Abstract
Ionic nutrition is essential for plant development. Many techniques have been developed to image and (or) measure ionic movement in plants. Nevertheless, most of them are destructive and limit the analysis. Here, we present the development of radioisotope imaging techniques that overcome such restrictions and allow for real-time imaging of ionic movement. The first system, called macroimaging, was developed to visualize and measure ion uptake and translocation between organs at a whole-plant scale. Such a device is fully compatible with illumination of the sample. We also modified fluorescent microscopes to set up various solutions for ion uptake analysis at the microscopic level. Both systems allow numerical analysis of images and possess a wide dynamic range of detection because they are based on radioactivity.
Keywords: real-time radioisotope imaging system, whole-plant imaging, 32P, 35S, 45Ca, 55Fe
1. Introduction
Imaging is now an indispensable analysis tool in understanding the biological activity of plants. Among the imaging methods, significant developments have occurred in the fluorescent imaging technique, which requires specific conditions (darkness) for signal acquisition. However, plants require light for physiological activity; therefore, the fluorescent technique cannot be easily applied to real-time imaging of nutrient uptake. Nuclear magnetic resonance (NMR) is another candidate for real-time imaging of nutrient uptake in plants [1,2]. However, for NMR, it is extremely difficult to control environmental conditions, such as humidity, temperature, and light, because the sample is prepared in a small test tube in a magnetic field. Then, what kind of imaging technique is suitable for analysing live plant activity at a high resolution? The use of radioisotopes offers a promising method because it allows imaging under light, and enables quantitative analysis of the image, as the imaging is based on radioactivity.
Positron emitters, which are widely used in the medical imaging technique of positron emission tomography, have been applied to plant research [3,4]. A positron emitted from the sample couples with an electron in the tissue and converts it into two identical γ-rays, a phenomenon called annihilation. Therefore, imaging using a positron emitter is based on γ-ray counting. However, because of the high energy of the positron emitted from the nuclide, tissue thickness of at least a few mm is required to reduce the energy and complete annihilation. If the tissue is less than 1 mm thick, such as that of a leaf, the positron escapes from the sample and couples with an electron outside the sample to produce γ-rays. The escape ratio of the positron is dependent on the thickness of the tissue. Therefore, when a positron emitter is supplied to a plant, the radioactivity count in the image of the stem is much higher than that of the leaf, the thickness of which is far less than that of the stem.
Several approaches have combined the positron-imaging system with other methods, such as magnetic resonance imaging and X-ray computed tomography [5,6]. However, as long as positron emitters are applied, positron escape from the sample will always remain an issue when they are used with plant samples. Recently, an optical imaging method using Cerenkov radiation from radioisotopes has been reported [7]. In order to obtain luminescent signal for imaging, the Cerenkov energy passing through the water in the sample should be higher than 262 keV, which limits the kinds of applicable isotopes. Therefore, it is preferable to use positron emitters, such as 18F, 64Cu or 68Ga. For example, in the case of phosphate imaging, considerable amounts of 32P are required, and 33P cannot be used because of its low radiation energy. Although Cerenkov radiation imaging allows numerical analysis of the image, it requires dark conditions because imaging is based on luminescence measurements.
Plants live on inorganic nutritional elements; therefore, to analyse live plant activity, it is crucial to study the real-time movement of elements: how they are taken up by the root and moved up to the above-ground parts. However, suitable tools have not yet been developed to visualize real-time nutrient uptake in plants. Because radioisotope imaging can be performed in both dark and light conditions, we developed a whole-plant imaging system using radioisotopes, and were able to obtain high sensitivity with β-ray emitters [8–10]. In this report, we present a real-time imaging system for plant nutrient uptake, using commercially available β-ray emitters, such as 35S, 45Ca and 32P, not only for the whole plant but also for microscopic images acquired with a radioisotope-fluorescence microscope.
Because the imaging is based on radioactivity counts, quantitative analysis is possible. Another advantage of applying a β-ray emitter is that it allows for the imaging of real-time ionic movement from the roots not only in a water culture solution but also in the soil. Without using radioisotopes, it is not possible to image the specific movement of ions from the soil towards the roots with such high sensitivity. Here, we present both the macroscopic and microscopic imaging systems.
