Abstract
Root system architecture plays an important role in determining nutrient and water acquisition and is modulated by endogenous and environmental factors, resulting in considerable developmental plasticity. The orientation of primary root growth in response to gravity (gravitropism) has been studied extensively, but little is known about the behaviour of lateral roots in response to this signal. Here, we analysed the response of lateral roots to gravity and, consistently with previous observations, we showed that gravitropism was acquired slowly after emergence. Using a lateral root induction system, we studied the kinetics for the appearance of statoliths, phloem connections and auxin transporter gene expression patterns. We found that statoliths could not be detected until 1 day after emergence, whereas the gravitropic curvature of the lateral root started earlier. Auxin transporters modulate auxin distribution in primary root gravitropism. We found differences regarding PIN3 and AUX1 expression patterns between the lateral root and the primary root apices. Especially PIN3, which is involved in primary root gravitropism, was not expressed in the lateral root columella. Our work revealed new developmental transitions occurring in lateral roots after emergence, and auxin transporter expression patterns that might explain the specific response of lateral roots to gravity.
Keywords: lateral root, gravitropism, auxin transporters, vascular connection, PIN3, AUX1
1. Introduction
Root system architecture is an important parameter in plant growth and survival. It influences the efficiency of nutrient and water provision, as well as anchoring the plant to the substrate [1,2]. Root system architecture results from the combination of the growth of the existing roots and their tropism (direction of growth), as well as the rate and position of emergence of lateral roots [1]. Primary root growth originates from the activity of the root apical meristem. In this structure, the stem cells, located around the quiescent centre, give rise to all root cell types, including the root cap that protects the root apex [3]. In contrast, lateral roots develop from pericycle cells in the primary root, although recent data have shown that this process is also initiated in the apical meristem [4,5]. Modulation of root elongation and branching provides the root system architecture with considerable plasticity in response to a heterogeneous and constraining environment [1,6]. Modifications to root tropism may also optimize the foraging capacity of the root system [2,7].
Root growth is not only mainly orientated along the gravity vector (positive gravitropism), but is also influenced by light, the presence of water, nutrients and the presence of an obstacle (phototropism, hydrotropism, chimiotropism and thigmotropism, respectively [8]). The action of these factors and the balance between the roles they play has been studied mainly in primary roots. Yet the behaviour of lateral roots with regard to these environmental cues is of great importance in the elaboration of the overall root system architecture [8].
In the primary root of Arabidopsis thaliana, reorientation of root growth following gravistimulation occurs within a few hours [9]. Gravity perception occurs in specific cells called statocytes [10]. In the primary root meristem of A. thaliana, cells in the central root cap (columella) act as statocytes [10]. These cells are polarized: the nucleus is on the root meristem side while the endoplasmic reticulum is located around the cell. In these cells, the perception of gravity depends on specialized amyloplasts called statoliths, which sediment according to gravity. While starch accumulation is not absolutely necessary, it is needed to obtain the full response to gravity [10]. It is thought that sedimenting statoliths that come into contact with the endoplasmic reticulum trigger the release of Ca2+ from the endoplasmic reticulum [10]. The signal transduction pathway induced by changes in statolith sedimentation is still largely unknown [10]. However, it is well known that graviperception eventually results in changes in auxin distribution in the root tip [11,12]. Upon gravistimulation of the root the auxin efflux carrier PIN3, which is expressed in the columella, quickly changes its cellular localization leading to asymmetric auxin transport [13]. More auxin is transported to the bottom side of the root [11]. This asymmetric distribution is then transferred via the lateral root cap to epidermal cells in the elongation zone by PIN2 and AUX1 activity [14]. Asymmetric auxin distribution in the epidermis leads to differential cell elongation and eventually to root bending [14].
While gravitropism has been extensively studied in the primary root, the response of lateral roots to gravitropic stimuli has poorly been described. It has been assumed that the molecular mechanisms are similar to what occurs in primary roots. However, this response is species-dependent: in some species lateral roots are unresponsive to gravity [15] while in others lateral roots grow at a set angle relative to the gravity vector, a response known as plagiogravitropism [16]. This indicates that lateral roots have an endogenous (genetic) programme for gravitropism that has been selected to optimize root system architecture depending on each species' ecological niche and lifecycle.
