Abstract
Studying the specific effects of water and nutrients on plant development is difficult because changes in a single component can often trigger multiple response pathways. Such confounding issues are prevalent in commonly used laboratory assays. For example, increasing the nitrate concentration in growth media alters both nitrate availability and osmotic potential. In addition, it was recently shown that a change in the osmotic potential of media alters the plant's ability to take up other nutrients such as sucrose. It can also be difficult to identify the initial target tissue of a particular environmental cue because there are correlated changes in development of many organs. These growth changes may be coordinately regulated, or changes in development of one organ may trigger changes in development of another organ as a secondary effect. All these complexities make analyses of plant responses to environmental factors difficult to interpret. Here, we review the literature on the effects of nitrate, sucrose and water availability on root system growth and discuss the mechanisms underlying these effects. We then present experiments that examine the impact of nitrate, sucrose and water on root and shoot system growth in culture using an approach that holds all variables constant except the one under analysis. We found that while all three factors also alter root system size, changes in sucrose and osmotic potential also altered shoot system size. In contrast, we found that, when osmotic effects are controlled, nitrate specifically inhibits root system growth while having no effect on shoot system growth. This effectively decreases the root : shoot ratio. Alterations in root : shoot ratio have been widely observed in response to nitrogen starvation, where root growth is selectively increased, but the present results suggest that alterations in this ratio can be triggered across a wide spectrum of nitrate concentrations.
Keywords: root system, root : shoot ratio, nitrate, osmotic potential, sucrose
1. Overview
Hundreds or even thousands of individual roots, branching in a myriad of ways and stretching in nearly all directions, may comprise the root system of a single adult plant. What appears to be amazing complexity is dwarfed by a remarkably humble origin. For many plants (including the model plant Arabidopsis thaliana), this complex adult root system originates from a single embryonic root. From this root develop new autonomous lateral roots, which give rise to more roots in turn. This process is neither fixed nor random, but highly dependent on the integration of developmental cues and environmental conditions [1].
It makes intuitive sense that the plant root system is responsive to its heterogeneous surroundings. Soils are complex and ever-changing. They consist of gases, liquids and solids, nutrients of organic and inorganic origin, and living creatures and dead ones. All of these components are unequally distributed throughout the soil, and some move or diffuse. As a result, plants must expand their root systems in the direction where resources accumulate. To accomplish this, plants incorporate signals from their surrounding environment to guide decisions on when and where to form new lateral roots.
Here, we review the current understanding of the mechanisms by which plants regulate post-embryonic development of the root system in response to environmental nitrogen (also reviewed in earlier studies [2–6]). We further discuss changes in root system architecture in response to water availability, and the effect of alterations in sugars in the aerial tissues on root architecture. Finally, we discuss problems with laboratory assays that have been used in the past to dissect these responses, and present experiments that differentiate between osmotic effects and specific effects of increased nitrogen or sucrose. We then extend these experiments to differentiate environmental effects on overall plant growth (shoot and root) from effects that are specific to the root system. Our results demonstrate that while nutrient nitrogen, nutrient sucrose and osmotic potential all impact root system growth, only nitrogen exerts a specific effect on the root system without altering whole plant growth.
2. Acquisition of nitrogen from the soil
Plants must extract many nutrients from the soil in order to sustain growth. To make DNA, amino acids and other complex molecules, plants need to obtain nitrogen, phosphorous and sulphur. For the proper function of proteins, iron and magnesium are needed as well. It is therefore not surprising that plants have evolved to optimize their growth plan in order to maximize the uptake of these elements. Indeed, all of the elements listed above have been demonstrated to have an effect on plant root systems (reviewed in earlier works [3,4,7,8]).
Nitrogen is the most abundant element in the Earth's atmosphere; yet, it is a limiting nutrient to plant growth [8]. This is because plants cannot assimilate the diatomic form of nitrogen found abundantly in the air, but rather must acquire soluble nitrogen from the soil. Whereas soil nitrogen can be found and acquired in many forms, including ammonia and amino acids, nitrate is the most common form absorbed and assimilated by plants. Nitrate is in low abundance in the soil, in part, due to its high solubility and predisposition to leaching, and its rapid acquisition by bacteria and fungi [2,8].
The ability of a plant to acquire nitrogen from its surroundings is dependent on two major characteristics of the root system: the amount of root area in contact with soluble nitrogen in the soil, and the efficiency by which any given amount of root can transport nitrogen from its surroundings into the plant. The former is dependent on the formation, placement and growth of post-embryonic roots. The latter depends on the expression of nitrate and ammonium transporters that shuttle nitrogen into the plant. All of these processes are dramatically affected by the amount of nitrogen within the plant and the amount and distribution of nitrogen in the surrounding environment. Here, we will focus solely on changes in root system architecture in response to environmental nitrogen. (For recent reviews of changes in nitrogen uptake and assimilation, readers are referred to reviews [6,9,10].)
3. Root system responses to environmental nitrogen
Root systems demonstrate three clearly separable responses to environmental nitrogen: (i) overall high concentrations of nitrate repress lateral root formation; (ii) nitrogen starvation leads to increased primary and lateral root growth as a component of a shift in the ratio of shoot-to-root biomass; and (iii) patches of high external nitrate in an otherwise low nitrate environment lead to local stimulation of lateral root proliferation.
(a). Alterations in root system growth in response to ubiquitous high nitrogen supply
Exposure to a uniformly high level of nitrate results in reversible suppression of lateral root development [11,12]. An Arabidopsis mutant carrying mutations in two nitrate reductases was even more sensitive to nitrate, suggesting that accumulation of nitrate itself, rather than downstream metabolites, signals this response [11]. Split-root experiments demonstrate that the shoot plays a critical role in repression of root system growth (see below). The root-to-shoot part of this systemic signaling pathway involves feedback inhibition by N-assimilates, and also root-to-shoot signaling via the xylem, with nitrate and cytokinin acting as signals to effect changes in shoot growth [2,3]. In contrast, very little is known about the shoot-to-root signalling pathay(s) that modulate the response of the root system to an N-replete shoot status. Phloem-translocated amino acids, sucrose, miRNAs, auxin and cytokinin have all been suggested as signals [2,7,13–17]. Nitrate-mediated repression of lateral root is mimicked by application of abscisic acid (ABA) [18,19]. Mutants have been identified that can overcome both ABA and high nitrate, suggesting a single response pathway [5]. Interestingly, growth of Arabidopsis plants on ubiquitously high nitrate results in a reduction in auxin concentration in the root and a concomitant increase in auxin in the shoot [2]. Since auxin concentrations are intimately connected with all stages of lateral root formation [20], this offers a potential mechanism for nitrate repression of root system growth.