2. Material and methods
(a). Macroscopic imaging system
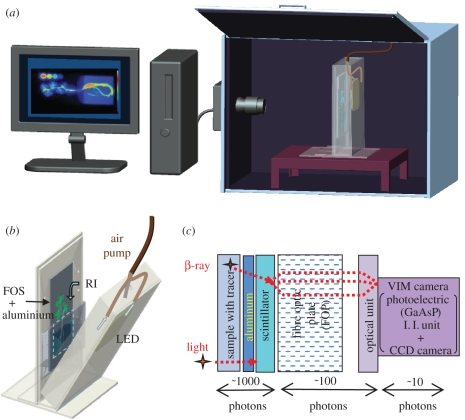
Figure 1 shows the schematic illustration of the real-time radioisotope imaging system. The system was composed of a CsI (Tl) scintillator deposited on a fibre optic plate (FOP) and a highly sensitive charge-coupled device (CCD) camera with an image intensifier unit (I. I. unit C8600-04, Hamamatsu Photonics Co., Japan). The scintillator was deposited on an FOP, 100 μm in thickness, which was grown into a needle-like crystal (product for fibre optic scintillator (FOS), J6675, Hamatsu Photonics Co., Japan). The filling factor of the CsI (Tl) scintillator was 80 per cent. When a radioisotope was applied to the plant, the β-rays emitted from the sample were converted to weak light by a CsI (Tl) scintillator. Then, the weak scintillation light was electronically multiplied by a multi-channel plate (MCP) with a GaAsP photoelectric surface (I. I. unit C8600-04, Hamamatsu Photonics Co., Japan) and photographed by a CCD camera (C3077-70, Hamatsu Photonics Co., Japan). The ultra-sensitive camera system consisted of an MCP and a CCD camera, which collected images every few minutes and produced successive images. The AQUACOSMOS software developed by Hamamatsu Photonics Co., Japan, was used to analyse the series of CCD images.
Figure 1.
Schematic of the macroimaging system for real-time isotope imaging and example of use. The plant was placed in a box, and light was applied to the aerial part of the plant. The plant root, placed in a container in the box, could grow for several days. Airflow was continuously introduced into the plant box to regulate temperature and humidity. The plant box was placed in a box kept in the dark. A fibre optic scintillator (FOS) comprising a CsI scintillator deposited on a fibre optic plate (FOP) converted the β-ray emitted from the isotope in the sample into light. This signal was electronically multiplied by a multi-channel plate (MCP) with a GaAsP photoelectric surface, and was guided to a highly sensitive charge-coupled device (CCD) camera. An aluminium film was used to isolate the plant sample from the detection system to allow the use of an illumination source (provided by white light emitting diode (LED) here). (a) System, (b) plant box and (c) schematic of image acquisition.
To image the sample under light, the plant box container was prepared using aluminium plates, and was set in the imaging box. Figure 1b shows a picture of the plant container equipped with 100 light-emitting diodes (LEDs), capable of emitting 120 µmol m−2 s−1 onto the sample plant. By completely covering the plant and the light source, the container prevented external light from producing a noise during the imaging process. Because the scintillator allows light to permeate, its surface facing the container was covered with an aluminium plate, 50 μm in thickness. With this condition, light emitted by the LEDs was completely shielded, and only the β-ray penetrated the aluminium plate and reached the scintillator without noticeable deterioration. The size of the scintillator working surface on the FOP was 10 × 20 cm. The LEDs can be switched on and off. We simulated day and night by turning the LEDs on and off to apply 16 L : 8 D cycle to the above-ground parts of the plant; the roots were kept in the dark. To control temperature and humidity in the box, airflow was introduced into the box from the upper part.
(b). Microscopic imaging system
We developed a radioisotope-fluorescence microscope using the same principle as that of the macroimaging apparatus. The vertical lens of the microscope was equipped with a taper FOS (J6757-5-25, Hamamatsu Photonics Co., Japan) whose diameter was changed from 3 to 15 μm to magnify the image five times. The CsI (Tl) scintillator was deposited at a thickness of 50 μm on one side of the FOS surface (5 μm Ø). The β-rays from the sample were converted into light by the scintillator, and were amplified via the GaAsP I. I. unit made by Hamamatsu Photonics Co., Japan. The I. I. unit is a light-amplifying device equipped with a photoelectric surface, and it allows the capture of weak scintillation light with a CCD camera (C3077-70, Hamamatsu Photonics Co., Japan). The image resolution was about 100 μm. For imaging Arabidopsis roots, a combination of a plate FOS (J6675MOD, Hamamatsu Photonics Co., Japan) and an optical lens, instead of the taper FOS, was applied to obtain a 40-fold increase in image magnification. To obtain linearity between image quantification and radioactivity, 1 μl of a standard solution of 32P was mounted on a filter paper and imaged for 2 min. Then the counts per minute of the image was calculated and plotted against the radioactivity of the 32P standard solution.
