Abstract
The formation of lateral roots (LRs) is a key driver of root system architecture and developmental plasticity. The first stage of LR formation, which leads to the acquisition of founder cell identity in the pericycle, is the primary determinant of root branching patterns. The fact that initiation events occur asynchronously in a very small number of cells inside the parent root has been a major difficulty in the study of the molecular regulation of branching patterns. Inducible systems that trigger synchronous lateral formation at predictable sites have proven extremely valuable in Arabidopsis to decipher the first steps of LR formation. Here, we present a LR repression system for cereals that relies on a transient water-deficit treatment, which blocks LR initiation before the first formative divisions. Using a time-lapse approach, we analysed the dynamics of this repression along growing roots and were able to show that it targets a very narrow developmental window of the initiation process. Interestingly, the repression can be exploited to obtain negative control root samples where LR initiation is absent. This system could be instrumental in the analysis of the molecular basis of drought-responsive as well as intrinsic pathways of LR formation in cereals.
Keywords: lateral root initiation, water deficit, barley, maize
1. Introduction
The formation of lateral roots (LRs) is among the major processes shaping root systems and impacting soil resources capture. In addition to their contribution to soil exploration, LRs are largely involved in the plastic response of root architecture to local environmental constraints, which make them essential players in plant adaptation [1]. While the plasticity and ecological significance of root branching were mainly established based on important species from agricultural or natural ecosystems [2,3], the wealth of data on the molecular mechanisms underlying LR formation has been gained using Arabidopsis thaliana [4].
LR formation is considered a multi-stage process, embracing LR primordium (LRP) initiation, LRP development and patterning, LRP emergence and meristem activation [5]. The existence of these stages is supported by the isolation of mutations and environmental cues that inhibit specific steps in LR formation. We focus here on the initiation stage, which can be considered the primary determinant of LR spacing along the parent root. LR initiation culminates with the first formative (asymmetric) division of two adjacent pericycle cells which, in Arabidopsis, take place in the basal part of the elongation zone (EZ) [6]. There exists, however, compelling evidence that this stage involves a cascade of signalling processes and potentially comprises several (sub-) stages [7].
The molecular analysis of the early stages of LR initiation is hampered by the fact that initiation occurs at positions and with a timing that are difficult to predict [8]. In addition, the morphological consequences of a mutation (or treatment) affecting early events only amount to a lack or reduction of LR initials. Although it is possible to develop reporter lines on a gene-by-gene basis, LR initiation remains recalcitrant to transcriptome-wide approaches as one will fail to isolate appropriate, synchronized samples of mutant and wild-type individuals. This issue has been successfully addressed by two experimental systems that trigger the initiation of new LRs, thereby ensuring a strictly controlled timing of early events. The LR inducible system (LRIS) manipulates indole-3-acetic acid transport and supply to induce LR formation along the whole primary root [9], while the gravitropic stimulus-based system exploits the correlation between LR formation and root bending to induce a single LR formation event at a predictable location [10,11].
The LRIS appears to be the only system that has been validated in maize [12]. Owing to the small LR spacing in maize and other cereals, the gravitropic stimulus-based system may be inappropriate as several LRs are likely to appear in sequence in the outer side of the bend. For the same reason, it is virtually impossible to sample for negative control root segments where LRs are absent. Treating roots with N-1-naphthylphthalamic acid prevents LR initiation on the whole root [12], but this is likely to affect many other developmental processes as well. Given the complex nature of the regulatory processes of LR formation, which involves an interplay of intrinsic and response pathways [13], experimental systems manipulating LR formation in different ways are certainly needed to reach a more integrated understanding of the molecular mechanisms that regulate the sites of LRs.
Here, we first report that transient water-deficit (WD) treatments in barley and maize grown in aeroponics block LR initiation before the first asymmetric division. This repression occurs presumably during a unique developmental step and can be exploited to obtain negative control root samples where LR initiation is absent. We also describe a time-lapse methodology for the analysis of the dynamics of this repression along growing roots. These results are the basis for a novel LR repression system that could be instrumental in the analysis of the molecular basis of drought-responsive as well as intrinsic pathways of LR formation in cereals.