High nitrate repression of lateral roots in Arabidopsis can be mimicked by an equimolar concentration of mannitol, which imposes a mild osmotic stress [21]. The mannitol-mediated repression of lateral roots also operates via an ABA-dependent mechanism [21]. However, further experiments demonstrated that, in laboratory assays, both high nitrate and equimolar mannitol prevented the leaves from absorbing sucrose from the media on which the plants were grown [22]. In culture, the cotyledons and/or leaves are usually in physical contact with the media, and the consequent sucrose absorption by the cotyledon/leaf tissue is highly stimulatory to lateral root formation (see §5). Indeed, the lrd2 mutant, which was isolated based on its ability to produce lateral roots under high nitrate and mild osmotic stress conditions [21], was found to have a defective gene for an acyl CoA synthetase with a known role in cuticle formation [22]. Subsequently, a large number of mutants with defects in leaf cuticle formation were demonstrated to have the same root system phenotype. All these mutants absorbed sucrose in culture even under high nitrate conditions and therefore produced high numbers of lateral roots in the presence of high nitrate [22]. Furthermore, ABA mutants were shown to absorb increased amounts of sucrose in culture under high nitrate conditions [22]. Sucrose uptake from the media does not approximate any condition that would be expected to occur in nature. Therefore, it appears that the model of nitrate-mediated repression of lateral root formation in an ABA-dependent manner must be re-examined, as some of the results leading to this model may have been associated with osmotic- or ABA-mediated changes in sucrose uptake from the nutrient media.
(b). Alterations of root-to-shoot mass ratio in response to nitrogen starvation
When plants are starved of nitrogen, they are presented with a conundrum. They must limit their growth, but in doing so they are less capable of exploring the surrounding soil for additional sources of nitrogen. Turner [23] was one of the first to point out that, when starved of nitrogen, barley plants preferentially expand their root system at the expense of shoot growth. Since nitrogen is acquired solely by the root system, an increase in the root-to-shoot ratio thus allows these plants to increase their chances of obtaining nitrogen to sustain growth. Many experiments have confirmed an increase in root growth versus shoot growth in response to nitrogen in a wide range of species, including maize [24], cotton [25], soya bean [25–27], rape [28], Plantago [29], tobacco [30,31], birch [32,33] and Arabidopsis [34,35]. Split root experiments again demonstrate a critical role for the shoot, and suggest that the N-depleted status of the shoot results in promotive systemic signalling. Very recent work was able to separate the repressive and promotive systemic signalling pathways that regulate root system growth based on their differential requirement for cytokinin [17].
(c). Alterations in root system growth in response to a localized nitrogen supply
In contrast to the situations described above, root systems normally encounter heterogeneous soils in which nitrate is unequally distributed [4]. In this case, some parts of the root system may be in contact with high nitrate while others are in contact with low nitrate, with the internal plant concentration of nitrogen reflecting some intermediate level. Given this complex scenario, one can imagine two possibilities: each region of the root system could respond independently to local nitrate concentrations, or the root system could be regulated in an integrated manner in response to the overall picture of nitrate distribution and internal nitrogen status. In fact, both of these possibilities appear to be true, as described below. The prevalent model is that root system growth is regulated by a balance between systemic signals that reflect the endogenous N state of the plant (replete or deficient) and the presence or absence of local stimulatory effects of nitrate at the root system.
(i). Differential growth within the root system
Some of the most stunning examples (as well as the earliest) of the extent to which a plant root system responds to environmental nitrogen were presented by Drew and co-workers in the 1970s [36–38]. The authors set out to determine how roots of barley plants respond to local increases in nitrate availability. By constructing specialized growth chambers that were separated into three compartments, the authors were able to expose separate parts of the root system to solutions containing different concentrations of nitrate. These experiments showed beautifully that the sections of root exposed to high concentrations of nitrate showed a large increase in lateral root formation and growth. Scheible et al. [31] obtained similar results in Nicotiana. Using a ‘split-root’ system—where the roots of a single plant are separated into two or three sections and exposed to different environments—the authors showed that sections of root exposed to higher concentrations of nitrate exhibited increased root growth. The same response was found when Arabidopsis was grown on agar medium supplied with unequally distributed amounts of nitrate [39]. These results show that plants optimize the growth and development of their organs in a highly controlled manner that optimizes nutrient uptake in areas of high nitrate availability (for review see Hodge [4]).
(ii). The long-distance signals regulating root system response to unequal distribution of nitrate
To determine how differential growth within the root system is regulated, Scheible et al. [31] studied the growth of roots in a split-root system with roots exposed to either low or high nitrate concentrations. These authors found that growth of roots in a low-nitrogen environment was inhibited when the plant was supplied with nitrogen from the other half of the root system. This is consistent with lateral root inhibition in response to uniformly high nitrate levels, as discussed in §3a, and suggests that a signal of the replete condition may be moving from the nitrogen replete roots to the roots undergoing nitrogen starvation conditions. Since signals originating in one half of the split-root system must traverse the shoot before arriving at the other half, this may suggest that the shoot is relaying signals between various parts of the root system. More likely, the nitrogen supplied to one part of the root system creates a replete condition in the shoot, and the shoot signals its status to the low nitrogen roots.
The split-root experiments presented above suggest that long distance signals downstream of changes in external nitrate—mediated by the shoot—integrate the rate at which roots form in various parts of the root system [31,40–42]. One possibility is that nitrate or its downstream metabolites act as the signal reporting nitrogen status in the plant. After acquisition from the environment, nitrate is reduced via nitrate and nitrite reductases to ammonia before being further converted to downstream metabolites. Mutants in these reductases can therefore be employed to see if plant responses to alterations in nitrate availability depend on nitrate itself or downstream metabolites.
Schieble et al. [31] found that, in tobacco, nitrate reductase mutants showed an increase in root-to-shoot ratio upon exposure to high nitrate (similar to wild-type plants grown in the presence of high nitrate), suggesting that nitrate itself regulates root-to-shoot ratio. Similarly, Zhang & Forde [39] found that local induction of lateral root formation is unaffected in nitrate reductase mutants, again suggesting that nitrate itself regulates environmental responses to local changes in environmental nitrate supply. These results suggest that lateral root formation is dependent on the perception of nitrate signals, although a caveat inherent in these experiments is that nitrate reductase mutants may be altered in many processes unrelated to signalling, such as amino acid synthesis.
(iii). Molecular mechanisms underlying root system responses to localized nitrate
One of the few molecules identified to play a role in the interaction of localized soil nitrogen and root system architecture is the MADS-box transcription factor ANR1. A mutant in ANR1 does not show a localized increase in lateral root growth in response to a localized patch of nitrate [39]. While the function of ANR1 is unknown, data suggest that it acts downstream of the nitrate transporter NRT1.1. Mutants in NRT1.1 showed a similar phenotype to anr1 mutants, and the level of ANR transcripts was greatly reduced in nrt1.1. Interestingly, no reduction in nitrate uptake was observed in nrt1.1 mutants, nor could the lateral root phenotype be rescued by nitrogen metabolites, suggesting that NRT1.1 may function as a nitrate sensor, stimulating localized lateral root growth via an ANR1-mediated pathway [43].
Recently, Krouk et al. [44] showed that NRT1.1 redistributes auxin away from lateral root tips at low but not high nitrate concentrations. The authors speculate that localized nitrate stimulation of lateral root growth might therefore be explained by a relatively higher level of auxin at the lateral root meristems [44]. Since NRT1.1 is induced by nitrate, this model describes a complete hypothetical circuit that may connect nitrate sensing with lateral root growth.
Two additional regulatory circuits have been described that may connect external nitrate with root system development via auxin. In one case [13], AUXIN RESPONSE FACTOR 8 (ARF8) was upregulated in the pericycle in response to high nitrate, while the microRNA that targets ARF8 was downregulated in the same tissue. The authors used a bioassay where plants were grown on ammonium and then supplied with ubiquitously high nitrate, and reported that the nitrate represses lateral root formation in this assay. Perturbation of the ARF8/miR167 auto-regulatory loop prevents Arabidopsis plants from repressing lateral root emergence in response to nitrate. Similarly, the auxin receptor AFB3 and the microRNA miR393 form an auto-regulatory circuit, except that in this case AFB3 is induced by nitrate and the microRNA which degrades AFB3 is induced by nitrogen metabolites [14]. Perturbation of this loop interferes with nitrate-induced changes in architecture.