(c). Plant preparation
(i). Oryza sativa L. ‘Nipponbare’
The Nipponbare cultivar of O. sativa was used in all experiments. To compare the 32P-phosphate uptake between water culture and soil culture, 3-day-old seedlings, which were about 7 cm in length, were used for imaging. One of the rice plants was grown in a 22.5 ml water culture solution (Hoagland medium) containing 1.5 MBq of 32P-phosphate. The other sample was grown in nursery soil for rice seedlings (Kumiai Baido, Kasanen Industry Co., Japan, 3.1 g phosphate per 20 kg). Then, 15 ml of water culture solution containing 1.5 MBq of 32P-phosphate was mixed well with 20 ml of soil (32 g) and packed in the container with the plant. The concentration of phosphate in the water culture solution was 100 μM. Successive 32P-phosphate uptake images of Nipponbare were collected for 60 h.
(ii). Arabidopsis thaliana L. ‘Columbia’
An Arabidopsis plant was grown in nutrient solution (MGRL) [11]. In the case of 45Ca imaging, 45CaSO4 solution (1 MBq per 0.5 ml in MGRL water culture solution) was supplied to the root of a 13-day-old seedling for 1 h. The plant was washed well, and 45Ca images of the leaves were collected for 10 h. In the case of 35S imaging, an Arabidopsis plant grown for 43 days in MGRL solution was used. The Na235SO4 solution (5 MBq per 0.5 ml in MGRL water culture solution) was supplied via the root for 48 h. The plants were washed well, and images of 35S distribution in the leaves were collected for 10 h. In the case of 32P imaging, a 10-day-old seedling grown in 1/10 Murashige and Skoog (MS) medium was placed on a glass slide after a pulse supply of 100 Bq µl−1 32P-phosphate solution (10 µl of 10 µM Pi solution containing 3 kBq of 32P) for 5 min. Root images were collected for 3 h using the radioisotope-fluorescence microscope.
In the case of 55Fe uptake, 100 kBq of 55FeCl2 solution was supplied to a 3-day-old seedling grown in MS medium containing 500 μM phosphate. The root tip was kept in MS solution containing 55FeCl2, and the other part was placed on an MS agar medium. The sample was covered and imaged for 120 min.
To generate a phosphate-deficient plant, Arabidopsis was grown for 10 days in 1/10 MS medium lacking phosphate. For comparison, another plant was grown for 10 days in 1/10 MS medium containing 500 µM phosphate. Then, 33P-phosphate solution (6 kBq µl−1) was supplied for 5 min, and the root was imaged for 5 min. After imaging, the root tip (2 mm) was digested with 0.1 N HNO3 solution, ACSII scintillator (Amersham Biosciences) was added, and the radioactivity of 33P was measured by a liquid scintillation counter (LSC 6100, Aloka Co., Japan).
3. Results and discussion
(a). Real-time macroscopic imaging
The principle of the imaging system is as follows: when the radioisotope is supplied to the plant at the root level, the radioisotope is absorbed and transferred to the above-ground parts with time. The β-ray emitted from the plant is converted to visible light by a CsI (Tl) scintillator plate placed close to the sample to produce a light image of the whole plant based on the distribution of the β-rays in the plant. Then, the light image is recorded by a CCD camera (figure 1).
This imaging system provided much higher sensitivity than that of an imaging plate (IP), and the resolution was about 100 μm. In the case of the macroscopic imaging system, using a drop of standard (using 32Pi solutions) mounted on a filter paper, it was possible to achieve linearity between the radioactivity count and the mounted 32P activity, which ranged from 10 Bq to 2 kBq mm−2 min−1. In-depth details and illustrations are further provided in §3b. The sensitivity of the imaging system was more than four times higher for the 32P image and 10 times higher for both 45Ca and 14C images than the corresponding images obtained with an IP, with about the same resolution [8]. This high imaging sensitivity offers the opportunity to use short integration times for each frame of successive imaging. For example, in the case of 32P, 3 min was sufficient to acquire one frame in real-time imaging.