Lateral roots develop inside the parent root from an internal tissue, the pericycle [17]. In A. thaliana, lateral roots initially emerge perpendicular to the main root, i.e. nearly horizontal, and they slowly change their growth to a near vertical orientation [18]. This progressive acquisition of gravitropism has been correlated with the formation and polar localization of starch grains in lateral root columella cells [18]. This response was still present but was slower in the pgm (starchless) mutant than in wild-type plants [18]. However, in some species, lateral root have sedimented amyloplasts in their columella cells but are either irresponsive to gravity [15] or weakly graviresponsive [19], thus showing that amyloplasts are necessary but not sufficient for positive gravitropism in lateral roots. Three A. thaliana mutants impaired in lateral root gravitropic response but not in primary root gravitropism, named gsa1, gsa2 and gsa3 (gravitropic set-point angle), have been isolated [16]. This clearly demonstrates that lateral root gravitropism can, at least in part, be genetically dissociated from primary root gravitropism. Interestingly, gsa2 was found to be allelic to rhd3, a mutant defective in root hair development [20]. RHD3 encodes a putative GTP-binding protein involved in endoplasmic reticulum organization [20,21]. Mutations to rhd3 may therefore have an indirect impact on the distribution of plasma membrane-localized auxin transporters in the root tip of lateral root primordia [16]. Alternatively, as the endoplasmic reticulum is thought to play an important role in gravity-sensing in statocytes [6], rhd3 mutation could prevent the proper organization of the endoplasmic reticulum and therefore its function in lateral root columella cells.
These previous studies have provided useful insight into the gravitropic behaviour of lateral roots, but as it was difficult to track lateral root growth from emergence and later, the kinetics of lateral root tropism coupled to its growth were not taken into consideration. Here, we took advantage of a lateral root induction system [22] to study the behaviour of synchronized young lateral roots from emergence onwards. We showed that the acquisition of gravitropism in lateral roots occurs progressively after emergence, and is variable. We then performed time course analyses of the acquisition of known actors for gravitropism, namely statoliths, vascular connections and auxin transporters. This study revealed new developmental transitions that occur in lateral roots after emergence.
2. Material and methods
(a). Plant material
Wild-type (Col-0) seeds were obtained from the NASC (Loughborough, UK). SUC2::GFP seeds (C24 background) were obtained from Dr M. Crespi (CNRS Gif/Yvette, France). AUX1::AUX1:YFP, PIN1::PIN1:GFP, PIN2::PIN2:GFP, PIN3::PIN3:GFP, PIN4::PIN4:GFP, and PIN7::PIN7:GFP seeds (Col-0 background) were provided by Prof. M. Bennett (University of Nottingham, UK).
Plants were grown on vertical Petri dishes as previously described [23]. Lateral root formation was induced by subjecting the plates to a 90° gravistimulus 4 days after germination [22]. Plates were rotated back to the initial orientation 24 h after gravistimulation (hag).
(b). Histology
Lugol's staining of A. thaliana roots was performed as described [24], with minor modifications to the protocol. Seedlings were incubated with the Lugol's solution for 10 min in vacuum to facilitate its penetration into the young lateral roots. Roots were then rinsed and mounted on a chloral hydrate : glycerol : water solution (8 : 1 : 2, p : v : v) and visualized using a Leica DMRB microscope equipped with DIC optics. Pictures were taken using an Evolution MP Color (Media Cybernetics) charge-coupled device (CCD) camera.
(c). Microscopy
Lateral root emergence and growth were monitored using a Leica MZFLIII binocular. Images were taken using a Micropublisher v. 5.0 RTV (QImaging) CCD camera and the QCapture Pro software (QImaging). Root length was measured using the QCapture Pro software and the angle of the root apex respective to the gravity vector was measured using the ImageJ-derived software Fiji.
Expression of the GFP constructs was monitored using a Zeiss Axio Imager A1 epifluorescence microscope, coupled to a Colibri LED illumination system (Zeiss) and a Retiga-SRV (QImaging) CCD camera. Precise analyses of the expression pattern were performed using a Zeiss LSM510 confocal microscope. Roots were counterstained with a solution of propidium iodide (200 μg ml−1). After excitation by the 488 nm Ar laser beam, GFP emission was collected using a BP 505–530 emission filter. After excitation by the 543 nm HeNe laser beam, propidium iodide emission was collected using a BP 560–615 emission filter. Image selection, merging and analyses were performed using the software Fiji.
