Abstract
Rootless concerning crown and seminal roots (Rtcs) encodes a LATERAL ORGAN BOUNDARIES domain (LBD) protein that regulates shoot-borne root initiation in maize (Zea mays L.). GREEN FLUORESCENT PROTEIN (GFP)-fusions revealed RTCS localization in the nucleus while its paralogue RTCS-LIKE (RTCL) was detected in the nucleus and cytoplasm probably owing to an amino acid exchange in a nuclear localization signal. Moreover, enzyme-linked immunosorbent assay (ELISA) experiments demonstrated that RTCS primarily binds to LBD DNA motifs. RTCS binding to an LBD motif in the promoter of the auxin response factor (ARF) ZmArf34 and reciprocally, reciprocal ZmARF34 binding to an auxin responsive element motif in the promoter of Rtcs was shown by electrophoretic mobility shift assay experiments. In addition, comparative qRT-PCR of wild-type versus rtcs coleoptilar nodes suggested RTCS-dependent activation of ZmArf34 expression. Consistently, luciferase reporter assays illustrated the capacity of RTCS, RTCL and ZmARF34 to activate downstream gene expression. Finally, RTCL homo- and RTCS/RTCL hetero-interaction were demonstrated in yeast-two-hybrid and bimolecular fluorescence complementation experiments, suggesting a role of these complexes in downstream gene regulation. In summary, the data provide novel insights into the molecular interactions resulting in crown root initiation in maize.
Keywords: crown root, LOB, maize, RTCL, RTCS, shoot-borne roots
1. Introduction
Maize (Zea mays) root system architecture is complex and involves several root types formed during embryogenesis or postembryonically to secure water and nutrient uptake and provide mechanical stability [1]. The embryonic phase of root development is defined by the primary root and a variable number of seminal roots [2]. Postembryonically, crown roots are initiated from consecutive shoot nodes below the soil level, whereas brace roots are formed from above-ground shoot nodes [3]. The dense crown root system makes up the major backbone of the adult maize root stock system, conferring lodging resistance [4], and is important for grain yield [2].
The maize mutant rootless concerning crown and seminal roots (rtcs) is impaired in the initiation of embryonic seminal and all postembryonic shoot-borne roots [5]. Hence, the root system of the mutant rtcs consists only of the primary root with its lateral roots. The primary root displays auxin-related defects such as a reduced gravitropic response [6]. However, the root initiation defect cannot be rescued by exogenous application of auxin [5]. Map-based cloning of the Rtcs gene revealed that it encodes for a member of the plant-specific LATERAL ORGAN BOUNDARIES (LOB) domain (LBD) protein family [6]. In line with its mutant phenotype, expression of Rtcs was detected in emerging crown root primordia and is auxin-inducible [6]. Coincidently, Rtcs is not expressed in lateral roots and the mutant rtcs is not affected in lateral root formation.
Proteins encoded by LBD genes display an N-terminal-conserved LOB domain that comprises a C-motif probably responsible for DNA binding, a conserved glycine residue and a putative leucine zipper-like oligomerization domain [7,8]. On the basis of this structure, LOB domain proteins are suggested to act as transcription factors [9]. AtLOB, one of the founder proteins of the LBD family, was demonstrated to bind to the LBD motif 5′ GCGGCG 3′, and its activity is post-translationally repressed by interaction with a bHLH protein [9].
In Arabidopsis and maize, the LBD gene family consists of 43 members [8], whereas in rice (Oryza sativa) 35 members have been reported [10]. The LBD gene family can be divided into two classes according to the structure of their leucine zipper-like motif [7]. Proteins with a complete leucine zipper-like motif belong to class I, whereas class II LOB domain proteins are characterized by an incomplete leucine zipper indicating divergent functions of these protein classes [7]. Class I LOB domain proteins are typically expressed at the base of lateral organs and are involved in the lateral organ formation [11–13]. Rtcs and its paralogue Rtcl are class I LOB domain proteins [6]. Maize Rtcs, its rice orthologue CRL1/ARL1 [11,13] and its closest Arabidopsis thaliana relative LBD29 [14] are involved in different aspects of root development such as shoot-borne root formation in maize and rice and lateral root development in Arabidopsis. Moreover, Arabidopsis LBD18, in conjunction with LBD16, plays a role in the initiation and emergence of lateral roots [15]. Other class I LOB domain genes affect adaxial–abaxial patterning [16–19], proximal–distal patterning [20], embryo development [21], tracheary element differentiation [22], inflorescence development [23] and nutrient metabolism [24]. Several class I LOB domain proteins such as AS2 or JLO influence the expression of KNOX genes, thereby regulating the separation of lateral organs from the surrounding tissue and patterning of the plant [12,21]. AtLBD37, AtLBD38 and AtLBD39 are the only class II LOB domain proteins functionally characterized thus far [24]. They are not involved in lateral organ formation but in anthocyanin synthesis [24].
Auxin response factor (Arf) genes are induced by auxin [25,26]. ARF proteins bind to auxin responsive elements (AuxREs) in the promoters of early auxin response genes [27]. In Arabidopsis, AtARF7, which interacts with AtMYB77 [28] and AtARF19, directly regulates AtLBD16 and AtLBD29, hence controlling lateral root formation [14]. Moreover, LBD16 and LBD18 that are involved in lateral root initiation and emergence also function downstream of AtARF7 and AtARF19 [15]. In monocots, ARF function in crown root formation was demonstrated by ARF1 binding to the promoter of the LBD gene, OsCRL1 [11]. While multiple developmental functions have been associated with LBD genes by genetic analyses, little is known about the molecular interactions of LBD genes. Here, we present novel insights related to the molecular interactions of RTCS and its paralogue RTCS-LIKE (RTCL).