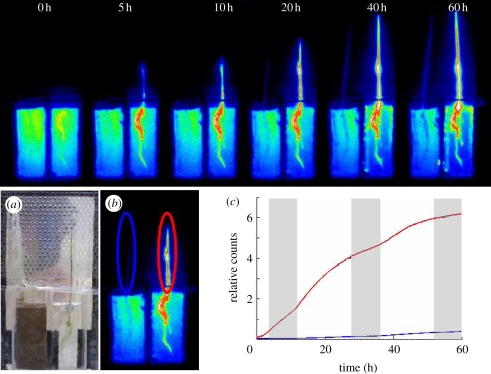
The thin (50 µm) aluminium foil placed between the sample and the scintillator allows plant illumination without interfering with the radiation measurement. This provides an opportunity to analyse the impact of circadian rhythm on ion influx in real time. Additionally, because the radioactive solution can be applied to both artificial (in vitro) and soil media, such a device also offers the opportunity to analyse the behaviours of ion influx in a natural environment. To illustrate this, rice plants grown in a plant box containing water or soil cultures were analysed. Figure 2 and the electronic supplementary material, video S1 show the successive 32P-phosphate uptake images of a rice plant during 60 h of culture (visualized by adding pseudocolour to the image). The integration time for each imaging frame was 3 min. Because of the high β-ray energy of 32P (1.7 MeV), it was possible to measure 32Pi activity in the soil. In such an environment, Pi is weakly mobile owing to a very low coefficient of diffusion (10−12 to 10−15 m2 s−1). Therefore, Pi uptake by the plants creates a depleted area around the roots [12]. This phenomenon is illustrated in figure 2a for the sample grown in soil, where a depletion zone was observed around the root within a few hours (figure 2a, sample on the left). This clearly impacted Pi uptake, as revealed by Pi uptake measurements (figures 2b, c). In plants grown in soil culture, there was hardly any 32Pi activity in the above-ground parts, even after 20 h. The plant growing in liquid culture solution continuously absorbed a large amount of 32P-phosphate from the solution.
Figure 2.
Comparison of 32P-phosphate uptake between water and soil cultures of Oryza sativa cv. Nipponbare. Successive images of 32P-phosphate uptake by rice seedlings during 60 h of water and soil cultures are shown. The integration time for each imaging frame was 3 min. For each record, the sample grown in soil is on the left, and the one grown in water culture solution on the right. (a) Images of the sample. (b) Radioisotope imaging of 32P after 60 h; area used for measurements is indicated. (c) Measurement of 32P-phosphate radioactivity taken up by the aerial part of the samples grown in soil (blue line) or nutrient solution (red line). The grey columns in (c) are the dark period. Pseudocolour was added to the image according to the intensity of radioactivity.
This equipment also offers the opportunity to investigate the effect of light and/or circadian rhythms without interfering with plant growth, as there is no need to remove the plants from their environment to measure ion uptake. This may be a key parameter in experiments, as many biological activities are finely and rapidly tuned to environmental modifications (for an example of light effect on mineral nutrition, see [13]). In the present study, when 32P-phosphate uptake was measured over a few days with 16 L : 8 D cycle, a clear increase in Pi uptake was observed during daytime (figure 2c). Similar observations have been made with other plants, such as Lotus japonicas (data not shown). Indeed, light [14] or circadian rhythms [15] have been found to directly or indirectly affect ion uptake, and such phenomena could have multiple and complex origins.