3. Results
(a). Progressive acquisition of gravitropism in emerging lateral roots
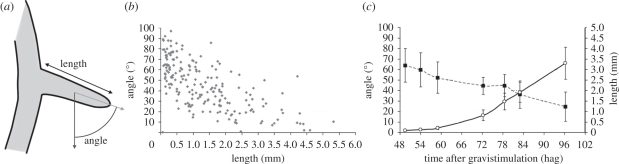
In order to study the response of lateral roots to gravity, fifteen 10-day-old A. thaliana plants grown on vertical plates were scanned and the length of the lateral roots and angle of the lateral root apex respective to the gravity vector were measured (figure 1a). As previously reported [18], we found that lateral roots emerged perpendicularly to the parent root (and therefore the gravity vector) and progressively reorientated their growth towards the gravity vector (figure 1b). This behaviour shows two interesting features: (i) the angle of the lateral roots respective to the gravity vector is highly variable; and (ii) it remains greater than 10° for most of the lateral roots from 0 to 4 mm in length. Lateral root gravitropic behaviour is thus different from the primary root gravitropic response, which is visible within 3 h [9] and leads in about 12 h to almost complete reorientation of the primary root tip along the gravity vector [25]. This analysis does not, however, take into account the growth rate of the lateral root after emergence.
Figure 1.
Length and angle of lateral roots after emergence. (a) Schematic of how lateral root length and angle were measured. (b) Length and apex angle of a total of 197 lateral roots were measured on 15 non-gravistimulated Col-0 wild-type plants, at 10 days after germination. The angle is 0° if the root apex is aligned with the gravity vector. (c) Length (open circles) and angle (filled squares) kinetics of 32 lateral roots induced by gravistimulation on Col-0 wild-type plants (mean ± s.d.).
We then took advantage of a lateral root induction system [22] to follow the gravitropic response of young lateral roots after emergence. We monitored the length and angle respective to gravity of a population of 32 synchronized lateral roots from 48 hag (emergence) to 96 hag (figure 1c). After emergence, root growth increased exponentially up to 72 hag and then reached a more steady rate of approximately 0.1 mm h−1. As the lateral root was induced at or slightly up from the turn in the primary root generated by the gravistimulus [22], and as the whole plant was rotated back to the initial orientation only 24 h after gravistimulation, the lateral root emerged at an angle of about 64° (figure 1c). After emergence, the root apex angle decreased progressively and in a discontinuous way (figure 1c). Analyses of individual curves revealed fluctuations in the lateral root angle, with periods during which the lateral root tip angle tended to decrease more slowly and even increase rather than decrease (data not shown). This behaviour could be due to endogenous or exogenous circadian rhythms, but more frequent time points and a larger population will be needed to address this issue. As in the previous experiment, at the short time points the lateral root angle was highly variable while lateral root length was not. Finally, 2 days after lateral root emergence (96 hag), the mean lateral root tip angle was still 25° while lateral root length was 3.3 mm on average. Thus, consistent with previous results [16,18], this experiment confirmed that lateral roots do not behave like primary roots in response to the gravitropic stimulus. In addition, this time course experiment revealed the heterogeneity and instability in lateral root tropism up to 48 h after emergence. We then used the same experimental design to study the acquisition and characteristics of known actors in primary root gravitropism.
(b). Statoliths appear between 12 and 24 h after emergence
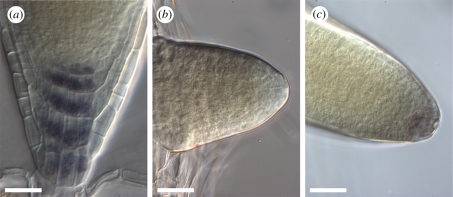
The progressive acquisition of gravitropic growth in lateral roots in A. thaliana has been linked to statolith formation in the columella [18]. We therefore monitored statolith differentiation in our experimental system using Lugol staining. In contrast to the main root apex (figure 2a), we could not detect any Lugol staining in emerging lateral roots up to 60 hag, which corresponds to 12 h post-emergence (figure 2b, n = 6/7). By contrast, at 72 hag (24 h post-emergence) all the lateral roots tested displayed Lugol stained amyloplasts in their columella (figure 2c, n = 10/10). At these stages in our experimental system, the average lateral root length increases from 210 µm (60 hag) to 828 μm (72 hag, figure 1c). This result is in agreement with previous studies using electron microscopy which found that amyloplasts with a few starch grains appeared in lateral roots when they reached a length of about 500 μm [18]. We conclude that statoliths are formed in the columella of lateral roots between 12 and 24 h post-emergence.
Figure 2.
Statolith detection using Lugol staining in lateral roots. (a) Statoliths are detected in the columella cells of the primary root meristem. (b) No Lugol staining is observed at the apex of lateral roots at 60 hag (n = 6/7). (c) Lugol staining of statoliths at the apex of lateral roots at 72 hag (n = 10/10). Scale bars, 25 μm.
