Abstract
New methods are needed to eradicate or prevent Chlamydia trachomatis infections. Blockade of epithelial membrane protein 2 (EMP2) by genetic silencing or neutralizing polyclonal antibody reduced chlamydial infectivity in vitro. This study tests the prediction that recombinant anti-EMP2 diabody could reduce early chlamydial infection of the genital tract in vivo. In a murine infection model, pretreatment with anti-EMP2 diabody, as compared to control diabody, significantly reduced bacterial load, tissue production of inflammatory cytokines, recruitment of polymorphonuclear leukocytes, and local tissue inflammation. These findings support EMP2 as a potential preventative and therapeutic target for genital chlamydial infection.
Keywords: Chlamydia muridarum, epithelial membrane protein 2 (EMP2), infection, antibody therapy
Introduction
Chlamydia trachomatis is an obligate gram-negative intracellular pathogen that causes recurring or persisting infection in mucosal tissues of multiple organs (Dautry-Varsat et al., 2004). Chronic Chlamydia infection in females correlates with severe sequelae such as pelvic inflammatory disease (PID), ectopic pregnancy, infertility, and chronic abdominal pain (Paavonen & Lehtinen, 1996). There are more than 4 million new cases of Chlamydia infection reported annually in the United States alone, and the annual costs for the management of chlamydial infections and related diseases are estimated to be more than $10 billion (Beagley & Timms, 2000). Antibiotic treatment abrogates genital immunity (Su et al., 1999) and increases the rate of recurrent infections (Brunham et al., 2005), emphasizing the need for a vaccine. Despite recent efforts to develop chlamydial vaccines, several obstacles such as short-lived, serovar specific immunity (Grayston et al., 1963; Dhir et al., 1967; Katz et al., 1987; Brunham et al., 1996) and induced immune –associated tissue pathology (Grayston et al., 1963; Dhir et al., 1967; Woolridge et al., 1967) make the creation of safe and effective vaccines challenging. Thus, alternate modality for early prevention and management during asymptomatic infection is needed to reduce C. trachomatis infection and its associated morbidity.
Epithelial membrane protein 2 (EMP2) is a member of the PMP22/GAS3 transmembrane protein family and known to be highly expressed at sites of Chlamydia infection, including the genital tract and eye. EMP2 is also known to orchestrate the trafficking of various surface molecules including specific integrin isoforms, MHC class I molecules, immunoglobulin superfamily members (e.g., CD54), and GPI-linked molecules (Wadehra et al., 2002; Wadehra et al., 2003; Wadehra et al., 2004). In the endometrium, EMP2 is required for blastocyst adhesion during the implantation period, in part through its role in αvβ3 integrin function (Wadehra, Daval, Mainigi, Ord, Iyer, Braun & Williams 2006; Wadehra, Natarajan, Seligson, Williams, Hummer, Hedvat, Braun & Soslow, 2006). Therefore as EMP2 orchestrates trafficking of surface proteins, EMP2 also seems well poised to mediate pathogen entry into host cells. Recently, we reported that EMP2 expression was indeed critical for host-Chlamydia interaction (Shimazaki et al., 2007). By genetic manipulation, levels of EMP2 surface expression were positively associated with chlamydial infectivity in vitro (Shimazaki et al., 2007). Furthermore, blockade of EMP2 by polyclonal anti-EMP2 antibody prior to infection successfully reduced Chlamydia internalization and infection in vitro, indicating a significant role for EMP2 in host-Chlamydia interaction (Shimazaki et al., 2007).
This in vitro study raised two questions that prompted the present investigation; 1) can blockade of EMP2 control initial chlamydial infection in vivo; 2) can monoclonal anti-EMP2 antibodies be developed with efficacy against chlamydial infection? Accordingly, the present study was developed to evaluate the use of antibody fragments with specificity for EMP2 in a murine genital tract infection model using the murine C. trachomatis strain, C. muridarum. In addition, monoclonal anti-mouse EMP2 and control non-specific recombinant diabodies were developed using the filamentous bacteriophage library selection method (Holliger et al., 1993). Diabodies are small bivalent biospecific antibody fragments with high avidity and specificity (Holliger et al., 1993). A high signal to noise ratio due to a better specificity and fast blood clearance show its potential for diagnostic and therapeutic targeting of specific antigen (Olafsen et al., 2004).
Here, we show that the treatment of animals with anti-EMP2 diabody prior to C. muridarum infection reduces early chlamydial infectivity. This was evidenced by reduced bacterial load (IFU counts) and host immune response, including decreased cytokine expression (IFN-γ, TNF-α, and GM-CSF) and tissue polymorphonuclear leukocytes (PMNs). Histopathologic analysis also showed significantly lessened inflammation and tissue damage. These findings demonstrate that the pre-treatment of the genital tract with anti-EMP2 diabody can substantially decrease chlamydial infection, and support the concept that targeting surface EMP2 using anti-EMP2 reagents may be a potential preventative or therapeutic agents in chlamydial infection.
Methods
Chlamydiae, antibodies and diabodies
For bacterial load (IFU) measurement, chlamydial inclusions were detected using immunofluorescence microscopy with an anti-Chlamydia LPS mouse antibody (clone EV1-H1), provided by Dr. Harlan Caldwell (Laboratory of Intracellular Parasites, Rocky Mountain Laboratories, National Institutes of Health, Hamilton, MT) (Zhang et al., 1987) and a FITC-conjugated goat anti-mouse IgG secondary antibody (Southern Biotech, Birmingham, AL) (Maxion et al., 2004).
