Abstract
Objectives
Research implicates the A1 allele of the dopamine D2 receptor gene (DRD2) Taq1A polymorphism in the development of depression and anxiety.
Furthermore, recent papers suggest that children with A1 allele of this gene may receive less positive parenting, and that the effects of this gene on child symptoms may be moderated by parenting. We sought to replicate and extend these findings using behavioral measures in a nonclinical sample of young children.
Methods
In a sample of 473 preschool-aged children and their mothers, structured clinical interview measures and maternal reports of child symptoms were collected, and standardized observations of parent–child interactions were conducted.
Results
An association was detected between the DRD2 A1 allele and symptoms of depression and anxiety indexed using interview and parent report methods. As found in previous reports, children with the DRD2 A1 allele received less supportive parenting and displayed higher levels of negative emotionality during parent–child interactions. Tests of mediation and moderation were conducted.
Conclusion
We found associations between the DRD2 A1 allele and early-emerging anxious and depressive symptoms in a community sample of preschool-aged children, and evidence of a gene–environment correlation and moderation of the main effect of child genotype on child symptoms by parenting.
Keywords: anxiety, depression, dopamine D2 receptor gene, parenting
Introduction
The dopamine D2 receptor gene (DRD2), located on chromosome 11q, encodes the D2 subtype of the dopamine receptor. This gene has a restriction fragment length polymorphism called Taq1A (rs1800497) with two alleles referred to as A1 and A2 (Eisenberg et al., 2007); the A1 allele is less common and is usually treated as dominant in genetic studies. This polymorphic variant seems to have functional effects on dopamine receptor density (Jönsson et al., 1999); for example, a post-mortem study found fewer D2 dopamine receptors in the brains of those with the DRD2 A1 allele than in those without this allele (Noble et al., 1991), and studies using in-vivo autoradiography and positron emission tomography (Thompson et al., 1997; Pohjalainen et al., 1998; Jönsson et al., 1999) have supported this finding. Recent studies have implicated this gene in shaping regulatory aspects of behavior related to approach and reward processing (Bowirrat and Oscar-Berman, 2005; Althaus et al., 2009); these findings, considered along with studies of humans and animals linking dopamine D2 receptor binding and DRD2 genotype to depression, anxiety, and impaired social functioning (Shively et al., 1997; Schneier et al., 2001; Lawford et al., 2006), suggest the potential importance of this gene in the development of depressive and anxiety disorders.
Some have posited that the influence of the DRD2 genotype on adaptive development emerges early in life (Lawford et al., 2006), although the majority of research on links between this gene and psychopathology has been conducted in samples of adults. Exceptions include a study conducted by Marino et al. (2004), who reported an association between the DRD2 A1 allele and social dysfunction in a small sample of children. In another study of this gene in youths, Althaus et al. (2009) linked the A1 allele to enhanced sensitivity to negative feedback and reduced sensitivity to positive feedback under probabilistic learning conditions indexed by standardized laboratory tasks. This same allele has been linked to greater child negative emotionality during a parent–child interaction task (Mills-Koonce et al., 2007). Considering the relevance of negative emotionality, interpersonal functioning, and sensitivity to affective stimuli in depressive and many forms of anxiety disorders (Clark et al., 1994; Erickson and Newman, 2007; Starr and Davila, 2008; Joiner and Timmons, 2009; Joorman, 2009; McNally and Reese, 2009), these findings further support the potential etiological significance of the DRD2 gene for the development of such forms of psychopathology.
In addition to influencing the development of symptoms, a potentially complementary line of research implicates this gene in gene–environment correlation (rGE). It was recently reported that infants with the A1 allele had mothers who were rated as less sensitive during parent–child tasks by independent observers (Propper et al., 2008), providing compelling evidence for a rGE between this gene and parenting behavior. The authors did not report tests of possible mechanisms underlying this relationship; for example, the reported rGE might be accounted for by the influence of the DRD2 genotype on child behavior that, in turn, elicited less sensitive maternal behavior (i.e., an evocative rGE, Rutter, 2007). In this same sample, it was also found that DRD2 genotype moderated the association between maternal sensitivity and child affective problems (Mills-Koonce et al., 2007), such that children with the A1 allele exhibited fewer affective symptoms when mothers were highly sensitive. Hence, although only limited attention has been directed toward these questions, the DRD2 gene may be linked to environmental variables that further exacerbate risk for psychopathology, and its effects on symptoms may be moderated by contextual influences. It therefore seems important to examine links between this gene and early environmental variables, whether mechanistic processes (i.e., mediators) can be identified that account for any rGE obtained, and whether environmental factors either amplify or mitigate the vulnerability that the DRD2 A1 allele appears to confer.