4. Changes in root system growth in response to water
(a). Root system growth towards increasing water availability
Plants possess the ability to direct the growth of organs towards numerous stimuli, including sunlight (phototropism), gravity (gravitropism) and water (hydrotropism). The last had been the subject of much debate over the eighteenth and nineteenth centuries (reviewed by Hooker [45]), and was neglected during much of the twentieth century until the identification of a pea mutant with altered gravitropism and a strong hydrotropic response [46]. Gravitropism is the single largest hindrance to the study of hydrotropism, as the effect of gravitropism is roughly one order of magnitude stronger. Researchers have gone to great lengths to overcome this limitation—literally by travelling to outer space [47]. Yet, for all the effort, very little is known about the molecular mechanisms guiding hydrotropism by the root system [48].
In The power of movement in plants (1880), Darwin et al. [49] presented evidence that hydrotropism functions in the root tip. More than 100 years later, Takahashi et al. [50] validated this observation by showing that osmotic stress increases hydrotropic response by stimulating the degradation of amyloplasts in root tip collumella cells that are required for gravitropic response [50]. Mutant screens have been employed to identify genetic regulators of hydrotropism, and have managed to identify several mutants with defects in hydrotropic response [48]. In only one case has a causal gene been identified [51]. This gene, MIZ1, encodes a protein of unknown function that is conserved among terrestrial plants. MIZ1 is expressed in the primary root tip (as well as in sections of the mature root, and in hydathodes in leaf tissues), adding additional evidence that the root tip is important in sensing environmental water supply.
(b). Alterations in root system growth in response to water limitation
Plants respond to water limitation by complex changes in root system growth. The most extensive literature concerns changes in primary root growth. It is well known that plants can sense water limitation, and in response produce the hormone ABA to act as a long-distance signal communicating environmental water availability [52]. Maize root cell elongation is inhibited by water stress in regions distal to the root apex, but at the apex elongation is maintained at a rate similar to non-water-stressed plants. Elongation maintenance at the apex is ABA-dependent [53]. Other root system responses to osmotic stress are indirect, and are due to stomatal closure and consequent limiting of photosynthesis, and limited expansion of young leaves. This in turn may reduce root system growth (for review see [54,55]).
Several groups have found that lateral root formation is repressed when Arabidopsis seedlings are grown on agar medium with reduced osmotic potential, which imposes a mild osmotic stress [21,56,57]. This reduction of lateral root formation is recapitulated with addition of exogenous ABA [57], and mutants in ABA biosynthesis show increased lateral root formation under mild osmotic stress [21,57]. These results suggest that ABA produced by roots may act as a signal to repress lateral root formation in response to reduced water availability.
To identify genes regulating lateral root formation in response to altered water availability, mutant screens were performed in Arabidopsis on medium with reduced osmotic potential [21,57,58]. From these screens, the authors reported the isolation of three mutants: lrd2, dig3 and Atcyt-inv1, respectively. Each mutant showed an increase in lateral root formation under mild osmotic stress, and all three were dwarfed when grown on soil [21,57,58]. The causal mutations in lrd2 and Atcyt-inv1 were cloned and characterized at the molecular level [22,58]. LRD2 encodes a gene responsible for the proper deposition of the cuticle on aerial tissues. The authors found that lrd2 exhibits increased lateral root formation owing to an increase in the ability to obtain sucrose through its aerial tissues from the growth medium (which contains sucrose). The authors also showed that the ABA biosynthesis mutants previously shown to have increased lateral root formation under mild osmotic stress also show an increase in the ability to acquire sucrose from the surrounding growth medium. The results above bring into question the hypothesis that ABA regulates lateral root formation in response to mild osmotic stress. Rather, it appears that altered acquisition of sucrose in the assay system, not reduced sensing of a mild osmotic stress, is the mechanism allowing increased lateral root formation of certain mutants on agar medium with reduced osmotic potential. In agreement with the findings of MacGregor et al. [22], AtCYT-INV1 was found to encode an invertase responsible for converting sucrose to glucose and fructose [58]. The Atcyt-inv1 mutant showed an increase in sucrose levels under mild osmotic stress, further adding support for the role of sucrose in regulating lateral root formation in these assays. As stated above, modulation of sucrose uptake is not expected to mirror any naturally occurring process outside the Petri dish environment. A molecular understanding of root system regulation in response to osmotic stress may have been confounded by artefacts of the plate assays, and a true understanding of osmotic stress responses is therefore lacking.
5. Sucrose as a stimulant of lateral root formation
While uptake of sucrose from nutrient media is not a naturally occurring process, sucrose itself is a major product of photosynthesis. Therefore, altering sucrose levels by exogenous application of sucrose may reveal the effects of increased photosynthetic activity on plant growth and development. Mutants that take up more sucrose from an agar medium show an increase in lateral root formation. Not surprisingly, wild-type seedlings supplemented with increasing exogenous sugar also show increased lateral root formation and primary root elongation [22,59,60], and this effect persists if the exogenous sucrose is applied specifically to the aerial tissues [22]. Furthermore, the sugar-induced increase in lateral root formation is correlated with the amount of sugars found in the root [59]. These results and others suggest that the sugar concentration in roots, presumably delivered from the shoot, may be a positive determinant of lateral root growth (for review see [7,61]). Consistent with this idea, Arabidopsis plants grown under conditions that stimulate photosynthesis have been shown to stimulate lateral root formation as well. Freixes et al. [59] showed that under high light conditions with no exogenous sucrose, Arabidopsis seedlings elongate primary and lateral roots as quickly as when grown on medium containing 2 per cent sucrose under low light conditions. Similarly, under elevated CO2 levels (which promote photosynthesis), Arabidopsis seedlings make more lateral roots [60]. These findings show that lateral root formation is responsive to increased plant sugar status, regardless of whether it is produced by the plant or supplied exogenously. Plants harvest sunlight in order to provide energy for processes related to plant growth. Therefore, it makes intuitive sense that when plant energy status is increased, so is the rate of growth of the root system. Sucrose can also function as a signal [62]. However, sucrose-stimulated lateral root formation was unaffected in hexokinase mutants [22]. Furthermore, the phosphorylated, non-metabolized sucrose analogue 3-O-methyl-glucose could not substitute for sucrose, while glucose and fructose could [22]. These results indicate that sucrose acts as a nutrient rather than a signal to regulate lateral root formation.
6. Dissecting root system responses to environmental conditions in laboratory assays
Since the soil environment is highly heterogeneous, it is common for researchers to use an artificial medium to dissect out the effects of specific environmental factors on plant growth and development. This also allows for easy visualization of the root system. As discussed above, this approach has lead to gene discovery through the isolation of mutants in root system responses. Forward genetic screens have been primarily conducted in Arabidopsis, where mutant identification and subsequent identification of the causal mutation is fast and relatively straightforward.
