HL-60 cells via MMP changes and caspase-3 activation. Anticancer Res. 2006;26:4361–4371. [PubMed] [Google Scholar]
- 7.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and Ikappa B alpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 8.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 9.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- 10.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem. 2007;102:580–592. doi: 10.1002/jcb.21500. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh M, Singh ATK, Sulchek T, Gordon LI, Ryan RO. Curcumin nanodisks: formulation and characterization. Nanomedicine. 2011;7:162–167. doi: 10.1016/j.nano.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redmond KA, Nguyen T-S, Ryan RO. All-trans-retinoic acid nanodisks. Int J Pharm. 2007;339:246–250. doi: 10.1016/j.ijpharm.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan RO. Nanodisks: hydrophobic drug delivery vehicles. Expert Opin Drug Deliv. 2008;5:343–351. doi: 10.1517/17425247.5.3.343. [DOI] [PubMed] [Google Scholar]
- 16.Iovannisci DM, Beckstead JA, Ryan RO. Targeting nanodisks via a single chain variable antibody–apolipoprotein chimera. Biochem Biophys Res Commun. 2009;379:466–469. doi: 10.1016/j.bbrc.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr Purif. 2003;27:98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 18.Singh AT, Evens AM, Anderson RJ, et al. All trans retinoic acid nanodisks enhance retinoic acid receptor mediated apoptosis and cell cycle arrest in mantle cell lymphoma. Br J Haematol. 2010;150:158–169. doi: 10.1111/j.1365-2141.2010.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 20.Kim SW, Oleksyn DW, Rossi RM, et al. Protein kinase C-associated kinase is required for NF-κB signaling and survival in diffuse large B-cell lymphoma cells. Blood. 2008;111:1644–1653. doi: 10.1182/blood-2007-05-088591. [DOI] [PubMed] [Google Scholar]
- 21.Dijkers PF, Medema RH, Pals C, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27 (KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rummel MJ, de Vos S, Hoelzer D, et al. Altered apoptosis pathways in mantle cell lymphoma. Leuk Lymphoma. 2004;45:49–54. doi: 10.1080/1042819031000151112. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal B, Naresh KN. Bcl-2 family of proteins in indolent B-cell non-Hodgkin’s lymphoma: study of 116 cases. Am J Hematol. 2002;70:278–282. doi: 10.1002/ajh.10139. [DOI] [PubMed] [Google Scholar]
- 24.Rudelius M, Pittaluga S, Nishizuka S, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108:1668–1676. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DS, Lee MK, Kim JH. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009;29:5039–5044. [PubMed] [Google Scholar]
- 26.Uddin S, Hussain AR, Manogaran PS, et al. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene. 2005;24:7022–7030. doi: 10.1038/sj.onc.1208864. [DOI] [PubMed] [Google Scholar]
- 27.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Galán P, Roué G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 29.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 30.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 31.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–3875. [PubMed] [Google Scholar]
- 32.Ma Z, Haddadi A, Molavi O, Lavasanifar A, Lai R, Samuel J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Res. 2008;86:300–310. doi: 10.1002/jbm.a.31584. [DOI] [PubMed] [Google Scholar]