In an effort to strengthen and extend existing research on the role of the DRD2 gene in psychopathology, we planned to examine associations between the DRD2 gene and early-emerging symptoms of depression and anxiety, predicting that the DRD2 A1 allele would be associated with elevated rates of these symptoms in children. To potentially replicate previous studies, we also examined whether this allele was associated with the behavior of children and parents during parent–child interactions. More specifically, we planned to examine whether the DRD2 A1 allele was associated with child emotional behavior during parent–child interactions (Mills-Koonce et al., 2007), and whether this allele was associated with either decreased positive or increased negative parenting (Propper et al., 2008). The DRD2 A1 allele has been associated with both depressive and anxious symptoms and with the parenting that children receive; therefore, to the extent that similar relationships were found in our sample, we planned to test whether links between this gene and child symptoms were direct, mediated (or moderated) by parenting, or whether a combination of the two processes was evident.
Methods
The sample was 473 children (251 males) from an original sample of 559 children and their parents from a suburban area. The mean age of the children was 42.2 months [standard deviation (SD)=3.1]. Potential participants were identified through a commercial mailing list. Eligible families had a child between 3 and 4 years of age, with no significant medical conditions or developmental disabilities, and at least one English-speaking biological parent. Most of the participants came from middle-class families, as measured by Hollingshead’s Four Factor Index of Social Status (mean=44.8, SD=10.9) (Hollingshead, 1975). The vast majority of children (96.0%) came from two-parent homes, and children were of average cognitive ability as indexed by the Peabody Picture Vocabulary Test (mean=103.1, SD=13.7) (Dunn and Dunn, 1997). Most children were White (85.9%). The second largest ethnic group in the sample was Hispanic (4.9%), and the remaining children were from an array of other ethnic backgrounds, including multiple races/ethnicities. This research was approved by the Committee on Research Involving Human Subjects at Stony Brook University.
When participants came to the laboratory for the behavioral assessments, buccal cells were collected for genetic analysis by rubbing the inside of each child participant’s cheek with two swabs. Of the 559 children participating in a larger study of depression vulnerability, 475 had parental consent to give samples for genetic assessment. The Qiagen DNA Micro Kit (Qiagen Valencia, California, USA) was used to extract genomic DNA from buccal swab samples according to the manufacturer’s instructions. Extracts were kept at 4°C when being analyzed, and were held at − 80°C for long-term storage. Genomic DNA was successfully extracted from 473 of the 475 children who provided buccal swabs for analysis and had laboratory data. Polymerase chain reaction (PCR) was carried out using the Applied Biosystems thermal cycler Gene Amp 9700 (Applied Biosystems, Foster City, California, USA), and PCR products were separated on polyacrylamide gels, stained with ethidium bromide, and visualized and documented by a UV imaging system (BioRad Labs, Mississauga, Ontario, Canada).
For the detection of the polymorphism in the Taq1A site, oligonucleotide primers 5′-CACGGCTGGCCAAGTTGT CTA-3′ (forward) and 5′-CACCTTCCTGAGTGTCAT CAA-3′ (reverse) were used to amplify a 300-bp region comprising the Taq1A site (Grandy et al., 1993). The PCR conditions used were initial denaturation for 5 min at 95°C followed by 30 cycles of 30 s denaturation at 94°C, 30 s annealing at 58°C, and a 30 s extension at 72°C, followed by a 5 min final extension at 72°C. The 300 bp PCR product was digested overnight with 1U of TaqαI restriction enzyme (New England BioLabs, Massachusetts, USA). The A1 allele is uncut by the restriction enzyme, whereas the A2 allele generates 125 and 175 bp fragments. Eleven children had the A1A1 genotype, 152 children were heterozygous (A1A2) and 310 had the A2A2 genotype. These genotype frequencies were consistent with Hardy–Weinberg equilibrium, χ2(1)=2.35, P=0.13. All genotyping was performed by research technicians blind to other study data. Consistent with most published research, and considering the rarity of the A1A1 genotype, groups for data analysis were formed based on whether children had (N=163) or did not have (N=310) an A1 allele.