For flow cytometry analysis, purified antibodies were purchased as fluorophore conjugates. Antibodies against CD16/32 (2.42G) were purchased from BD Pharmingen (San Jose, CA). Antibodies against CD3ε (145-2C11) and Gr-1 (RB6-8C5) were purchased from eBioscience (San Diego, CA).
Anti-EMP2 diabodies against the second extracellular loop of mouse EMP2 were produced using mouse peptides as previously described (Shimazaki et al., 2008). Anti-EMP2 diabody (KS83) and control diabody (A10) were used in this study (Shimazaki et al, 2008). Preparations of diabody were expressed and purified according to published protocol (Marks & Bradbury, 2004).
C. muridarum was used as purified infectious elementary bodies (Ramsey et al., 1988) or inactivated by UV-irradiation as previously described (Caldwell et al., 1981).
Chlamydial infection
Five-week-old female BALB/c mice (Charles River) were used for the infection model. All animal protocols were approved by the University of California, Los Angeles, Chancellor’s Animal Research Committee. For genital tract infection, animals were subcutaneously injected with 2.5mg of progesterone (Depo-provera; Pfizer, New York, NY) 7 days before the intravaginal infection. Prior to the infection, each animal underwent an intraperitoneal injection with 2.5mg Ketamine (Phoenix Pharmaceutical, St. Joseph, MO) and 1.25mg Xylazine (Phoenix Pharmaceutical) mixture. The cervico-vaginal region was swabbed with Dacron swabs (Copan Diagnostics, Murrieta, CA). The animals each received an intravaginal pre-treatment with either 10μl of PBS (no diabody control), KS83 (0.5μg/animal; anti-EMP2 diabody) or A10 (0.5μg/animal, control diabody) 20 minutes prior to infection. Intravaginal infection was performed using 20μl of C. muridarum (1.5 × 105 IFU/mouse) as previously described (Maxion & Kelly, 2002). Vaginal swabs were collected every 3 days following infection, and inclusion forming units (IFU) was monitored via infection of McCoy cell monolayer to ensure successful and uniform infection within the treatment groups (Kelly et al., 1996).
Immunohistochemistry
Three animals per group were subcutaneously injected with Depo-provera 7 days prior to treatment. The animals were anesthetized with the ketamine/xylazine mixture as stated above, and then treated with the diabodies. Twenty minutes after the intravaginal treatment with either 10μl of PBS, KS83 (0.5μg/animal) or A10 (0.5μg/animal), genital tracts were removed en bloc, fixed in 10% formalin overnight and subsequently, 70% ethanol. Paraffin-embedded genital tract sections cut longitudinally (4 μm), deparaffinized, blocked for endogenous peroxidase activity with 3% hydrogen peroxide, and heated for 25 minutes with citrate buffer. The tissue samples were blocked in a humidified chamber using an Avidin/Biotin Blocking kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol. The tissues samples were blocked with goat serum for 30 minutes and stained with rabbit anti-mouse EMP2 polyclonal antibody (1:500) in a humidified chamber. The sections were developed by incubation with a biotinylated secondary antibody from the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol, followed by, a diaminobenzidine tetrahydrocholride (DAB) substrate solution (Pierce, Rockford, IL). The data are representative of 2 independent experiments with 3 animals per group.
Bacterial load (IFU) measurement
Genital tracts from 7 animals per group were removed 4 days post-infection, separated into oviducts without ovaries (OD), uterine horns (UH), and cervico-vaginal (CV) regions. Each tissue section was homogenized with a tissue homogenizer (Omni International, Marrieta, GA) in 1ml protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) (Maxion & Kelly, 2002). The genital tract homogenates were used to infect 5×104 cells/well of overnight cultured McCoy cell monolayer plated on a 96-well plate. The cultures were spun at 1900 × g for 1 hour at 35 °C immediately after infection, incubated for 32 hours in a 5% CO2 incubator at 35°C, and fixed with ice-cold methanol at −20 °C for 20 minutes. Chlamydial inclusion bodies were visualized with mouse anti-chlamydial LPS antibody, followed by FITC-conjugated goat anti-mouse IgG secondary antibody (see above sections). Inclusion bodies were counted within 20 randomly selected fields at 40x under the Olympus IX70 fluorescent microscope (Olympus, Hamburg, Germany) and the IFU/ml was calculated for each sample. The observer was masked as to the group identity of the samples analyzed. The data are expressed as the mean ± SEM from 2 independent experiments with 7 animals per group.
Cytokine protein analysis
Genital tracts from 7 animals per group were isolated from infected mice 4 days post-infection, and regionally divided into oviducts without ovaries (OD), uterine horns (UH), and cervico-vaginal (CV) regions. following previously published protocols (Maxion & Kelly, 2002). Tissues were homogenized, spun, and the supernatant was filtered through 0.22μm filters (Millipore, Bedford, MA). Secreted cytokines and chemokines in these preparations were analyzed using enzyme-linked immunosorbent assay (ELISA) kit for IFN-γ, TNF-α, and GM-CSF (eBioscience, San Diego, CA), following the manufacturer’s instructions. Protein concentration of each preparation was determined according to manufacturer’s protocol via commercially available BCA Protein Assay kit (Pierce, Rockford, IL) for normalization. Data are expressed as the mean ± SEM of three independent experiments with 7 mice for each treatment group.