(b). Microscopic imaging
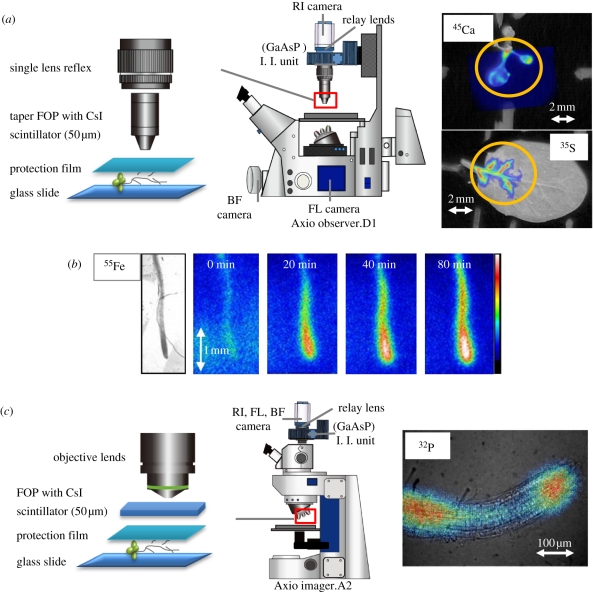
In the case of microscopic imaging, different technical solutions have been used according to the desired level of magnification. The first system (figure 3a) used taper FOS, which allowed a five times magnification. Illustrations of the distribution of different radioisotopes (45Ca, 35S and 55Fe) in various tissues of Arabidopsis can also be found in figure 3a,b. The radioisotope images (pseudocolours) were superposed on the corresponding light images. With the 45CaSO4 solution, images were obtained within 1 h after supplying the solution to roots. A higher accumulation of 45Ca was observed in younger leaves. On the other hand, in the case of 35S, it took hours before the 35S was detected in leaves. (The image of 35S distribution in figure 3 was obtained after 48 h of Na2 35SO4 supply, and 35S was observed to accumulate along the leaf vein.) The last example illustrates real-time imaging by the microsystem with successive images acquired every 20 min after supplying a 3-day-old seedling with 55FeCl2 solution (100 kBq per 25 μl). Each imaging frame resulted from a 2-min integration time. It illustrates the accumulation of 55Fe in the root tip (figure 3b).
Figure 3.
Schematic of microimaging systems and examples of use. (a) The first setup is based on the use of taper FOS, which allowed five times magnification of the sample. Its use is illustrated with images of 45Ca and 35S accumulation in Arabidopsis leaves. In the case of 45Ca imaging, 45CaSO4 solution (1 MBq per 0.5 ml in MGRL solution) was supplied to the root of a 13-day-old seedling for 1 h. The Na2 35SO4 solution (5 MBq per 0.5 ml in MGRL solution) was supplied to the root for 48 h. In both plants, the imaging was performed for 10 h. (b) The capacity of the system for real-time imaging is shown. To obtain successive images of iron uptake, 55FeCl2 solution (100 kBq per 25 μl) was supplied to a 3-day-old seedling; 55Fe accumulation was observed in the root tip. (c) Second setup used to achieve higher magnification (optical lens: 20 times). The image showing 32P distribution in the root of an Arabidopsis plant grown for 10 days in 1/10 MS medium was acquired using this lens system. The resolution is seen with 32P labelling of the root. The 32P-phosphate solution (100 Bq µl−1) was applied for 5 min and the sample was imaged for 3 h. The isotope image was superposed on the corresponding light image, and pseudocolour was added according to the intensity of radioactivity.
The resolution of this microsystem was tested with a 109Cd standard solution supplied to an inkjet printer to draw a grid [16]. Figure S1 in the electronic supplementary material shows the light and radioactive images, both of which clearly revealed the printed 50 μm lines.
To achieve a higher magnification, a combination of an FOS without taper and an optical lens was used, which magnified the image 20 or 40 times depending on the lens used.
Figure 3c shows an image of 32Pi distribution superposed on the light image of Arabidopsis root immediately following a 5-min labelling pulse. The integration time varies depending on the amount of radioisotopes used (2–5 min on average). In the past, IPs were used to analyse Pi uptake locations [17]. These experiments identified the root tips as an important area for Pi uptake. Nevertheless, the resolutions in these experiments were far from that obtained here, where it was possible to clearly visualize the labelling of the meristem area, distinct from the uptake at the level of root hairs above the area of differentiation. This clearly illustrates the importance of the role of the PHT1 family of phosphate transporters, which have recently been shown to be strongly expressed in the root cap of Arabidopsis [18].