2. Material and methods
(a). Subcellular localization
C-terminal GFP fusions were generated by amplifying the full-length coding sequence of Rtcs (GRMZM2G092542_P01) without the stop codon with the oligonucleotide primers Rtcs-gw-fw-m-atg and Rtcs-gw-rv-oh-stop (electronic supplementary material); this introduced attB recombination sites for the Gateway cloning system (Invitrogen, Carlsbad, CA, USA). The PCR product was cloned into a 35S-Gateway-GFP vector provided by Claus Schwechheimer (TU Munich, Germany) resulting in a 35S::Rtcs-GFP construct. Similarly, a C-terminal GFP fusion was generated by amplifying the full-length coding sequence of Rtcl (AC149818.2 FG009) excluding the stop codon with the oligonucleotide primers Rtcl-BamH1-fw-m-atg and Rtcl-BspH1-rv-oh-stop (electronic supplementary material); it was then ligated into the BamHI and BspHI restriction sites of the vector pCF203 provided by Karin Schumacher (University of Heidelberg) yielding a 35S::Rtcl-GFP construct. Subcellular localization experiments were performed by transiently transforming the plasmids 35S::Rtcs-GFP, 35S::Rtcl-GFP, the control constructs 35S::HMGA-GFP [29] and 35S::GFP into A. thaliana Col-0 protoplasts according to Li et al. [30]. Transformed protoplasts were examined and documented with an HCX PL APO 63 × /1.2 W CORR water immersion objective (Leica Microsystems, Wetzlar, Germany) in a TCS SP2 AOBS confocal microscope (Leica Microsystems). GFP was excited at 488 nm with an argon laser, and the emitted fluorescence was detected with a bandpass 509 nm filter. Image processing was performed with Leica Confocal Software (Leica Microsystems). Brightfield images were taken from the same protoplasts that were analysed for green fluorescence localization.
(b). Protein expression and electrophoretic mobility shift assays
For N-terminal GST fusions, the open reading frame of Rtcs was amplified with the oligonucleotide primers (Rtcs-8-BamHI-fw and Rtcs-EcoRI-9-rv), whereas the B3-DNA-binding domain (amino acids 129–231) of ZmARF34 (GRMZM2G160005_P01) was amplified using the oligonucleotide primers ZmArf34_B3-8-BamHI-fw and ZmArf34_B3-8-EcoRI-rv (electronic supplementary material). Both PCR products were introduced into the BamHI and EcoRI sites of the vector pGEX-6P-1 [31], which was expressed in Escherichia coli BL21-DE3 cells. After induction with 1 mM IPTG, cultures were grown for 3 h at 37°C and lysed in sonication buffer (50 mM Tris, pH 7.5, 50 mM NaCl, 5 mM MgCl2, 1 mM PMSF, 0.08 µl ml−1 β-ME, 0.02% NP40). The soluble fractions with the recombinant proteins were used for electrophoretic mobility shift assay (EMSA) experiments. In contrast to ZmARF34, the crude extract of GST–RTCS was isolated in the presence of 0.25 per cent sarcosyl because of inclusion body formation [32].
EMSAs were performed according to Promega (Madison, WI, USA) manual TB110 (www.promega.com/tbs/tb110/tb110.pdf). A 59 bp sequence of the Rtcs promoter containing the AuxRE-238 motif (pRtcs-AuxRE-238) was amplified with the oligonucleotide primers EF051732-238-fw and EF051732-238-rv (electronic supplementary material), and a 64 bp sequence of the ZmArf34 promoter containing the LBD-277 motif (pZmArf34-LBD-277) was amplified with the oligonucleotide primers GRMZM2G160005-fw and GRMZM2G16005-rv (electronic supplementary material). Amplified DNA fragments were labelled with [γ-32P]-dATP (Hartmann Analytic, Braunschweig, Germany) and T4 polynucleotide kinase (Fermentas, St Leon-Roth, Germany). In total, 20 μg of the crude protein extract containing recombinant fusion proteins was incubated with [γ-32P]-dATP-labelled DNA fragments and 1 μg of poly(dI-dC) in 10× buffer (100 mM Tris pH 7.5, 500 mM NaCl, 10 mM EDTA, 10 mM DTT) in a total volume of 40 μl per experiment. The reaction products were analysed on 4 per cent non-denaturing polyacrylamide gels. The specificity of protein binding was controlled by using either 50-fold excess of specific competitor DNA, i.e. unlabelled-specific template, or unspecific competitor DNA, i.e. sonicated herring sperm DNA (Promega), providing a mixture of distinct DNA motifs of the appropriate size. The band shift of the radioactively labelled DNA probes was detected by exposition of the dried gels to X-ray films (Agfa, Duesseldorf, Germany).
(c). Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) are designed to quantify protein (antigen) amounts in a sample. GST–RTCS fusion proteins were overexpressed as described earlier in E. coli BL21-DE3 cells. Biotin-labelled oligonucleotides were fixed to a streptavidin-coated 96-well plate. The raw bacterial protein lysate was applied to the plate, and the binding was detected via the GST antibody–HRP conjugate anti-GST-HRP (RPN1236, Amersham Biosciences, UK) [33]. The 18 bp sequence including a LBD motif 5′ CAA AAA GCG GCG GCA GCA 3′ and the reverse complementary sequence labelled with biotin at the 5′ end were annealed by heating to 95°C and slowly cooling down to room temperature. The mutated oligonucleotide sequence was 5′ CAA AAA TTT TTT GCA GCA 3′.
(d). Yeast-two-hybrid assay
Yeast-two-hybrid assays were performed with the Matchmaker system (Clontech). Full-length open reading frames of Rtcs (GRMZM2G092542_P01) and Rtcl (AC149818.2 FG009) were amplified with the oligonucleotide primer combinations BD/AD-Rtcs-NdeI-fw, BD/AD-Rtcs-BamHI-rv and BD/AD-Rtcl-EcoRI-fw, BD/AD-Rtcl-BamHI-rv, respectively, that introduced NdeI, EcoRI and BamHI restriction sites (electronic supplementary material). Subsequently, the PCR products were introduced into the corresponding restriction sites of the vectors pGBKT7 (BD) and pGADT7 (AD). BD plasmids of truncated RTCS (encoding for amino acids (aa) 1–183) and RTCL (encoding for aa 1–167) were constructed using the oligonucleotide primers BD/AD-Rtcs-NdeI-fw, BD-Rtcs-del-BamHI-rv, and BD/AD-Rtcl-EcoRI-fw and BD-Rtcl-del-BamHI-rv, respectively (electronic supplementary material). The BD and AD constructs were co-transformed into yeast strain AH109 and selected on quadruple dropout (QDO) medium. The interaction between the pGBKT7(BD)-p53 and pGADT7(AD)-SV40 large T-antigen (provided in the Matchmaker system) served as a positive control, and the interaction between pGBKT7(BD)-lam and pGADT7(AD)-SV40 as a negative control.