Isolation of and FACS analysis of genital tract leukocytes
Genital tracts from 8 mice per group were removed at day 4 and divided into OD, UH, and CV sections as described above (Maxion & Kelly, 2002). The tissues were processed to generate single cell suspension following published protocols (Kelly et al., 1996; Maxion et al., 2004). Briefly, separated genital tracts were minced and treated with digestion buffer (5mg/ml collagenase and 0.2 mg/ml DNase I in Hanks balanced salt solution; Sigma-Aldrich, St. Louis, MO) for 1 hour at 37°C on a rotator. Single cell suspensions were obtained by filtering the digests through 70 μm filters (Becton Dickinson, Franklin Lakes, NJ) in isolation medium (Hank’s balanced salt solution containing 5mM EDTA, 0.5% bovine serum albumin). After filtration, cell suspensions were spun and subjected to red blood cell lysis with ACK lysing buffer (Cambrex, Charles City, IA) for 3 minutes at room temperature and washed. All subsequent FACS staining procedures were at 4°C. Single cell suspensions were first stained with CD16/32 (BD Bioscience) for 20 minutes to minimize non-specific binding. PMN populations were examined by staining for anti-Gr-1-FITC and CD3ε-APC for 20 minutes, and analyzed by FACS by gating on live GR-1+CD3ε- cells. Error bars indicate SEM from 3 independent experiments with 8 mice for each treatment group.
Histopathology
Genital tracts from 4 animals per treatment group were removed at day 7 and 14 post-infection, fixed in 10% formalin overnight, and subsequently, 70% ethanol. Tissues were embedded en bloc in paraffin, sectioned (5 μm), and stained with either hematoxylin & eosin or trichrome. Three regions of the genital tract: OD, UH, and CV regions were masked and independently scored for the thickening, redness, presence of lymphocytes and fibrosis in 0-4 scaling system as previously described (Darville et al., 1997; Darville et al., 2001). Scores from each category were added to evaluate the overall changes in histopathology. Data are representative of three independent experiments with four mice per treatment group.
Statistical Analysis
The histopathology score is non-parametric and was analyzed using the Mann-Whitney U test. Cytokine protein analysis studies, groups were analyzed by two-tailed Student’s paired t-test, with a confidence level of p≤0.05. For the bacterial load (IFU) measurement studies, log-transformed data were analyzed by two-tailed Student’s paired t-test, one-way ANOVA, and Fisher’s exact test, with a confidence level of p≤0.05. For the FACS data, log-transformed data were analyzed by two-tailed Student’s paired t-test, with a confidence level of p≤0.05.
Results
Treatment with anti-EMP2 diabody reduces overall expression of EMP2 in the genital tract
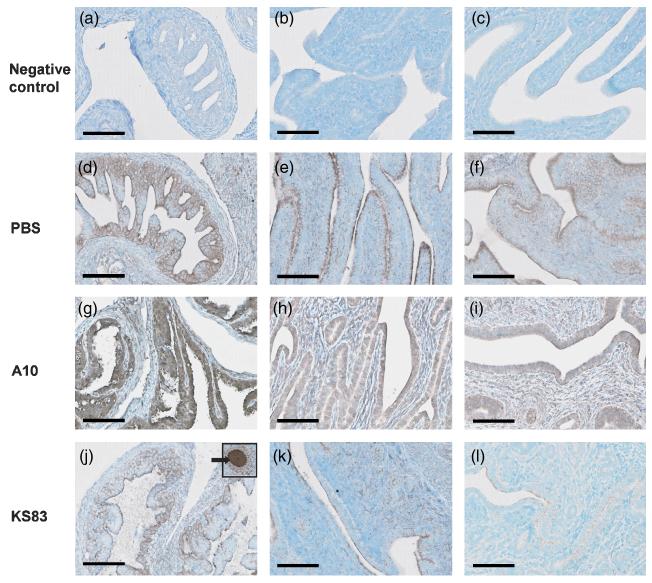
Examination of the effect of anti-EMP2 diabody on EMP2 expression level was tested in the genital tract of animals that were intravaginally treated with either PBS, control diabody (A10), or anti-EMP2 diabody (KS83). After a 20 minute incubation period, genital tracts were removed and EMP2 expression was examined by immunohistochemistry using a rabbit anti-mouse EMP2 polyclonal antibody. Figure 1 illustrates that animals treated with anti-EMP2 diabody (Fig. 1, j-l) showed a significant decrease in surface and intracellular expression of EMP2 in the endometrium, compared to PBS (Fig. 1, d-f) and control diabody treated animals (Fig. 1, g-i). Although the decrease in EMP2 expression was observed in all parts of the genital tract, it was more prominent in the cervico-vaginal (CV) region (Fig. 1, l) than the uterine horns (UH) or the oviducts (OD) (Fig. 1, j and k). Regardless of the treatment, EMP2 expression in the oocytes remains unchanged (Fig. 1, j, inset shown with arrow).
Figure 1. Blockade of epithelial membrane protein 2 (EMP2) reduces expression of EMP2.
After intravaginal treatment for 20 minutes with either PBS, control diabody (A10), or anti-EMP2 diabody (KS83) EMP2 expression was detected in the oviducts (OD; a, d, g, j), uterine horn (UH; b, e, h, k), and cervico-vaginal region (CV; c, f, i, l). EMP2 expression was decreased in the various genital tract regions treated with KS83 (j-l), but not in those treated with PBS (d-f) or A10 diabody (g-i). EMP2 expression in oocytes of the upper tract remains unchanged despite diabody treatment (inset, arrow in j). Scale bar, 100 μm. Magnification, 400x.
