Abstract
In Arabidopsis thaliana, lateral root (LR) formation is regulated by multiple auxin/indole-3-acetic acid (Aux/IAA)–AUXIN RESPONSE FACTOR (ARF) modules: (i) the IAA28–ARFs module regulates LR founder cell specification; (ii) the SOLITARY-ROOT (SLR)/IAA14–ARF7–ARF19 module regulates nuclear migration and asymmetric cell divisions of the LR founder cells for LR initiation; and (iii) the BODENLOS/IAA12–MONOPTEROS/ARF5 module also regulates LR initiation and organogenesis. The number of Aux/IAA–ARF modules involved in LR formation remains unknown. In this study, we isolated the shy2-101 mutant, a gain-of-function allele of short hypocotyl2/suppressor of hy2 (shy2)/iaa3 in the Columbia accession. We demonstrated that the shy2-101 mutation not only strongly inhibits LR primordium development and emergence but also significantly increases the number of LR initiation sites with the activation of LATERAL ORGAN BOUNDARIES-DOMAIN16/ASYMMETRIC LEAVES2-LIKE18, a target gene of the SLR/IAA14–ARF7–ARF19 module. Genetic analysis revealed that enhanced LR initiation in shy2-101 depended on the SLR/IAA14–ARF7–ARF19 module. We also showed that the shy2 roots contain higher levels of endogenous IAA. These observations indicate that the SHY2/IAA3–ARF-signalling module regulates not only LR primordium development and emergence after SLR/IAA14–ARF7–ARF19 module-dependent LR initiation but also inhibits LR initiation by affecting auxin homeostasis, suggesting that multiple Aux/IAA–ARF modules cooperatively regulate the developmental steps during LR formation.
Keywords: auxin, lateral root formation, SHY2/IAA3, Arabidopsis
1. Introduction
In vascular plants, branched root systems arise through the production of lateral and adventitious roots, thereby allowing the plants to absorb water and nutrients from the soil and to sustain the aerial shoots [1]. In most eudicot plants, lateral roots (LRs) initiate from asymmetric, anticlinal cell divisions in the xylem pole pericycle cells of the parental roots [1,2]. These divided pericycle cells undergo periclinal cell divisions to produce a young LR primordium. Subsequent cell divisions and cell differentiation lead to the establishment of a mature LR primordium with a root apical meristem. Finally, the LR emerges through the outer cortical and epidermal cell layers of parental roots [3,4]. Recent molecular genetic and physiological studies in several model plants have shown that LR formation is dependent on auxin [4–6]. In Arabidopsis, auxin regulates most of the developmental steps during LR formation; LR founder cell specification, LR initiation, LR primordium development and LR emergence [4–9].
In plant cells, auxin regulates transcription of many auxin-responsive genes dependent on auxin/indole-3-acetic acid (Aux/IAA)–AUXIN RESPONSE FACTOR (ARF) auxin-signalling modules [10]. In Arabidopsis, there are 22 functional ARFs and 29 Aux/IAA proteins [11,12]. ARFs directly activate or repress the transcription of their target genes that contain AuxREs, auxin responsive elements in the promoter. In the absence of auxin, the Aux/IAA protein interacts with its partner ARF, thereby inactivating ARF activity. In the presence of auxin, the Aux/IAA protein is degraded through ubiquitination by the SCFTIR1/AFBs E3 ubiquitin ligase complex that contains the auxin receptor TIR1/AFBs [13–16]. Activated ARFs positively or negatively regulate target genes, resulting in ARF-dependent auxin responses. Gain-of-function mutations in domain II of Aux/IAAs stabilize the protein even in the presence of auxin, thereby constitutively inactivating ARF activity as well as affecting auxin-mediated growth and development [12,17]. ARFs and Aux/IAAs have distinct and overlapping functions in plant growth and development [18–23]. Particularly, molecular genetic studies with mutants defective in LR formation have shown that several Aux/IAA–ARF modules play important roles in the developmental steps during LR formation. For example (i) the IAA28–ARFs module regulates the LR founder cell specification in the root basal meristem [7]; (ii) the SOLITARY-ROOT (SLR)/IAA14–ARF7–ARF19 module regulates nuclear migration and asymmetric cell divisions of LR founder cells for LR initiation [9,18,24,25]; and (iii) the BODENLOS (BDL)/IAA12-MONOPTEROS (MP)/ARF5 module also participates in LR initiation after the SLR/IAA14–ARF7–ARF19 module acts [10,26]. Among these auxin-signalling modules, the SLR/IAA14–ARF7–ARF19 module regulates LR initiation by activating several auxin-responsive genes [9,18,20,24,25,27]. The gain-of-function slr-1 mutation in the SLR/IAA14 gene blocks pericycle cell division for LR initiation, resulting in a solitary-root phenotype [9,27]. In contrast, the arf7 arf19 loss-of-function double mutant also has a few LRs, but the arf7 and arf19 single mutants do produce LRs, indicating that ARF7 and ARF19 have redundant functions for LR formation [18,20]. The SLR/IAA14, ARF7 and ARF19 genes are co-expressed in root tissues, including the pericycle, and SLR/IAA14 interacts with ARF7 and ARF19 in a yeast two-hybrid system [18,24]. These results strongly suggested that the stabilized mutant IAA14 constitutively inhibits the activity of ARF7 and 19, thereby repressing the downstream genes for LR initiation. Therefore, auxin was proposed to promote the degradation of SLR/IAA14 and the other Aux/IAAs, resulting in the activation of ARF7/19-dependent transcription of the target genes involved in LR initiation [18,20,24]. In fact, ARF7 and ARF19 were recently shown to regulate LR initiation via activating LATERAL ORGAN BOUNDARIES-DOMAIN (LBD)/ASYMMETRIC LEAVES2-LIKE (ASL) genes such as LBD16/ASL18 [25].