(c). Vascular connection occurs between 12 and 24 h after emergence
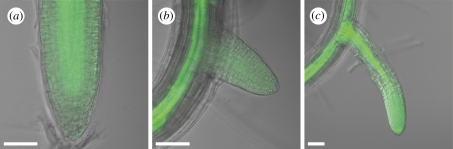
As carbon sources and certain phytohormones are transported with the phloem stream, we next analysed at what time young lateral roots were connected to the vascular system of the parent root. Previous studies have shown that vascular cells start to differentiate in lateral root primordia as early as stage VI, i.e. before lateral root emergence [4]. However, this does not prove that the lateral root vascular cells are truly connected to the parent root vasculature. In order to monitor when lateral roots become connected to the main root phloem stream, we used the SUC2::GFP molecular marker. The AtSUC2 gene encodes a sucrose-proton symporter and is expressed specifically in companion cells [26]. Analyses of the SUC2::GFP plants demonstrated that the 27 kD GFP protein can travel from companion cells into sieve elements and migrate within the phloem [26]. The GFP therefore marks the phloem stream and can also be unloaded from the phloem into sink tissues where it can travel from cell to cell by means of symplastic transport [26]. In the main root, strong GFP fluorescence was visible in the vasculature and a weaker, diffuse signal was also observed in the meristem (figure 3a). In the early stages of lateral root development, a weak, diffuse GFP signal was visible throughout the lateral root primordia and later in the lateral root meristem up to 60 hag (figure 3b, n = 17/17), thus indicating that phloem unloading was taking place but that the lateral root phloem was not differentiated and/or connected to the main root phloem vessels. Yet later, at 72 hag, a strong vascular GFP signal was detected in most of the lateral roots (figure 3c, n = 21/22). The connection of lateral root vasculature to the main root vasculature thus occurs between 60 and 72 hag.
Figure 3.
SUC2::GFP expression pattern in young lateral roots after emergence. (a) SUC2::GFP signal is diffuse in the primary root apical meristem and very strong in the primary root vasculature. (b) SUC2::GFP signal is weak and diffuse in the lateral root apex up to 60 hag (n = 17/17). (c) Strong vascular SUC2::GFP signal is detected in lateral roots at 72 hag (n = 21/22). Scale bars, 25 μm.
Interestingly, this event is correlated with the appearance of statoliths in the columella of lateral roots. Connection of the lateral root phloem to the main root phloem might lead to an increase in carbon and phytohormone availability for growth, tropic response and starch formation in the columella.
(d). Expression of auxin transporter genes in lateral roots
Asymmetric auxin transport is responsible for gravitropic curvature in primary roots [14]. Several auxin transporters have been implicated in this process [12,27]. We therefore compared the expression pattern of AUX1, PIN1, 2, 3, 4 and 7 in the primary root and in synchronized lateral roots using translational fusions to fluorescent proteins.
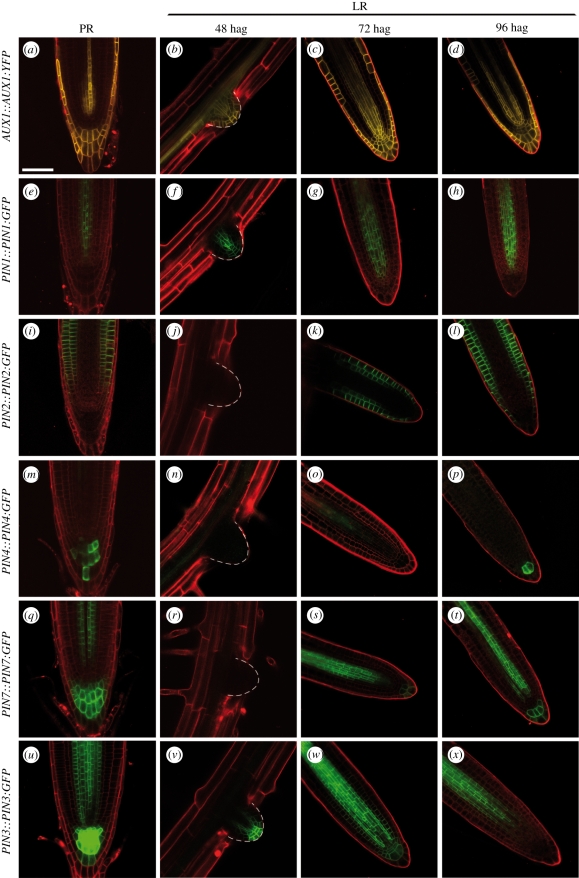
The AUX1::AUX1:YFP reporter was expressed in lateral root primordia before lateral root emergence, as previously described [28] (data not shown). After emergence, AUX1:YFP fluorescence was detected in the provascular tissue, quiescent centre, root cap and epidermal cells at the apex of the lateral root (figure 4b). This pattern was similar to that found in the primary root (figure 4a), except that AUX1:YFP was detected in the quiescent centre and the inner cells of the lateral root columella whereas it was absent from this region in the primary root meristem. As the lateral root grew, this pattern was maintained at least up to 96 hag (figure 4c,d).