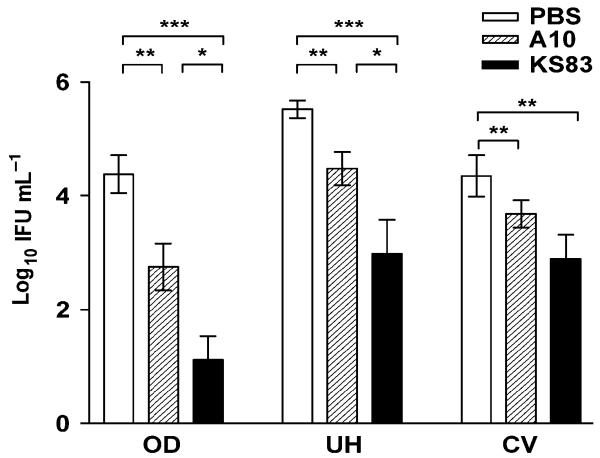
Blockade of EMP2 significantly decreases bacterial load (IFU) in the genital tract
Using quantitation of bacterial load (IFU), we determined whether treatment with anti-EMP2 diabody corresponded to a reduction in bacterial infection as it was previously shown in vitro (Shimazaki et al., 2007). At day 4, genital tracts of infected animals were removed and separated into oviducts without ovaries (OD), uterine horns (UH), and cervico-vaginal (CV) region sections. Tissues were homogenized, and assayed for bacterial load in each sample. As figure 2 illustrates, there was a significant decrease in bacterial load for animals treated with KS83. The effect of anti-EMP2 diabody was greater in the upper versus lower genital tract, indicating that anti-EMP2 diabody particularly reduced ascension of chlamydial infection. Although a decrease in IFU was also observed in control diabody-treated animals, the effect was much smaller compared to the anti-EMP2 diabody-treated samples (Fig. 2). These results were analyzed by a two-tailed Student’s t-test, which yielded statistical significance in the decrease of bacterial load in animals treated with anti-EMP2 diabody. Also, a Fisher’s exact test was performed to examine the significance between infected and non-infected OD, UH, and CV. The Fisher’s exact test confirmed with statistical significance that treatment with anti-EMP2 diabody reduced the chlamydial infection in OD (p= 0.024) and UH (p = 0.030) as compared with the control diabody.
Figure 2. Anti-EMP2 diabody reduces chlamydial infectivity in genital Chlamydia infection.
Genital tracts of animals pre-treated with either PBS, control diabody (A10), or anti-EMP2 diabody (KS83) were removed at day 4 post-infection. Tissues homogenates were plated on monolayer McCoy cells, and inclusion bodies were detected by immunofluorescent staining of Chlamydia LPS for the determination of bacterial load recovery (IFU). A significant decrease in chlamydial infectivity was observed in KS83 treated animals, and the reductive effect was more prominent in the upper genital tract. Statistical analysis of the IFU measurements were performed using a Student’s t-test (*p-value<0.05, **p-value<0.005, ***p-value<0.0001). Data are expressed as the mean ± SEM from two independent experiments, with n=7 for each experiment.
Blockade of EMP2 decreases production of proinflammatory cytokines during early Chlamydia infection
The acute response in genital tract tissues following vaginal infection with Chlamydia is seen by the elevation of various tissue associated cytokines and chemokines, forming a complex signaling network to facilitate an appropriate host immune response (Darville et al., 2001). We therefore tested how the reduction of bacterial load by anti-EMP2 diabody correlated with features of the host immune response. In particular, we hypothesized whether anti-EMP2 reduced such host responses (due to the abrogation of infection), or increased them (e.g., by a response to foreign diabody protein) so as to non-specifically reduce bacterial load and histopathology.
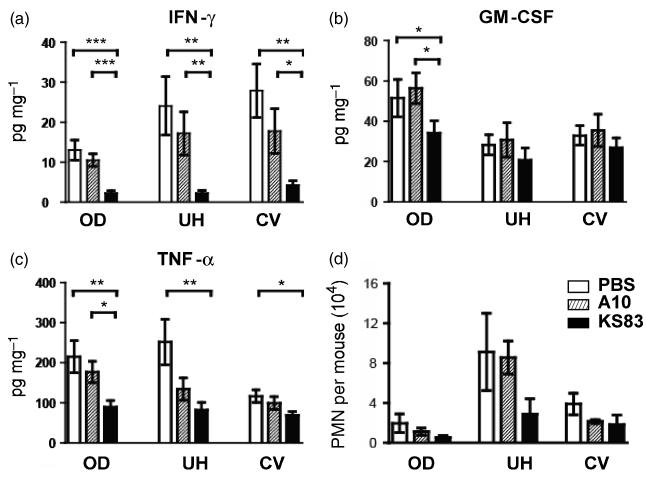
At day 4, IFN- γ production was significantly reduced in all tested genital tract segments in the EMP2-specific diabody KS83-treated animals as compared to either vehicle (PBS)-treated animals or to the non-specific diabody A10-treated animals (Fig. 3a). Although there was a trend toward reduction of IFN- γ by the non-specific diabody A10, there was no statistical difference in IFN- γ as compared to PBS-treated control animals (Fig. 3a). Consistent with the IFU data, this suppressive effect of anti-EMP2 diabody was more evident in the OD and UH. Pre-treatment using KS83 diabody, as compared to either the PBS control or A10 diabody, also showed significant reduction in TNF-α in the OD region of the genital tract (Fig. 3b). TNF-α secretion in the UH region of the genital tract was decreased by pre-treatment using both the KS83 diabody and the A10 diabody, however, the effect of the EMP2-specific diabody was greater (Fig 3b). A small effect of anti-EMP2 was also observed on GM-CSF secretion, although this only reached significance compared to controls in the OD region of the genital tract (Fig. 3c).
Figure 3. Anti-EMP2 diabody reduces host immune response in genital Chlamydia infection.
