Abstract
Purpose
Breast cancer survivors (BCS) taking aromatase inhibitors (AIs) are at an increased risk for decreased bone density and fractures. Given the role vitamin D plays in bone metabolism, we examined the prevalence of and risk factors for vitamin D deficiency in a study of postmenopausal BCS on AIs.
Methods
We collected data on 391 postmenopausal women with stage I–III breast cancer on AI therapy. Vitamin D levels were measured by radioimmunoassay from patients' sera; deficiency was defined as a level < 30 ng/mL. Multivariate models were created to assess risk factors for deficiency.
Results
The median vitamin D level was 35 ng/mL (range 6.78–93.15), and 35% of women were vitamin D deficient. When adjusting for age and vitamin D supplementation, minority participants were more likely to be vitamin D deficient than white women, (adjusted odds ratio [AOR] 2.18, 95% confidence interval [CI]1.22-3.89, p=0.009). Both overweight (AOR 3.05, 95% CI 1.72-5.41, p<0.001) and obese participants (AOR 3.21, 95% CI 1.79-5.78, p<0.001) had higher deficiency rates than did normal weight participants.
Conclusions
Hypovitaminosis D is common in BCS, and those who are nonwhite or overweight are at a higher risk of deficiency despite taking vitamin D supplements.
Introduction
There has been growing awareness of the widespread prevalence of vitamin D deficiency. It is estimated that 30%–50% of the adult U.S. population is vitamin D deficient.1–3 For breast cancer survivors (BCS), vitamin D status is of special concern for a number of reasons. First, there is a possible relationship between vitamin D deficiency and increased rates of breast cancer recurrence and mortality4; however, the results have not been replicated.5 Second, this population is also vulnerable to bone loss and musculoskeletal symptoms in the setting of exposure to aromatase inhibitor (AI) therapy. Data show that BCS have a 15% higher fracture risk than women without a history of breast cancer.6 Vitamin D deficiency is also independently associated with an increased chance of hip fractures7,8 and falls.9 Lastly, there may be a relationship between vitamin D levels and AI-associated arthralgia.10,11
Vitamin D is a fat-soluble vitamin that regulates bone modeling and calcium absorption. Although moderate amounts of the vitamin from such foods as milk and fatty fish are absorbed from the gastrointestinal tract, the majority of vitamin D is produced in the body when 7-dehydrocholesterol in the skin is exposed to ultraviolet B (UVB) radiation to produce vitamin D3 (cholecalciferol). Vitamin D3 then undergoes two hydroxylation steps, first in the liver to form 25-hydroxyvitamin D (25-OHD), the major circulating metabolite, and then in the kidney to produce 1,25-dihydroxyvitamin D (1,25-(OH)2D), the biologically active form. Vitamin D is also produced in a variety of tissues, including the breast, prostate, and colon, where binding of 1,25-(OH)2D to the vitamin D receptor regulates gene expression, inhibits cell proliferation, induces cell differentiation, promotes apoptosis, and decreases angiogenesis.13–16
Known risk factors for vitamin D deficiency include older age,17 darker skin pigmentation, obesity,18 low dietary intake, renal or liver disease, and sun avoidance behaviors. As a result, vitamin D deficiency is quite prevalent in the general population, particularly among minorities.19 Melanin is an effective filter of UVB irradiation, which leads to a higher prevalence of vitamin D deficiency among blacks.17,19
A previous study demonstrated a large percentage of women undergoing breast cancer treatment were vitamin D deficient,20 although the prevalence has not been examined in postmenopausal BCS taking AIs. Therefore, our study aims to describe the prevalence of vitamin D deficiency in this population and identify risk factors for deficiency. Because little is known about the practice behaviors of oncologists in diagnosing and treating vitamin D deficiency, we also examined the prevalence of a documented serum vitamin D level in the clinical charts of these patients.