Figure 4.
Expression pattern of auxin transport genes in the emerging lateral roots, compared with the primary root meristem. Expression of AUX1::AUX1:YFP (a–d), PIN1::PIN1:GFP (e–h), PIN2::PIN2:GFP (i–l), PIN4::PIN4:GFP (m–p), PIN7::PIN7:GFP (q–t), and PIN3::PIN3:GFP (u–x) in the primary root (PR) meristem (a,e,i,m,q,u), and in the lateral root apex at 48 hag (b,f,j,n,r,v), 72 hag (c,g,k,o,s,w), and 96 hag (d,h,l,p,t,x). The orientation in the pictures does not reflect the angle of lateral root relative to the primary root. Scale bars, 50 μm.
Similarly, PIN1::PIN1:GFP was expressed very early on in the lateral root primordia as previously described [29] (data not shown). After emergence, PIN1:GFP fluorescence in the provascular tissues quickly organized in the lateral root as in the primary root (figure 4e–h). Weak expression of PIN1:GFP remained detectable in the base of lateral root columella whereas it did not seem to be expressed in that part of the primary root (figure 4h). In both AUX1:YFP and PIN1:GFP cases, we cannot rule out the hypothesis that these discrepancies could be due to GFP proteins remaining in certain cells after the expression of the construct has been switched off. The discrete boundaries of domains with and without GFP fluorescence nevertheless suggest that these could be distinct functional domains.
PIN2 is expressed in the lateral root cap, epidermis and in the cortex of the primary root, where it plays an important role in the primary root gravitropic response [30] (figure 4i). Arabidopsis thaliana pin2 mutant has an agravitropic root [14]. PIN2::PIN2:GFP was not expressed in the lateral root primordia nor the emerging lateral roots (figure 4j), but PIN2:GFP fluorescence was detected soon after emergence at the flanks of the growing lateral root (data not shown). From 72 hag onwards, PIN2:GFP was detected in the same tissues in the lateral root as in the primary root (figure 4k,l).
While PIN4 and PIN7 are expressed in the vascular tissues and the columella of the primary root [31] (figure 4m,q), PIN4::PIN4:GFP and PIN7::PIN7:GFP were not expressed in the lateral root primordia or the emerging lateral root (figure 4n,r). Weak expression of these two transgenes began between 48 and 72 hag in the provascular tissues (figure 4o,q), and expression in the columella was detected from 96 hag onwards (figure 4p,t). Thus, 2 days after emergence, PIN4:GFP and PIN7:GFP had similar expression patterns in emerged lateral roots as in the primary root.
In the primary root, PIN3 is expressed in the provasculature and in columella cells where it is thought to play a central role in gravitropism [13] (figure 4u). We found that PIN3 was also expressed in lateral root primordia very early on (data not shown). After emergence, PIN3:GFP fluorescence was detected in the provascular tissues, endodermis, quiescent centre and columella (figure 4v). This expression pattern, which is similar to the PIN3::PIN3:GFP expression pattern in the primary root, was observed up to 72 hag (figure 4w). In contrast, GFP fluorescence in the quiescent centre and the columella was no longer detectable in most lateral roots from 96 hag onwards (figure 4x, n = 17/18). This striking loss of PIN3::PIN3:GFP expression in the columella of lateral roots was confirmed in older lateral roots and up to 168 hag (n = 7/8, data not shown). Thus, while PIN3 has been implicated in initiating the asymmetric lateral distribution of auxin in gravistimulated primary roots [13], this protein is not expressed in most of the lateral root columellas from 96 hag onwards, which corresponds to the time when PIN4 and PIN7 expression in the columella was detected. In addition to PIN3, PIN7 may be involved in the primary root gravitropic response [32]. Thus, it may be possible that PIN7 mediates the gravitropic behaviour of lateral roots in the absence of PIN3.