Genital tracts of animals treated with either PBS, control diabody (A10), or anti-EMP2 diabody (KS83) were removed at day 4 post-infection. Tissues homogenates were filtered, and the supernatants were used for the detection of various cytokines, (a) IFN-γ, (b) TNF-α, and (c) GM-CSF. Statistical analysis of the levels of cytokine secretion among treatment groups were performed using a Student’s t-test (*p-value<0.05, **p-value<0.005, ***p-value<0.0001). Recruitment of PMNs was also quantified (d). Single cell suspension was obtained as described above, and examined for PMNs using FACS by gating on live, Gr-1+CD3ε- cells. Error bars indicate SEM from three independent experiments with at least eight mice for each treatment group. Cytokine data are expressed as the mean ± SEM of three independent experiments with at least seven mice for each treatment group.
Anti-EMP2 diabody treatment decreases influx of PMNs to the genital tract
Our results demonstrate that anti-EMP2 diabody treatment significantly decreased both the bacterial load and proinflammatory response to infection in the OD. Clearance of chlamydial infection requires a cellular immune response, which early infection is evidenced by acute inflammation dominated by polymorphonuclear neutrophils (PMN) (Maxion et al., 2004). Therefore we sought to determine whether anti-EMP2 treatment would affect PMN influx in the chlamydial-infected GT. On day 4, we observed that PMN numbers were decreased following KS83 treatment (Fig. 3d). Specifically, those animals that received the KS83 diabody showed fewer numbers of PMN throughout the GT as compared with either the A10 diabody or the PBS treated groups, and this decreased influx was most prevalent in the UH.
Blockade of EMP2 significantly suppresses early host response to chlamydial infection
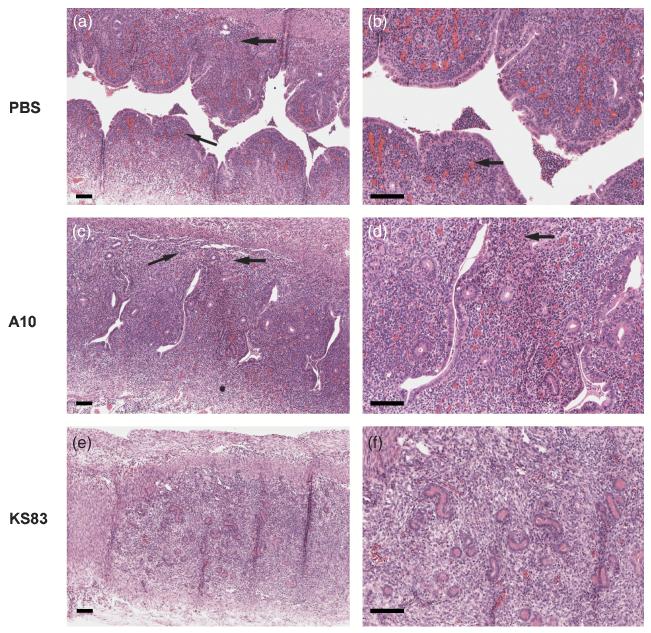
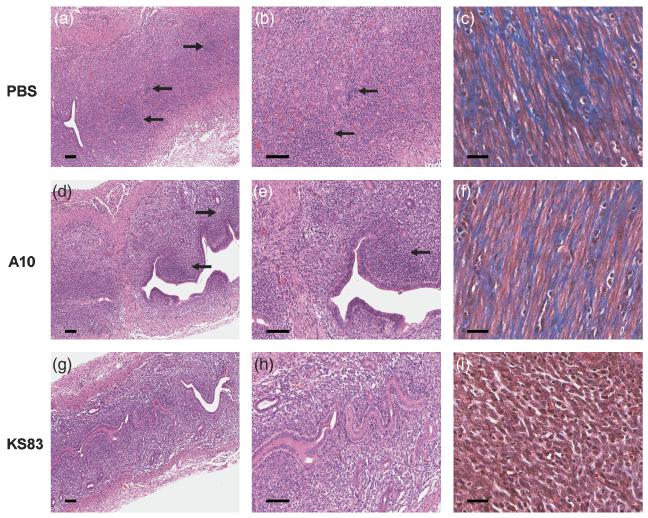
In order to determine the overall effect of anti-EMP2 diabody on Chlamydia infection, mice were treated and infected as described above. Gross and histologic examination of the UH region were performed 7 days post-infection to examine the early signs of inflammation. Animals treated with PBS or the A10 diabody showed progressive tissue thickening and increased redness over the course of infection (data not shown). In the PBS and A10 treated animals, similar levels of lymphocytic infiltrates were observed at day 7 (Fig. 4: a-d, infiltrates shown in arrows). Lymphocyte infiltration was present but diminished in anti-EMP2 treated animals (Fig. 4, e-f).
Figure 4. Histopathological examination of uterine horn 7 days post-chalmydial infection.
Genital tracts of animals treated with either PBS, control diabody (A10), or anti-EMP2 diabody (KS83) were removed at day 7 and stained with H&E. A decrease in lymphocyte infiltration was observed in KS83 (e and f) treated animals compared to the PBS (a and b) and A10 (c and d) treated genital tracts, as indicated by the arrows. Scale bars, 100 μm. Magnification, 100x (a, c, and e) and 200x (b, d, and f).