Materials and Methods
Study population
We conducted a cross-sectional study of women taking AIs at the Rena Rowan Breast Cancer Center of the Abramson Cancer Center of the University of Pennsylvania (Philadelphia). Potential study participants included postmenopausal women with a history of histologically confirmed, stage I–III, hormone receptor-positive breast cancer who were currently taking a third-generation AI (anastrozole, letrozole, or exmestane) and visited the clinic between March 2008 and August 2009. Additional inclusion criteria were completion of chemotherapy or radiotherapy at least 1 month before enrollment in the study, approval of the patient's primary oncologist, and the patient's ability to understand and provide informed consent in English. After informed consent was obtained, each participant completed a self-administered survey. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Primary study outcome: Vitamin D deficiency
Serum samples were stored in aliquots at −80°C until measurement. Samples were analyzed for 25-OHD by radioimmunoassay (RIA) as previously described (Diasorin, Stillwater, MN) in the Clinical Research Center of the University of Pennsylvania.21 Interassay precision at 10 ng/mL was 1.57%, and the intra-assay coefficient of variation (CV) was 8.42%. Although other researchers have trichotomized vitamin D levels into sufficient, deficient, and insufficient,11,21 we dichotomized the groups to increase the power of our analysis. We defined vitamin D deficiency as a serum 25-OHD level < 30 ng/mL, which is consistent with previous articles cited.10
Demographic and clinical Information
Patients completed a survey that queried demographic and medical variables, including age, race/ethnicity, body mass index (BMI), marital status, education level, employment status, and such medical comorbidities as osteopenia and osteoporosis. Chart abstraction was performed for data about such variables as breast cancer stage, chemotherapy and tamoxifen use, vitamin D supplementation, and previously documented serum vitamin D levels.
Statistical methods
Descriptive analyses for demographics, clinical characteristics, and serum vitamin D levels were performed. We used chi-square analyses to assess the differences between patients who were found to be vitamin D deficient vs. those who were vitamin D sufficient. We then performed multivariate analyses to examine the relationship between demographic and clinical variables and vitamin D status. We selected variables that met entry criteria in univariate analysis with p value < 0.1. We forced age into the model, although it did not make the entry criteria because other research showed that age was a predictor of vitamin D deficiency. Also, we examined patterns of vitamin D level documentation by the oncologist using chi-square analyses. All analyses were two-sided, with values < 0.05 indicating significance. Data analysis was performed using STATA 10.0 for Windows (STATA Corp, College Station, TX).
Results
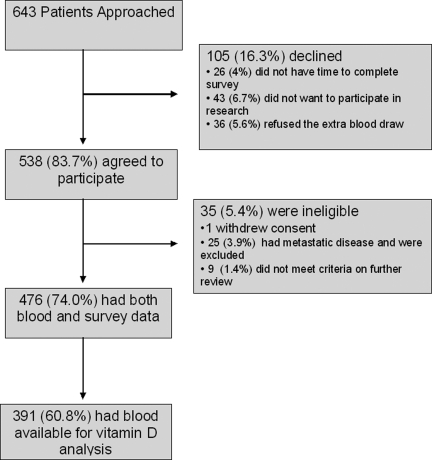
Of 643 consecutive patients screened, 538 (83.7%) agreed to participate. (Fig. 1). Among 105 who declined (16.3%), the main reasons were lack of time to complete the survey (n=26, 4%), lack of desire to participate in research (n=43, 6.7%), and refusal to have an extra blood draw (n=36, 5.6%). Additionally, 1 subject withdrew consent, and 9 subjects (1.4%) were further disqualified because they did not meet eligibility criteria upon further review. Of 528 subjects who returned questionnaire data (82.1%), 501 (77.9%) had both an evaluable survey and a blood sample; 25 subjects were further excluded after chart review revealed metastatic disease (3.9%), resulting in the final sample of 476. This study was part of a larger cohort of BCS on AI therapy; therefore, only 391 had adequate serum samples that could be used for vitamin D analysis. Among the 391 study participants, the mean age was 61 years, ranging from 33 to 91 years. Table 1 includes further demographic and clinical information.
FIG. 1.
A consort diagram of our cohort recruitment.
Table 1.