2. Material and methods
(a). Seeds, solutions and plant culture
Seeds of Hordeum vulgare cv. Derkado and of Zea mays B83 inbred line were used in this study. Barley and maize seeds were left to germinate on a vertical filter paper for 72 h in the dark at 18°C and 22°C, respectively. After germination, the root system of barley seedlings was reduced to a single seminal root, preferably the most vertical one. Germinated seeds were transferred to 40 × 60 × 60 cm aeroponic systems containing 5 l of nutrient solution sprayed for 15 s every 5 min [14]. Barley and maize were grown in a half-strength Hewitt solution (2 mM Ca(NO3)2 · 4H2O, 2 mM KNO3, 0.75 mM MgSO4 · 7H2O, 0.67 mM NaH2PO4 · 2H2O, 0.05 mM FeEDTANa, 0.03 µM (NH4)6Mo7O24 · 4H2O, 50 µM NaCl, 25 µM H3BO3, 5 µM MnCl2 · 4H2O, 0.5 µM CuSO4 · 5H2O, 0.5 µM ZnSO4 · 7H2O, 0.6 mM Na2SiO3 · 5H2O; pH 5.8) and a modified Hoagland solution (2 mM KNO3, 2 mM Ca(NO3)2 · 4H2O, 2 mM MgSO4 · 7H2O, 1 mM NH4NO3, 1 mM KH2PO4, 10 µM MnCl2 · 4H2O, 50 µM CuSO4, 1 µM ZnSO4 · 7H2O, 0.2 µM H3Mo4; pH 5.0), respectively. All experiments were conducted in a PGV36 growth chamber (Conviron, Winnipeg, Canada) with a day : night temperature regime of 18°C : 16°C for barley and 22°C : 20°C for maize, a relative humidity of 70 per cent, a 16 h long photoperiod and a photosynthetically active radiation of 350 µmol m−2 s−1.
In each experiment, half of the seeds were subjected to a transient WD treatment 3 days after the transfer to the aeroponic system. The treatment was applied by interrupting the nutrient supply for 8 (or 4) h during the night phase. This treatment cannot be applied during the day because the amount of water retained on the root surface is insufficient to sustain the transpiration demand and seedlings do not survive the WD. It follows that the effect of the WD is confounded with the effect of the night and their interactions.
(b). Morphological measurements
Greyscale images of all seedlings (control and WD) were captured repeatedly using a modified 600 dots per inch flatbed transparency scanner (Medion, Germany). Images were obtained at least at the onset and at the end of the WD treatment and 7 days later. They were analysed using SmartRoot [15] to estimate the length of the barley seminal or maize primary root and the longitudinal position of individual LRs on selected segments of seminal or primary roots. The SmartRoot database was imported in SAS software for the computation of root growth rate and for data manipulation preceding statistical analysis.
(c). Kinematic analysis
Root growth components were quantified using the GROW Map-Root method based on digital image sequence processing [16,17]. Time-lapse image sequences of growing seminal root tips were acquired with a high-resolution camera (Point Grey, Richmond, Canada) and near-infrared illumination (880 nm). In the acquired image sequences, cellular growth resulted in changes of local grey value structures that were used to calculate profiles of relative elemental growth rate (REGR) along the root growth zone (for more details see [18]). A linear regression was then fitted on the declining basal part of the REGR profiles of five roots. The basal end of the EZ was obtained as the position along the profile where the predicted REGR equals zero.
(d). Light microscopy
Roots were fixed in standard FAA solution (formaldehyde–acetic acid–ethanol) for 24 h and dehydrated in a series of 70, 80, 96 and 96 per cent ethanol solutions (1 h each). Fragments were embedded in Technovit 7100 resin (Heraeus Kulzer, Wehrheim, Germany), sliced to 5–8 µm and stained with 0.5 per cent toluidine blue. The initiation sites and the morphology of LR primordia were observed under light microscopy.
(e). Statistical analysis
Regression and variance analysis were performed with the GLM or MIXED procedures of SAS/STAT software v. 9.2. Possible covariance between cell measurement obtained from the same root sample was taken into account using mixed models. Statistical inference to compare specific conditions and time points was carried out through the estimation of appropriate linear combinations of model parameters using CONTRAST statements.