4. Conclusion and outlook
Lateral roots have a specific response to gravity. This response is species-specific and is one part of the genetic programme controlling root system architecture. This allows different plants to exploit different spatial niches, and therefore to reduce the extent of competition with other plants from the same species or from different species. Indeed, computer simulations show that lateral and adventitious root gravitropism has a significant impact on the efficiency of nutrient acquisition and competition between roots both from the same plant and from neighbouring plants [7,33]. Despite its great importance in shaping the plant root system, lateral root gravitropism has been the subject of very few studies. This manuscript reviews our current knowledge on this interesting and puzzling problem and the data we present suggest testable hypotheses and ways forward.
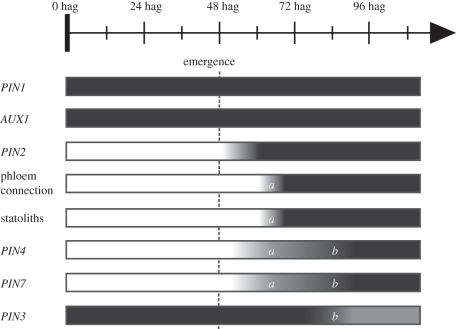
In the model plant A. thaliana, young developing lateral roots slowly reorientate their growth towards the orientation of the gravity vector. The mechanisms that control this behaviour are poorly understood. By comparison, the behaviour of very young primary root has not been studied in detail in A. thaliana, but it has been shown in flax that gravisensitivity in the main root occurs prior to germination and that the germinating root grows towards the gravity vector [34]. Here, we used a lateral root induction system to define a schedule of events occurring after lateral root emergence. We did not study the behaviour of longer lateral roots (more than 1 cm long). Altogether, our data indicate that a major transition occurs in lateral root development between 12 and 24 h post-emergence (figure 5). This transition is characterized by the connection of the lateral root to the main root vasculature and by the formation of detectable stalotliths in the lateral root columella cells. In addition, PIN4 and PIN7 expression begins in the provascular tissues of the lateral root during the first day after emergence. A second transition occurs between 24 and 48 h post-emergence, while PIN4 and PIN7 expression appears in the root cap. Strikingly, at this transition, PIN3 expression is turned off in lateral root columella cells. PIN3 is known to be a major player in primary root gravitropism [13]. This interesting feature of lateral roots might partly explain their specific response to gravity. It might, for instance, change auxin redistribution in the root tip and therefore the orientation of root growth. Experiments to measure the dynamic of auxin distribution in the lateral root tip will be needed to test this hypothesis. Interestingly, it was reported that in the pin3 mutant, lateral roots reorientate their growth towards the gravity vector more quickly than control plants [35]. However, it is difficult to address the role of individual PIN genes in lateral root gravitropism using mutants owing to pleiotropic effects and functional redundancy.
Figure 5.
Main transitions and putative actors for lateral root gravitropism after emergence. PIN1 and AUX1 are expressed throughout the lateral root primordium development and after emergence in the lateral root apex in a pattern close to the one observed in primary root meristem (see text). PIN2 expression is switched on early after lateral root emergence. Between 60 and 72 hag (respectively, 12 and 24 h post-emergence, (a)), phloem connection is established and statoliths appear in the columella. PIN4 and PIN7 expression also begins in lateral root vascular tissues between 48 and 72 hag. Between 72 and 96 hag (respectively, 24 and 48 h post-emergence, (b)), PIN4 and PIN7 expression starts in the lateral root columella while PIN3, which was previously expressed in the same tissues as in the primary root meristem, disappears from the lateral root columella.
Clearly, more work is needed in order to understand the plagiogravitropic behaviour of lateral roots. For instance, our work was done on first-order lateral roots. However, we did not test whether or not the behaviour of higher-order lateral roots was different. The characterization of the gsa mutants impaired in lateral root gravitropism but not in primary root gravitropism [16] should provide some interesting information. The cellular function of RHD3 (allelic to GSA2) has been well studied [21] and tools are available to address its role in lateral root gravitropism. Finally, because of its quantitative nature, the study of lateral root gravitropism would greatly benefit from a system biology approach combining modelling with experimental biology.
Acknowledgements
We thank the Montpellier Rio Imaging platform for help with imaging. Work in our laboratory was supported by the IRD, the Université Montpellier 2 (grant from the Conseil Scientifique to S.G. and L.L.), the Région Languedoc-Roussillon (grant ‘Chercheur d'Avenir’ to L.L. and S.G.), and the Agropolis Fondation (‘Rhizopolis’ Federative Grant). F. Perrine-Walker was supported by a postdoctoral fellowship from Agropolis Fondation (no. 07024).