Child emotional and behavioral problems
The Preschool Age Psychiatric Assessment (PAPA; Egger et al., 2006) Version 1.4 was used to assess symptoms of psychopathology as defined by Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria relevant to children in this age group. The PAPA is the first published diagnostic interview to assess parent-reported psychopathology in children between the ages of 2 and 5 years. It uses a structured format and an interviewer-based approach. Symptoms occurring 3 months prior the interview are rated to enhance accurate recall. Adequate test–retest reliability has been reported [range of intraclass correlations (ICC) for dimensional symptom scores=0.56–0.80, median=0.66, Egger et al., 2006].
For this study, we used PAPA indices of depressive and anxious symptoms, and selected symptoms of an externalizing disorder common in preschoolers, oppositional defiant disorder (ODD), to determine the relative specificity of the DRD2 genotype for depressive and anxious symptoms. We created dimensional scores by summing the ratings of all items included in the algorithms created by Egger et al. (2006) to derive depressive and anxiety disorders and ODD diagnoses. Interviews were conducted by advanced graduate students in clinical psychology who received training on the administration of the PAPA from a member of the PAPA group. To examine interrater reliability, a second rater independently rated audiotapes of 21 PAPA interviews. ICCs for the symptom scales used in this study were as follows: depression (0.85), anxiety (1.00), and ODD (0.99). Internal consistency (α) was calculated for each symptom scale and indicated good reliability for depression (α=0.75), anxiety (α=0.83), and ODD (α=0.84).
As an additional measure of children’s symptoms, we collected maternal reports on the Child Behavior Checklist/4–18 (CBCL, Achenbach, 1991). The CBCL consists of 118 items measuring children’s emotional and behavioral problems over the past 6 months, which are rated on a scale from 0 (not true) to 2 (very or often true). The CBCL yields two broadband factors assessing internalizing and externalizing problems, and several subscales, which include withdrawn, somatic complaints, anxious/depressed, social problems, delinquent behavior, and aggressive behavior. The anxious/depressed subscale was used to test associations between the DRD2 gene and these symptoms. To examine specificity of the effects of this gene on depressive and anxious symptoms, and because the DRD2 A1 allele has also been linked to externalizing problems (Lu et al., 2001), we also examined whether the A1 allele of the DRD2 gene was associated with scores on the aggressive behavior subscale.
Teaching tasks
Of the sample of 473 children, 443 children and a primary caregiver (usually the mother, N=412, 87.1%) participated in a modified version of the Teaching Tasks battery (Egeland et al., 1995). The primary caregiver and child participated in a 30-min series of six standardized parent–child interaction tasks designed to elicit different parenting and child behaviors and interaction styles. The Teaching Tasks battery called for parents and children to read a book together, play with blocks, and work on a matching task and maze, among other activities. For coding, a single rating was made for each variable in each episode based on all relevant behaviors, and ratings were then averaged across tasks. To index parenting practices with relevance for psychopathology vulnerability (McLeod et al., 2007a, 2007b; Van der Bruggen et al., 2008), ratings of parent supportive presence and intrusiveness were used, and relevant behaviors were coded in all the tasks. In addition, ratings of child positive and negative affect (PA, NA) were coded in each task. Ratings of parent supportive presence (α=0.88) were based on the parent’s expression of positive regard and emotional support to the child, and intrusiveness ratings (α=0.61) were made based on the parent’s failure to permit autonomous child behavior. Ratings of child PA (α=0.82) and NA (α=0.73) were based on the frequency and intensity of the child’s expression of relevant facial, vocal, and bodily indicators (e.g., facial expressions, vocalizations). Interrater ICCs for parent supportive presence, intrusiveness, and child positive and negative affect were 0.85, 0.70, 0.87, and 0.90 (N=55), respectively.
Statistical analyses
Analyses were structured with the goal of replicating and extending existing findings on links between child DRD2 genotype, child psychiatric symptoms, and parenting behaviors. First, we examined bivariate associations between DRD2 genotypes and all major study variables, including symptoms of child psychopathology and parenting measures. These analyses were carried out to test for main effects of DRD2 genotypes on child symptoms, and to reveal any associations between child genotype and parenting behavior (rGE).