3. Results
(a). Repression of lateral root formation in barley
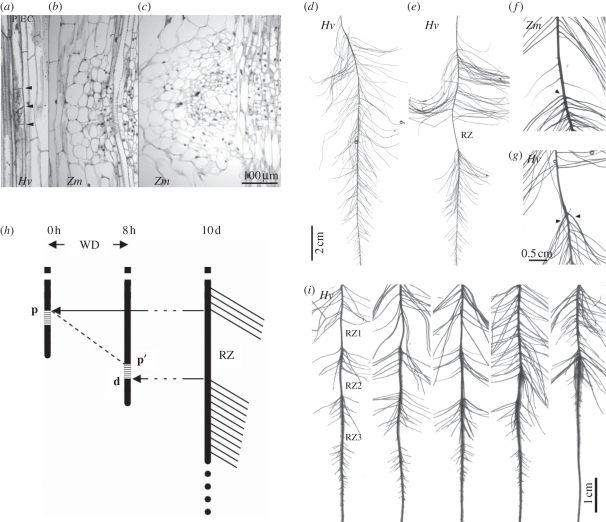
In the repression system, germinated barley seedlings with a single, 1 cm long seminal root are transferred to an aeroponics device under standard conditions. These seedlings are considered to be at time point 0. Three days later, the seminal root is 10–15 cm long and LRs from the acropetal sequence have been initiated with an average density of ca 15 LR primordia cm−1 (estimated from subsequent counts of LRs after emergence). Formative divisions in the pericycle are visible at 1–2 cm from the tip, and LR primordia near the seminal root base are at about 1 day from emergence (figure 1a). An 8 h long WD treatment is then applied by interrupting the nutrient solution supply. During this period, a drop of solution remains attached to the root tip, contributing to the maintenance of a film of solution at the surface of the whole root by capillarity. The WD is applied during the night as the amount of solution retained at the root surface is insufficient to sustain the transpiration demand during the day. At day 10, the seminal root is 30–40 cm long and all LRs initiated between days 0 and 6 are fully emerged. The branching pattern of WD seminal roots is identical to that of control seedlings except for a characteristic segment where LRs are completely absent (figure 1d,e). This segment is 1.44 ± 0.41 cm long (n = 15) and is located close to the previous position of the root tip at the onset of the WD treatment (10–15 cm from the seminal root base).
Figure 1.
(a–c) Observations of barley seminal and maize primary roots in light microscopy. (a) Barley: formative divisions in the pericycle, 1–2 cm from the tip (arrows delimit the two small daughter cells). (b,c) Maize: LR primordia blocked at an early or late stage, sometimes observed in the repression zone. P, pericycle; E, endodermis; C, cortex. (d,e) Branching patterns of 10-day old roots in LR repression experiments. (d) Barley control. (e) Barley root exposed to an 8 h long WD during the night of day 3. The basal and apical zones of the roots are not shown. (f,g) Close view of a repression zone in maize and barley, respectively. Arrows point to the first LR, usually more vigorous, initiated after the end of the WD. (h) Principle of the time-lapse approach used to estimate the position and size of the target window where LR repression occurs. Roots are imaged at the onset of WD (0 h), at the end of the WD (8 h) and 7 days later (10 days) and the resulting images are aligned on the root base. Arrows connect the boundaries of the repression zone (RZ) to their corresponding positions p and d, respectively at the onset (0 h) and at the end (8 h) of the WD episode. The dashed line draws the trajectory of p during the WD, ending at p′ at 8 h. This trajectory is assumed to follow the rate at which the pp′ segment was laid down. The p′d segment gives the position and length of the target window. (i) Branching pattern of barley roots in IBA experiments. Plants were exposed to WD over three successive nights, generating three repression zones (RZ1–RZ3). IBA was applied for 1 h before the onset of the first WD. From left to right: control, 10−7, 10−6, 10−4 and 10−3 M IBA.
The pericycle layer was examined using light microscopy to determine the presence of LR precursors (initial cells or primordia) in 10-day old repressed segments. Only four out of a set of 20 segments showed asymmetric division figures (similar to figure 1a) and in these, not more than three figures were observed. This has to be compared with an expected count of ca 20 initiation sites per segment. As these could correspond to late- or slowly forming LR structures, we performed a separate experiment in which seedlings were maintained in aeroponics for 30 days after the WD treatment. As no LRs emerged from the repressed segments, we concluded that the WD treatment blocks LR formation before or, at very low frequency, just after the first asymmetric divisions.