Despite the success of laboratory assays, it is always important to bear in mind that there are artefacts associated with analysis of root systems grown on Petri dishes. The roots tend to be exposed to the light, for instance. Many laboratories including our own have sealed plates with parafilm to maintain sterility; this and other non-porous tapes cause an unnatural accumulation of gases, including the gaseous hormone ethylene, in the plates [63,64]. Sucrose is generally included in plant medium, and both leaves and roots are directly in contact with this sucrose. MacGregor et al. [22] showed that leaf contact with sucrose-containing medium has a profound promotive effect on lateral root formation, and this effect can be eliminated by placing a parafilm barrier between the leaf and the medium. MacGregor et al. [22] also demonstrated that different mutants (i.e. ABA synthesis mutants, cuticle mutants) have differential leaf permeability to molecules in the medium, allowing greater uptake of dye molecules from their environments than wild-type plants. These results highlight the need for caution in interpreting laboratory assays.
Despite the inherent problems associated with laboratory assays, they still provide the best opportunity to dissect plant responses to distinct environmental cues in detail, especially when the proper controls are included. Here, we present a set of experiments designed to independently characterize the responses of plants to alterations in water, sucrose and nitrogen in laboratory assays. In these experiments, we altered only one parameter at a time, allowing the effects of sucrose and nitrogen as osmotica to be distinguished from their effects as nutrients or signalling molecules. Furthermore, we measured both root system development and shoot development in response to altered nitrogen, sucrose and water to see if changes in root and shoot growth were separable. Finally, since it has been shown that certain growth conditions can alter sucrose uptake from the medium in laboratory assays, we also evaluated this parameter for each component. Our findings demonstrate that nitrate, when isolated from all other factors, reduces lateral root formation without altering shoot growth or sucrose uptake.
7. Results and discussion
(a). Both shoot and root system growth are altered when sucrose and nitrate concentrations are increased
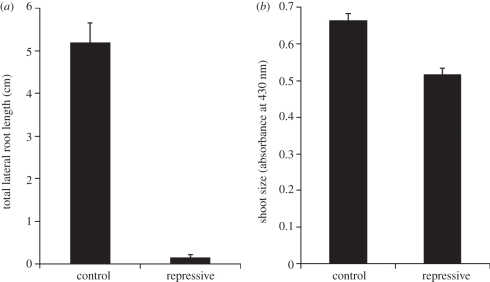
As a starting point, we replicated conditions demonstrated in Deak et al. [21] and MacGregor et al. [22] to strongly repress lateral root formation. In this laboratory assay, control conditions were defined as a low nitrate variant of Murashige and Skoog (MS) medium, containing 1× MS basal salts, 5 mM each KNO3 and NH4NO3 and 1 per cent sucrose (see §9). Repressive conditions were defined as 1× MS basal salts with 20 mM each KNO3 and NH4NO3 and 4.5 per cent sucrose. Seedlings grown on repressive medium have been previously reported to show a dramatic decrease in lateral root formation and shoot size compared with seedlings grown on control medium [22]. We repeated this result using a chlorophyll extraction protocol to quantify shoot size (see §9) and a measurement of total lateral root length (TOT) to quantify root system size. Indeed, seedlings grown on repressive medium showed a dramatic reduction in TOT, and also exhibited a decrease in shoot size (figure 1).
Figure 1.
(a) Lateral root formation and (b) shoot size for Ws seedlings grown for 15 days on control and repressive media. Bars represent average of 50 individuals for control media and 47 individuals for repressive media (p ≪ 0.01 using Student's paired t-test for both total lateral root length (TOT) and shoot size). Standard error is shown.
Since control and repressive media contain different quantities of sucrose and nitrate salts, and therefore also have different osmotic potentials, it was possible that changes in TOT and shoot size may have been regulated by a combination of nitrate, sucrose and osmotic responses. To dissect these responses and determine if changes in TOT could be separated from changes in shoot size, we studied the effect of each repressive medium component individually. It should also be noted that ionic strength may affect plant growth, and this is not tested for in our experiments. It should also be noted that levels of K were not normalized between media in these experiments, although K availability is generally not reported to affect lateral rooting or root/shoot ratio [7].
(b). Osmotica and sucrose alter root and shoot system growth in a coordinated manner
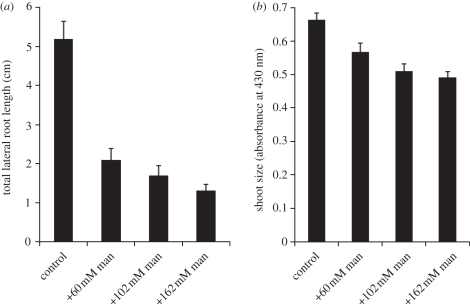
Previous work has shown that addition of mannitol, a poorly metabolized sugar, reduces the osmotic potential of media and represses lateral root formation [21,22]. We confirmed this result using a series of media containing varying amounts of mannitol (figure 2). Compared with control medium, repressive medium contained an additional 30 mM of nitrate salts (equivalent to 60 mM solute owing to dissociation of the salts) and 102 mM sucrose. We therefore tested media containing an additional mannitol concentration of 60 mM (nitrate salt equivalent), 102 mM (sucrose equivalent) and 162 mM (nitrate salt plus sucrose equivalent). Addition of increasing amounts of mannitol resulted in a reduction in lateral root length (figure 2), as previously reported. We then examined the effects of osmotica on shoot size and found that the reduction in TOT is accompanied by a reduction in shoot size (figure 2). The reductions in both TOT and shoot size are consistent with the idea that changes in osmotica alter overall plant growth characteristics rather than specifically targeting root system development.
Figure 2.
(a) Lateral root formation and (b) shoot size for Ws seedlings grown for 15 days on control media supplemented with varying amounts of the non-metabolizable sugar mannitol. n = 46–50 for all data points. For shoot size, comparison between control and all other mannitol concentrations was statistically significant at p < 0.01 using Student's paired t-test. For TOT, comparison between control and all other mannitol concentration was statistically significant at p ≪ 0.01 using Student's paired t-test. Standard error is shown.
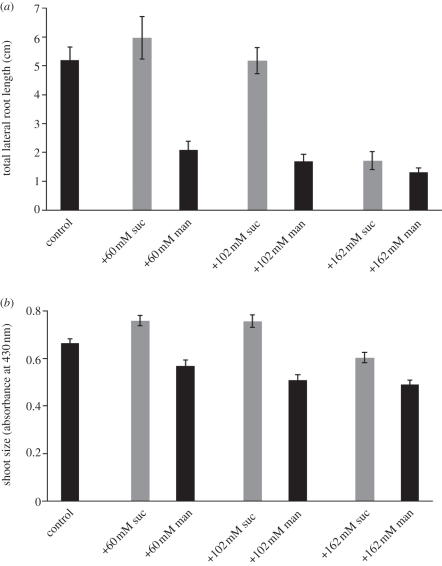
We next tested the effect of alteration in sucrose availability on TOT and shoot size. Sucrose can act both as a signal, a nutrient and as an osmolyte [7,61,62]. To test the specific effect of sucrose as a nutrient, seedlings were grown on medium containing increasing amounts of sucrose, and compared with seedlings grown on medium containing equivalent molar amounts of mannitol. Sucrose was added in the same amounts (in addition to the 30 mM sucrose present in the control medium) as was mannitol in the previous experiment. In each of three experimental sets (figure 3, e.g. compare +60 mM mannitol and +60 mM sucrose), the seedlings with added sucrose showed an increase in TOT and shoot size compared with seedlings grown with the equivalent amount of mannitol (the increase in TOT at 162 mM sucrose was not significant in this repetition). These results indicate that sucrose as a nutrient (rather than an osmolyte) leads to an increase in both shoot size and TOT, again consistent with a model in which sucrose affects overall plant growth characteristics.