At present, the number of Aux/IAA–ARF modules involved in LR formation is unknown. In addition to the mutations in IAA28, SLR/IAA14 and BDL/IAA12, gain-of-function mutations in other Aux/IAA members, including AUXIN RESISTANT5 (AXR5)/IAA1, SHORT HYPOCOTYL2/SUPPRESSOR OF HY2 (SHY2)/IAA3, CRANE/IAA18, MASSUGU2 (MSG2)/IAA19 also decrease the number of LRs, indicating that auxin signalling dependent on these Aux/IAAs is necessary for LR formation [6,28]. The aux/iaa mutants do have phenotypic differences in LR formation. For example, the slr-1 mutant has no LRs [9], whereas the other mutants including axr5/iaa1, shy2/iaa3, crane/iaa18, msg2/iaa19 and iaa28 have a decreased number of LRs but retain the ability to form LRs [9,29–34]. In addition, because these aux/iaa mutants were isolated and characterized by several laboratories using different growth conditions, how their LR phenotypes differ in terms of the frequency of LR initiation, the positioning of LRs and the emergence of LRs is unknown. Furthermore, because a few of the aux/iaa mutants (shy2-2, shy2-3 and iaa28-1) are isolated in genetic backgrounds other than the Columbia (Col) accession, it is necessary to carefully characterize and compare the LR phenotype among the aux/iaa mutants isolated from these different accessions.
Previous studies on the shy2-2/iaa3 allele, isolated in the Landsberg erecta (Ler) accession, have shown that SHY2/IAA3-mediated auxin signalling is important for LR emergence because shy2-2 has a decreased number of emerged LRs and an increased number of non-emerged LR primordia. In contrast, shy2-24, a loss-of-function allele, has an increased number of emerged LRs and a decreased number of non-emerged LR primordia. These results indicate that SHY2/IAA3 negatively regulates LR emergence [30,35]. Expression analyses using the SHY2 promoter-GUS line have shown that SHY2/IAA3 is expressed in the root endodermis [35], indicating that SHY2/IAA3-mediated auxin signalling for LR emergence occurs in the endodermal tissue. However, how SHY2/IAA3-mediated auxin signalling affects LR initiation and interacts with the other Aux/IAA–ARF modules such as the SLR/IAA14–ARF7–ARF19 module during LR formation is unknown.
In this study, we isolated the shy2-101 mutant, a new gain-of-function allele of shy2/iaa3 in the Col accession background, and characterized the LR phenotype in detail. We demonstrated that the shy2-101/iaa3 mutation strongly inhibited LR primordium development and LR emergence as observed in the shy2-2 mutant in the Ler accession background, but the shy2-101/iaa3 mutation significantly increased LR initiation sites with the activation of LBD16/ASL18, a target gene of the SLR/IAA14–ARF7–ARF19 module. Genetic analysis revealed that the enhanced LR initiation in the shy2-101 mutant depended on the SLR/IAA14–ARF7–ARF19 module. In addition, we showed that the shy2 mutations strongly affect auxin homeostasis in the roots. Our results indicate the critical role of the SHY2/IAA3–ARFs module in LR formation after SLR/IAA14–ARF7–ARF19-dependent LR initiation, suggesting that multiple Aux/IAA–ARF-signalling modules cooperatively regulate the developmental steps during LR formation.
2. Material and methods
(a). Plant materials and growth conditions
Arabidopsis thaliana accessions Columbia (Col-0) and Landsberg erecta (Ler) were used in this study. The shy2-101 mutant line was isolated as a mutant with fewer LRs from ethyl methanesulphonate (EMS)-mutagenized M2 Col seeds that were purchased from LEHLE SEEDS (http://www.arabidopsis.com/). The slr-1, arf7-1, arf19-1 and pLBD16::GUS lines have been described previously [18,25]. The shy2-2 mutant seeds (Ler accession) were kindly provided by Jason W. Reed (University of North Carolina, USA) [30]. Seeds were germinated under sterile conditions on 1× Murashige–Skoog medium with 1 per cent sucrose. Plants were grown at 23°C under continuous light as described previously [9]. The number of LRs and root length were determined using a dissecting microscope and ImageJ software (NIH).
(b). Microscopy
β-Glucuronidase (GUS) staining, fixation and whole-mount clearing of roots were performed essentially as described earlier [3], and samples were observed with a Leica DM6000 microscope equipped with Nomarski optics (Leica Microsystems, Wetzlar, Germany).