Based on earlier studies (Propper et al., 2008), we expected that we might detect associations between child genotype and parenting behavior; however, the mechanisms that potentially account for such relationships remain unclear. We therefore planned to explore any such links further through tests of mediation, with the goal of determining whether any child genotype-parenting associations could be mechanistically accounted by the effects of child genotype on child behavior. To test mediation models, we used the bootstrap sampling procedure and companion macro developed by Preacher and Hayes (2008a, 2008b). This procedure yields estimates of mean direct (c) and indirect (i.e., mediated, c′) effects and confidence intervals (CIs) derived from multiple samples (5000 in the present case) drawn from a data set. When estimated CIs yielded by the bootstrapping procedure contain the value ‘zero’ within them, the estimated effect is not statistically significant at P value less than 0.05. This strategy is comparable and conceptually similar to multiple regression, but with numerous advantages over more traditional approaches to testing mediation (e.g., robustness with respect to smaller sample sizes and violations of normality, see Preacher and Hayes (2008a, 2008b) for an extensive discussion and validation of this method).
Finally, based on previously reported data (Mills-Koonce et al., 2007), we planned to examine whether any associations between DRD2 genotypes and child symptoms were moderated by parenting styles. Moderation was tested using multiple regression (Aiken and West, 1991). Predictor variables were centered as appropriate (i.e., converted into deviation score form) to minimize multicollinearity, and interaction terms were formed as the product of the two predictors. Variables were entered in the first step and the interaction term (the product of the two predictors) was entered in step 2. Statistically significant interactions were subsequently probed using the techniques outlined by Aiken and West. In this study, for example, significant DRD2-parenting interactions were probed by regressing parenting behavior on child symptoms for each child genotype.
Results
Associations between DRD2 genotype and major study variables
Associations between DRD2 genotype and all study variables are shown in Table 1 (to examine whether ethnicity influenced the findings obtained, parallel analyses excluding non-White children and families were conducted. These yielded similar results to those presented here. In addition, we initially conducted analyses treating child sex, cognitive ability, and socioeconomic status as covariates; as no substantive differences between the adjusted and unadjusted models were found, we do not consider these further). Children with and without an A1 allele were not significantly different on sex proportion, socioeconomic status, or Peabody Picture Vocabulary Test scores. Consistent with the hypothesis that the A1 allele may increase risk for depressive and anxious symptoms, children with an A1 allele had higher scores on the CBCL anxious/depressive subscale. In contrast, the two genotype groups did not differ significantly on aggressive behavior indexed by the CBCL. Children with the A1 allele also had a greater number of anxiety symptoms as indexed by the PAPA. The two groups did not differ in terms of depressive symptoms from the PAPA, although mean differences were in the predicted direction, with the A1 group having slightly higher levels of depressive symptoms. There were no significant group differences on PAPA measures of ODD symptoms. Male and female children did not differ on any major study variables (data not in table, all P>0.22). Consistent with earlier reports of gene–environment correlation, children with an A1 allele received less support from parents during the Teaching Tasks battery. However, the groups did not differ in terms of intrusive behavior displayed by caregivers. Furthermore, at the level of a trend, children with an A1 allele expressed greater levels of NA during the Teaching Tasks.
Table 1.