Vitamin D Status and Clinical Characteristics (n=391)
| Total number of participants | Vitamin D replete | Vitamin D deficient | p value | |
|---|---|---|---|---|
| Age, years | 0.841 | |||
| <55 | 24% (94) | 68.1% (64) | 31.9% (30) | |
| 55–65 | 45% (177) | 65% (117) | 35% (66) | |
| >65 | 31% (120) | 66.5% (84) | 33.5% (41) | |
| Race/ethnicity | 0.003 | |||
| White | 82% (321) | 69.8% (224) | 30.2% (97) | |
| Nonwhite | 18% (70) | 51.4% (36) | 48.6% (34) | |
| BMI | <0.001 | |||
| <25 | 41% (161) | 80.8% (130) | 19.3% (31) | |
| 25–30 | 31% (122) | 57.4% (70) | 42.6% (52) | |
| >30 | 28% (108) | 55.6% (60) | 44.4% (48) | |
| Education level | 0.311 | |||
| High school or less | 19% (76) | 59.2% (45) | 40.8% (31) | |
| College | 45% (174) | 67.2% (117) | 32.8% (57) | |
| Graduate or professional school | 36% (140) | 69.3% (97) | 30.7% (43) | |
| Employment | 0.067 | |||
| Full-time | 41% (158) | 60.8% (96) | 39.2% (62) | |
| Part-time | 13% (50) | 78% (39) | 22% (11) | |
| Not currently employed | 46% (180) | 67.8% (122) | 32.2% (58) | |
| Season | 0.379 | |||
| October–March | 34% (134) | 69.4% (93) | 30.6% (41) | |
| April–September | 66% (257) | 65% (167) | 35% (90) | |
| Cancer stage | 0.262 | |||
| Stage I/0 | 39% (151) | 70.9% (107) | 29.1% (44) | |
| Stage II | 48% (191) | 64.9% (124) | 35.1% (67) | |
| Stage III | 13% (49) | 59.2% (29) | 40.8% (20) | |
| Chemotherapy | 0.105 | |||
| No chemotherapy | 39% (152) | 71.1% (108) | 29% (44) | |
| Chemotherapy, no taxane | 23% (90) | 57.8% (52) | 52.2% (48) | |
| Taxane chemotherapy | 38% (149) | 67.1% (100) | 32.9% (49) | |
| Vitamin D supplementation | p<0.001 | |||
| No | 27% (104) | 48.1% (50) | 51.9% (54) | |
| Yes | 73% (287) | 72.2% (210) | 26.8% (77) | |
| Bone health | 0.014 | |||
| Neither | 54% (211) | 60.2% (127) | 39.8% (84) | |
| Osteopenia | 31% (122) | 75.4% (92) | 24.6% (30) | |
| Osteoporosis | 15% (58) | 70.7% (41) | 29.3% (17) |
BMI, body mass index.
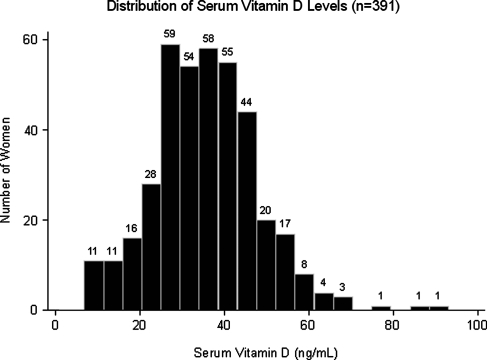
Vitamin D distribution
The mean vitamin D level was 35 ng/mL, standard deviation (SD) 12 ng/mL. Vitamin D levels were normally distributed in our population (Fig. 2). Using the aforementioned definitions of vitamin D deficiency, we found that 35% of patients were vitamin D deficient.
FIG. 2.
Histogram depicting the distribution of serum vitamin D levels in our cohort.