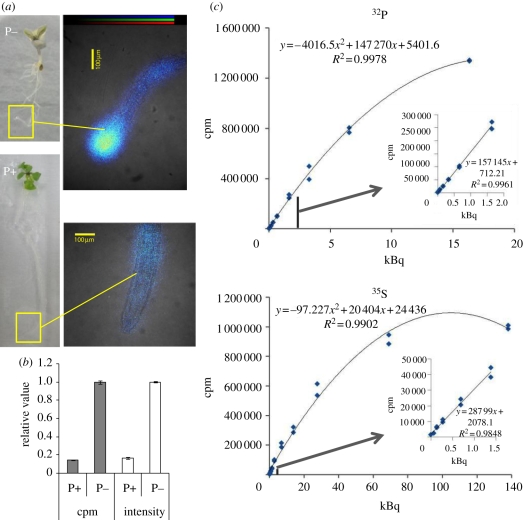
As mentioned above, the system can be used to obtain quantitative data from both macro- and microimaging. Figure 4 illustrates the capacity of the microsystem to provide absolute or relative measurements. For relative measurements, plants grown in Pi-deficient or Pi-sufficient medium were used. Under conditions of Pi deficiency, plants induce high-affinity phosphate transporters to increase Pi uptake. In such conditions, a higher amount of 33P-phosphate was taken up, and an accumulation of 33P was observed in the root tip (figure 4a). On the other hand, when the plants were grown in medium containing 500 µM phosphate, the amount of 33P absorbed by the root was low, and 33P accumulation was not observed in the root tip. When the root tips (2 mm) of both plants were cut and the radioactivity was measured, the amount of 33P in the phosphate-deficient root was about 7–8 times higher than that of the Pi-sufficient one (figure 4b). The relative values of radioactivity measured using a scintillation counter (grey column) and image analysis (white column) were very similar, demonstrating the possibility of using both measurement systems. Absolute measurements can also be easily performed. To achieve this, the radioactivity of the images of standard radioactive solution spots were measured and compared with the radioactivity of the standard solution used with 14C, 55Fe, 32P, 35S and 109Cd (figure 4c provides examples for 32P and 35S). In all cases, the linearity of the signal was conserved between the counts of the image (cpm) and the radioactivity of the mounted standard solution, to facilitate imaging over a broad range of concentrations. Curves indicate conservation of signal linearity from 0 to 6.5 kBq μl−1 (32P) or even 28 kBq μl−1 (35S). In all cases, such signal dynamics far exceed the signals used in experiments, and are thus applicable to a broad range of experiments. For example, in the case of 32P solution, it is possible to detect the image of a 1 μl spot containing only 16 Bq in 2 min.
Figure 4.
Capacities of the system to perform absolute or quantitative measurements. (a) An example of the difference in 33P uptake by plants grown in sufficient or deficient conditions. The radioisotope image was superposed on the light image and illustrates higher uptake capacities of plants grown in low Pi solution. (b) Measurements of plant radioactivity in the root tip (2 mm). The grey and white columns represent the radioactivity counts in the root tip measured by a scintillation counter and image analysis, respectively. (c) Calibration curves of the image count (cpm) (N = 2) versus the standard solution (N = 3).
4. Conclusions and outlook
Various real-time radioisotope imaging systems were developed to image nutrient uptake in plants, using conventional β-ray emitters. Robustness and flexibility of these solutions were tested here with several elements (35S, 45Ca, 32P and 33P). Table 1 provides a list of additional elements which can also be analysed with this equipment. It may be noticed that other radioisotopes, such as gamma-ray or X-ray emitters (109Cd and 55Fe), can also interact with the scintillator, offering an even broader choice of elements. However, a higher dose of radioactivity may be required to compensate for their lower energy. Interestingly, a radioisotope provides specific and direct imaging possibilities for many ions where no alternative solution with fluorescent probes exists (e.g. phosphorus, sulphur, arsenic, cadmium, manganese).
Table 1.