Previous studies have shown an increase in inflammatory infiltrates at 14 days post-infection in the UH (Darville et al., 1997). Lymphocyte aggregates were observed in PBS (Fig. 5: a-b, aggregates shown in arrows) and A10 diabody (Fig. 5: d-e, aggregates shown in arrows) treated UH. However, aggregates are absent in KS83 treated UH, although lymphocytic infiltration is present (Fig. 5, g-h). Fibrotic tissue is not traditionally studied at day 14, but the presence of collagen was detected in the UH of control animals (Fig. 5: c and f, collagen shown in blue), and absent in the UH of anti-EMP2 treated animals (Fig. 5, i). Pyosalpinx can occur as early as 14 days post-infection (Shah et al., 2005), however, day 14 is early in the course of infection for the appearance of hydrosalpinx. It was interesting to note that on gross examination, all of the control animals, but none of the KS83 diabody treated animals, demonstrated pyosalpinx (data not shown). In the control animals, unilateral pyosalpinx was observed in 50% percent and bilateral pyosalpinx was observed in 50% percent.
Figure 5. Histopathological examination of uterine horn 14 days post-chalmydial infection.
Genital tracts of animals treated with either PBS, control diabody (A10), or anti-EMP2 diabody (KS83) were removed at day 14 and stained with H&E or trichrome. Multiple lymphocyte aggregates are present in PBS (arrows, a and b) and A10 (arrows, d and e), treated animals, but are lacking in those treated with KS83 (g and h). Tissue fibrosis as indicated by trichrome staining was greatly decreased in anti-EMP2 diabody treated (i) animals compared to the PBS (c) and A10 (f) treatment. Scale bars, 100 μm. Magnification, 100x (a, d, and g) and 200x (b, c, e, f, h, and i).
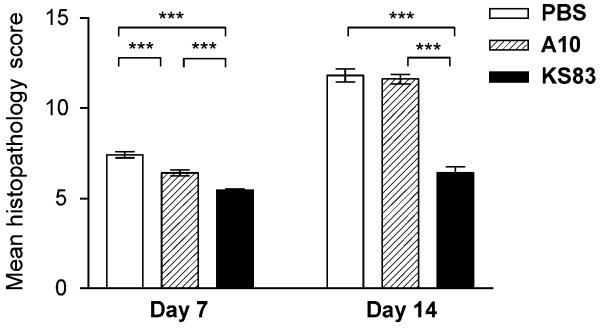
Compared to animals that were treated with PBS or the control diabody, masked examination to produce a quantitative histopathology score revealed a marked reduction in inflammation in the UH of KS83 treated animals (Fig. 6). Despite it being early in inflammation, average histopathology scores for anti-EMP2 diabody treated animals were significantly lower compared to the controls at day 7 (Fig. 6). Similarly, at day 14, although scores indicated an increase in mean histopathology scores across all groups, the average scores on anti-EMP2 diabody treatment group was approximately half that of the control groups (Fig. 6).
Figure 6. Blockade of EMP2 reduces histopathology in uterine horns during Chlamydia infection.
A significant reduction in thickness, recruitment of lymphocytes, and fibrosis was observed at day 7 and day 14 of anti-EMP2 diabody treated animals compared to control animals. Mean histopathologic scores at day 7 and day 14 post-infection were lower in anti-EMP2 diabody treated animals compared to control groups. Statistical analysis of the mean histology scores were performed using Mann-Whitney U test (*p-value<0.05, **p-value<0.005, ***p-value<0.0001). Data are representative of two independent experiments with at least four mice for each treatment group.
Discussion
This study demonstrates that blockade of EMP2 using anti-EMP2 diabody suppresses early chlamydial infection in vivo. This was evidenced by significantly reduced bacterial load and inflammation. Protection was associated with reduced tissue associated cytokines, and decreased recruitment of PMNs throughout the genital tract. These findings suggest that the protective effect of anti-EMP2 on chlamydial disease involves its direct interference with the ascension of infection in the genital tract, which thereby reduces inflammation and tissue damage highlighting its importance in preventing upper tract infection and subsequent sequelae.
We tested the production of multiple cytokines including IFN-γ, TNF-α, and GM-CSF. We chose these cytokines because IFN- γ is a cytokine that is known to play a critical role in inhibition of chlamydial growth, and TNF-α is an early cytokine which facilitates secretion of adhesion molecules that are important for leukocyte extravasation in infected tissue (Darville et al., 2001). GM-CSF has been reported to induce neutrophil migration and activation, and its secretion is one of the early indicators of Chlamydia infection (Darville et al., 2001). Thus, detection of these three cytokines should be suitable for characterization of early chlamydial infection and corresponding host immune response.
Inoculation of animals with a lower dosage of Chlamydiae results in inefficient resolution of the bacteria, and consequently leads to frequent bacterial ascension and oviduct pathology including hydrosalpinx and dilation of oviduct (Maxion et al., 2004). In our present study, the protective effect of EMP2 blockade in chlamydial infection was more significant in the upper versus lower regions of the genital tract. This tendency was especially demonstrated by bacterial load recovery and cytokine data, suggesting that the preventative effect of diabody resulted in a decrease in the ascension of Chlamydia infection. A decrease in overall histopathology and infrequent observation of pyosalpinx in oviducts, which usually becomes apparent 14 days post-infection (Shah et al., 2005), was also observed in the anti-EMP2 diabody treated group, further supporting the effect of anti-EMP2 diabody treatment in preventing overall infection and resultant inflammation.
Overall inflammation and histopathologic evidence for active disease showed a dramatic reduction in the animals that were exposed to anti-EMP2 diabody prior to infection. However, pathologic evidence for disease changes over time with sequential cell influx of PMN and their activation of proteolytic molecules, followed by lymphocytes (Shah et al., 2005; Ramsey et al., 2005). Although our data support a reduction in infectious burden, a longitudinal study should be performed to examine the late effect on future scaring, the course of infection, and infertility, a subject beyond the scope of this study.