Further analyses were carried out to assess whether the repression system is acting locally and strictly within the duration of the WD treatment. On the one hand, LR spacing was estimated in regions proximal and distal to the repressed segments in 10-day old WD plants (based on the insertion position of 324 LRs observed on 16 roots). LR spacings were 0.63 ± 0.10 mm and 0.53 ± 0.10 mm, respectively, proximal and distal to the repression zone. The small decrease of LR spacing after WD was only bordering significance (mixed model, F-test, p = 0.057). In a separate experiment, the WD treatment was repeated during 15 successive nights, each WD episode generating an additional inhibited segment. The successive segments were evenly spaced along the seminal root and their length did not show any significant trend (F-test on slope effect, n.s.), indicating that the repression does not act in a cumulative way. Together, these results suggest that the repression system in barley acts locally and within the duration of the WD.
(b). Repressed segments extend acropetally during water deficit
In a first attempt to uncover the dynamics of LR repression during WD, we estimated the length of the repressed zone in barley plants submitted to a 4 h long WD treatment. Compared with 8 h WD, the repression zone was approximately reduced by a factor of two (0.78 ± 0.24 cm, n = 15) indicating that it is roughly proportional to the duration of the treatment. Using root scans obtained at the onset and at the end of WD and at day 10, we estimated the longitudinal distance separating the proximal boundary of the inhibited segment from the position of the seminal root apex at the onset of WD (figure 1h). This distance was on average 1.38 ± 0.03 cm and was not significantly different among roots from 4 and 8 h long treatments (comparison of means, t-test, n.s.). These results demonstrate that the proximal boundary of inhibited segments is established at the onset of WD and that their distal boundary extends acropetally during the WD episode.
In the acropetal sequence of LR formation, developmental steps of LR formation occur at predictable distances from the tip and, therefore, are displaced acropetally as the tip grows. The fact that both LR repression and LR formation occured acropetally suggests that the repression is likely to target a specific developmental window of the LR formation programme (referred hereafter as target window).
From the same experiments, we estimated the distance between the distal boundary of repressed segments and the position of the seminal root apex at the end of the WD treatment (0.91 ± 0.02 cm; n = 17). A kinematic analysis was performed to exclude the possibility that this location resided in the EZ, as this would have implied that longitudinal distances were time-dependent. The decreasing part of five independent REGR profiles was analysed using a linear regression model and the intersection of the regression line with the horizontal axis was estimated with 95% confidence limits. This analysis established that, in our experimental conditions, the EZ extended to 0.8 ± 0.13 cm from the tip. Therefore, it follows that LR repression under the WD treatment occurs when LR precursors leave the EZ, which provides a first approximation of the position of the target window. Interestingly, the distal boundary of the root segment where asymmetric divisions were observed is located 1 cm from the tip (see above).
(c). The repression targets a unique developmental step of lateral root initiation
We then attempted to define the boundaries of the target window (figure 1h). The preceding section established that the repression initiated at 1.38 cm from the tip at the onset of WD (point p on figure 1h, estimated from the position of the proximal boundary) and reached 0.91 cm from the tip at the end of WD (point d on figure 1h, estimated from the position of the distal boundary). In a first approximation, the target window thus spans a region of 1.38 – 0.91 = 0.47 cm.
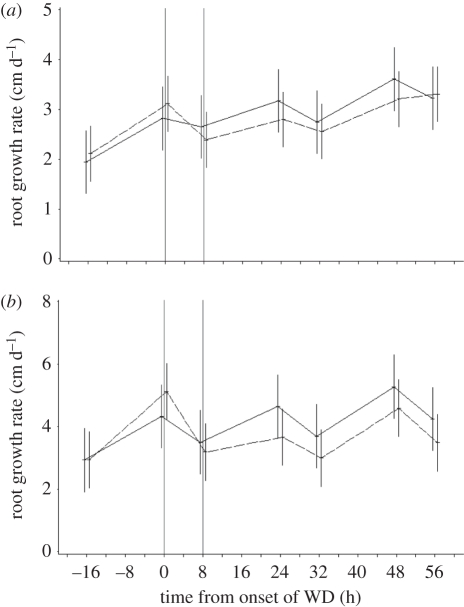
This approximation is valid provided the growth rate of the seminal is constant and the displacement of the target window during the WD strictly follows the rate of root extension. Using time-lapse experiments, we found that the seminal root growth rate was transiently reduced during the WD treatment (contrast comparing the reduction of the root growth rate during the night of day 3, in control versus WD plants; p < 0.03; figure 2a). This effect resulted from reduced cell proliferation as no difference was observed between the length of cortical cells (in the layer adjacent to the endodermis) from an inhibited segment and from a neighbour segment (n = 96, F-test, p = 0.32). It is therefore likely that the proximal boundary of the target window at the end of the WD was closer to the tip than expected.