Figure 3.
(a) Lateral root formation and (b) shoot size for Ws seedlings grown for 15 days on control media supplemented with varying amounts of sucrose or equivalent amount of mannitol. Bars represent average of 44–50 individuals for all data points. Differences between sucrose and mannitol addition is statistically significant at p < 0.01 using Student's paired t-test in all cases except for TOT at 162 mM solutes. Standard error is shown.
(c). Nitrate specifically regulates lateral root formation without affecting shoot size or leaf permeability
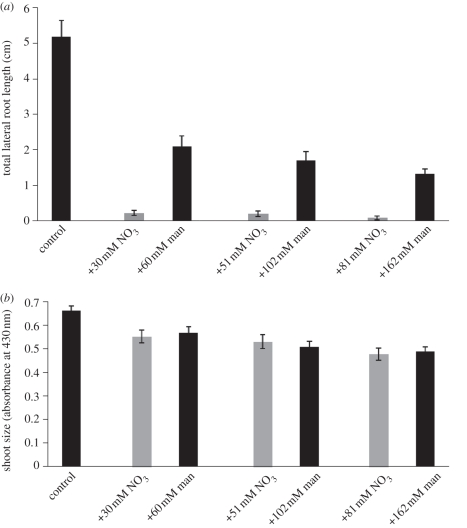
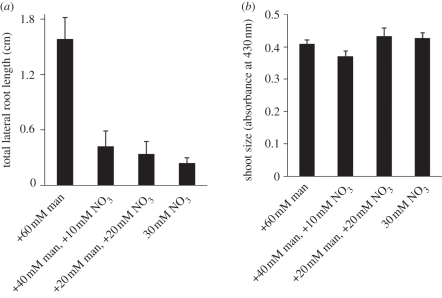
As with sucrose, nitrate salts can act as a signal, a nutrient and/or as an osmolyte (see §7b). To test the effect of nitrate salts as a nutrient and/or signal, seedlings were grown on medium containing varying amounts of nitrate salts and compared with seedlings grown on medium containing equivalent amounts of mannitol. Increasing nitrate concentration resulted in a strong repression of lateral root formation, but surprisingly had no significant effect on shoot size when compared with equivalent concentrations of mannitol (figure 4). These results indicate that increasing nitrate salt concentration acts as a nutrient or signal to repress TOT independently of shoot size. The strongest repression of TOT occurred upon addition of 30 mM nitrate salts to the control medium. To further validate the effect that nitrate salts have on repressing TOT, but not shoot size, another experiment was performed. In this experiment, seedlings were grown on medium containing 30 mM nitrate salts or a series of media where mannitol was substituted for equivalent 10 mM decreases in nitrate salts. Thus, all media had the same osmotic potential, but varying amounts of nitrate salts. Again, an increase in nitrate salt concentration resulted in a decrease in TOT, but not in shoot size (figure 5). These results indicate that even 10 mM increments of nitrate salts can have a profound effect on TOT, while having no significant effect on shoot size, and these effects are not caused by changes in osmotic potential. Together, the present findings indicate that plants specifically repress lateral root formation irrespective of whole-plant growth in response to increases in environmental nitrate. Similar results have been previously reported in the literature [11,65], but neither group measured responses in shoot growth. The present results replicate their findings, and add an extra level of support to the conclusions presented in those studies.
Figure 4.
(a) Lateral root formation and (b) shoot size for Ws seedlings grown for 15 days on control media supplemented with varying amounts of nitrate salts or equivalent amount of mannitol. Bars represent average of 42–50 individuals for all data points. For all comparisons, differences in TOT are statistically significant, at p ≪ 0.01 using Student's paired t-test, but differences in shoot size are not. Standard error is shown.
Figure 5.
(a) Lateral root formation and (b) shoot size for Ws seedlings grown for 14 days on control media supplemented with varying amounts of nitrate salts and mannitol such that osmotic potential is constant. Bars represent average of 38–56 individuals for all data points. For TOT, difference between +60 mM mannitol and all other conditions is significant at p ≪ 0.01 using Student's paired t-test. For shoot size, only the difference between +40 mM mannitol and +20 mM mannitol is statistically significant at p < 0.05 using Student's paired t-test. Standard error is shown.
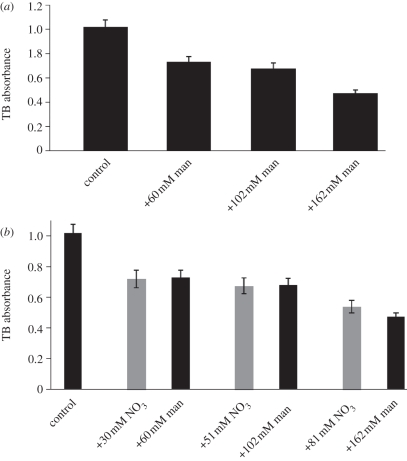
Decreased osmotic potential in growth medium results in a visual decrease in permeability of wild-type seedling leaves, as measured by reduced uptake of Toluidine Blue (TB; see §9) [22]. However, it was never tested whether nitrate acting as a nutrient/signal (rather than an osmolyte) affects permeability. This is important since alterations in permeability lead to alterations in the uptake of medium components, including stimulatory sucrose. To confirm the effect of osmotic potential and quantify permeability changes, we measured TB staining in seedlings grown on medium containing increasing amounts of mannitol. With increasing mannitol, seedlings showed a decrease in TB staining (figure 6a), consistent with the observations reported previously [22]. To test the effect of nitrate salts independently of their effect on osmotic potential, seedlings were grown on medium containing increasing amounts of nitrate and compared with seedlings grown on medium containing equivalent osmotic potential owing to mannitol. The results showed that nitrate had no effect on the permeability of shoot tissues to TB (figure 6b). Since nitrate salts show a strong repressive effect on lateral root formation and have no effect on TB staining, this suggests that nitrate repression of lateral root formation is independent of mechanisms controlling the acquisition of sucrose from the medium by shoot tissues.
Figure 6.
Toluidine Blue (TB) absorbance decreases with decreasing osmotic potential but is not specifically altered by nitrate. (a) TB absorbance decreases with addition of mannitol. All data points are statistically significantly different from each other (p ≪ 0.01 using Student's paired t-test), except for the comparison of +60 mM mannitol and +102 mM mannitol. Bars represent average of 46–50 individuals for all data points. (b) TB absorbance is not altered between additions of nitrate or equivalent osmotic amounts of mannitol. Bars represent average for 42–50 individuals for all data points. No significant difference using Student's paired t-test. Standard error is shown.