Physical features of radioisotope imaging-compatible elements.
| element | radionuclide | energy (keV) | half-life | note |
|---|---|---|---|---|
| carbon | 14C | 156 (β−) | 5730 year | a |
| aluminium | 26Al | 1170 (β+), 511 (γ) | 740 000 year | |
| phosphorus | 32P | 1710 (β−) | 14.3 day | a |
| phosphorus | 33P | 249 (β−) | 25.3 day | a |
| sulphur | 35S | 167 (β−) | 87.5 day | a |
| calcium | 45Ca | 257 (β−) | 162.6 day | a |
| chromium | 51Cr | 329 (γ), 5 (X) | 27.7 day | |
| manganese | 54Mn | 835 (γ), 5.4 (X) | 312 day | |
| iron | 55Fe | 5.9 (X), 6.5 (X) | 2.73 year | a |
| nickel | 59Ni | 6.9 (X), 7.7 (X) | 76 000 year | |
| copper | 67Cu | 576 (β−) | 61.8 hours | |
| zinc | 65Zn | 1120 (γ), 511 (γ), 8 (X) | 244 day | |
| arsenic | 73As | 9.9 (X), 11 (X) | 80.3 day | |
| cadmium | 109Cd | 22.2 (X) | 462 day | a |
aNuclide used for imaging by our imaging system.
Such a system offers a direct, quantitative and non-destructive access to mineral nutrition. The quality of the numerical analysis offers a wide dynamic range of detection. Therefore, by manipulating the amount of radioactivity introduced into the culture medium it has been possible to study ion influx over time periods as short as 1 or 2 min, or over a period of a few days. This provides opportunities to analyse both short- and long-term effects of mineral nutrition, and conduct several types of pulse chase experiments. For the latter, it is noteworthy that radioisotope imaging offers the opportunity to trace an element, whereas this cannot be performed using fluorescence techniques, which are mostly restricted to distribution analyses.
Furthermore, both techniques developed here also offer specific features. The main advantage of the macroimaging system is the possibility of performing real-time radioisotope imaging experiments without impacting the growth conditions of the plant, because such a device is compatible with a broad range of substrates (liquid, solid, artificial or natural) and does not interfere with sample illumination.
The resolution of the microimaging system offers the opportunity to investigate labelling of sub-millimetre areas (at 50–100 µm resolution), and can be combined with light microscopic imaging to investigate the response of specific plant tissues. Furthermore, it should be noted that this solution is also compatible owing to additional equipment (objectives and illumination source) mounted on the microscope to proceed to acquisition of signals with light, fluorescence of luminescence, which could be combined with radioactive imaging. This offers a broad range of applications, such as measuring the effects of genetic manipulation of transporters labelled with green fluorescent protein or luciferase on ion transport in specific plant tissues.
The development of these two radioisotope imaging systems (macroscopic and microscopic systems) will facilitate the systematic analysis of real-time uptake of various macro- and micronutrients, from the macroscopic level to the microscopic one, i.e. from whole plant to cellular level. Because these visualizations allow for numerical analysis of the image, we expect that the isotope images will open a new avenue in plant physiology.
References
- 1.Bottomley P. A., Rogers H. H., Foster T. H. 1986. NMR imaging shows water distribution and transport in plant root systems in situ. Proc. Natl Acad. Sci. USA 83, 87–89 10.1073/pnas.83.1.87 (doi:10.1073/pnas.83.1.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishida N., Koizumi M., Kano H. 2000. The NMR microscope: a unique and promising tool for plant science. Ann. Bot. 86, 259–278 10.1006/anbo.2000.1181 (doi:10.1006/anbo.2000.1181) [DOI] [Google Scholar]