Figure 2.
Evolution of the seminal root growth rate of barley (a) and maize (b) during 3 days. The origin of the time axis corresponds to the onset of WD. Solid line, control plants; dashed line, WD plants.
A recent study suggested that the speed of the tip at a given position (relative to the root base) sets the speed of the progression front of subsequent morphological events (e.g. root hair formation, exudation or LR emergence) at that same position [19]. Using this rule, we calculated a corrected position of the proximal boundary of the inhibited zone. This position would have been 1.12 cm from the tip at the onset of WD, giving a target window size of 1.12 – 0.91 = 0.23 cm. This value is about one-fourth of the distance separating the first and second round of asymmetric divisions (A. Babé & X. Draye 2010, unpublished data). It seems therefore likely that the LR repression that occurs in barley in the repression system targets a unique developmental step within the LR formation programme.
(d). A role for auxin supply in the lateral root repression
Interestingly, in most WD experiments we observed two or three additional LRs forming synchronously and immediately after the WD treatment (figure 1g). These LRs shared the same characteristic features as LRs that are formed after excision of the root apex (large diameter and high growth rate). This result appears consistent with a stimulatory signal accumulating during the WD upstream of the LR initiation domain and being released upon the relief of WD.
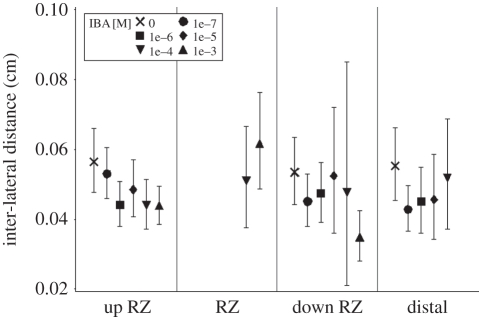
This observation led to a set of experiments, in which roots were treated with an auxin analogue for 1 h before the onset of the WD. Indole-3-butyric acid (IBA) was chosen based on its stimulatory effect on LR formation at concentrations that do not affect root elongation in Arabidopsis [20]. We verified the effect of IBA in barley using a series of concentrations: 0, 10−7, 10−6, 10−5, 10−4 and 10−3 M IBA (figures 1i and 3). According to IBA concentrations, observed root elongation rates were, respectively, 2.00a, 2.07ab, 1.99ab, 1.89ab, 1.87ab and 1.56b cm d−1, while LR spacings were, respectively, 0.056a, 0.053a, 0.044b, 0.049ab, 0.044b and 0.044b cm (numbers with the same suffix letter are not significantly different at p < 0.05). LR formation was thus stimulated above 10−6 M, while root elongation was reduced only above 10−3 M. The stimulatory effect of IBA on LR formation was less pronounced distal to the repression zone, in tissues that were laid down after the treatment (figures 1i and 3).
Figure 3.
Inter-lateral distance observed in the IBA experiments (barley) in three successive segments located upstream (up RZ), in (RZ) and downstream (down RZ) of the first repression zone, and in a distal segment located between RZ2 and RZ3. Values are lacking at zones and IBA concentrations for which LR were not (or not systematically) present. See legend of figure 1i.
These experiments revealed that IBA concentrations above 10−5 M promote the formation of LRs in the root segment where it is normally repressed by the WD (figure 1i). This demonstrates that the inhibited zone did not lose its competence for LR formation and suggests that the latter was limited by auxin supply. The observations from these experiments thus converge into the hypothesis that WD impairs auxin supply to the LR initiation domain.
(e). Extension to the maize case
Most experiments described for barley were carried out with maize and are summarized hereafter. Firstly, the transient WD episode led to a characteristic LR repression zone, except that a few LR primordia at different stages (figure 1b,c) and up to three abnormally short and tiny LRs were found occasionally in repressed segments (figure 1f). The length of the repression zone in maize was 1.3 ± 0.04 cm (n = 58). Secondly, the primary root elongation was significantly reduced during the WD episode (contrast comparing the reduction of the root growth rate during the night of day 3, in control versus WD plants; p < 0.02; figure 2b). Finally, LR spacings, estimated from 839 LRs on 12 roots, were 0.60 ± 0.11 and 0.49 ± 0.10 mm, respectively, proximal and distal to the inhibited segments. The lowering of LR spacing after WD was significant in maize (mixed-model ANOVA with F = 6.51, p = 0.011), though it remained quantitatively small and similar to that observed in barley.