(e). Bimolecular fluorescence complementation and FACS
Bimolecular fluorescence complementation (BiFC) experiments were performed as described by Walter et al. [34]. Fusion proteins of RTCS, RTCL and their respective LOB domains were generated with the C- or N-terminal parts of YFP. The sequences of full-length Rtcs (GRMZM2G092542_P01) and Rtcl (AC149818.2 FG009) lacking the stop codon and the corresponding LOB domain sequences (encoding for aa 1–116 of RTCS and aa 1–115 of RTCL) were amplified. For all four constructs, the forward oligonucleotide primer Rtcs/Rtcl-SPY-atg-XbaI-fw was used. For full-length constructs, the reverse primers Rtcs-SPY-no-stop-SmaI-rv and Rtcl-SPY-no-stop-SmaI-rv were employed, whereas for LOB domain amplification, the reverse primers Rtcs LOB-SPY-SmaI-rv and Rtcl LOB-SPY-SmaI-rv were used (electronic supplementary material). PCR products were introduced into the XbaI/SmaI restriction sites of the BiFC vector pUC-SPYCE [34] and the modified vector pUC-SPYNE-152 [30]. The constructs were co-transformed into A. thaliana Col-0 protoplasts according to Li et al. [30].
Flow cytometry was performed in a MoFlo (Modular Flow; Beckman Coulter, Brea, CA, USA) as previously described [35]. BiFC YFP fluorescence was excited with a 488 nm (50 mW) argon laser, and its principle emission was captured in FL1 (510–554 nm) and plotted against autofluorescence in FL2 (565–605 nm). Arabidopsis protoplast transformations and FACS analyses were performed in three biological replicates.
(f). Transient luciferase expression assays in Arabidopsis protoplasts
The effector plasmid containing the GAL4 DNA-binding domain (GAL4DB), and the reporter and reference plasmids containing firefly luciferase (LUC) and renilla LUC were prepared as previously described [35]. The full-length coding sequence of Rtcs (GRMZM2G092542_P01; oligonucleotide primers: Rtcs/Rtcl-LUC-atg-SmaI-fw and Rtcs-LUC-stop-SacI-rv) and Rtcl (AC149818.2 FG009; oligonucleotide primers: Rtcs/Rtcl-LUC-atg-SmaI-fw and Rtcl-LUC-stop-SacI-rv), and the middle region of ZmARF34 (encoding for aa 377–941, GRMZM2G160005_P01; oligonucleotide primers: ZmArf34_MR-LUC-atg-SmaI-fw and ZmArf34_MR-LUC-stop-SacI-rv) were amplified (electronic supplementary material) and subsequently introduced into the SmaI/SacI sites of the effector plasmid. The effector, reporter and reference plasmids were transiently co-transformed into A. thaliana Col-0 protoplasts according to Li et al. [30]. LUC assays were performed with the dual-luciferase reporter assay system (Promega) using a TriStar multimode microplate reader LB 941 (Berthold, Bad Wildbad, Germany). LUC activity was measured three times for each transformant, and the values were normalized with the corresponding renilla LUC values. The experiment was repeated three times with independent transformants.
(g). qPCR
Total RNA was isolated from 5 and 10 day-old wild-type and rtcs mutant coleoptilar nodes in three independent biological replicates via the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). cDNA was synthesized with qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA). qPCR was performed in three technical replications for each of the three biological replicates using the MESA Green qPCR Master Mix Plus for SYBR Assay no ROX kit (Eurogentec, Cologne, Germany) in a CFX384 real-time PCR Detection System (Bio-Rad, Munich, Germany). Primer efficiency was tested in a dilution series (1, 1/2, 1/4, 1/8, 1/16, 1/32, 1/64, 1/128). Transcript levels were normalized to the expression levels of the heavy chain of the housekeeping gene myosin (GenBank accession: 486090G09.x1; oligonucleotide primers: 486090G09.x1-fw and 486090G09.x1-rv; electronic supplementary material), which was previously used as a qPCR standard [36]. The oligonucleotide primers ZmArf34-TGA-50-fw and ZmArf34-TGA-200-rv (electronic supplementary material) were used for the amplification of 3′ untranslated regions of the ZmARF34 gene (GRMZM2G160005_P01). Differential gene expression was determined by Student's t-test (p ≤ 0.05).
3. Results
(a). Subcellular localization of RTCS and its paralogue RTCL
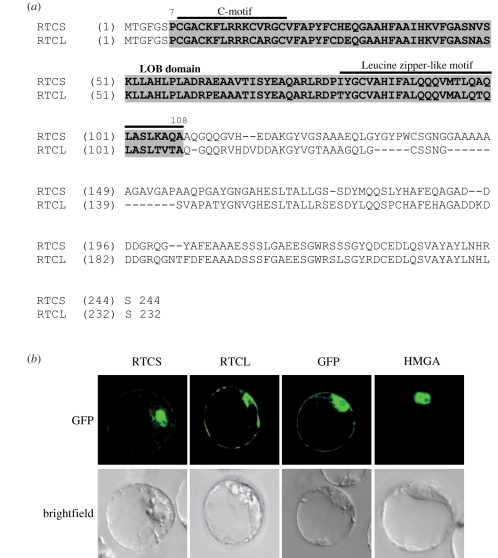
As previously reported, RTCS and RTCL are closely related paralogues that share an overall protein identity of 72 per cent and an 88 per cent identity of the LOB domain (figure 1a) [6]. To study their subcellular localization, C-terminal GFP fusion proteins of RTCS and RTCL were expressed in A. thaliana Col-0 protoplasts (figure 1b). Consistent with the predicted role of RTCS as a transcription factor, GFP fluorescence of RTCS–GFP was primarily detected in the nucleus with faint fluorescence in the cytoplasm. In contrast, RTCL–GFP fluorescence was present in both the nucleus and the cytoplasm, with less nuclear specificity than RTCS. Control experiments demonstrated that the GFP tag alone was ubiquitously expressed in Arabidopsis protoplasts, whereas the chromatin associated HMGA protein was exclusively expressed in the nucleus (figure 1b).
Figure 1.
(a) Alignment of the RTCS and RTCL full-length protein sequences. (b) Subcellular localization of RTCS and RTCL in Arabidopsis thaliana protoplasts. GFP and HMGA-GFP were used as controls.