(c). LC–ESI–MS/MS analysis of indole-3-acetic acid
IAA analysis was performed as described by Mashiguchi et al. [36] with slight modifications. For analysis of IAA in Arabidopsis seedlings, fresh plant tissues (30–40 mg) were homogenized in 80 per cent acetone/H2O (0.5–1 ml) containing 13C6-IAA (Cambridge Isotope Laboratories), and purified by high performance liquid chromatography as described previously [37]. The IAA fraction was redissolved in 10 per cent methanol/H2O with 1 per cent acetic acid (1 ml) and fractionated to an Oasis HLB column (1 cc; Waters). The column was washed with 20 per cent methanol/H2O with 1 per cent acetic acid (1 ml), and IAA was eluted with 70 per cent methanol/H2O with 1 per cent acetic acid (1 ml) and evaporated to dryness by using a Speed-Vac. The IAA fraction was redissolved in 1 per cent acetic acid/H2O (10–20 μl) and injected into an LC–ESI–MS/MS. MS/MS analysis conditions were as follows: capillary, 3.00 kV; source temperature, 100°C; desolvation temperature, 500°C; collision energy, 10 V; sampling cone voltage, 16 V; scan time, 0.6 s per scan (delay, 0.05 s); and MS/MS transition (m/z), 176.1/130.1 for unlabelled IAA and 182.1/136.1 for 13C6-IAA, respectively. Ultra-performance liquid chromatography conditions and quantification of IAA were the same as in previous methods [37].
3. Results
(a). The shy2-101 mutation inhibits lateral root primordium development and lateral root emergence but increases the number of lateral root founder cells
The shy2-101 mutant was screened from EMS-mutagenized Col M2 seedlings and selected based on having fewer LRs than wild-type (figure 1a). Genetic and sequence analyses showed that shy2-101 has a gain-of-function mutation in domain II of IAA3 that changes the 70th amino acid from proline to serine (P70S), thereby stabilizing the IAA3 protein in a manner similar to the shy2-2 mutation [38] (figure 1c). As observed in the other gain-of-function shy2 mutants (shy2-2, shy2-3) isolated from the Ler background [30], shy2-101 showed a dwarf shoot phenotype with curled-up leaves and an abnormal gravitropic response in the root (data not shown). Because most of the auxin-related mutants and reporter lines in Arabidopsis are produced in the Col accession, the shy2-101 allele will be useful in comparing phenotype and reporter expression with other mutants isolated in the Col background as described below.
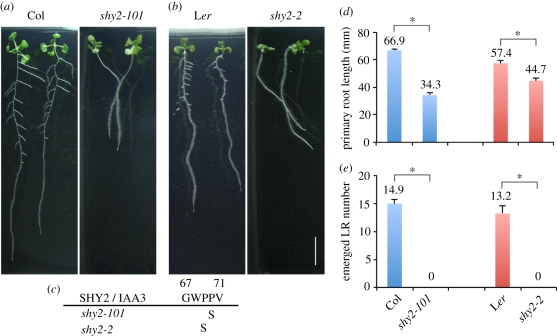
Figure 1.
Lateral root (LR) formation is inhibited by the gain-of-function shy2/iaa3 mutants. (a,b) 10-day-old seedlings of Col and shy2-101 (a), and those of Ler and shy2-2 (b). Scale bar = 10 mm. (c) Amino acid sequences of the conserved region in domain II of the SHY2/IAA3 protein. The shy2-101 mutation changes the 70th amino acid from proline to serine (P70S), whereas the shy2-2 mutation changes the 69th amino acid from proline to serine (P69S) [30]. (d,e) Primary root length (d) and emerged LR number (e) of Col, shy2-101, Ler and shy2-2 seedlings at 10 days after germination (n = 15). Error bars indicate standard error of the mean. Asterisks indicate a statistical difference (*p < 0.01 by a two-sided t-test).
First, we compared the primary root length and number of LRs in 10-day-old wild-type Col and shy2-101 seedlings grown under standard conditions (constant light, 1× MS medium). Compared with wild-type Col, the primary root length of shy2-101 seedlings was shorter (figure 1a,d). In addition, shy2-101 seedlings had no emerged LRs, whereas the wild-type seedlings had many LRs (figure 1a,e). These shy2-101 phenotypes were similar to those of the shy2-2 mutant in Ler (figure 1b,d,e); however, the degree of primary root growth inhibition was higher in shy2-101 (root growth was 51.3% of Col root length) than in the shy2-2 (root growth was 77.9% of Ler root length), suggesting an accession-specific effect on the shy2 mutant phenotype. The LR density (number of emerged LR per portion of the primary root where LRs are present) was zero in both shy2 mutant alleles, indicating that SHY2/IAA3-mediated auxin signalling is important for LR formation.
Next, we examined whether LR primordium formation occurred in the shy2-101 mutant. The 12-day-old seedlings were cleared, and non-emerged LR primordia were observed microscopically. In the Col, a few LR initiation sites were observed in the distal primary root region where asymmetric cell divisions occur (figure 2a). In contrast, the shy2-101 roots had more LR initiation sites in the same distal root region but subsequent periclinal cell divisions were inhibited (figure 2b). Interestingly, in the proximal regions of the shy2-101 primary root, many early-stage LR primordia were observed but LR emergence was significantly inhibited (figure 2c,d). These results strongly suggest that the shy2-101 mutation increases the number of LR initiation events with anticlinal cell divisions of xylem pole pericycle, but inhibits/retards subsequent LR primordium development and LR emergence. Similar LR phenotypes were also observed in shy2-2 (data not shown), confirming that SHY2/IAA3-mediated auxin signalling is important for LR primordium development and LR emergence in both Col and Ler genetic backgrounds.