Using the above method, the boundaries of the target window in maize were positioned at 1.08 ± 0.03 and 1.51 ± 0.04 cm (n = 12) from the tip. This region perfectly matches previous observations of LR initiation in maize that occurs between 1.2 and 1.5 cm from the tip [21]. The repression system is therefore acting at very early stages of LR formation in maize as observed in barley.
The origin of structures observed in repressed segments is less easy to determine for maize when compared with barley, because LR initiation in maize deviates from a strict acropetal sequence [22]. Besides initiation that occurs near the tip and which is repressed by the WD, several late initiation events have been described between 5 and 8 cm from the tip [22]. For such late LR primordia or LRs to appear in repressed segments in the repression system, they were inevitably initiated after the WD treatment. Therefore, as the fate of all LR primordia and LRs observed in repressed segments had been disrupted by the WD, we assumed that they originated from founder cells specified in the target window during the WD episode. It follows that the barley and maize responses were similar, in the sense that only a few founder cells were able to complete the first asymmetric division during the WD, but differed in the extent of the subsequent development of those cells, which were blocked at the asymmetric division stage in barley and at various later stages in maize.
4. Discussion
(a). A novel system for monitoring early events of lateral root formation in cereals
The present results indicate that it is possible to use transient WD to repress LR initiation locally with an almost 100 per cent success rate in barley and, to a lesser extent, in maize. As such, the system could be useful in studies on the short-term effects of WD on root system development in controlled conditions, without recourse to osmotic compounds that are integral to drought experiments with agar-grown plants. Given the spatial and temporal dynamics of soil water content, short-term and local depletion of water is very likely to occur around individual roots and to affect their development, well in advance of the occurrence of a long-term deficit [23].
The LR repression system presented here offers an alternative to LR induction systems based on auxin [9] or on a gravitropic stimulus [11] to study intrinsic pathways of LR formation [13]. In general, induction systems use treatments or stimuli to induce or synchronize LR formation and monitor the course of cellular events, thereby manipulating the normal sequence of LR formation. The gravitropic stimulus-based system, for example, exploits the correlation between root bending and LR formation to trigger a single LR initiation event [10], but this is thought to override an endogenous control of LR spacing [11]. In the repression system, on the other hand, it is possible to monitor the course of cellular events during an undisturbed acropetal sequence of LR formation, using the repressed zone of the WD roots as a ‘no-LR’ control. As such, the system constitutes a novel tool to disentangle response and intrinsic pathways of LR formation.
In general, the study of the early stages of LR formation preceding the first asymmetric divisions is hampered by the fact that LR initiation is an asynchronous process. Even when it occurs in an acropetal sequence, as in Arabidopsis and barley, the precise timing and positioning of LR initiation is difficult to predict [8]. Auxin-based LR induction systems avoid this issue by triggering synchronized LR initiation over almost the whole primary root [9]. The situation is different in the gravitropic stimulus-based and the repression systems where LR induction or repression occurs locally. Here, a precise knowledge of where induction or repression takes place is also required to enable the appropriate sampling of tissues for cellular and molecular analysis following the gravitropic stimulus or the onset of the WD treatment.
The repression system could be used to study LR response to other environmental cues such as phosphate availability that impact LR growth and branching [24]. However, if the external signal would disrupt LR initiation at a stage that occurs within the root EZ, the precise positioning of the target developmental window may require the recourse to kinematic analysis [16,25] to match the position of the developmental window with that of the repressed zone.
(b). An early water deficit responsive pathway involving auxin transport and/or sensitivity
To our knowledge, the dramatic response of LR initiation to WD observed here has not been reported before. However, the effects of WD on LR initiation have been poorly studied in general [13]. A reduction in LR number has been reported for Arabidopsis subjected to osmotic stress (polyethylene glycol), but only emerged and visually detectable LRs were taken into account, which made it unclear at what stage LR formation was (partially) disrupted [26]. Further experiments revealed that osmotic stress blocks LR formation at the emergence stage in Arabidopsis [27]. It was also suggested in maize that desiccation blocks LR development and not initiation [28]. In the latter experiment, however, rehydration was found to trigger the activation of a very small number of LRs, which suggests, as in the repression system, that many other LRs did not form or did not succeed in reaching a mature primordium stage. Finally, in a later study in maize and wheat, LR densities did not respond to soil drying [29].