Demographic and study variables by child DRD2 genotype
| Variable | Child DRD2 genotype
|
|||||
|---|---|---|---|---|---|---|
| DRD2 A2A2 (N=310)
|
DRD2 A1A2/A1A1 (N= 163)
|
|||||
| Mean | SD | N | Mean | SD | N | |
| Child sex, male | 165 (53%) | 86 (53%) | ||||
| PPVT | 103.57 | 13.51 | 102.31 | 13.99 | ||
| SES | 44.96 | 11.20 | 44.43 | 10.45 | ||
| CBCL anxiety/depression** | 2.42 | 2.03 | 3.14 | 2.59 | ||
| CBCL aggress | 10.59 | 6.52 | 10.80 | 6.53 | ||
| PAPA anxiety* | 8.85 | 7.49 | 10.66 | 9.69 | ||
| PAPA depression | 3.92 | 3.95 | 4.41 | 4.93 | ||
| PAPA ODD | 5.05 | 6.99 | 5.10 | 7.03 | ||
| PCI support* | 4.52 | 0.54 | 4.38 | 0.62 | ||
| PCI intrusion | 1.44 | 0.40 | 1.49 | 0.44 | ||
| PCI child PA | 2.62 | 0.79 | 2.59 | 0.74 | ||
| PCI child NA† | 1.34 | 0.49 | 1.44 | 0.52 | ||
CBCL aggress, Child Behavior Checklist Aggressive Behavior Subscale; CBCL anxiety/depression, Child Behavior Checklist Anxious/Depressed Subscale; DRD2, dopamine D2 receptor gene; NA, negative affect; ODD, oppositional defiant disorder; PA, positive affect; PAPA, Preschool Age Psychiatric Assessment; PCI, parent–child interaction task; PPVT, Peabody Picture Vocabulary Test; SD, standard deviation; SES, socioeconomic status, as indexed by Hollingshead’s Four Factor Index of Social Status (Hollingshead, 1975).
P<0.05.
P<0.01.
P<0.10.
Mediation analyses
As DRD2 genotype was associated with children’s depressive and anxious symptoms, and with parent and child behavior during parent–child interaction tasks (parental support and child NA, respectively), it is possible that child NA mediated the link between child genotype and parenting, and that parenting mediated the link between child DRD2 genotype and symptoms of depression and anxiety. As CBCL anxious/depressed subscale scores and PAPA anxiety symptoms were substantially correlated with one another (r=0.52, P<0.0001), we z-transformed and averaged the two measures and used this composite index of child symptoms for these analyses.
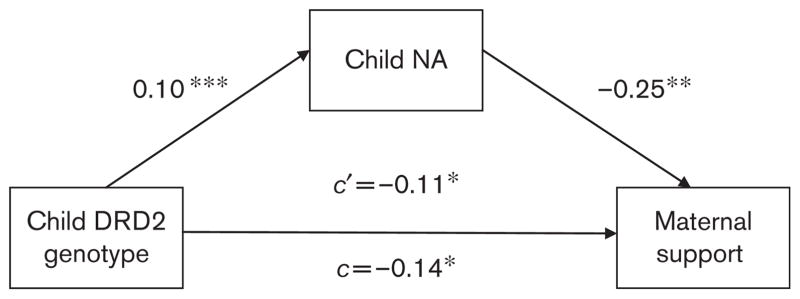
The first model examined whether links between DRD2 genotype and parenting were mediated by the effect of this gene on child NA during parent–child interactions, as a means of accounting for child genotype-parenting associations. As a precondition for testing mediation, associations must be present between the predictor and the outcome, the predictor and the hypothesized mediator, and the hypothesized mediator and the outcome (Baron and Kenny, 1986). Table 1 shows the associations between DRD2 genotype and parental support, and child NA during the Teaching Tasks. After confirming the significant association between parental support and child NA (r= −0.28, P<0.001), we proceeded with the mediation analysis. The bootstrapping procedure yielded a marginally significant estimate of the indirect effect of DRD2 genotype on parental support as mediated by child NA, mean= −0.02, 95% CI: −0.06 to −0.00. The direct association between DRD2 genotype and parental support was also significant, indicating that the DRD2 genotype-parenting link was only partially mediated by child NA (Fig. 1).
Fig. 1.
Mediated effect of child dopamine D2 receptor gene (DRD2) genotype on parental support by child negative affect (NA). **P<0.05, *P<0.01, ***P<0.10. Note: Child DRD2 genotype coded as 0=A2A2 genotype, 1=A1A2 or A1A1 genotype; maternal support coded during parent–child interaction task; c=coefficient for direct path between child DRD2 genotype and parental support; c′ =coefficient for path between child DRD2 genotype and parental support, mediated by child NA.
Previous work indicates links between child DRD2 genotype, parenting, and affective symptoms (Mills-Koonce et al., 2007). There was a modest but significant association between parental support and child symptoms (r= −0.10, P<0.05). We therefore examined whether the association between DRD2 genotype and depressive and anxious symptoms was mediated by parental support. Bootstrapping estimates of the indirect effect of DRD2 genotype as mediated by child NA were not significant, mean=0.00, 95% CI: −0.02 to −0.02; the estimated path coefficients between DRD2 genotype and parental support were virtually identical before and after accounting for the effects of parental support, indicating that the DRD2 genotype-child symptoms association was not mediated by parental support.