(b). RTCS primarily binds to the LBD DNA motif
In order to demonstrate DNA binding of RTCS, an ELISA was designed (see §2) to determine the specificity of RTCS binding to a LBD motif in the context of the ZmArf16 promoter (figure 2a). The experiment demonstrated that GST–RTCS displayed a 7.1-fold increase in binding capacity to an 18 bp sequence containing a central LBD 5′ GCGGCG 3′ motif compared with the negative control, in which the LBD motif was replaced by a hexathymidine stretch. As an additional negative control, binding to the GST tag was tested. The binding capacity was reduced by 67 per cent when the LBD motif in the 18 bp oligonucleotide was replaced by a 5′ TTTTTT 3′. Nevertheless, the hexathymidine stretch oligonucleotide still displayed an interaction with GST–RTCS significantly above the GST negative control. These results suggest that the conserved LBD motif is important for the binding capacity of RTCS but that the sequence context, in which the LBD motif is embedded, also significantly influences RTCS binding.
Figure 2.
(a) Specificity of RTCS binding (GST–RTCS) to an 18 bp oligonucleotide sequence (LBD) representing the LBD motif 5′ GCGGCG 3′ assayed via ELISA. In a control experiment (LBD-mutated) the LBD motif of the 18 bp sequence was replaced by the oligonucleotide sequence (5′ TTTTTT 3′). In another control binding of GST (without RTCS) was assayed (**p ≤ 0.01). (b) Binding of RTCS to a [γ-32P]-ATP labelled 64 bp ZmArf34 promoter fragment containing the LBD-277 motif (lane 2). A 50× excess of unlabelled 64 bp probe was used as specific competitor (lane 3), whereas a 50× excess of λ-DNA was used as unspecific competitor (lane 4). Escherichia coli protein extract without RTCS was used as a negative control (lane 5). (c) Transcript levels of ZmArf 34 in 5- and 10-day-old coleoptilar nodes of wild-type and the rtcs mutant assayed by qPCR (*p ≤ 0.05). (d) Binding of ZmARF34 to a [γ-32P]-ATP labelled 59 bp Rtcs promoter fragment containing the AuxRE-238 motif. Controls as described in 2b. (e) RTCS, RTCL and ZmARF34 transient co-transformation assay. Relative luciferase activities after co-transformation of Arabidopsis protoplasts with the reporter construct GAL4-LUC and the effector constructs GAL4DB (control), GAL4DB-RTCS, GAL4DB-RTCL and GAL4-ZmARF34MR were tested. All luciferase activities are expressed relative to values obtained with the GALDB control (with GALDB set arbitrarily to 1). Error bars indicate ± s.d. (f) Model suggesting mutual control of ZmArf34 and Rtcs expression via a feedback loop.
(c). RTCS binds to the LBD motif of ZmARF34 promoter
ZmArf34 (GRMZM2G160005_P01) is the closest maize homologue of the Arabidopsis genes AtArf7 and AtArf19. Proteins encoded by these genes interact with the promoter of AtLBD16 and AtLBD29, and regulate root formation in Arabidopsis. Sequence analysis demonstrated that ZmArf34 includes five LBD motifs in its 1 kb promoter sequence upstream of the ATG start codon. The LBD motif −277 bp upstream of ZmArf34 start codon was selected, and its affinity to RTCS was tested by EMSA (figure 2b). RTCS binding capacity was demonstrated by shifting radioactively labelled promoter fragments ZmArf34-277 upon interaction with RTCS (figure 2b, lane 2). Specificity of RTCS binding was attested by a 50-fold excess of unlabelled LBD promoter motif, which outcompeted the radioactively labelled sequence, and did not lead to a shift (figure 2b, lane 3). In contrast, band shifting was not affected by a 50-fold excess of unlabelled herring sperm DNA as unspecific competitor (figure 2b, lane 4). Furthermore, binding of any other protein of the BL21-DE3 cells to the LBD promoter motif was excluded (figure 2b, lane 5).
(d). ZmArf34 expression is RTCS-dependent
Direct interaction of RTCS with ZmArf34 promoter elements as demonstrated in figure 2b can influence the expression of ZmArf34. Therefore, expression of ZmARF34 was studied in 5- and 10-day-old coleoptilar nodes of wild-type and rtcs seedlings via qPCR. In line with RTCS-dependent regulation of ZmArf34, gene expression was significantly reduced in both 5- and 10-day-old coleoptilar nodes of the rtcs mutant (*p ≤ 0.05, figure 2c). These results suggest an activation of ZmArf34 expression by RTCS during the early stages of coleoptilar node development.
(e). ZmARF34 binds to the auxin responsive element motif of the Rtcs promoter
ARFs can bind to specific AuxREs [25]. Sequence analysis of the Rtcs promoter revealed a canonical AuxRE 5′ TGTCTC 3′ −238 bp upstream of the ATG start codon. Binding of ZmARF34 fusion protein to the AuxRE-238 motif of the Rtcs promoter was tested by EMSAs using the B3-DNA-binding domain of ZmARF34. This experiment demonstrated that ZmARF34 can bind to the AuxRE motif of the Rtcs promoter (figure 2d, lane 2). A 50-fold excess of unlabelled AuxRE sequence was used as specific competitor (figure 2d, lane 3) and 50-fold excess of unlabelled herring sperm as unspecific competitor (figure 2d, lane 4). Binding of any unspecific protein of E. coli BL21-DE3 cell extracts to AuxRE promoter elements was excluded (figure 2d, lane 5).
(f). RTCS, RTCL and ZmARF34 function as transcriptional activators
To examine the ability of RTCS, RTCL and ZmARF34 to regulate transcription of downstream genes, transient co-transfection assays were performed in Arabidopsis protoplasts. The effector plasmids consisted of the yeast GAL4DB as a control or the GAL4DB fused in-frame with the coding regions of Rtcs (GAL4DB-RTCS) or Rtcl (GAL4DB-RTCL) driven by a dual 35S promoter (figure 2e). The reporter plasmid included the LUC gene driven by the minimal TATA box of the cauliflower mosaic virus 35S promoter with GAL4-binding sites immediately upstream (figure 2e). LUC activity was induced by the overexpression of GAL4DB-RTCS 54-fold and by GAL4DB-RTCL 56-fold compared with the GAL4DB control (figure 2e), suggesting a strong transcriptional activation of downstream genes by RTCS and RTCL.