Risk factors for vitamin D deficiency
In bivariate analysis (Table 1), race, BMI, part-time employment, and vitamin D supplementation were all significantly associated with vitamin D deficiency. Bisphosphonate use (p=0.12) and length of time since initiating AI therapy (p=0.71) were not significant. There was no significant difference in vitamin D levels obtained from April through September vs. October through March (p=0.28). In the multivariate model incorporating clinical and demographic factors (Table 2), BCS who were overweight (adjusted odds ratio [AOR] 3.07, 95% confidence interval [CI] 1.77- 5.33, p<0.001) or obese (AOR 2.88, 95% CI 1.64-5.06, p<0.001) had a significantly higher risk of being vitamin D deficient. Nonwhite BCS also had a significantly higher risk of being vitamin D deficient (AOR 2.00, 95% CI 1.14-3.51, p=0.016). Employment became nonsignificant in this model.
Table 2.
Multivariate Model of Vitamin D Status (Deficient Yes/No) Versus Clinical and Demographic Factors
| |
Bivariate analysis |
Multivariate model 1a |
Multivariate model 2a |
|||
|---|---|---|---|---|---|---|
| Characteristic | OR (95% CI) | p | AOR (95% CI) | p | AOR (95% CI) | p |
| Age, years | ||||||
| <55 (reference) | - | |||||
| 55–65 | 1.15 (0.68-1.96) | 0.607 | 0.99 (0.56-1.75) | 0.964 | 0.98 (0.54-1.77) | 0.949 |
| >65 | 1.03 (0.58-1.83) | 0.928 | 0.97 (0.47-1.98) | 0.93 | 1.16 (0.55-2.43) | 0.694 |
| Race/Ethnicity | ||||||
| White (reference) | - | |||||
| Nonwhiteb | 2.18 (1.29-3.69) | 0.004 | 2.00 (1.14-3.51) | 0.016 | 2.18 (1.22-3.89) | 0.009 |
| BMI | ||||||
| <25 (reference) | - | |||||
| 25–30 | 3.12 (1.83-5.30) | <0.001 | 3.07 (1.77-5.33) | <0.001 | 3.05 (1.72-5.41) | <0.001 |
| >30 | 3.35 (1.94-5.79) | <0.001 | 2.88 (1.64-5.06) | <0.001 | 3.21 (1.79-5.78) | <0.001 |
| Employment | ||||||
| Full-time (reference) | ||||||
| Part-time | 0.44 (0.21-0.92) | 0.029 | 0.50 (0.23-1.08) | 0.079 | 0.40 (0.18-0.92) | 0.03 |
| Not currently employed | 0.74 (0.47-1.15) | 0.179 | 0.70 (0.40-1.21) | 0.199 | 0.66 (0.37-1.17) | 0.152 |
| Vitamin D supplementation | ||||||
| No (reference) | ||||||
| Yes | 0.34 (0.21-0.54) | <0.001 | 0.29 (0.17-0.48) | <0.001 | ||
Model 1 is the basic model that incorporates clinical characteristics; Model 2 additionally adjusts for vitamin D supplementation.
All nonwhite participants were grouped for the purpose of analysis.
AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Impact of vitamin D supplementation on vitamin D deficiency
In our cohort, 73.4% had vitamin D supplementation documented in their charts. They were significantly less likely to be vitamin D deficient compared to those women not on vitamin D supplementation (26.8% vs. 51.9%, p<0.001). To further elaborate this finding, we created a second multivariate model that incorporated vitamin D supplementation into the previous multivariate model (Table 2). As expected, vitamin D supplementation was associated with a lower risk of vitamin D deficiency (AOR 0.29, 95% CI 0.17-0.48, p<0.001). Interestingly, the risk for BCS who were overweight (AOR 3.05, 95% CI 1.72- 5.41, p<0.001) or obese (AOR 3.21, 95% CI 1.79-5.78, p<0.001) increased when adjusting for vitamin D supplementation. Nonwhite BCS also had a greater AOR of being deficient when adjusting for vitamin D supplementation (AOR 2.18, 95% CI1.22-3.89, p=0.009).