References
- 1.Nibau C., Gibbs D. J., Coates J. C. 2008. Branching out in new directions: the control of root architecture by lateral root formation. New Phytol. 179, 595–614 10.1111/j.1469-8137.2008.02472.x (doi:10.1111/j.1469-8137.2008.02472.x) [DOI] [PubMed] [Google Scholar]
- 2.Hodge A. 2006. Plastic plants and patchy soils. J. Exp. Bot. 57, 401–411 10.1093/jxb/eri280 (doi:10.1093/jxb/eri280) [DOI] [PubMed] [Google Scholar]
- 3.Bennett T., Scheres B. 2010. Root development—two meristems for the price of one? Curr. Top. Dev. Biol. 91, 67–102 10.1016/S0070-2153(10)91003-X (doi:10.1016/S0070-2153(10)91003-X) [DOI] [PubMed] [Google Scholar]
- 4.Malamy J. E., Benfey P. N. 1997. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44 [DOI] [PubMed] [Google Scholar]
- 5.De Smet I., et al. 2007. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134, 681–690 10.1242/dev.02753 (doi:10.1242/dev.02753) [DOI] [PubMed] [Google Scholar]
- 6.Hodge A. 2009. Root decisions. Plant Cell Environ. 32, 628–640 10.1111/j.1365-3040.2008.01891.x (doi:10.1111/j.1365-3040.2008.01891.x) [DOI] [PubMed] [Google Scholar]
- 7.Ge Z., Rubio G., Lynch J. 2000. The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant Soil. 1, 159–171 10.1023/A:1014987710937 (doi:10.1023/A:1014987710937) [DOI] [PubMed] [Google Scholar]
- 8.Malamy J. E. 2005. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 28, 67–77 10.1111/j.1365-3040.2005.01306.x (doi:10.1111/j.1365-3040.2005.01306.x) [DOI] [PubMed] [Google Scholar]
- 9.Rosen E., Chen R., Masson P. H. 1999. Root gravitropism: a complex response to a simple stimulus? Trends Plant Sci. 4, 407–412 [DOI] [PubMed] [Google Scholar]
- 10.Morita M. T. 2010. Directional gravity sensing in gravitropism. Annu. Rev. Plant Biol. 61, 705–720 10.1146/annurev.arplant.043008.092042 (doi:10.1146/annurev.arplant.043008.092042) [DOI] [PubMed] [Google Scholar]
- 11.Ottenschläger I., Wolff P., Wolverton C., Bhalerao R. P., Sandberg G., Ishikawa H., Evans M., Palme K. 2003. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl Acad. Sci. USA 100, 2987–2991 10.1073/pnas.0437936100 (doi:10.1073/pnas.0437936100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi H., Miyazawa Y., Fujii N. 2009. Hormonal interactions during root tropic growth: hydrotropism versus gravitropism. Plant Mol. Biol. 69, 489–502 10.1007/s11103-008-9438-x (doi:10.1007/s11103-008-9438-x) [DOI] [PubMed] [Google Scholar]
- 13.Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. 2002. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809 10.1038/415806a (doi:10.1038/415806a) [DOI] [PubMed] [Google Scholar]
- 14.Swarup R., Kramer E. M., Perry P., Knox K., Leyser H. M., Haseloff J., Beemster G. T., Bhalerao R., Bennett M. J. 2005. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat. Cell Biol. 7, 1057–1065 10.1038/ncb1316 (doi:10.1038/ncb1316) [DOI] [PubMed] [Google Scholar]
- 15.Ransom J., Moore R. 1983. Geoperception in primary and lateral roots of Phaseolus vulgaris (Fabaceae). I. Structure of columella cells. Am. J. Bot. 70, 1048–1056 [PubMed] [Google Scholar]
- 16.Mullen J. L., Hangarter R. P. 2003. Genetic analysis of the gravitropic set-point angle in lateral roots of Arabidopsis. Adv. Space. Res. 31, 2229–2236 10.1016/S0273-1177(03)00249-7 (doi:10.1016/S0273-1177(03)00249-7) [DOI] [PubMed] [Google Scholar]
- 17.Péret B., De Rybel B., Casimiro I., Benková E., Swarup R., Laplaze L., Beeckman T., Bennett M. J. 2009. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14, 399–408 10.1016/j.tplants.2009.05.002 (doi:10.1016/j.tplants.2009.05.002) [DOI] [PubMed] [Google Scholar]
- 18.Kiss J. Z., Miller K. M., Ogden L. A., Roth K. K. 2002. Phototropism and gravitropism in lateral roots of Arabidopsis. Plant Cell Physiol. 43, 35–43 10.1093/pcp/pcf017 (doi:10.1093/pcp/pcf017) [DOI] [PubMed] [Google Scholar]