Consistent with a decrease in bacterial load, anti-EMP2 treatment decreases proinflammatory cytokines, recruitment of innate cells, and acute inflammation in the genital tract early during infection. This is in agreement with previous findings, where the infectious dose positively correlates with cellular influx of innate immune cells (Maxion et al., 2004). However, it is interesting to speculate that EMP2 may also act to modulate the host immune response such that immunopathological responses are averted. In this context, it is possible that anti-EMP2 diabody treatment promotes a regulatory response which inhibits pathology. Given the results shown, and the immune role in the sequalae of chlamydial infection, this possibility warrants future examination.
In this study, our focus was to examine the changes in early chlamydial infection. It is likely that cytokine production and recruitment of immune cells may change over the later course of infection. Further examination of the effect of anti-EMP2 on regional recruitment of immune cell populations and cytokine and chemokine production at later time points of infection would be beneficial for understanding of overall effect on chlamydial infection pathology and will be subject to future investigation. The correlation between decreased overall EMP2 expression and reduced chlamydial infection further supports the hypothesis that EMP2 is an important host protein in early chlamydial pathogenesis, however determination of the precise mechanism of decreased infectivity requires additional investigation.
Treatment with control diabody also reduced chlamydial infection and host immune response, though to a smaller degree than anti-EMP2 diabody. One explanation may be a non-specific effect of administered lumenal protein in the normally serous, protein-poor secretions of the endometrial lumen (Roberts & Parker, 1974). Accordingly, such protein could potentially impact the initial reversible host-chlamydial interaction (Zhang & Stephens, 1992; Su et al., 1996; Chen & Stephens, 1997; Davis & Wyrick, 1997; Gutierrez-Martin et al., 1997; Taraktchoglou et al., 2001; Wuppermann et al., 2001). Alternatively, bioactive contaminants in the diabody preparations may elicit local epithelial or inflammatory responses that interfere with infection. However diabodies alone did not elicit tissue inflammatory cytokines or recruitment of leukocytes that might reflect the presence of such contaminants (unpublished data). Further characterization of diabody interaction with the mucosal surface, and further measures to assure highly purified diabody protein, are needed to evaluate the nature of this non-specific diabody effect.
Antibody targeting against infectious disease is an area of emerging interest. Antibodies against bacterial toxin, first discovered in the 1890s, were the earliest effective therapy for infectious disease (Behring & Kitasato; 1890), but were replaced by the 1930s with other anti-microbial drugs, particularly antibiotics. The increase in antibiotic-resistant microbes, emerging new organisms, and inefficiency of antibiotic treatment in immune compromised individuals led to recent re-visitation of antibody-based therapy (Casadevall et al., 2004). Antibody targeting using human monoclonal antibodies is a mature area of current biological therapeutics, and offers novel targeting strategies, such as blocking host interaction molecules in microbial pathogenesis.
Acknowledgments
This work was supported by NIH CA86306 (AMW, JB), CA009120 (MW), 2-T32-AI-07323 (KS), AI026328 (KK) and CA016042 (UCLA Jonsson Comprehensive Cancer Center flow cytometry core); Iris Cantor Foundation (MW), the Giannini Family Foundation (MW), the Oppenheimer Family Foundation Grant- Center for the Prevention of Eye Disease (LKG, JB), and Richard and Barbara Braun. We are grateful to Dr. Harlan Caldwell for gift of reagent (anti-Chlamydia LPS mouse antibody) and to the helpful comments of Dr. Wei Bo.
Supported by: Oppenheimer Family Foundation Grant- Center for the Prevention of Eye Disease
References
- Beagley KW, Timms P. Chlamydia trachomatis infection: incidence, health costs and prospects for vaccine development. J Reprod Immunol. 2000;48:47–68. doi: 10.1016/s0165-0378(00)00069-3. [DOI] [PubMed] [Google Scholar]
- Behring EA, Kitasato S. Ueber das austandekommen der diptherie-immunitat und der tetanus-immunitat bei thieren. Deutch. Med. Woch. 1890;49:1113–1114. [Google Scholar]
- Brunham RC, Kimani J, Bwayo J, et al. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J Infect Dis. 1996;173:950–956. doi: 10.1093/infdis/173.4.950. [DOI] [PubMed] [Google Scholar]
- Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of Chlamydia trachomatis infection control program on susecptibility to reinfection. J Infect Dis. 2005;192:1836–1844. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- Chen JC, Stephens RS. Chlamydia trachomatis glycosaminoglycan-dependent and independent attachment to eukaryotic cells. Microb Pathog. 1997;22:23–30. doi: 10.1006/mpat.1996.0087. [DOI] [PubMed] [Google Scholar]
- Darville T, Andrews CW, Jr, Laffoon KK, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville T, Andrews CW, Jr, Sikes JD, Fraley PL, Rank RG. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–3561. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A, Balana ME, Wyplosz B. Chlamydia--host cell interactions: recent advances on bacterial entry and intracellular development. Traffic. 2004;5:561–570. doi: 10.1111/j.1398-9219.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- Davis CH, Wyrick PB. Differences in the association of Chlamydia trachomatis serovar E and serovar L2 with epithelial cells in vitro may reflect biological differences in vivo. Infect Immun. 1997;65:2914–2924. doi: 10.1128/iai.65.7.2914-2924.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir SP, Agarwal LP, Detels R, Wang SP, Grayston JT. Field trial of two bivalent trachoma vaccines in children of Punjab Indian villages. Am J Ophthalmol. 1967;63(Suppl):1639–1644. doi: 10.1016/0002-9394(67)94157-8. [DOI] [PubMed] [Google Scholar]