Moderation analyses
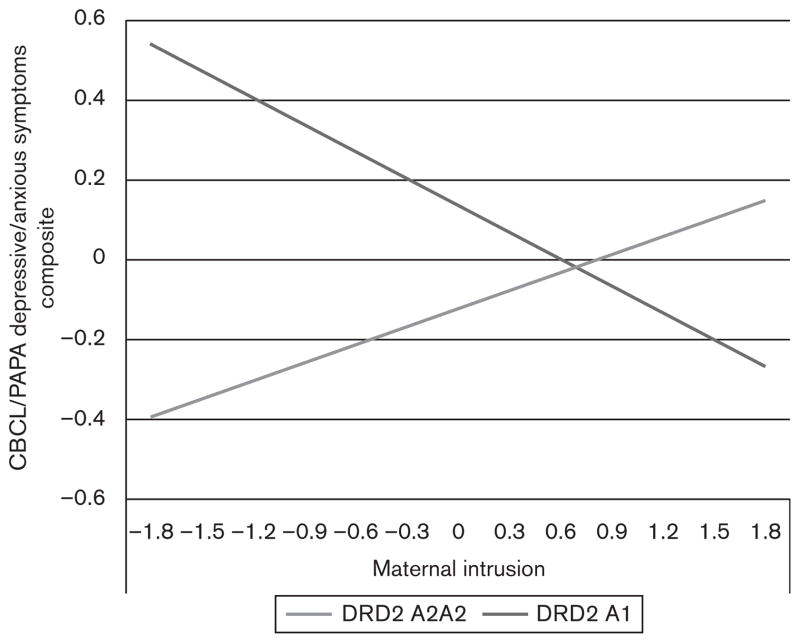
Finally, we examined whether the main effect of the DRD2 gene on child depressive and anxious symptoms was moderated by either parental intrusion or support (Mills-Koonce et al., 2007). The interaction term for DRD2 genotype and parental support was not significant (b=0.04, standard error=0.14, partial correlation=0.01, P=0.79); however, there was a marginally significant interaction between DRD2 genotype and parental intrusion (b= −0.37, standard error=0.19, partial correlation, −0.09, P=0.05), indicating that the DRD2-child symptoms association differed depending on the extent to which parenting was intrusive. To understand the nature of the interaction, we plotted estimated levels of child depressive and anxious symptoms across estimated levels of parental intrusion separately for the two DRD2 genotypes (Fig. 2). Children without an A1 allele of the DRD2 gene showed increasing levels of symptoms as parental intrusiveness increased; in contrast, for children with at least one copy of the A1 allele, parental intrusion and symptoms were negatively associated, although the slopes for each genotype group did not attain significance (P>0.16).
Fig. 2.
Relationship between maternal intrusion and child depressive and anxious symptom composite by child dopamine D2 receptor gene (DRD2) genotypes. CBCL, Child Behavior Checklist, PAPA, preschool age psychiatric assessment; CBCL/PAPA Depressive/Anxious Symptoms Composite is derived from the z-transformed average of CBCL anxious/depressed subscale scores and PAPA anxiety symptoms; hence, some children’s scores were negative; maternal intrusion coded during parent–child interaction task.
Discussion
We examined whether the DRD2 gene was associated with early-emerging symptoms of depression and anxiety in a community sample of young children. Findings were supportive: the DRD2 A1 allele was associated with children’s depressive and anxious symptoms measured using both maternal report and interview methods. Our findings suggest specificity of the effects of this gene for depressive and anxious symptomatology, as no links between this candidate gene and externalizing psychopathology were detected.
We also found evidence of a rGE in our sample, in that child DRD2 genotype was associated with parenting behavior exhibited during laboratory tasks. This finding, along with the modest association we found between DRD2 genotype and child NA, is an approximate replication of findings from Propper et al. (2008). In addition, Mills-Koonce et al. (2007) similarly reported a link between the DRD2 A1 allele and child negative mood during parent–child interactions. The association between child genotype and parenting behavior is interesting, considering the relative rarity of reported rGE’s in the literature (Jaffee and Price, 2007). Tests of mediation provided limited support for the presence of an evocative rGE, in that the association between child genotype and parenting was partially, albeit weakly, mediated by the association between the DRD2 gene and child NA during parent–child interactions. As far as other plausible mechanisms for the association between parenting and child genotype are concerned, it is certainly possible that this gene influences other child behaviors not measured in this study that act to reduce supportive parenting. It is also possible that some of the variance in this rGE is ‘passive’ (Rutter, 2007), such that certain parenting styles result from the parent’s own genetic variants, which are inherited by their children.