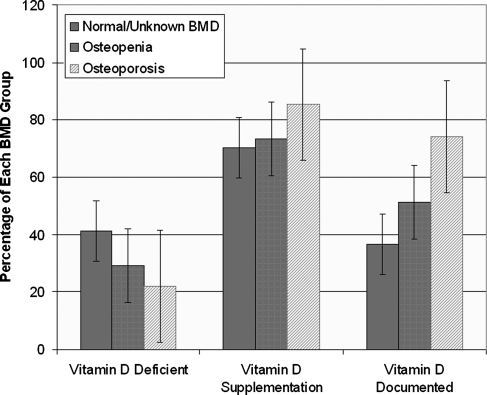
Vitamin D supplementation and bone health
From our chart review, we found that 43% of our cohort had previously had a vitamin D level documented. The rate of vitamin D supplementation or vitamin D documentation did not differ by body size or race after controlling for differences in practitioner behavior. However, there was a significant relationship between bone health and vitamin D deficiency (Fig. 3). Participants with a history of osteoporosis or osteopenia were significantly less likely to be vitamin D deficient, 22% and 29.2%, respectively, vs. 41.2% in patients with normal or unknown bone density (p=0.015). There was no significant difference in vitamin D supplementation among the three groups, but patients with osteoporosis were significantly more likely to have a vitamin D level documented in their chart (p=0.001), even when controlling for different healthcare providers (AOR 2.44, 95% CI1.08-5.52, p=0.033).
FIG. 3.
Comparison of vitamin D deficiency, supplementation, and documentation rates among women with a diagnosis of osteopenia, osteoporosis, or normal/unknown bone mineral density (BMD).
Discussion
In this study among a cohort of postmenopausal BCS on AI, we found that 35% of women were vitamin D deficient, and of those, 58.8% were already receiving supplementation. Nonwhite women and women who had a BMI > 25 regardless of race were more likely to be vitamin D deficient. In our cohort, despite the fact that one third of BCS have vitamin D deficiency, only 43% of our cohort had a vitamin D level documented in their chart. Women with osteoporosis were less likely to be vitamin D deficient and more likely to have vitamin D levels ordered and documented in their chart by their oncologist compared to other BCS.
Our findings are consistent with numerous studies that have demonstrated that the vitamin D deficiency rate among breast cancer patients is >30%.22,23 A recent study by Crew et al.20 found a deficiency rate of 74% among breast cancer patients on chemotherapy. The difference in deficiency rates may be due to the difference in racial composition of the two studies; 49% of participants in the Crew study were nonwhite vs. 18% of our cohort. The difference may also be explained by differing rates of vitamin D supplementation between the two cohorts or differences in diet or UV exposure.
Surprisingly, 26.8% of the women currently receiving vitamin D supplementation were vitamin D deficient. This finding raises the possibility that some levels of supplementation may not be adequate. A previous study found that after supplementation with 400 IU daily for 1 year, <15% of white and Hispanic women and no black women achieved sufficient vitamin D levels.20 The current Institute of Medicine (IOM) recommended daily allowance (RDA) for vitamin D is 600 IU for women aged 51–70 and 800 IU for women aged ≥70.24 Although we were unable to determine from the chart the exact number of international units of vitamin D that each participant was taking, these data suggest that the amount being taken may not be sufficient for some individuals to correct vitamin D deficiency in this cohort of women.
In our cohort, nonwhite patients were about twice as likely to be vitamin D deficient as their white counterparts. A number of previous studies have documented this relationship between race and vitamin D levels.3,25,26 Data from the third National Health and Nutrition Examination Survey (NHANES) showed that serum 25-OHD levels were significantly different among women of different races. The mean levels were 30 ng/mL for white women, 23 ng/mL for Hispanic women, and 18 ng/mL for black women (p<0.001).27 This relationship between nonwhite race and vitamin D deficiency may be a result of increased melanin levels in darker skin, which block the UV conversion of 7-dehydrocholesterol to cholecalciferol.28 Given that lower serum vitamin D levels have been associated with increased risk of breast cancer recurrence,4,5 this raises the question whether discrepancy in vitamin D levels may offer one explanation for why minority patients have worse breast cancer outcomes.