The effects of abscisic acid (ABA) on LR formation have attracted comparatively more attention than those of WD [30]. There is now a body of evidence that ABA blocks the emergence of LRs before meristem activation [31] and that the osmotic-responsive mechanism which blocks LR emergence requires ABA [27]. In a recent study of abscisic acid insensitive 4 (ABI4), which encodes an ABA-regulated AP2 domain transcription factor, it was shown that ABA signalling in control conditions downregulates the number of LRs (including LR primordia) by 25 per cent [32]. ABI4 is thought to mediate ABA and cytokinin inhibition of LR formation via reduction of polar auxin transport and the resulting decrease in root auxin.
The binary response observed in the repression system for barley and maize contrasts with most results on ABA signalling and osmotic treatments which, at best, partially reduce LR densities. The promoting effect of IBA on LR neo-formation between established LRs of the acropetal sequence indicates that auxin is sufficient to trigger novel LR initiation in barley, as in Arabidopsis [33]. The fact that those additional LR initiation sites were not recruited in the normal acropetal sequence suggests that auxin supply was limiting in the LR initiation zone or that inhibitory signals like ABA (see above) or cytokinins [34] were preventing LR initiation from taking place close to the last established primordia.
The fact that IBA supply before the WD treatment was able to overcome LR repression is compatible with the hypothesis of a limitation in auxin supply. The early stages of LR formation indeed depend on basipetal auxin transport from the root tip [10,11] and there are several reports on reorientation of auxin efflux carriers in response to intrinsic or external signals [35]. The above statement that ABA reduces polar auxin transport would also support this hypothesis. However, the WD treatment may have reduced the sensitivity of LR initiation to auxin, which was already shown to occur in the pericycle in response to the availability of inorganic phosphate [36]. Alternatively, the WD treatment may have increased the sensitivity of LR initiation to a negative signal such as cytokinins, also known to interact with ABA and auxins [32,34].
The almost immediate and synchronized flush of LRs that occurred after the end of the WD treatment can be analysed in the framework of the auxin-based model of LR initiation proposed recently [37]. In this model, auxin supply progressively fills an exploitable auxin pool at a constant rate. When the quantity of auxin in the pool is greater than a threshold value, LR initiation occurs and the auxin pool is consumed. A change of auxin sensitivity is abstracted in the model as a modification of the threshold. In the hypothesis that auxin supply is limited during WD, the auxin flux upstream of the pool would be impaired, the pool would not be filled and auxin would accumulate on the route upstream of the pool. When the WD is released, the stock of auxin would be delivered to the pool at a higher rate than normal and generate the flush of LRs. Alternatively, in the hypothesis that sensitivity to auxin is decreased by WD, the threshold would be increased to a very high level and auxin would accumulate in the pool as long as the WD condition is maintained, without reaching the level that triggers LR formation.
(c). New perspectives for the determination of lateral root growth rate potential
The observation that, in the repression system, almost all LR events were blocked before the asymmetric division is in line with the concept that LR formation is a multi-stage process [5]. However, since a cascade of signalling pathways operates before the formative division in Arabidopsis [7], further molecular analysis is still required to uncover the WD-responsive regulatory mechanisms.
However, the presence of few asymmetric division figures (in barley), or LR primordia and tiny LRs (in maize) in inhibited segments suggests that founder cells have unequal abilities to respond to the WD condition. A major feature of maize, for which this effect is more pronounced, is the lack of a strict acropetal sequence of LR formation [22]. As nothing is known on the temporal sequence of founder cell specification in maize, one can imagine that founder cell specification occurs in a well-defined region of the root, as in Arabidopsis and barley, but that the rate of evolution of founder cells varies among them, leading to the occurrence of the first asymmetric divisions up to 8.0 cm from the tip [22]. One could then speculate that founder cells with a slow evolution may have a higher probability to overcome the WD treatment if they reach the WD-responsive stage after the WD treatment.
Interestingly, the fact that conditions prevailing at the WD-responsive stage, when LR precursors comprise two founder cells, are able to affect a diverse array of developmental and growth events that occur several days later calls for the existence of a quantitative imprinting of founder cells (or their cellular environment). Such a mechanism is also supported by the synchronous formation of few thick and fast-growing LRs when WD is released, which indicates that transient conditions can act on founder cells in such a way that the resulting LR primordia will give rise to fast-growing LRs. That the growth rate potential of LRs is determined very early in development was already proposed 40 years ago [38], but, despite its significance, this important topic has not attracted much attention ever since.