Figure 2.
The shy2-101 mutation inhibits lateral root (LR) primordium development but increases the number of LR founder cells expressing pLBD16::GUS reporter. (a–d) Nomarski images of Col (a) and shy2-101 (b–d) primary roots at 12 days after germination (DAG). Three panels of shy2-101 (b–d) show the different mature root regions, respectively: distal (b), proximal (c) and more proximal regions (d). Black arrowheads indicate LR primordia. Scale bar = 100 µm. (e,f) Expression of pLBD16::GUS in the mature root region of Col (e) and shy2-101 (f) seedlings at 7 DAG. Black arrowheads indicate LR initiation sites expressing pLBD16::GUS. Scale bar = 100 µm.
(b). The shy2-101 mutation increases the number of lateral root initiation sites expressing LBD16/ASL18, a key gene for lateral root initiation
To determine whether the shy2-101 mutation increases the activity of LR initiation genes, we monitored the expression of the pLBD16::GUS reporter that was constructed in the Col background [25]. LBD16/ASL18 is one of the direct target genes activated by ARF7 and ARF19 and functions in LR formation downstream of ARF7/ARF19 [25]. In 7-day-old wild-type Col seedlings, pLBD16::GUS was specifically expressed in the LR founder cells and LR initiation sites along the xylem pole pericycle (figure 2e) [25]. This strong GUS activity was never observed in the slr-1/iaa14 mutant (Goh et al. 2011, unpublished results). Surprisingly, 7-day-old shy2-101 seedlings already had many LR initiation sites with pLBD16::GUS activity (figure 2f). These results indicate that the shy2-101 mutation significantly increases LR initiation events with the activation of LBD16, suggesting that SHY2/IAA3-mediated auxin signalling is necessary for inhibiting LR initiation in the pericycle cells.
The shy2-101 mutation also inhibited LR primordium development and LR emergence after LR initiation in 12-day-old seedlings (figure 2), whereas at 15 days post-germination the shy2-101 mutation often caused ‘clustered LRs’, where numerous LRs emerged from a limited region of the primary root (figure 3a). We hypothesize that these clustered LRs are already initiated at a younger age and eventually develop LR primordia and emerge at an older age.
Figure 3.
Genetic interactions between shy2-101 and slr-1, arf7-1 and arf19-1. (a) 15-day-old seedlings of shy2-101, shy2-101 slr-1, and shy2-101 arf7-1 arf19-1 mutants. Scale bar = 10 mm. (b) Nomarski images of roots from shy2-101, shy2-101 slr-1, and shy2-101 arf7-1 arf19-1 mutants at 9 days after germination. Enhanced lateral root (LR) initiation in shy2-101 is blocked by the slr-1 mutation or the arf7 arf19 double mutation. Blue arrowheads indicate LR initiation sites, and black arrowheads show pericycle cell layers adjacent to the xylem pole. Scale bar = 100 µm.
(c). Enhanced lateral root initiation in shy2-101 is dependent on activation of the SLR/IAA14–ARF7–ARF19 auxin-signalling module
The SLR/IAA14–ARF7–ARF19 auxin-signalling module is necessary for LR initiation [9,18,20,24,25]. To determine whether enhanced LR initiation in shy2-101 depends on the SLR/IAA14–ARF7–ARF19 auxin-signalling module, we constructed the shy2-101 slr-1 double and shy2-101 arf7-1 arf19-1 triple mutants in the Col background, and analysed their LR phenotype. As shown in figure 3, the shy2-101 slr-1 double-mutant seedlings had no LR initiation sites, resulting in the slr-1-like LR phenotype in 15-day-old seedlings (figure 3a,b). These observations indicate that the slr-1 mutation blocks the enhanced LR initiation in shy2-101. Similarly, the 15-day-old shy2-101 arf7-1 arf19-1 triple-mutant seedlings had no LRs, whereas the shy2-101 seedlings had clustered LRs (figure 3a). There were no LR initiation sites in either shy2-101 slr-1 or shy2-101 arf7-1 arf19-1 primary roots (figure 3b). These results also indicate that the enhanced LR initiation in the shy2-101 mutant is dependent on activation of the SLR/IAA14–ARF7–ARF19 signalling module, suggesting that the shy2-101 mutation acts on LR formation downstream of SLR/IAA14–ARF7–ARF19 module-dependent LR initiation.