8. Conclusions and outlook
Tremendous insights into plant processes can be gained using laboratory assays in which plants are grown on defined medium. However, correct controls must be included to tease out the effects of medium components, and to check for indirect effects of additives (i.e. osmotic effects) and unequal uptake of medium components. Here, we have revisited the role of nitrate, water and sucrose in controlling root system growth and shoot system growth. Our results largely confirm those of previous authors, and demonstrate that osmotic changes in medium alter leaf permeability to sucrose and, hence, sucrose uptake; this plays a major role in subsequent plant growth. In the absence of osmotic changes, we demonstrate that sucrose stimulates both root and shoot system growth. A novel finding of this study is that ubiquitous increases in nitrate decrease root system growth without altering shoot system growth. Since only root system size is altered under varying nitrate supply, this indicates that there is a fundamental decrease in root-to-shoot ratio with increasing nitrogen conditions. Many studies have identified increases in root-to-shoot ratio upon nitrogen starvation. However, this is the first study to our knowledge to show alterations in root-to-shoot ratio in conditions where nitrogen is plentiful. This suggests that plants can regulate their root-to-shoot ratio over a much wider nitrogen range than previously thought. If these results can be translated into more physiological growth conditions, they predict that plants regulate root-to-shoot ratio not specifically in response to nitrogen starvation, but as a general mechanism to tailor their growth to environmental nitrogen supply.
9. Methods
(a). Plant growth conditions
Seeds were surface sterilized in 100 per cent bleach plus Tween-20 for 3 min while vortexing, and rinsed three times using sterile water. Seeds were vernalized for two or more days at 4°C and planted on agar medium (described below) approximately 2 cm from the top edge of a 100 × 100 cm square Petri plate, with nine seeds per plate. Plates were wrapped with parafilm and placed vertically in a growth chamber with 16 L : 8 D cycles at 22°C. Plants were grown for 15 days unless specified otherwise.
(b). Media composition
Control medium contained the following components per litre of medium: 10 g sucrose, 0.5 g MES [2-(N-morpholino)-ethanesulphonic acid], 100 ml Murashige and Skoog Basal Salt Micronutrient Solution (10× stock from Sigma Aldrich, catalogue no. M0529), 5 ml of 1 M KNO3, 5 ml of 1 M NH4NO3, 0.33 g CaCl2·6H2O, 0.1807 g MgSO4, 0.17 g KH2PO4 and 7.0 g agar. Medium was brought to a pH of 5.7 using 1 M KOH prior to addition of agar, and autoclaved for 30–45 m.
Other media were made by supplementing control medium with varying amounts of mannitol, sucrose, KNO3 and NH4NO3. Repressive medium contained an additional 35 g l−1 sucrose, 15 ml of 1 M KNO3 and 15 ml of 1 M NH4NO3, compared with control medium.
(c). Determination of total lateral root length
Digital images of plant roots (taken using a Canon SD1000 digital camera) were traced by hand using ImageJ. For each seedling, the length of all lateral roots was summed to give the TOT value.
(d). Measurement of shoot size
Two-week-old seedlings generally had a fresh weight of the order of 1 μg. Given the difficulties of obtaining reproducible measurements in this weight range, we used chlorophyll content as a proxy for biomass. Seedlings were cut at the root–shoot junction, and the aerial tissue was transferred to a 1.5 ml tube containing 0.5 ml anhydrous ethanol. The aerial tissue was allowed to incubate in the ethanol for at least 15 h, after which 0.2 ml of solution from each sample was transferred to 96-well plates. The absorption of the samples was analysed using a plate reader (Tecan Safire II) at 430 nm. In studies where multiple seedlings were pooled and weighed, and chlorophyll extracted as above, chlorophyll content was nearly perfectly correlated with the mass of the seedlings on our media conditions in the presence and absence of osmotic stress (r2 = 0.904, y = 21.38x, n = 37, data not shown). The effect of altered sucrose or nitrogen on chlorophyll content was not determined.
(e). Determination of shoot permeability
Up to eight plates at a time were laid down horizontally such that the seedlings were on top of the agar, and the plate was angled slightly such that the root tip was higher than the aerial tissue. A solution of 5 mg TB per 10 ml water was poured over the aerial tissues such that the aerial tissues were completely submerged. The dye solution was decanted after 10 min, and the plate was submerged under water for approximately 10 s. After staining, seedlings were cut at the root–shoot junction, and the aerial tissue was transferred to a 1.5 ml tube containing 0.5 ml ethanol. After 15+ h, 0.2 ml of solution from each sample was transferred to 96-well plates. The absorption of the samples was analysed using a plate reader (Tecan Safire II) at 625 nm.
Note that absorbance readings at 430 nm and 625 nm did not interfere with each other (data not shown). Hence, permeability and size can be evaluated for the same seedling.
Acknowledgements
The authors thank Paul Ingram and Dana MacGregor for helpful discussions. This work was funded by NSF grant IOS-0951302 to J.E.M.
References
- 1.Malamy J. E. 2005. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 28, 67–77 10.1111/j.1365-3040.2005.01306.x (doi:10.1111/j.1365-3040.2005.01306.x) [DOI] [PubMed] [Google Scholar]
- 2.Walch-Liu P., Ivanov I. I., Filleur S., Gan Y., Remans T., Forde B. G. 2006. Nitrogen regulation of root branching. Ann. Bot. 97, 875–881 10.1093/aob/mcj601 (doi:10.1093/aob/mcj601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walch-Liu P., Filleur S., Gan Y., Forde B. G. 2005. Signaling mechanisms integrating root and shoot responses to changes in the nitrogen supply. Photosyn. Res. 83, 239–250 10.1007/s11120-004-2080-9 (doi:10.1007/s11120-004-2080-9) [DOI] [PubMed] [Google Scholar]
- 4.Hodge A. 2006. Plastic plants and patchy soils. J. Exp. Bot. 57, 401–411 10.1093/jxb/eri280 (doi:10.1093/jxb/eri280) [DOI] [PubMed] [Google Scholar]
- 5.Zhang H., Rong H., Pilbeam D. 2007. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J. Exp. Bot. 58, 2329–2338 10.1093/jxb/erm114 (doi:10.1093/jxb/erm114) [DOI] [PubMed] [Google Scholar]
- 6.Kraiser T., Gras D. E., Guitierrez A. G., Gonzalez B., Gutuerrez R. A. 2011. A holistic view of nitrogen acquisition in plants. J. Exp. Bot. 62, 1455–1466 10.1093/jxb/erq425 (doi:10.1093/jxb/erq425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermans C., Hammond J. P., White P. J., Verbruggen N. 2006. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 11, 610–617 10.1016/j.tplants.2006.10.007 (doi:10.1016/j.tplants.2006.10.007) [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Bucio J., Cruz-Ramirez A., Herrera-Estrella L. 2003. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 6, 280–287 10.1016/S1369-5266(03)00035-9 (doi:10.1016/S1369-5266(03)00035-9) [DOI] [PubMed] [Google Scholar]