After controlling for individual oncologist behavior, there was no significant difference in the rates of clinical vitamin D documentation or vitamin D supplementation between white and nonwhite BCS. This suggests that oncologists may not be aware of the amplified risk of vitamin D deficiency in the nonwhite population. The 2005 Dietary Guidelines for Americans recommends that groups at high risk for vitamin D deficiency, including older adults, people with dark skin, and those exposed to insufficient UV radiation, should consume 1000 IU vitamin D daily.29 In our study, we found that controlling for vitamin D supplementation in our multivariate model increased the odds for nonwhite women to be vitamin D deficient, suggesting that white women are more easily able to achieve sufficient levels with recommended levels of supplementation. One study found that postmenopausal African American women required 2000 IU daily to achieve a sufficient serum vitamin D level, highlighting that for vitamin D supplementation, one size may not fit all.30 Given the uncertainty surrounding the appropriate vitamin D supplementation dosage for minority patients, it is not surprising that a large percentage of them remain vitamin D deficient.
Obesity is another well-documented risk factor for vitamin D deficiency. Numerous studies have demonstrated a relationship between obesity and serum vitamin D levels, irrespective of race.18,31–33 This is consistent with our findings, in which both overweight (BMI 25–30) and obese (BMI > 30) women were about three times as likely to be vitamin D deficient as those BCS with a BMI ≤ 25. In addition, we found that controlling for vitamin D supplementation in our multivariate model increased the AOR of vitamin D deficiency for overweight and obese women, suggesting that women with a BMI < 25 are more easily able to achieve sufficient levels with recommended levels of supplementation. The exact mechanism for this phenomenon is still unknown. One theory is based on the fact that vitamin D is lipophilic, leading to increased fat uptake of vitamin D among more obese women. Mower et al. found that radiolabeled cholecalciferol injected intravenously into adipose tissue was rapidly cleared, suggesting that vitamin D is sequestered in adipose tissue in overweight and obese individuals and, thereby, not bioavailable.34,35 Other researchers theorize that overweight women may feel more self-conscious about exposing their skin, thereby decreasing their UV exposure and endogenous production of vitamin D. It is also possible that increased weight may merely be the result of a nutritionally poor diet that is low in sources of vitamin D. These interesting hypotheses need to be explored in future research.
Although overweight and obese patients are at a lower risk for decreased bone density, obesity is a negative prognosis factor for several events related to breast cancer, including overall survival (hazard ratio [HR] 1.43 [1.28-1.60]).36 It is unclear if vitamin D deficiency plays a causal role for these increased risks, but correcting a vitamin D deficiency may have the potential to improve outcomes for these BCS. This requires greater awareness on the part of physicians to diagnose and treat vitamin D deficiency in overweight or obese women, but we found no increase in vitamin D level documentation or supplementation in this subpopulation of our cohort. This clinical scenario is complicated by the fact that patients with increased BMI may require increased supplementation compared to BCS of normal weight.37 Unfortunately, there is uncertainty about what constitutes adequate supplementation for overweight or obese patients.
Although we found that patients with self-reported osteopenia or osteoporosis had lower rates of vitamin D deficiency compared to their counterparts, the deficiency rates were still quite high, 22% and 29.2%, respectively. Vitamin D status is a special concern for BCS on AIs because of its well-established relationship to bone mineral density (BMD).7,22 Women on AIs are at an increased risk of decreased BMD and fracture. One study of a cohort of BCS found that after 5 years of anastrozole, there was a 6.08% decrease in median BMD in the lumbar spine and a 7.24% drop in the total hip.38 BCS on AIs also have an AOR of 2.03 for any type of fracture compared to nonusers.39 Given evidence that vitamin D may prevent osteoporotic fractures,40,41 it may be important to understand how much vitamin D supplementation may help prevent osteoporosis and fracture in this population.
Our study has a number of strengths, including the size of the cohort and its relative ethnic diversity. Our population is also relatively diverse in terms of season of blood draw, BMI, and socioeconomic status. We used an assay that takes into account both exogenous sources and endogenous production and had a high degree of precision. Limitations to our study include the lack of information about UV exposure and nonendogenous sources of vitamin D. Additionally, we were not able extract the exact dosage of vitamin D supplementation and compliance with supplementation, as this information is usually not documented in sufficient detail in a clinical chart. Further, given the lack of consistent documentation of BMD and diagnoses of osteopenia and osteoporosis, we had to rely on self-report for these variables. Lastly, we did not collect data on waist circumference, which has been found to be a more accurate representation of fat mass.