- 3.Fujimaki S., Suzui N., Ishioka N.S., Kawachi N., Ito S., Chino M., Nakamura S. 2010. Tracing cadmium from culture to spikelet: non-invasive imaging and quantitative characterization of absorption, transport and accumulation of cadmium in an intact rice plant. Plant Physiol. 152, 1796–1806 10.1104/pp.109.151035 (doi:10.1104/pp.109.151035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoneyama T., Suzui N., Ishioka N. S., Fujimaki S. 2011. A quick incorporation of 13N into the soluble high-molecular compound in rice (Oryza sativa L.) roots by application of 13N-labeled nitrate/nitrite. Soil Sci. Plant Nutr. 57, 279–282 10.1080/00380768.2011.576399 (doi:10.1080/00380768.2011.576399) [DOI] [Google Scholar]
- 5.Melkus G., et al. 2011. Dynamic 13C/1H NMR imaging uncovers sugar allocation in the living seed. Plant Biotechnol. J. 9, 1022–1037 10.1111/j.1467-7652.2011.00618.x (doi:10.1111/j.1467-7652.2011.00618.x) [DOI] [PubMed] [Google Scholar]
- 6.Jahnke S., et al. 2009. Combined MRI-PET dissects dynamic changes in plant structures and functions. Plant J. 59, 634–644 10.1111/j.1365-313X.2009.03888.x (doi:10.1111/j.1365-313X.2009.03888.x) [DOI] [PubMed] [Google Scholar]
- 7.Park J. C., et al. 2011. Luminescence imaging using radionuclides: a potential application in molecular imaging. Nuclear Med. Biol. 38, 321–329 10.1016/j.nucmedbio.2010.09.003 (doi:10.1016/j.nucmedbio.2010.09.003) [DOI] [PubMed] [Google Scholar]
- 8.Kanno S., Rai H., Ohya T., Hayashi Y., Tanoi K., Nakanishi T. M. 2007. Real-time imaging of radioisotope labeled compounds in a living plant. J. Radioanal. Nuclear Chem. 272, 565–570 10.1007/s10967-007-0625-z (doi:10.1007/s10967-007-0625-z) [DOI] [Google Scholar]
- 9.Nihei N., Masuda S., Rai H., Nakanishi T. M. 2008. Imaging analysis of direct alanine uptake by rice seedlings. Radioisotopes 57, 361–366 10.3769/radioisotopes.57.361 (doi:10.3769/radioisotopes.57.361) [DOI] [Google Scholar]
- 10.Yamawaki M., Kanno S., Ishibashi H., Noda A., Hirose A., Tanoi K., Nakanishi T. M. 2009. The development of real-time RI imaging system for plant under light environment. J. Radioanal. Nuclear Chem. 282, 275–279 10.1007/s10967-009-0344-8 (doi:10.1007/s10967-009-0344-8) [DOI] [Google Scholar]
- 11.Fujiwara T., Hirai Y. H., Chino M., Komeda Y., Naoto S. 1992. Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol. 99, 263–268 10.1104/pp.99.1.263 (doi:10.1104/pp.99.1.263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schachtman D. P., Reid R. J., Ayling S. M. 1998. Phosphorus uptake by plants: from soil to cell. Plant Physiol. 116, 447–453 10.1104/pp.116.2.447 (doi:10.1104/pp.116.2.447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nussaume L., Vincentz M., Meyer C., Boutin J. P., Caboche M. 1995. Post-transcriptional regulation of nitrate reductase by light is abolished by an N-terminal deletion. Plant Cell 7, 611–621 10.1105/tpc.7.5.611 (doi:10.1105/tpc.7.5.611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marten I., Deeken R., Hedrich R., Roelfsema M. R. 2010. Light-induced modification of plant plasma membrane ion transport. Plant Biol. 12, 64–79 10.1111/j.1438-8677.2010.00384.x (doi:10.1111/j.1438-8677.2010.00384.x) [DOI] [PubMed] [Google Scholar]
- 15.Haydon M. J., Bell L. J., Webb A. A. 2011. Interactions between plant circadian clocks and solute transport . J. Exp. Bot. 62, 2333–2348 10.1093/jxb/err040 (doi:10.1093/jxb/err040) [DOI] [PubMed] [Google Scholar]
- 16.Sato Y., Hino Y., Yamada T., Matsumoto M. 2004. The new fabrication method of standard surface sources. Appl. Radiat. Isotop. 60, 543–546 10.1016/j.apradiso.2003.11.074 (doi:10.1016/j.apradiso.2003.11.074) [DOI] [PubMed] [Google Scholar]
- 17.Misson J., Thibaud M. C., Bechtold N., Raghothama K., Nussaume L. 2004. Transcriptional regulation and functional properties of Arabidopsis Pht1;4, a high affinity transporter contributing greatly to phosphate uptake in phosphate deprived plants. Plant Mol. Biol. 55, 727–741 10.1007/s11103-004-1965-5 (doi:10.1007/s11103-004-1965-5) [DOI] [PubMed] [Google Scholar]
- 18.Bayle V., Arrighi J. F., Creff A., Nespoulous C., Vialaret J., Rossignol M., Gonzalez E., Paz-Ares J., Nussaume L. 2011. Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 23, 1523–1535 10.1105/tpc.110.081067 (doi:10.1105/tpc.110.081067) [DOI] [PMC free article] [PubMed] [Google Scholar]