Similarly, the role of ZmARF34 on downstream gene expression was determined (figure 2e). The middle region of ZmARF34 from the C-terminal end of the DNA-binding domain to the N-terminal end of the AUX/IAA-interacting domains III and IV (aa 377–941) was inserted into the effector plasmid (GAL4DB-ARF34MR; figure 2e). Co-expression of the reporter plasmid with GAL4DB-ARF34MR resulted in a 316-fold increase of luminescence in comparison to the GAL4DB control experiment (figure 2e) suggesting a strong transcriptional activation of downstream target genes by ZmARF34.
(g). Homo- and hetero-interactions of RTCS and RTCL
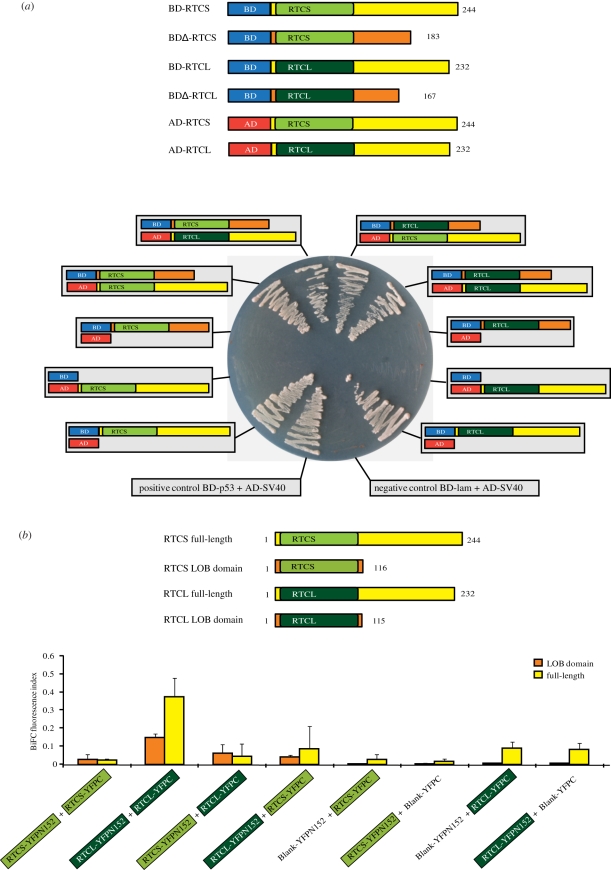
Class I LOB domains contain a leucine zipper-like domain, suggested to be involved in protein dimerization [37]. Homo- and hetero-interactions of RTCS and RTCL were tested via yeast-two-hybrid and BiFC analyses.
Owing to the putative roles of RTCS and RTCL as transcription factors, BD-RTCS and BD-RTCL plasmids encoding for full-length fusion proteins were co-transformed with control AD plasmids into yeast cells to test their self-activation capacity (figure 3a). Positive colonies suggested that both can self-activate the expression of reporter genes in yeast. Therefore, 61 aa at the C-terminus of RTCS and 65 aa of RTCL were deleted (BD-RTCSΔ and BD-RTCLΔ). These were the shortest deletions that prevented self-activation. BD-RTCSΔ and BD-RTCLΔ were used for subsequent yeast-two-hybrid analyses. Co-transformation of BD-RTCSΔ and BD-RTCLΔ with AD-RTCS and AD-RTCL in all combinations revealed homo and hetero-interactions of RTCS and RTCL in yeast (figure 3a).
Figure 3.
Homo- and hetero-interaction of RTCS and RTCL. (a) Yeast-two-hybrid interaction of RTCS and RTCL proteins fused to a GAL4 activation domain (AD: red) and a Gal4 DNA-binding domain (BD: blue). LOB domains are highlighted in green, full-length variable regions are coloured in yellow. Truncated variable regions without the activation domain are marked in orange. The interaction between the pGBKT7 (BD)-p53 and pGADT7 (AD)-SV40 large T-antigen was used as a positive control, and the interaction between pGBKT7(BD)-lam and pGADT7(AD)-SV40 as a negative control. (b) Interaction of RTCS and RTCL LOB domains and full-length proteins in the bimolecular fluorescence complementation (BiFC) system in Arabidopsis thaliana protoplasts. BiFC was quantified by flow cytometry.
To quantify RTCS and RTCL interactions, BiFC experiments were performed in plant cells (figure 3b). The quantification of three biological replicates per experiment in figure 3b revealed homo-interactions of RTCL LOB domains and of full-length RTCL/RTCL proteins. Significant homo-interactions of RTCS and hetero-interactions between both proteins were detected only for isolated LOB domains but not for the full-length proteins in the BiFC system (electronic supplementary material). This might imply that the variable C-terminus of RTCS might inhibit RTCS homo-interactions and hetero-interactions with RTCL.
4. Discussion
Rtcs, which controls crown and seminal root initiation in maize, encodes a LBD protein [6]. LBD proteins are suggested to act as transcription factors [9]. Nuclear localization of RTCS is consistent with the subcellular localization of other LBD proteins such as AtAS2 [7], OsARL1 [13], ZmRA2 [23], AtLOB [9], AtLBD16 and AtLBD29 [14], AtASL9 [38], AtJLO [21] and AtLBD18 [15]. In contrast, RTCL was expressed ubiquitously in the nucleus and the cytoplasm. Proteins that are targeted to the nucleus typically carry a nuclear localization signal (NLS) [39]. NLS sequences are often characterized by short stretches of the positively charged amino acids arginine (R) and lysine (K). RTCS contains a RRK stretch in its C-motif, whereas RTCL displays the sequence RRR at the same position (figure 1a). Sequence alignment of RTCL with the LBD proteins in Arabidopsis, rice and maize, for which a nuclear localization has been demonstrated, revealed that all known nuclear LBD proteins identified thus far contain a RRK sequence (electronic supplementary material, figure S3a). Hence, the K to R exchange in RTCL might result in its ubiquitous localization in the nucleus and cytoplasm. Similarly, the LOB domain protein RA2 and the mutant protein ra2-mum4 display an R to H amino acid change, which led to the suggestion that this might affect its subcellular localization [23]. Interestingly, among 35 class I LBD proteins in maize, 18 contain the sequence RRK whereas 15 display RRR (electronic supplementary material, 3b). In contrast, all eight class II maize LBD proteins display the sequence RKG (electronic supplementary material, 3b). The subcellular localization of these proteins needs to be determined. In addition to difference in subcellular localization, Rtcs was demonstrated to be expressed in the primary root and in the coleoptilar node, whereas Rtcl was preferentially expressed in primary roots [6]. Differences in subcellular localization and tissue-specific expression patterns suggest diverse functions of RTCS and RTCL in maize root development.