- Grayston JT, Woolridge RL, Wang SP, Yen CH, Yang CY, Cheng KH, Chang IH. Field studies of protection from infection by experimental trachoma virus vaccine in preschool-aged children on Taiwan. Proc Soc Exp Biol Med. 1963;112:589–595. doi: 10.3181/00379727-112-28112. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Martin CB, Ojcius DM, Hsia R, Hellio R, Bavoil PM, Dautry-Varsat A. Heparin-mediated inhibition of Chlamydia psittaci adherence to HeLa cells. Microb Pathog. 1997;22:47–57. doi: 10.1006/mpat.1996.0090. [DOI] [PubMed] [Google Scholar]
- Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz BP, Batteiger BE, Jones RB. Effect of prior sexually transmitted disease on the isolation of Chlamydia trachomatis. Sex Transm Dis. 1987;14:160–164. doi: 10.1097/00007435-198707000-00008. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Robinson EA, Rank RG. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JD, Bradbury A. Selection of human antibodies from phage display libraries. Methods Mol Biol. 2004;248:161–76. doi: 10.1385/1-59259-666-5:161. [DOI] [PubMed] [Google Scholar]
- Maxion HK, Kelly KA. Chemokine expression patterns differ within anatomically distinct regions of the genital tract during Chlamydia trachomatis infection. Infect Immun. 2002;70:1538–1546. doi: 10.1128/IAI.70.3.1538-1546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxion HK, Liu W, Chang MH, Kelly KA. The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection. Infect Immun. 2004;72:6330–6340. doi: 10.1128/IAI.72.11.6330-6340.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsen T, Cheung CW, Yazaki PJ, et al. Covalent disulfide-linked anti-CEA diabody allows site-specific conjugation and radiolabeling for tumor targeting applications. Protein Eng Des Sel. 2004;17:21–27. doi: 10.1093/protein/gzh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen J, Lehtinen M. Chlamydial pelvic inflammatory disease. Hum Reprod Update. 1996;2:519–529. doi: 10.1093/humupd/2.6.519. [DOI] [PubMed] [Google Scholar]
- Ramsey KH, Soderberg LS, Rank RG. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KH, Sigar IM, Schripsema JH, Shaba N, Cohoon KP. Expression of matrix metalloproteinases subsequent to urogenital Chlamydia muridarum infection of mice. Infect Immun. 2005;73:6962–6973. doi: 10.1128/IAI.73.10.6962-6973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GP, Parker JM. Macromolecular components of the luminal fluid from the bovine uterus. J Reprod Fertil. 1974;40:291–303. doi: 10.1530/jrf.0.0400291. [DOI] [PubMed] [Google Scholar]
- Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis. 2005;32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Wadehra M, Forbes A, Chan AM, Goodglick L, Kelly KA, Braun J, Gordon LK. Epithelial membrane protein 2 modulates infectivity of Chlamydia muridarum (MoPn) Microbes Infect. 2007;9:1003–1010. doi: 10.1016/j.micinf.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Lepin EJ, Wei B, et al. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clinical Cancer Research. 2008 doi: 10.1158/1078-0432.CCR-08-1016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Raymond L, Rockey DD, Fischer E, Hackstadt T, Caldwell HD. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc Natl Acad Sci U S A. 1996;93:11143–11148. doi: 10.1073/pnas.93.20.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Morrison R, Messer R, Whitmire W, Hughes S, Caldwell HD. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J Infect Dis. 1999;180:1252–1258. doi: 10.1086/315046. [DOI] [PubMed] [Google Scholar]
- Taraktchoglou M, Pacey AA, Turnbull JE, Eley A. Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate. Infect Immun. 2001;69:968–976. doi: 10.1128/IAI.69.2.968-976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadehra M, Iyer R, Goodglick L, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002;277:41094–41100. doi: 10.1074/jbc.M206868200. [DOI] [PubMed] [Google Scholar]
- Wadehra M, Su H, Gordon LK, Goodglick L, Braun J. The tetraspan protein EMP2 increases surface expression of class I major histocompatibility complex proteins and susceptibility to CTL-mediated cell death. Clin Immunol. 2003;107:129–136. doi: 10.1016/s1521-6616(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Wadehra M, Goodglick L, Braun J. The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins. Mol Biol Cell. 2004;15:2073–2083. doi: 10.1091/mbc.E03-07-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadehra M, Dayal M, Mainigi M, Ord T, Iyer R, Braun J, Williams CJ. Knockdown of the tetraspan protein epithelial membrane protein-2 inhibits implantation in the mouse. Dev Biol. 2006;292:430–441. doi: 10.1016/j.ydbio.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Wadehra M, Natarajan S, Seligson DB, Williams CJ, Hummer AJ, Hedvat C, Braun J, Soslow RA. Expression of epithelial membrane protein-2 is associated with endometrial adenocarcinoma of unfavorable outcome. Cancer. 2006;10:90–98. doi: 10.1002/cncr.21957. [DOI] [PubMed] [Google Scholar]
- Woolridge RL, Grayston JT, Chang IH, Yang CY, Cheng KH. Long-term follow-up of the initial (1959-1960) trachoma vaccine field trial on Taiwan. Am J Ophthalmol. 1967;63(Suppl):1650–1655. doi: 10.1016/0002-9394(67)94159-1. [DOI] [PubMed] [Google Scholar]
- Wuppermann FN, Hegemann JH, Jantos CA. Heparan sulfate-like glycosaminoglycan is a cellular receptor for Chlamydia pneumoniae. J Infect Dis. 2001;184:181–187. doi: 10.1086/322009. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Stephens RS. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Stewart S, Joseph T, Taylor HR, Caldwell HD. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987;138:575–581. [PubMed] [Google Scholar]