- 19.Stoker R., Moore R. 1984. Structure of columella cells in primary and lateral roots of Helianthus annuus (Compositae). New Phytol. 97, 205–212 10.1111/j.1469-8137.1984.tb04123.x (doi:10.1111/j.1469-8137.1984.tb04123.x) [DOI] [Google Scholar]
- 20.Wang H., Lockwood S. K., Hoeltzel M. F., Schiefelbein J. W. 1997. The ROOT HAIR DEFECTIVE3 gene encodes an evolutionarily conserved protein with GTP-binding motifs and is required for regulated cell enlargement in Arabidopsis. Genes Dev. 11, 799–811 10.1101/gad.11.6.799 (doi:10.1101/gad.11.6.799) [DOI] [PubMed] [Google Scholar]
- 21.Chen J., Stefano G., Brandizzi F., Zheng H. 2011. Arabidopsis RHD3 mediates the generation of the tubular ER network and is required for Golgi distribution and motility in plant cells. J. Cell Sci. 124, 2241–2252 10.1242/jcs.084624 (doi:10.1242/jcs.084624) [DOI] [PubMed] [Google Scholar]
- 22.Lucas M., Godin C., Jay-Allemand C., Laplaze L. 2008. Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. J. Exp. Bot. 59, 55–66 10.1093/jxb/erm171 (doi:10.1093/jxb/erm171) [DOI] [PubMed] [Google Scholar]
- 23.Laplaze L., et al. 2005. GAL4-GFP enhancer trap lines for genetic manipulation of lateral root development in Arabidopsis thaliana. J. Exp. Bot. 56, 2433–2442 10.1093/jxb/eri236 (doi:10.1093/jxb/eri236) [DOI] [PubMed] [Google Scholar]
- 24.Willemsen V., Wolkenfelt H., de Vrieze G., Weisbeek P., Scheres B. 1998. The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development. 125, 521–531 [DOI] [PubMed] [Google Scholar]
- 25.Stanga J., Baldwin K., Masson P. H. 2009. Joining forces: the interface of gravitropism and plastid protein import. Plant Signal. Behav. 4, 933–941 10.4161/psb.4.10.9470 (doi:10.4161/psb.4.10.9470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imlau A., Truernit E., Sauer N. 1999. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 11, 309–322 10.1105/tpc.11.3.309 (doi:10.1105/tpc.11.3.309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peer W. A., Blakeslee J. J., Yang H., Murphy A. S. 2011. Seven things we think we know about auxin transport. Mol. Plant 4, 487–504 10.1093/mp/ssr034 (doi:10.1093/mp/ssr034) [DOI] [PubMed] [Google Scholar]
- 28.Lucas M., et al. 2011. Short-root regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiol. 155, 384–398 10.1104/pp.110.165126 (doi:10.1104/pp.110.165126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 10.1016/S0092-8674(03)00924-3 (doi:10.1016/S0092-8674(03)00924-3) [DOI] [PubMed] [Google Scholar]
- 30.Vieten A., Vanneste S., Wisniewska J., Benková E., Benjamins R., Beeckman T., Luschnig C., Friml J. 2005. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132, 4521–4531 10.1242/dev.02027 (doi:10.1242/dev.02027) [DOI] [PubMed] [Google Scholar]
- 31.Blilou I., et al. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44 10.1038/nature03184 (doi:10.1038/nature03184) [DOI] [PubMed] [Google Scholar]
- 32.Palme K., Dovzhenko A., Ditengou F. A. 2006. Auxin transport and gravitational research: perspectives. Protoplasma 229, 175–181 10.1007/s00709-006-0216-9 (doi:10.1007/s00709-006-0216-9) [DOI] [PubMed] [Google Scholar]
- 33.Rubio G., Walk T., Ge Z., Yan X., Liao H., Lynch J. 2001. Root gravitropism and below-ground competition among neighbouring plants: a modelling approach. Ann. Bot. 88, 929–940 10.1006/anbo.2001.1530 (doi:10.1006/anbo.2001.1530) [DOI] [Google Scholar]
- 34.Ma Z., Hasenstein K. H. 2005. The onset of gravisensitivity in the embryonic root of flax. Plant Physiol. 140, 159–166 10.1104/pp.105.073296 (doi:10.1104/pp.105.073296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullen J. L., Wolverton C., Hangarter R. P. 2005. Apical control, gravitropic signalling, and the growth of lateral roots in Arabidopsis. Adv. Space. Res. 36, 1211–1217 10.1016/j.asr.2005.03.103 (doi:10.1016/j.asr.2005.03.103) [DOI] [Google Scholar]