We also found evidence of a gene–environment interaction: while depressive and anxious symptoms and parental intrusiveness were positively associated in children without a DRD2 A1 allele, children with at least one copy of the A1 allele appeared to have low levels of symptoms when parenting was intrusive. Although this pattern may seem counterintuitive (i.e., the notion that genetic risk might be mitigated by seemingly undesirable parenting practices), it is not inconsistent with developmental theory and research on inhibition in children. More specifically, Kagan (1994) posited that inhibited children would be likely to benefit from less sensitive parenting, and an empirical test of this possibility by Park et al. (1997) was consistent, indicating that maternal intrusiveness led to decreased child inhibition. Similarly, our data suggest that the heightened risk for symptoms of depression and anxiety conferred by the DRD2 gene may be buffered by less supportive parenting; while speculative, this could be because such parents are less tolerant of children’s symptoms and therefore compel children to modify anxious/depressive behavior that they are genetically predisposed to exhibit. Our finding seems to run counter to the study by Mills-Koonce et al. (2007), who reported that children with the A1 allele exhibited fewer affective symptoms when mothers were highly sensitive. Confirmation of these findings is important, especially considering recent work indicating that gene–environment interactions may be less robust than previously thought (Risch et al., 2009).
Our study had several strengths, including our use of laboratory-based measures of parenting behavior and interview-based measures of child psychopathology. However, our study had a number of limitations; first, experts disagree on the extent to which population stratification, which can produce false positive associations, is a concern in studies such as ours using an ethnically homogenous sample (Hutchison et al., 2004). In addition, we cannot rule out the possibility that the DRD2 gene is in linkage disequilibrium with another gene that has functional effects on the variables we examined in this study. Although our sample size was large compared with other studies examining related research questions (e.g. Propper et al., 2008), it is still relatively small for a genetic association study. We did not have parent genotype data, which would have permitted more extensive analyses of rGEs between genes and parenting behavior. Finally, we report cross-sectional data in this paper, although causal links between these constructs (e.g. parenting, child behavior, child symptoms) are best understood by examining how associations between parenting and child behavior develop over time.
In conclusion, we found associations between the DRD2 A1 allele and early-emerging anxious and depressive symptoms in a community sample of preschool-aged children, and evidence of a rGE and moderation of the main effect of child genotype by parenting. We had only limited success in attempts to identify processes that accounted for associations between child genotype and parenting styles; however, this research contributes to an emerging body of work linking the DRD2 Taq1A polymorphism to both early-emerging vulnerability to psychopathology and adverse early environments.
Acknowledgments
This study was supported by a Young Investigator award from NARSAD to Elizabeth P. Hayden, a GCRC grant no. M01-RR10710 to Stony Brook University from the National Center for Research Resources and a National Institute of Mental Health grant R01 MH069942 to Daniel N. Klein.