The C-domain is a part of the LOB domain and consists of four cysteine residues with conserved spacing, which probably forms a zinc-finger-like motif [8]. Such structures are typical for DNA-binding domains of transcription factors [37]. It has been demonstrated that AtLOB specifically binds to a DNA motif 5′ GCGGCG 3′ designated the LBD motif [9]. In this study, an ELISA experiment demonstrated that RTCS binds primarily to the LBD motif.
Although some Arabidopsis LBD proteins such as AtAS2, AtJLO and AtLBD18 were reported to regulate the expression levels of KNOX [17,21], ANT and PLT genes [38], little is known about the transcriptional control of direct downstream target genes by LBD proteins [37]. In this study, transient co-expression assays in protoplasts demonstrated that RTCS and RTCL act as transcriptional activators. Moreover, as shown in a yeast-two-hybrid experiment, 61 aa (position 184–244) and 65 aa (position 168–232) C-terminal fragments of RTCS and RTCL were deleted in order to prevent self-activation as observed for full-length RTCS proteins. These experiments suggested the presence of activation domains in the C-terminus of RTCS and RTCL. In line with these results, a C-terminal fragment, without LOB domain, of CRL1/ARL1, the orthologue of RTCS in rice, acted as a transcription activator in yeast cells [13]. However, the full-length CRL1/ARL1 protein did not show any significant activating activity on the transcription of the reporter gene probably due to masking of the C-terminal activating domain by the N-terminal LOB domain [13]. The distinct abilities of full-length maize RTCS and RTCL proteins and rice CRL1/ARL1 protein on transcription control suggest distinct mechanisms of LBD action even between these closely related orthologues.
Crown root formation is regulated by the phytohormone auxin as suggested by auxin-induced crown root formation in rice and the inhibition of crown root formation by the auxin transport inhibitor NPA [11,40,41]. In Arabidopsis, it has been demonstrated that in response to auxin, AtARF7 and AtARF19 directly activate AtLBD16, AtLBD18 and AtLBD29 expression to promote lateral root formation [42]. Similarly in rice, the LOB domain protein OsCRL1, which is a positive regulator for crown and lateral root formation, is a direct target of an ARF protein [11]. The LOB domain gene Rtcs analysed here is an early auxin-inducible gene [6] and displays several AuxRE motifs in its promoter. One of these elements is the ARFAT motif 5′ TGTCTC 3′ [43] located close to the start codon. It was demonstrated that ZmARF34 binds to this motif and functions as a transcriptional activator in plant cells. Taken together, these results suggested an activation of Rtcs expression by ZmARF34. Remarkably, it was recently demonstrated that ZmARF34 interacts with RUM1, a monocot-specific AUX/IAA protein that controls seminal and lateral root initiation in maize [35]. This might suggest that ZmARF34 possibly functions as a general regulator in maize embryonic seminal and postembryonic crown and lateral root formation.
While the Arabidopsis proteins ARF7 and ARF19 function as transcriptional activators of downstream LBD genes [14], the LOB domain gene AtAS2 directly or indirectly represses its target gene AtARF3 [7]. In this study, direct interactions of RTCS with the ZmArf34 promoter and RTCS-dependent expression of ZmArf34 was demonstrated. Repression of ZmArf34 expression in mutant rtcs coleoptilar nodes was consistent with the role of RTCS as a transcription activator. Taken together, these results suggest a mutual feedback loop regulation of ZmArf34 and Rtcs transcription during coleoptilar node development and crown root formation in maize (figure 2f). As the starting signal for crown root formation, auxin induces the degradation of AUX/IAA proteins so that ZmARF34 and some other ARFs activate the expression of downstream targets such as RTCS. The induced RTCS proteins bind to the promoter of ZmArf34 and activate transcription. The fine-tuning of ZmARF34 levels promotes Rtcs expression, representing an amplified auxin-signalling cascade. This amplification cascade resulting from the positive feedback loop of ARFs and RTCS might be required for the formation of multiple shoot-borne roots from the same node. As shown in Arabidopsis, bHLH046 reduces the DNA-binding activity of AtLOB [9]. Similarly, a yet unknown factor might inhibit the feedback loop of RTCS and ARFs later in development, to control shoot-borne root initiation from a specific node.
LOB domains contain C-terminal leucine zipper-like sequences, required for homo and hetero-dimerization with other proteins [37]. Yeast-two-hybrid experiments employing constructs with a truncated C-terminal domain to avoid self-activation of the proteins demonstrated homo- and hetero-interactions of RTCS and RTCL. Similarly, the rice homologue of RTCS, CRL1/ARL1, formed homo-dimers in yeast [13]. In contrast to the yeast-two-hybrid system, BiFC experiments allow quantifying interactions with full-length proteins and LOB domains in living plant protoplasts [34]. In general, relative LOB domain homo- and hetero-interactions in BiFC experiments were stronger than those of full-length proteins suggesting that the variable C-terminus might negatively influence the interaction of LOB domain proteins. Among all interactions tested in the BiFC system, RTCL full-length proteins and RTCL LOB domains displayed the strongest interaction. In contrast to the yeast-two-hybrid results, neither RTCS full-length proteins nor the RTCS LOB domains displayed significant interactions at p ≤ 0.05. These results might be explained by not yet identified inhibitory plant-specific proteins not present in yeast that interfere with RTCS–RTCS interaction in BiFC experiments. Moreover, for full-length RTCS constructs, again the inhibitory action of the variable C-terminus of the protein might prevent interactions. Finally, BiFC experiments suggested a weak hetero-interaction of the LOB domains of RTCS and RTCL, whereas no interaction was demonstrated for the full-length proteins. The weak hetero-interaction of the LOB domains of RTCL and RTCS and missing hetero-interaction of full-length proteins might be explained by the distinct subcellular localization of RTCS and RTCL, their unique tissue specificity [6], inhibitory plant-specific proteins that might outcompete RTCS, or inhibition by the variable C-terminus for full-length proteins. Moreover, differences in their leucine zipper sequence might reduce binding affinity of RTCS and RTCL. The leucine zipper in the C-terminal part of the LOB domain consists of five hydrophobic amino acids, each separated by a stretch of six amino acids [37]. The leucine zippers in RTCS and RTCL are imperfect due to the replacement of the first N-terminal amino acid by isoleucine in RTCS and threonine in RTCL and the first C-terminal amino acid by alanine in both instances.