Conclusions
Despite the growing body of literature that suggests the importance of vitamin D for BCS, vitamin D deficiency rates remain relatively high in this population. Although the efficacy of vitamin D supplementation for reducing breast cancer mortality is still uncertain, there is a known benefit of vitamin D for maintaining BMD and decreasing the osteoporotic fracture rate.40,41 Given the documented risk of fractures associated with AI therapy,6 this is a particularly relevant clinical problem. Practitioners should be aware that overweight or obese women and ethnic minorities are at the highest risk for being vitamin D deficient. In addition, the amount of vitamin D supplementation recommended by IOM guidelines may not be enough to raise vitamin D levels to an appropriate level, especially in these high-risk groups. More research is needed to establish vitamin D supplementation guidelines for nonwhite and overweight patients. Clinicians should be aware that a one size fits all approach to supplementation may not be adequate.
Acknowledgments
This study is in part supported by a Penn Clinical Pharmacogenomic Epidemiology pilot grant (5P20RR020741), National Institutes of Health (NIH) grant R21 AT004695, and a Clinical and Translational Research Center at the University of Pennsylvania Clinical Research Award (no. 807602). C.F.F. is supported by the Doris Duke Clinical Research Foundation. J.J.M. is supported by the American Cancer Society (ACS) CCCDA-08-107-02 and NIH grant 1 K23 AT004112-03. H.I.S. is support by ACS grant MRSG-08-110-01-CCE and NIH grant HD-058799-01. The funding agencies had no role in the design and conduct of this study. We thank all the breast cancer survivors, physicians, nurse practitioners, and staff for their support. We thank Krupali Desai, Luke Velders, and Donna Chen for their dedication to the data collection and management process.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Tangpricha V. Pearce E. Chen T. Holick M. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lips P. Duong T. Oleksik A, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: Baseline data from the Multiple Outcomes of Raloxifene Evaluation Clinical Trial. J Clin Endocrinol Metab. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 3.Nesby-O'Dell S. Scanlon KS. Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin PJ. Ennis M. Pritchard KI. Koo J. Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 5.Freedman DM. Chang SC. Falk RT, et al. Serum levels of vitamin D metabolites and breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2008;17:889–894. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z. Maricic M. Bassford TL, et al. Fracture risk among breast cancer survivors: Results from the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165:552–558. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA. Giovannucci E. Wilett WC. Dietrich T. Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 8.Jackson RD. LaCroix AZ. Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 9.Robbins J. High-dose oral vitamin D supplements and active vitamin D prevent falls in older persons. Ann Intern Med. 2010;152:JC1-3. doi: 10.7326/0003-4819-152-2-201001190-02003. [DOI] [PubMed] [Google Scholar]
- 10.Waltman NL. Ott CD. Twiss JJ. Gross GJ. Lindsey AM. Vitamin D insufficiency and musculoskeletal symptoms in breast cancer survivors on aromatase inhibitor therapy. Cancer Nurs. 2009;32:143–150. doi: 10.1097/01.NCC.0000339262.44560.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan QJ. Reddy PS. Kimler BF, et al. Effect of vitamin D supplmentation on serum 25-hydroxyvitamin D levels, joint pain and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2009;119:111–118. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colston KW. Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer. 2002;9:45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 13.Nagpal S. Na S. Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 14.Mantell DJ. Owens PE. Bundred NJ. Mawer EB. Canfield AE. 1-Alpha,25-dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–220. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 15.Trump DL. Muindi J. Fakih M. Yu WD. Johnson CS. Vitamin D compounds: Clinical development as cancer therapy and prevention agents. Anticancer Res. 2006;26:2551–2556. [PubMed] [Google Scholar]