(d). The shy2 mutations strongly elevate endogenous indole-3-acetic acid level in the roots
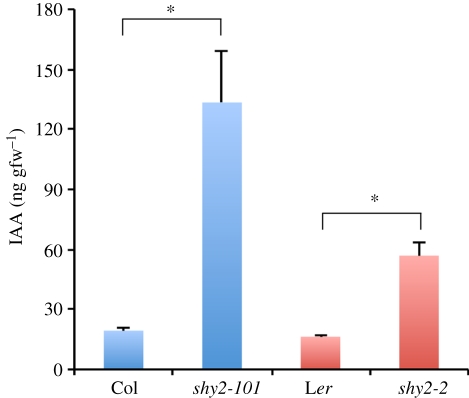
Our genetic evidence that enhanced LR initiation in the shy2-101 mutant depends on the SLR/IAA14–ARF7–ARF19 auxin-signalling module suggests the possibility that the shy2 mutant might contain higher auxin levels in the roots, thereby increasing the number of LR initiation sites. To clarify this point, we measured endogenous IAA levels in the roots of 7-day-old wild-type and shy2 seedlings by LC–ESI–MS/MS analysis (see §2). As shown in figure 4, both the shy2-101 (in Col background) and shy2-2 (in Ler background) mutants contained a higher level of endogenous IAA in the roots, compared with their corresponding wild-types. Particularly, the shy2-101 roots had a much higher IAA level than shy2-2, suggesting differences in the genetic background between the Ler and Col accessions (figure 4). These results indicate that the shy2 gain-of-function mutations increased endogenous auxin levels in roots, strongly suggesting that elevated auxin levels promoted LR initiation and resulted in the increased number of LR initiation sites in the shy2-101 mutant.
Figure 4.
Indole-3-acetic acid (IAA) levels in wild-type and shy2 mutant plants. IAA levels (ng gfw−1) in the roots of the 7-day-old wild-type (Col and Ler) and shy2 (shy2-101 and shy2-2) mutant seedlings are shown (n = 4). Error bars indicate standard error of the mean. Asterisks indicate a statistical difference (*p < 0.01 by a two-sided t-test). Experiments were repeated twice, and similar values were obtained in each experiment.
4. Discussion
In this study, we demonstrated that the shy2-101 mutation, a newly isolated gain-of-function mutant of shy2/iaa3 in the Col genetic background, strongly inhibits LR primordium development and LR emergence as well as also significantly increases LR initiation events with the activation of LBD16/ASL18, a key gene for LR initiation. Previous studies have reported that the shy2-2 mutant, isolated in the Ler background, also has a decreased number of emerged LRs and an increased number of non-emerged LR primordia, whereas shy2-24, a loss-of-function allele, has an increased number of emerged LRs and a decreased number of non-emerged LR primordia [35]. We observed that the shy2-101 mutation in the Col background also inhibits LR primordium development and LR emergence, showing a LR phenotype similar to that of shy2-2 in spite of their being in different genetic backgrounds. These results indicate that the SHY2/IAA3–ARFs module regulates LR primordium development and LR emergence (figure 5). Previous expression analyses have shown that SHY2/IAA3 is expressed in the root endodermis, where LAX3, an auxin influx carrier required for LR emergence, is also co-expressed [35]. A model was proposed in which auxin originating from the dividing xylem pole pericycle cells induces cell wall-remodelling gene expression in the adjacent endodermal cells by targeting the degradation of SHY2/IAA3, allowing the LR primordium to emerge through the outer cortex and epidermis by inducing LAX3 in the cortex and epidermis [35]. As the shy2-2 mutation is also thought to inhibit the SHY2/IAA3-mediated induction of cell wall-remodelling genes in the endodermis [35], it is possible that cell wall remodelling in the adjacent endodermal cells may produce a signal for the early LR primordium to stimulate subsequent LR primordium development and LR emergence. The shy2 mutation inhibits the expression of many kinds of genes [39], suggesting that the SHY2/IAA3–ARFs module positively regulates the genes necessary for LR primordium development and LR emergence (figure 5).
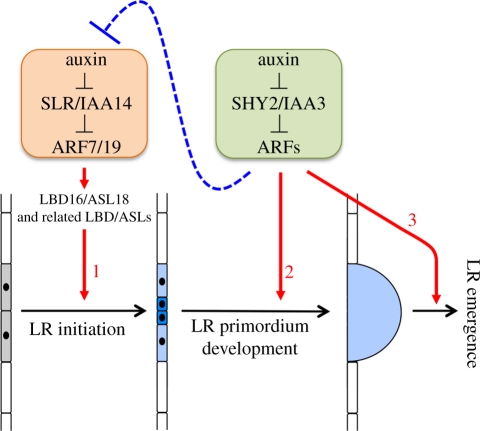
Figure 5.
Schematic of lateral root (LR) formation regulated by SLR/IAA14–ARF7–ARF19 and SHY2/IAA3–ARFs auxin-signalling modules. LR initiation is controlled by the SLR/IAA14–ARF7–ARF19 auxin-signalling module (orange box) by the activation of LBD16/ASL18 and its related LBD/ASL proteins (red arrow 1). After initiation, the SHY2/IAA3–ARFs signalling module (green box) plays a role not only for LR primordium development and LR emergence after the SLR/IAA14–ARF7–ARF19 module (red arrows 2 and 3) but also for inhibition of SLR/IAA14–ARF7–ARF19-dependent LR initiation in the xylem pericycle cells by affecting auxin homeostasis (blue dotted line).