In this study, it was demonstrated that RTCS, which is a major regulator of crown root formation in maize, displays typical attributes of a transcription factor including nuclear localization, DNA-binding and downstream gene activation. Moreover, it was suggested that RTCS is involved in a regulatory feedback loop involving ZmARF34 and that interaction between RTCS and its paralogue RTCL occurs.
Acknowledgements
We thank Angela Dressel and Caterina Brancato (ZMBP, University of Tuebingen) for excellent technical assistance, Karin Schumacher (University of Heidelberg) for the pCF203 GFP transformation vector, Claudia Oecking (University of Tuebingen) for the modified pUC-SPYNEN152 vector and Claus Schwechheimer (Technische Universität München) for a 35S-Gateway-GFP vector. This project was supported in part by Deutsche Forschungsgemeinschaft grant no. HO2249/4 to F.H. C.X. was supported by the China Scholarship Council.
References
- 1.Abbe E. C., Stein O. L. 1954. The origin of the shoot apex in maize: embryogeny. Am. J. Bot. 41, 285–293 10.2307/2438600 (doi:10.2307/2438600) [DOI] [Google Scholar]
- 2.Hochholdinger F., Tuberosa R. 2009. Genetic and genomic dissection of maize root development and architecture. Curr. Opin. Plant Biol. 12, 172–177 10.1016/j.pbi.2008.12.002 (doi:10.1016/j.pbi.2008.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Hochholdinger F., Park W. J., Sauer M., Woll K. 2004b. From weeds to crops: genetic analysis of root development in cereals. Trends Plant Sci. 9, 42–48 10.1016/j.tplants.2003.11.003 (doi:10.1016/j.tplants.2003.11.003) [DOI] [PubMed] [Google Scholar]
- 4.McCully M. E., Canny M. J. 1988. Pathways and processes of water and nutrient uptake in roots. Plant Soil 111, 159–170 10.1007/BF02139932 (doi:10.1007/BF02139932) [DOI] [Google Scholar]
- 5.Hetz W., Hochholdinger F., Schwall M., Feix G. 1996. Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. Plant J. 10, 845–857 10.1046/j.1365-313X.1996.10050845.x (doi:10.1046/j.1365-313X.1996.10050845.x) [DOI] [Google Scholar]
- 6.Taramino G., Sauer M., Stauffer J. L., Jr., Multani D., Niu X., Sakai H., Hochholdinger F. 2007. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 50, 649–659 10.1111/j.1365-313X.2007.03075.x (doi:10.1111/j.1365-313X.2007.03075.x) [DOI] [PubMed] [Google Scholar]
- 7.Iwakawa H., et al. 2002. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol. 43, 467–478 10.1093/pcp/pcf077 (doi:10.1093/pcp/pcf077) [DOI] [PubMed] [Google Scholar]
- 8.Shuai B., Reynaga-Pena C. G., Springer P. S. 2002. The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol. 129, 747–761 10.1104/pp.010926 (doi:10.1104/pp.010926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husbands A., Bell E. M., Shuai B., Smith H. M., Springer P. S. 2007. LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res. 35, 6663–6671 10.1093/nar/gkm775 (doi:10.1093/nar/gkm775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L., Yang L., Pi L. M., Liu Q. L., Ling Q. H., Wang H., Poethig R. S., Huang H. 2006. Genetic interaction between the AS1-AS2 and RDR6-SGS3-AGO7 pathways for leaf morphogenesis. Plant Cell Physiol. 47, 853–863 10.1093/pcp/pcj057 (doi:10.1093/pcp/pcj057) [DOI] [PubMed] [Google Scholar]
- 11.Inukai Y., et al. 2005. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17, 1387–1396 10.1105/tpc.105.030981 (doi:10.1105/tpc.105.030981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwakawa H., Iwasaki M., Kojima S., Ueno Y., Soma T., Tanaka H., Semiarti E., Machida Y., Machida C. 2007. Expression of the ASYMMETRIC LEAVES2 gene in the adaxial domain of Arabidopsis leaves represses cell proliferation in this domain and is critical for the development of properly expanded leaves. Plant J. 51, 173–184 10.1111/j.1365-313X.2007.03132.x (doi:10.1111/j.1365-313X.2007.03132.x) [DOI] [PubMed] [Google Scholar]
- 13.Liu H., Wang S., Yu X., Yu J., He X., Zhang S., Shou H., Wu P. 2005. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 43, 47–56 10.1111/j.1365-313X.2005.02434.x (doi:10.1111/j.1365-313X.2005.02434.x) [DOI] [PubMed] [Google Scholar]
- 14.Okushima Y., Fukaki H., Onoda M., Theologis A., Tasaka M. 2007. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19, 118–130 10.1105/tpc.106.047761 (doi:10.1105/tpc.106.047761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H. W., Kim N. Y., Lee D. J., Kim J. 2009. LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 151, 1377–1389 10.1104/pp.109.143685 (doi:10.1104/pp.109.143685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne M. E., Simorowski J., Martienssen R. A. 2002. ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development 129, 1957–1965 [DOI] [PubMed] [Google Scholar]
- 17.Lin W. C., Shuai B., Springer P. S. 2003. The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene ASYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial-abaxial patterning. Plant Cell 15, 2241–2252 10.1105/tpc.014969 (doi:10.1105/tpc.014969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ori N., Eshed Y., Chuck G., Bowman J. L., Hake S. 2000. Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 127, 5523–5532 [DOI] [PubMed] [Google Scholar]
- 19.Semiarti E., Ueno Y., Tsukaya H., Iwakawa H., Machida C., Machida Y. 2001. The asymmetric leaves2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 128, 1771–1783 [DOI] [PubMed] [Google Scholar]
- 20.Chalfun-Junior A., Franken J., Mes J. J., Marsch-Martinez N., Pereira A., Angenent G. C. 2005. ASYMMETRIC LEAVES2-LIKE1 gene, a member of the AS2/LOB family, controls proximal-distal patterning in Arabidopsis petals. Plant Mol. Biol. 57, 559–575 10.1007/s11103-005-0698-4 (doi:10.1007/s11103-005-0698-4) [DOI] [PubMed] [Google Scholar]