References
- Achenbach TM. Manual for the child behavior checklist/4-18 and 1991 profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Althaus M, Groen Y, Wijers AA, Mulder LJM, Minderaa RB, Kema IP, et al. Differential effects of 5-HTTLPR and DRD2/ANKK1 polymorphisms on electro-cortical measures of error and feedback processing in children. Clin Neurophysiol. 2009;120:93–107. doi: 10.1016/j.clinph.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neuro-transmission, alcoholism, and reward deficiency syndrome. Am J Med Genet. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994;103:103–116. [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test. 3. Circle Pines, Minnesota: American Guidance Service; 1997. [Google Scholar]
- Egeland B, Weinfield N, Hiester M, Lawrence C, Pierce S, Chippendale K, et al. Teaching tasks administration and scoring manual. Minneapolis, MN: University of Minnesota Institute of Child Development; 1995. [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the preschool age psychiatric assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA, MacKillop J, Modi M, Beauchemin J, Dang D, Lisman SA, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 Taq1 A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson TM, Newman MG. Interpersonal and emotional processes in generalized anxiety disorder analogues during social interaction tasks. Behav Ther. 2007;38:364–377. doi: 10.1016/j.beth.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Zhang Y, Civelli O. PCR detection of the TaqA RFLP at the DRD2 locus. Hum Mol Genet. 1993;2:2197. doi: 10.1093/hmg/2.12.2197-a. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975. Unpublished manuscript. [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: fatal threat or red herring? Psychol Bull. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner TE, Timmons KA. Depression in its interpersonal context. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 322–339. [Google Scholar]
- Jö nsson EG, Nö then MM, Grünhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Joorman J. Cognitive aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 299–321. [Google Scholar]
- Kagan J. Galen’s prophecy. New York: Basic Books; 1994. [Google Scholar]
- Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Lu R-B, Lee J-F, Ko H-C, Lin W-W. Dopamine D2 receptor gene (DRD2) is associated with alcoholism with conduct disorder. Alcohol Clin Exp Res. 2001;25:177–184. [PubMed] [Google Scholar]
- Marino C, Vanzin L, Giorda R, Frigerio A, Lorusso ML, Nobile M, et al. An assessment of transmission disequilibrium between quantitative measures of childhood problem behaviors and DRD2/Taq1 and DRD4/48bp-repeat polymorphisms. Behav Genet. 2004;34:495–502. doi: 10.1023/B:BEGE.0000038487.80597.7e. [DOI] [PubMed] [Google Scholar]
- McLeod BD, Wood JJ, Weisz JR. Examining the association between parenting and childhood anxiety: a meta-analysis. Clin Psychol Rev. 2007a;27:155–172. doi: 10.1016/j.cpr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- McLeod BD, Wood JJ, Weisz JR. Examining the association between parenting and childhood depression: a meta-analysis. Clin Psychol Rev. 2007b;27:986–1003. doi: 10.1016/j.cpr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- McNally RF, Reese HE. Information-processing approaches to understanding anxiety disorders. In: Antony MM, Stein MB, editors. Oxford handbook of anxiety and related disorders. Oxford library of psychology. New York, NY, US: Oxford University Press; 2009. pp. 136–152. [Google Scholar]
- Mills-Koonce WG, Propper CB, Gariepy J-L, Blair C, Garrett-Peters P, Cox MJ. Bidirectional genetic and environmental influences on mother and child behavior: the family system as the unit of analyses. Dev Psychopathol. 2007;19:1073–1087. doi: 10.1017/S0954579407000545. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Park S, Belsky J, Putnam S, Crnic K. Infant emotionality, parenting, and 3-year inhibition: exploring stability and lawful discontinuity in a male sample. Dev Psychol. 1997;33:218–227. doi: 10.1037//0012-1649.33.2.218. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K, Lehikoinen P, Anttila K, Syvalahti EKG, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008a;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2008b;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore GA, Mills-Koonce WR, Tucker Halpern C, Hill-Soderlung AL, Calkins SD, et al. Gene-Environment contributions to the development of infant vagal reactivity: the interaction of dopamine and maternal sensitivity. Child Dev. 2008;79:1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Gene-environment interdependence. Dev Science. 2007;10:12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Leibowitz MR, Abi-Dargham A, Zea-Ponce Y, Lyn SH, Laurelle M. Low dopamine D(2) receptor binding potential in social phobia. Am J Psychiatry. 2001;158:327–328. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression and brain dopamine in female cynomolgus monkeys. Ann NY Acad Sci. 1997;807:574–577. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- Starr LR, Davila J. Differentiating interpersonal correlates of depressive symptoms and social anxiety in adolescence: implications for models of comorbidity. J Clin Child Adolesc Psychol. 2008;37:337–349. doi: 10.1080/15374410801955854. [DOI] [PubMed] [Google Scholar]
- Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, et al. D2 dopamine receptor gene (DRD2) TaqI A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Van der Bruggen CO, Stams GJJM, Bogels SM. Research review: the relationship between child and parent anxiety and parental control: a meta-analytic review. J Child Psychol Psychiatry. 2008;49:1257–1269. doi: 10.1111/j.1469-7610.2008.01898.x. [DOI] [PubMed] [Google Scholar]