In addition to the inhibition of LR primordium development and emergence, the shy2-101 mutation significantly increased the number of LR initiation sites expressing pLBD16::GUS (figure 2), suggesting that the shy2-101 mutation enhanced the auxin response in the xylem pole pericycle, thereby increasing the number of LR founder cells. As hypothesized by Swarup et al. [35], shoot-derived auxin may hyper-accumulate in the root pericycle of the shy2-101 mutant, resulting in more LR initiation sites compared with the wild-type. In fact, we demonstrated that the shy2-101 and shy2-2 mutants contained higher levels of endogenous IAA in the roots (figure 4), strongly suggesting that the elevated auxin promoted LR initiation and resulted in the increased number of LR initiation sites in the shy2-101 mutant. The exact mechanism for SHY2/IAA3-mediated auxin homeostasis is unknown, but the shy2-2 mutation reduces the expression of several GH3 genes (GH3-3 and GH3-5) that are involved in the control of auxin homeostasis [39,40], suggesting that normal SHY2/IAA3-mediated auxin signalling negatively controls the number of LR initiation sites by decreasing the free IAA level in the roots. Alternatively, the possibility still remains that a newly initiated young LR primordium, or LR primordium development itself may act as a repressor of LR initiation in the adjacent pericycle cells, but the shy2-101 mutation may block such lateral inhibition of LRs. Transgenic plants expressing the stabilized bdl/iaa12 protein under the regulation of the BDL/IAA12 promoter, which is active in dividing pericycle cells, had clustered roots as a result of ectopic pericycle cell divisions during LR initiation as observed in the weak mp/arf5 mutant allele [10]. However, the shy2-101 phenotype is different from that of the bdl/iaa12-expressing plants because the shy2-101 mutation did not cause an aberrant pericycle cell division pattern during LR initiation (figure 2). Considering that SHY2/IAA3 is expressed in the endodermis [35], our results suggest that the SHY2/IAA3–ARFs module regulates the inhibition of SLR/IAA14–ARF7–ARF19-dependent LR initiation indirectly by affecting auxin homeostasis in the roots (figure 5).
We also observed phenotypic differences in both primary root growth and endogenous IAA levels between the shy2-2 (in Ler background) and shy2-101 (in Col background) mutants that may be due to differences in the genetic background between the Ler and Col accessions. We hypothesize that the enhanced shy2 inhibitory effect on primary root growth in the Col background might be due to the increased IAA level in the shy2-101 (in Col background) roots. Such accession-dependent effects on mutant phenotypes are also observed in the crane/iaa18 mutants; the iaa18-1 allele in the Ler background had defects in embryonic patterning, whereas the crane-2/iaa18 allele in the Col background had almost no effect on embryonic patterning [33,34]. Recent studies using the shy2-2 mutant have shown that primary root growth of Arabidopsis is regulated by complicated cross-talk among plant hormones, including auxin, cytokinin and gibberellin, whereas SHY2/IAA3 plays a key role for cell differentiation and division balance necessary for controlling root meristem size and root growth [41,42]. Thus, the shy2-101 allele in the Col background may be helpful for studying accession-dependent effects on root growth regulation.
SHY2/IAA3 has been hypothesized to pair with ARF7 and ARF19 for the auxin response in the root gravitropic response, because both the arf7 arf19 and shy2 mutants are defective in the root gravitropic response [43]. In addition, SHY2/IAA3 interacts with ARF19 in a yeast two-hybrid system [43]. However, because the LR phenotype of the shy2 mutants is different from that of the arf7 arf19 mutant in which LR initiation is inhibited [18,25], it is unclear whether SHY2/IAA3 forms pairs with ARF7 and ARF19 during LR formation. Because SHY2/IAA3 is expressed in the root endodermis [35], the corresponding ARFs should also be expressed in the endodermis. ARF19 is ubiquitously expressed in the root [18], suggesting the possibility that the SHY2/IAA3–ARF19 module might regulate LR formation.
5. Conclusions and outlook
Recent molecular genetic studies of the gain-of-function mutants in the Aux/IAA family and loss-of-function mutants in ARFs have revealed that the developmental events during LR formation are regulated by multiple Aux/IAA–ARF auxin-signalling modules. Before LR initiation, the LR founder cell specification occurs in the root basal meristem, which is regulated by the IAA28–ARFs module [7]. Then, nuclear migration and asymmetric cell divisions of the LR founder cells lead to LR initiation that depends on the SLR/IAA14–ARF7–ARF19 module [18,24,25,44]. In addition, the BDL/IAA12-MP/ARF5 module also regulates LR initiation and LR organogenesis by repressing ectopic pericycle cell divisions [10]. In addition to these three modules, the shy2-101/iaa3 LR phenotype strongly suggests that a fourth auxin-signalling module, SHY2/IAA3–ARFs, plays a role not only for LR primordium development and LR emergence but also for inhibition of LR initiation through affecting auxin homeostasis (figure 5). Identification of the corresponding ARFs for SHY2/IAA3-mediated signalling in LR formation and further study of the downstream genes will reveal the SHY2/IAA3–ARFs module-dependent molecular cascade that regulates LR initiation, LR primordium development and LR emergence. Furthermore, it will be important to determine the roles of the other Aux/IAA–ARF modules in LR formation, thereby contributing to our understanding of the mechanism that regulates plant root formation by multiple Aux/IAA–ARF auxin-signalling modules.
Acknowledgements
We wish to thank Jason W. Reed for providing shy2-2 mutant seeds. We also thank Masao Tasaka for supporting the mutant screening. We thank Yoshie Okamoto and Aya Ide for technical assistance. This research was supported by grants-in-aid for Scientific Research on Priority Areas to H.F. (no. 19060006) from The Ministry of Education, Culture, Sports, Science and Technology (Japan), and the strategic programmes for R&D (President's discretionary fund) of RIKEN to H.K.