- 9.Barber S. A. 1995. Soil nutrient bioavailability, 2nd edn New York, NY: John Wiley & Sons [Google Scholar]
- 10.Tsay Y., Ho C., Chen H., Lin S. 2011. Integration of nitrogen and potassium signaling. Annu. Rev. Plant Biol. 62, 207–226 10.1146/annurev-arplant-042110-103837 (doi:10.1146/annurev-arplant-042110-103837) [DOI] [PubMed] [Google Scholar]
- 11.Zhang H., Jennings A., Barlow P. W., Forde B. G. 1999. Dual pathways for regulation of root branching by nitrate. Proc. Natl Acad. Sci. USA 96, 6529–6534 10.1073/pnas.96.11.6529 (doi:10.1073/pnas.96.11.6529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Forde B. 2000. Regulation of Arabidopsis root development by nitrate availability. J. Exp. Bot. 51, 51–59 10.1093/jexbot/51.342.51 (doi:10.1093/jexbot/51.342.51) [DOI] [PubMed] [Google Scholar]
- 13.Vidal E. A., Araus V., Lu C., Parry G., Green P. J., Coruzzi G. M., Gutiérrez R. A. 2010. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 107, 4477–4482 10.1073/pnas.0909571107 (doi:10.1073/pnas.0909571107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gifford M. L., Dean A., Guiteirrez R. A., Coruzzi G. M., Birnbaum K. D. 2008. Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl Acad. Sci. USA 105, 803–808 10.1073/pnas.0709559105 (doi:10.1073/pnas.0709559105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., An X., Cheng L., Chen F., Bao J., Yuan L., Hang F., Mi G. 2010. Auxin transport in maize roots in response to localized nitrate supply. Ann. Bot. 106, 1019–1026 10.1093/aob/mcq202 (doi:10.1093/aob/mcq202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takei K., Takahashi T., Sugiyama T., Yamaya T., Sakakibara H. 2002. Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin. J. Exp. Bot. 53, 971–977 10.1093/jexbot/53.370.971 (doi:10.1093/jexbot/53.370.971) [DOI] [PubMed] [Google Scholar]
- 17.Ruffel S., Krouk G., Ristova D., Shasha D., Birnbaum K. D., Coruzzi G. M. 2011. Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc. Natl Acad. Sci. USA 108, 18 524–18 529 10.1073/pnas.1108684108 (doi:10.1073/pnas.1108684108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Signora L., De Smet I., Foyer C. H., Zhang H. 2001. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J. 28, 655–662 10.1046/j.1365-313x.2001.01185.x (doi:10.1046/j.1365-313x.2001.01185.x) [DOI] [PubMed] [Google Scholar]
- 19.De Smet I., Signora L., Beeckman T., Inze D., Foyer C. H., Zhang H. 2003. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 33, 543–545 10.1046/j.1365-313X.2003.01652.x (doi:10.1046/j.1365-313X.2003.01652.x) [DOI] [PubMed] [Google Scholar]
- 20.Fukaki H., Okushima Y., Tasaka M. 2007. Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 256, 111–137 10.1016/S0074-7696(07)56004-3 (doi:10.1016/S0074-7696(07)56004-3) [DOI] [PubMed] [Google Scholar]
- 21.Deak K. I., Malamy J. 2005. Osmotic regulation of root system architecture. Plant J. 43, 17–28 10.1111/j.1365-313X.2005.02425.x (doi:10.1111/j.1365-313X.2005.02425.x) [DOI] [PubMed] [Google Scholar]
- 22.Macgregor D. R., Deak K. I., Ingram P. A., Malamy J. E. 2008. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell 20, 2643–2660 10.1105/tpc.107.055475 (doi:10.1105/tpc.107.055475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner T. W. 1922. Studies of the mechanism of the physiological effects of certain mineral salts in altering the ratio of top growth to root growth in seed plants. Am. J. Bot. 9, 415–445 10.2307/2435424 (doi:10.2307/2435424) [DOI] [Google Scholar]
- 24.Brouwer R. 1962. Nutritive influences on the distribution of dry matter in the plant. Neth. J. Agric. Sci. 10, 399–408 [Google Scholar]
- 25.Raper C. D., Parsons L. R., Patterson D. T., Kramer P. J. 1977. Relationship between growth and nitrogen accumulation for vegetative cotton and soybean plants. Bot. Gaz. 138, 129–137 10.1086/336907 (doi:10.1086/336907) [DOI] [Google Scholar]
- 26.Rufty T. W., Jr, Raper C. D., Jr, Huber S. C. 1984. Alterations in internal partitioning of carbon in soybean plants in response to nitrogen stress. Can. J. Bot. 62, 501–508 10.1139/b84-074 (doi:10.1139/b84-074) [DOI] [PubMed] [Google Scholar]
- 27.Tolley-Henry L., Raper C. D. 1986. Nitrogen and dry-matter partitioning in soybean plants during onset of and recovery from nitrogen stress. Bot. Gaz. 147, 392–399 10.1086/337606 (doi:10.1086/337606) [DOI] [PubMed] [Google Scholar]
- 28.Bhat K., Brereton A., Nye P. 1979. The possibility of predicting solute uptake and plant growth response from independently measured soil and plant characteristics. Plant Soil 53, 169–191 10.1007/BF02181889 (doi:10.1007/BF02181889) [DOI] [Google Scholar]
- 29.Freijsen A., Otten H. 1984. The effect of nitrate concentration in a flowing solution system on growth and nitrate uptake of two Plantago species. Plant Soil 77, 159–169 10.1007/BF02182920 (doi:10.1007/BF02182920) [DOI] [Google Scholar]
- 30.Paul M. J., Stitt M. 1993. Effects of nitrogen and phosphorus deficiencies on levels of carbohydrates, respiratory enzymes and metabolites in seedlings of tobacco and their response to exogenous sucrose. Plant Cell Environ. 16, 1047–1057 10.1111/j.1365-3040.1996.tb02062.x (doi:10.1111/j.1365-3040.1996.tb02062.x) [DOI] [Google Scholar]
- 31.Scheible W., Lauerer M., Schulze E., Caboche M., Stitt M. 1997. Accumulation of nitrate in the shoot acts as a signal to regulate shoot–root allocation in tobacco. Plant J. 11, 671–691 10.1046/j.1365-313X.1997.11040671.x (doi:10.1046/j.1365-313X.1997.11040671.x) [DOI] [Google Scholar]
- 32.Ingestad T. 1979. Nitrogen stress in birch seedlings. Physiologia Plantarum 45, 149–157 10.1111/j.1399-3054.1979.tb01679.x (doi:10.1111/j.1399-3054.1979.tb01679.x) [DOI] [Google Scholar]
- 33.Agren G. I., Franklin O. 2003. Root : shoot ratios, optimization and nitrogen productivity. Ann. Bot. 92, 795–800 10.1093/aob/mcg203 (doi:10.1093/aob/mcg203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linkohr B. I., Williamson L. C., Fitter A. H., Leyser H. M. 2002. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 29, 751–760 10.1046/j.1365-313X.2002.01251.x (doi:10.1046/j.1365-313X.2002.01251.x) [DOI] [PubMed] [Google Scholar]
- 35.Ikram S., Bedu M., Daniel-Vedele F., Chaillou S., Chardon F. 2011. Natural variation of Arabidopsis response to nitrogen availability. J. Exp. Bot. 63, 91–105 10.1093/jxblerr244 (doi:10.1093/jxblerr244) [DOI] [PubMed] [Google Scholar]
- 36.Drew M. C. 1975. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol. 75, 479–490 10.1111/j.1469-8137.1975.tb01409.x (doi:10.1111/j.1469-8137.1975.tb01409.x) [DOI] [Google Scholar]