- 21.Borghi L., Bureau M., Simon R. 2007. Arabidopsis JAGGED LATERAL ORGANS is expressed in boundaries and coordinates KNOX and PIN activity. Plant Cell 19, 1795–1808 10.1105/tpc.106.047159 (doi:10.1105/tpc.106.047159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soyano T., Thitamadee S., Machida Y., Chua N. H. 2008. ASYMMETRIC LEAVES2-LIKE19/LATERAL ORGAN BOUNDARIES DOMAIN30 and ASL20/LBD18 regulate tracheary element differentiation in Arabidopsis. Plant Cell 20, 3359–3373 10.1105/tpc.108.061796 (doi:10.1105/tpc.108.061796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bortiri E., Chuck G., Vollbrecht E., Rocheford T., Martienssen R., Hake S. 2006. ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18, 574–585 10.1105/tpc.105.039032 (doi:10.1105/tpc.105.039032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin G., Tohge T., Matsuda F., Saito K., Scheible W. R. 2009. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 21, 3567–3584 10.1105/tpc.109.067041 (doi:10.1105/tpc.109.067041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulmasov T., Hagen G., Guilfoyle T. J. 1999. Activation and repression of transcription by auxin-response factors. Proc. Natl Acad. Sci. USA 96, 5844–5849 10.1073/pnas.96.10.5844 (doi:10.1073/pnas.96.10.5844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Deng D., Bian Y., Lv Y., Xie Q. 2010. Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays L.). Mol. Biol. Rep. 37, 3991–4001 10.1007/s11033-010-0058-6 (doi:10.1007/s11033-010-0058-6) [DOI] [PubMed] [Google Scholar]
- 27.Guilfoyle T. J., Hagen G. 2007. Auxin response factors. Curr. Opin. Plant Biol. 10, 453–460 10.1016/j.pbi.2007.08.014 (doi:10.1016/j.pbi.2007.08.014) [DOI] [PubMed] [Google Scholar]
- 28.Shin R., Burch A. Y., Huppert K. A., Tiwari S. B., Murphy A. S., Guilfoyle T. J., Schachtman D. P. 2007. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 19, 2440–2453 10.1105/tpc.107.050963 (doi:10.1105/tpc.107.050963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launholt D., Merkle T., Houben A., Schulz A., Grasser K. D. 2006. Arabidopsis chromatin-associated HMGA and HMGB use different nuclear targeting signals and display highly dynamic localization within the nucleus. Plant Cell 18, 2904–2918 10.1105/tpc.106.047274 (doi:10.1105/tpc.106.047274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M., Doll J., Weckermann K., Oecking C., Berendzen K. W., Schoffl F. 2010. Detection of in vivo interactions between Arabidopsis class A-HSFs, using a novel BiFC fragment, and identification of novel class B-HSF interacting proteins. Eur. J. Cell Biol. 89, 126–132 10.1016/j.ejcb.2009.10.012 (doi:10.1016/j.ejcb.2009.10.012) [DOI] [PubMed] [Google Scholar]
- 31.Smyth G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article 3. [DOI] [PubMed] [Google Scholar]
- 32.Frangioni J. V., Neel B. G. 1993. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 210, 179–187 10.1006/abio.1993.1170 (doi:10.1006/abio.1993.1170) [DOI] [PubMed] [Google Scholar]
- 33.Kirchler T., et al. 2010. The role of phosphorylatable serine residues in the DNA-binding domain of Arabidopsis bZIP transcription factors. Eur. J. Cell Biol. 89, 175–183 10.1016/j.ejcb.2009.11.023 (doi:10.1016/j.ejcb.2009.11.023) [DOI] [PubMed] [Google Scholar]
- 34.Walter M., et al. 2004. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438 10.1111/j.1365-313X.2004.02219.x (doi:10.1111/j.1365-313X.2004.02219.x) [DOI] [PubMed] [Google Scholar]
- 35.von Behrens I., Komatsu M., Zhang Y., Berendzen K. W., Niu X., Sakai H., Taramino G., Hochholdinger F. 2011. Rootless with undetectable meristem 1 encodes a monocot-specific AUX/IAA protein that controls embryonic seminal and post-embryonic lateral root initiation in maize. Plant J. 66, 341–353 10.1111/j.1365-313X.2011.04495.x (doi:10.1111/j.1365-313X.2011.04495.x) [DOI] [PubMed] [Google Scholar]
- 36.Hoecker N., Keller B., Muthreich N., Chollet D., Descombes P., Piepho H. P., Hochholdinger F. 2008. Comparison of maize (Zea mays L.) F1-hybrid and parental inbred line primary root transcriptomes suggests organ-specific patterns of nonadditive gene expression and conserved expression trends. Genetics 179, 1275–1283 10.1534/genetics.108.088278 (doi:10.1534/genetics.108.088278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majer C., Hochholdinger F. 2011. Defining the boundaries: structure and function of LOB domain proteins. Trends Plant Sci. 16, 47–52 10.1016/j.tplants.2010.09.009 (doi:10.1016/j.tplants.2010.09.009) [DOI] [PubMed] [Google Scholar]
- 38.Lee H. W., Kim J. 2010. Ectopic expression of LBD18/ASL20 results in arrest of plant growth and development with repression of AINTEGUMENTA and PLETHORA genes. J. Plant Biol. 53, 214–221 10.1007/s12374-010-9108-9 (doi:10.1007/s12374-010-9108-9) [DOI] [Google Scholar]
- 39.Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. 1984. A short amino acid sequence able to specify nuclear location. Cell 39, 499–509 10.1016/0092-8674(84)90457-4 (doi:10.1016/0092-8674(84)90457-4) [DOI] [PubMed] [Google Scholar]
- 40.McSteen P. 2010. Auxin and monocot development. Cold Spring Harb. Perspect. Biol. 2, a001479. 10.1101/cshperspect.a001479 (doi:10.1101/cshperspect.a001479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu M., Zhu L., Shou H., Wu P. 2005. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 46, 1674–1681 10.1093/pcp/pci183 (doi:10.1093/pcp/pci183) [DOI] [PubMed] [Google Scholar]
- 42.Okushima Y., et al. 2005. Functional genomic analysis of the auxin response factor gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444–463 10.1105/tpc.104.028316 (doi:10.1105/tpc.104.028316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulmasov T., Hagen G., Guilfoyle T. J. 1997. ARF1, a transcription factor that binds to auxin response elements. Science 276, 1865–1868 10.1126/science.276.5320.1865 (doi:10.1126/science.276.5320.1865) [DOI] [PubMed] [Google Scholar]