References
- 1.Charlton W. A. 1996. Lateral root initiation. In Plant roots: the hidden half, 2nd edn. (eds Waisel Y., Eshel A., Kafkafi U.), pp. 149–173. New York, NY: Marcel Dekker Inc. [Google Scholar]
- 2.Barlow P. W., Volkmann D., Baluška F. 2004. Polarity in roots. In Polarity in plants (ed. Lindsey K.), pp. 192–241 Oxford, UK: Blackwell Publishing Ltd [Google Scholar]
- 3.Malamy J. E., Benfey P. N. 1997. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44 [DOI] [PubMed] [Google Scholar]
- 4.Péret B., De Rybel B., Casimiro I., Benková E., Swarup R., Laplaze L., Beeckman T., Bennett M. J. 2009. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14, 399–408 10.1016/j.tplants.2009.05.002 (doi:10.1016/j.tplants.2009.05.002) [DOI] [PubMed] [Google Scholar]
- 5.Fukaki H., Okushima Y., Tasaka M. 2007. Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 256, 111–137 10.1016/S0074-7696(07)56004-3 (doi:10.1016/S0074-7696(07)56004-3) [DOI] [PubMed] [Google Scholar]
- 6.Fukaki H., Tasaka M. 2009. Hormone interactions during lateral root formation. Plant Mol. Biol. 69, 437–449 10.1007/s11103-008-9417-2 (doi:10.1007/s11103-008-9417-2) [DOI] [PubMed] [Google Scholar]
- 7.De Rybel B., et al. 2010. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 20, 1697–1706 10.1016/j.cub.2010.09.007 (doi:10.1016/j.cub.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 8.De Smet I., et al. 2007. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134, 681–690 10.1242/dev.02753 (doi:10.1242/dev.02753) [DOI] [PubMed] [Google Scholar]
- 9.Fukaki H., Tameda S., Masuda H., Tasaka M. 2002. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 29, 153–168 10.1046/j.0960-7412.2001.01201.x (doi:10.1046/j.0960-7412.2001.01201.x) [DOI] [PubMed] [Google Scholar]
- 10.De Smet I., et al. 2010. Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl Acad. Sci. USA 107, 2705–2710 10.1073/pnas.0915001107 (doi:10.1073/pnas.0915001107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remington D. L., Vision T. J., Guilfoyle T. J., Reed J. W. 2004. Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol. 135, 1738–1752 10.1104/pp.104.039669 (doi:10.1104/pp.104.039669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilfoyle T. J., Hagen G. 2007. Auxin response factors. Curr. Opin. Plant Biol. 10, 453–460 10.1016/j.pbi.2007.08.014 (doi:10.1016/j.pbi.2007.08.014) [DOI] [PubMed] [Google Scholar]
- 13.Dharmasiri N., Dharmasiri S., Estelle M. 2005. The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 10.1038/nature03543 (doi:10.1038/nature03543) [DOI] [PubMed] [Google Scholar]
- 14.Kepinski S., Leyser O. 2005. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446–451 10.1038/nature03542 (doi:10.1038/nature03542) [DOI] [PubMed] [Google Scholar]
- 15.Mockaitis K., Estelle M. 2008. Auxin receptors and plant development: a new signaling paradigm. Annu. Rev. Cell Dev. Biol. 24, 55–80 10.1146/annurev.cellbio.23.090506.123214 (doi:10.1146/annurev.cellbio.23.090506.123214) [DOI] [PubMed] [Google Scholar]
- 16.Dharmasiri N., Dharmasiri S., Weijers D., Lechner E., Yamada M., Hobbie L., Ehrismann J. S., Jurgens G., Estelle M. 2005. Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 9, 109–119 10.1016/j.devcel.2005.05.014 (doi:10.1016/j.devcel.2005.05.014) [DOI] [PubMed] [Google Scholar]
- 17.Reed J. W. 2001. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 6, 420–425 10.1016/S1360-1385(01)02042-8 (doi:10.1016/S1360-1385(01)02042-8) [DOI] [PubMed] [Google Scholar]
- 18.Okushima Y., et al. 2005. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444–463 10.1105/tpc.104.028316 (doi:10.1105/tpc.104.028316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overvoorde P. J., et al. 2005. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 17, 3282–3300 10.1105/tpc.105.036723 (doi:10.1105/tpc.105.036723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilmoth J. C., Wang S., Tiwari S. B., Joshi A. D., Hagen G., Guilfoyle T. J., Alonso J. M., Ecker J. R., Reed J. W. 2005. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 43, 118–130 10.1111/j.1365-313X.2005.02432.x (doi:10.1111/j.1365-313X.2005.02432.x) [DOI] [PubMed] [Google Scholar]
- 21.Hardtke C. S., Ckurshumova W., Vidaurre D. P., Singh S. A., Stamatiou G., Tiwari S. B., Hagen G., Guilfoyle T. J., Berleth T. 2004. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development 131, 1089–1100 10.1242/dev.00925 (doi:10.1242/dev.00925) [DOI] [PubMed] [Google Scholar]
- 22.Ellis C. M., Nagpal P., Young J. C., Hagen G., Guilfoyle T. J., Reed J. W. 2005. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132, 4563–4574 10.1242/dev.02012 (doi:10.1242/dev.02012) [DOI] [PubMed] [Google Scholar]
- 23.Nagpal P., et al. 2005. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132, 4107–4118 10.1242/dev.01955 (doi:10.1242/dev.01955) [DOI] [PubMed] [Google Scholar]