- 37.Drew M. C., Saker L. R. 1975. Nutrient supply and the growth of the seminal root system in barley. II. Localized compensatory increases in lateral root growth and rates of nitrate uptake when nitrate supply is restricted to only part of the root system. J. Exp. Bot. 26, 79–90 10.1093/jxb/26.1.79 (doi:10.1093/jxb/26.1.79) [DOI] [Google Scholar]
- 38.Drew M. C., Saker L. R., Ashley T. W. 1973. Nutrient supply and the growth of the seminal root system in barley. I. The effect of nitrate concentration on the growth of axes and laterals. J. Exp. Bot. 24, 1189–1202 10.1093/jxb/24.6.1189 (doi:10.1093/jxb/24.6.1189) [DOI] [Google Scholar]
- 39.Zhang H., Forde B. G. 1998. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279, 407–409 10.1126/science.279.5349.407 (doi:10.1126/science.279.5349.407) [DOI] [PubMed] [Google Scholar]
- 40.Robinson D. 1994. The responses of plants to nonuniform supplies of nutrients. New Phytol. 127, 635–674 10.1111/j.1469-8137.1994.tb02969.x (doi:10.1111/j.1469-8137.1994.tb02969.x) [DOI] [PubMed] [Google Scholar]
- 41.Tillard P., Passama L., Gojon A. 1998. Are phloem amino acids involved in the shoot to root control of NO–3 uptake in Ricinus communis plants? J. Exp. Bot. 49, 1371–1379 10.1093/jexbot/49.325.1371 (doi:10.1093/jexbot/49.325.1371) [DOI] [Google Scholar]
- 42.Gansel X., Munos S., Tillard P., Gojon A. 2001. Differential regulation of the NO–3 and NH+4 transporter genes AtNrt2.1 and AtAmt1.1 in Arabidopsis: relation with long-distance and local controls by N status of the plant. Plant J. 26, 143–155 10.1046/j.1365-313x.2001.01016.x (doi:10.1046/j.1365-313x.2001.01016.x) [DOI] [PubMed] [Google Scholar]
- 43.Remans T., Nacry P., Pervent M., Filleur S., Diatloff E., Mounier E., Tillard P., Forde B. F., Gojon A. 2006. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc. Natl Acad Sci. USA 103, 19 206–19 211 10.1073/pnas.0605275103 (doi:10.1073/pnas.0605275103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krouk G., et al. 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 18, 927–937 10.1016/j.devcel.2010.05.008 (doi:10.1016/j.devcel.2010.05.008) [DOI] [PubMed] [Google Scholar]
- 45.Hooker H. D., Jr 1915. Hydrotropism in roots of Lupinus albus. Ann. Bot. 29, 265–283 [Google Scholar]
- 46.Jaffe M. J., Takahashi H., Biro R. L. 1985. A pea mutant for the study of hydrotropism in roots. Science 230, 445–447 10.1126/science.230.4724.445 (doi:10.1126/science.230.4724.445) [DOI] [PubMed] [Google Scholar]
- 47.Takahashi H., et al. 1999. A spaceflight experiment for the study of gravimorphogenesis and hydrotropism in cucumber seedlings. J. Plant Res. 112, 497–505 10.1007/PL00013906 (doi:10.1007/PL00013906) [DOI] [PubMed] [Google Scholar]
- 48.Monshausen G. B., Gilroy S. 2009. The exploring root–root growth responses to local environmental conditions. Curr. Opin. Plant Biol. 12, 766–772 10.1016/j.pbi.2009.08.002 (doi:10.1016/j.pbi.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 49.Darwin C., Darwin F., Darwin C. 1880. The power of movement in plants. London, UK: John Murray [Google Scholar]
- 50.Takahashi N., Yamazaki Y., Kobayashi A., Higashitani A., Takahashi H. 2003. Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiol. 132, 805–810 10.1104/pp.018853 (doi:10.1104/pp.018853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi A., Takahashi A., Kakimoto Y., Miyazawa Y., Fujii N., Higashitani A., Takahashi H. 2007. A gene essential for hydrotropism in roots. Proc. Natl Acad. Sci. USA 104, 4724–4729 10.1073/pnas.0609929104 (doi:10.1073/pnas.0609929104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taiz L., Zeiger E. 2002. Plant physiology, 3rd edn Sunderland, MA: Sinauer Associates [Google Scholar]
- 53.Yamaguchi M., Sharp R. E. 2010. Complexity and coordination of root growth at low water potentials: recent advances from transcriptomic and proteomic analyses. Plant Cell Environ. 33, 590–603 10.1111/j.1365-3040.2009.02064.x (doi:10.1111/j.1365-3040.2009.02064.x) [DOI] [PubMed] [Google Scholar]
- 54.Munns R., Tester M. 2008. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681 10.1146/annurev.arplant.59.032607.092911 (doi:10.1146/annurev.arplant.59.032607.092911) [DOI] [PubMed] [Google Scholar]
- 55.Galvan-Ampudia C. S., Testerink C. 2011. Salt stress signals shape the plant root. Curr. Opin. Plant Biol. 14, 296–302 10.1016/j.pbi.2011.03.019 (doi:10.1016/j.pbi.2011.03.019) [DOI] [PubMed] [Google Scholar]
- 56.van der Weele C. M., Spollen W. G., Sharp R. E., Baskin T. I. 2000. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 51, 1555–1562 10.1093/jexbot/51.350.1555 (doi:10.1093/jexbot/51.350.1555) [DOI] [PubMed] [Google Scholar]
- 57.Xiong L., Wang R. G., Mao G., Koczan J. M. 2006. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 142, 1065–1074 10.1104/pp.106.084632 (doi:10.1104/pp.106.084632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi X., Wu Z., Li J., Mo X., Wu S., Chu J., Wu P. 2007. AtCYT-INV1, a neutral invertase, is involved in osmotic stress-induced inhibition on lateral root growth in Arabidopsis. Plant Mol. Biol. 64, 575–587 10.1007/s11103-007-9177-4 (doi:10.1007/s11103-007-9177-4) [DOI] [PubMed] [Google Scholar]
- 59.Freixes S., Thibaud M., Tardieu F., Muller B. 2002. Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant Cell Environ. 25, 1357–1366 10.1046/j.1365-3040.2002.00912.x (doi:10.1046/j.1365-3040.2002.00912.x) [DOI] [Google Scholar]
- 60.Lee-Ho E., Walton L. J., Reid D. M., Yeung E. C., Kurepin L. V. 2007. Effects of elevated carbon dioxide and sucrose concentrations on Arabidopsis thaliana root architecture and anatomy. Can. J. Bot. 85, 324–330 10.1139/B07-009 (doi:10.1139/B07-009) [DOI] [Google Scholar]
- 61.Hammond J. P., White P. J. 2011. Sugar signaling in root responses to low phosphorus availability. Plant Physiol. 156, 1033–1040 10.1104/pp.111.175380 (doi:10.1104/pp.111.175380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rolland F., Baena-Gonzalez E., Sheen J. 2006. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu. Rev. Plant Biol. 57, 675–709 10.1146/annurev.arplant.57.032905.105441 (doi:10.1146/annurev.arplant.57.032905.105441) [DOI] [PubMed] [Google Scholar]
- 63.Buer C. S., Wasteneys G. O., Masle J. 2003. Ethylene modulates root-wave responses in Arabidopsis. Plant Physiol. 132, 1085–1096 10.1104/pp.102.019182 (doi:10.1104/pp.102.019182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buer C. S., Sukumar P., Muday G. K. 2006. Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol. 140, 1384–1396 10.1104/pp.105.075671 (doi:10.1104/pp.105.075671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hermans C., Porco S., Verbruggen N., Bush D. R. 2010. Chitinase-like protein CTL1 plays a role in altering root system architecture in response to multiple environmental conditions. Plant Physiol. 152, 904–917 10.1104/pp.109.149849 (doi:10.1104/pp.109.149849) [DOI] [PMC free article] [PubMed] [Google Scholar]