- 16.Gombart AF. Luong QT. Koeffler HP. Vitamin D compounds: Activity against microbes and cancer. Anticancer Res. 2006;26:2531–2542. [PubMed] [Google Scholar]
- 17.Looker AC. Dawson-Hughes B. Calvo MS. Gunter EW. Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S. Massaro JM. Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zadshir A. Tareen N. Pan D. Norris K. Martins D. The prevalence of hypovitaminosis D among U.S. adults: Data from the NHANES III. Ethnicity Dis. 2005;15:S5-97–101. [PubMed] [Google Scholar]
- 20.Crew KD. Shane E. Cremers S. McMahon DJ. Irani D. Hershman DL. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol. 2009;27:2151–2156. doi: 10.1200/JCO.2008.19.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang-Gillam A. Miles DA. Hutchins LF. Evaluation of vitamin D deficiency in breast cancer patients on bisphosphonates. Oncologist. 2008;13:821–827. doi: 10.1634/theoncologist.2008-0013. [DOI] [PubMed] [Google Scholar]
- 23.Palmieri C. MacGregor T. Girgis S. Vigushin D. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. J Clin Pathol. 2006;59:1334–1336. doi: 10.1136/jcp.2006.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 25.Chan J. Jaceldo-Siegl K. Fraser G. Determinants of serum 25 hydroxyvitamin D levels in a nationwide cohort of blacks and non-Hispanic whites. Cancer Causes Control. 2010;21:501–511. doi: 10.1007/s10552-009-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egan KM. Signorello LB. Munro HM. Hargreaves MK. Hollis BW. Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: Implications for cancer disparities (United States) Cancer Causes Control. 2008;19:527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 27.McKinney K. Breitkopf CR. Berenson AB. Association of race, body fat and season with vitamin D status among young women: A cross-sectional study. Clin Endocrinol. 2008;69:535–541. doi: 10.1111/j.1365-2265.2008.03233.x. [DOI] [PubMed] [Google Scholar]
- 28.Clemens TL. Adams JS. Henderson SL. Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;319:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 29.Johnson MA. Kimlin MG. Vitamin D, aging, and the 2005 Dietary Guidelines for Americans. Nutr Rev. 2006;64:410–421. doi: 10.1111/j.1753-4887.2006.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 30.Talwar SA. Aloia JF. Pollack S. Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr. 2007;86:1657–1662. doi: 10.1093/ajcn/86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Compston JE. Vedi S. Ledger JE. Webb A. Gazet JC. Pikington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34:2359–2363. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 32.Lagunova Z. Porojnicu AC. Lindberg F. Hexeberg S. Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–3720. [PubMed] [Google Scholar]
- 33.Looker AC. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab. 2005;90:635–640. doi: 10.1210/jc.2004-1765. [DOI] [PubMed] [Google Scholar]
- 34.Mawer EB. Backhouse J. Holman CA. Lumb GA. Stanbury SW. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci. 1972;43:413–431. doi: 10.1042/cs0430413. [DOI] [PubMed] [Google Scholar]
- 35.Wortsman J. Matsuoka LY. Chen TC. Lu Z. Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 36.Majed B. Moreau T. Senouci K. Salmon RJ. Fourquet A. Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329–342. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- 37.Lee P. Greenfield JR. Seibel MJ. Eisman JA. Center JR. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med. 2009;122:1056–1060. doi: 10.1016/j.amjmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Eastell R. Adams JE. Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-Year results from the Anastrozole, tamoxifen, Alone or in Combination Trial 18233230. J Clin Oncol. 2008;26:1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 39.Vestergaard P. Rejnmark L. Mosekilde L. Effect of tamoxifen and aromatase inhibitors on the risk of fractures in women with breast cancer. Calcif Tissue Int. 2008;82:334–340. doi: 10.1007/s00223-008-9132-7. [DOI] [PubMed] [Google Scholar]
- 40.Bergman G. Fan T. McFetridge JT. Sen SS. Efficacy of vitamin D(3) supplementation in preventing fractures in elderly women: A meta-analysis. Curr Med Res Opin. 2010;26:1193–1201. doi: 10.1185/03007991003659814. [DOI] [PubMed] [Google Scholar]
- 41.Bischoff-Ferrari HA. Willett WC. Wong JB. Giovannucci E. Dietrich T. Dawson-Hughes B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]