- 24.Fukaki H., Nakao Y., Okushima Y., Theologis A., Tasaka M. 2005. Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J. 44, 382–395 10.1111/j.1365-313X.2005.02537.x (doi:10.1111/j.1365-313X.2005.02537.x) [DOI] [PubMed] [Google Scholar]
- 25.Okushima Y., Fukaki H., Onoda M., Theologis A., Tasaka M. 2007. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19, 118–130 10.1105/tpc.106.047761 (doi:10.1105/tpc.106.047761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Smet I. 2010. Multimodular auxin response controls lateral root development in Arabidopsis. Plant Signal. Behav. 5, 580–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanneste S., et al. 2005. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17, 3035–3050 10.1105/tpc.105.035493 (doi:10.1105/tpc.105.035493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overvoorde P., Fukaki H., Beeckman T. 2010. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2, a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X., et al. 2004. The IAA1 protein is encoded by AXR5 and is a substrate of SCFTIR1. Plant J. 40, 772–782 10.1111/j.1365-313X.2004.02254.x (doi:10.1111/j.1365-313X.2004.02254.x) [DOI] [PubMed] [Google Scholar]
- 30.Tian Q., Reed J. W. 1999. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126, 711–721 [DOI] [PubMed] [Google Scholar]
- 31.Tatematsu K., Kumagai S., Muto H., Sato A., Watahiki M. K., Harper R. M., Liscum E., Yamamoto K. T. 2004. MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16, 379–393 10.1105/tpc.018630 (doi:10.1105/tpc.018630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogg L. E., Lasswell J., Bartel B. 2001. A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 13, 465–480 10.1105/tpc.13.3.465 (doi:10.1105/tpc.13.3.465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ploense S. E., Wu M. F., Nagpal P., Reed J. W. 2009. A gain-of-function mutation in IAA18 alters Arabidopsis embryonic apical patterning. Development 136, 1509–1517 10.1242/dev.025932 (doi:10.1242/dev.025932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uehara T., Okushima Y., Mimura T., Tasaka M., Fukaki H. 2008. Domain II mutations in CRANE/IAA18 suppress lateral root formation and affect shoot development in Arabidopsis thaliana. Plant Cell Physiol. 49, 1025–1038 10.1093/pcp/pcn079 (doi:10.1093/pcp/pcn079) [DOI] [PubMed] [Google Scholar]
- 35.Swarup K., et al. 2008. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10, 946–954 10.1038/ncb1754 (doi:10.1038/ncb1754) [DOI] [PubMed] [Google Scholar]
- 36.Mashiguchi K., et al. 2011. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 18 512–18 517 10.1073/pnas.1108434108 (doi:10.1073/pnas.1108434108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugawara S., Hishiyama S., Jikumaru Y., Hanada A., Nishimura T., Koshiba T., Zhao Y., Kamiya Y., Kasahara H. 2009. Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc. Natl Acad. Sci. USA 106, 5430–5435 10.1073/pnas.0811226106 (doi:10.1073/pnas.0811226106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian Q., Nagpal P., Reed J. W. 2003. Regulation of Arabidopsis SHY2/IAA3 protein turnover. Plant J. 36, 643–651 10.1046/j.1365-313X.2003.01909.x (doi:10.1046/j.1365-313X.2003.01909.x) [DOI] [PubMed] [Google Scholar]
- 39.Tian Q., Uhlir N. J., Reed J. W. 2002. Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 14, 301–319 10.1105/tpc.010283 (doi:10.1105/tpc.010283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staswick P. E., Serban B., Rowe M., Tiryaki I., Maldonado M. T., Maldonado M. C., Suza W. 2005. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17, 616–627 10.1105/tpc.104.026690 (doi:10.1105/tpc.104.026690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dello Ioio R., Nakamura K., Moubayidin L., Perilli S., Taniguchi M., Morita M. T., Aoyama T., Costantino P., Sabatini S. 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322, 1380–1384 10.1126/science.1164147 (doi:10.1126/science.1164147) [DOI] [PubMed] [Google Scholar]
- 42.Moubayidin L., Perilli S., Dello Ioio R., Di Mambro R., Costantino P., Sabatini S. 2010. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 20, 1138–1143 10.1016/j.cub.2010.05.035 (doi:10.1016/j.cub.2010.05.035) [DOI] [PubMed] [Google Scholar]
- 43.Weijers D., Benková E., Jager K. E., Schlereth A., Hamann T., Kientz M., Wilmoth J. C., Reed J. W., Jürgens G. 2005. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J. 24, 1874–1885 10.1038/sj.emboj.7600659 (doi:10.1038/sj.emboj.7600659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goh T., Joi S., Mimura T., Fukaki H. 2012. The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139, 883–893 10.1242/dev.071928 (doi:10.1242/dev.071928) [DOI] [PubMed] [Google Scholar]