Acknowledgements
We thank the Communauté française de Belgique (ARC 0510-329 grant and FRIA fellowships to A.B. and T.L.) and the Belgian Science Policy (BARN project) for financial support. We are also grateful to Nathalie Wuyts and anonymous referees for constructive comments on a former version of the manuscript.
References
- 1.Walch-Liu P., Ivanov I. I., Filleur S., Gan Y. B., Remans T., Forde B. G. 2006. Nitrogen regulation of root branching. Ann. Bot. 97, 875–881 10.1093/aob/mcj601 (doi:10.1093/aob/mcj601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodge A., Robinson D., Griffiths B. S., Fitter A. H. 1999. Why plants bother: root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ. 22, 811–820 10.1046/j.1365-3040.1999.00454.x (doi:10.1046/j.1365-3040.1999.00454.x) [DOI] [Google Scholar]
- 3.Hodge A., Berta G., Doussan C., Merchan F., Crespi M. 2009. Plant root growth, architecture and function. Plant Soil 321, 153–187 10.1007/s11104-009-9929-9 (doi:10.1007/s11104-009-9929-9) [DOI] [Google Scholar]
- 4.Peret B., De Rybel B., Casimiro I., Benkova E., Swarup R., Laplaze L., Beeckman T., Bennett M. J. 2009. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14, 399–408 10.1016/j.tplants.2009.05.002 (doi:10.1016/j.tplants.2009.05.002) [DOI] [PubMed] [Google Scholar]
- 5.Malamy J. E., Benfey P. N. 1997. Down and out in Arabidopsis: the formation of lateral roots. Trends Plant Sci. 2, 390–396 10.1016/S1360-1385(97)90054-6 (doi:10.1016/S1360-1385(97)90054-6) [DOI] [Google Scholar]
- 6.Casimiro I., Beeckman T., Graham N., Bhalerao R., Zhang H. M., Casero P., Sandberg G., Bennett M. J. 2003. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 8, 165–171 10.1016/S1360-1385(03)00051-7 (doi:10.1016/S1360-1385(03)00051-7) [DOI] [PubMed] [Google Scholar]
- 7.De Rybel B., et al. 2010. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 20, 1697–1706 10.1016/j.cub.2010.09.007 (doi:10.1016/j.cub.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 8.Dubrovsky J. G., Gambetta G. A., Hernandez Barrera A., Shishkova S., Gonzalez I. 2006. Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Ann. Bot. 97, 903–915 10.1093/aob/mcj604 (doi:10.1093/aob/mcj604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Himanen K., Boucheron E., Vanneste S., Engler J. D., Inze D., Beeckman T. 2002. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14, 2339–2351 10.1105/tpc.004960 (doi:10.1105/tpc.004960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Smet I., et al. 2007. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134, 681–690 10.1242/dev.02753 (doi:10.1242/dev.02753) [DOI] [PubMed] [Google Scholar]
- 11.Lucas M., Godin C., Jay-Allemand C., Laplaze L. 2008. Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. J. Exp. Bot. 59, 55–66 10.1093/jxb/erm171 (doi:10.1093/jxb/erm171) [DOI] [PubMed] [Google Scholar]
- 12.Jansen L., Roberts I., de Rycke R., Beeckman T. 2012. Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Phil. Trans. R. Soc. B 367, 1525–1533 10.1098/rstb.2011.0239 (doi:10.1098/rstb.2011.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malamy J. E. 2005. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 28, 67–77 10.1111/j.1365-3040.2005.01306.x (doi:10.1111/j.1365-3040.2005.01306.x) [DOI] [PubMed] [Google Scholar]
- 14.de Dorlodot S., Bertin P., Baret P., Draye X. 2005. Scaling up quantitative phenotyping of root system architecture using a combination of aeroponics and image analysis. Aspects Appl. Biol. 73, 41–54 [Google Scholar]
- 15.Lobet G., Pages L., Draye X. 2011. A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiol. 157, 29–39 10.1104/pp.111.179895 (doi:10.1104/pp.111.179895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter A., Spies H., Terjung S., Kusters R., Kirchgessner N., Schurr U. 2002. Spatio-temporal dynamics of expansion growth in roots: automatic quantification of diurnal course and temperature response by digital image sequence processing. J. Exp. Bot. 53, 689–698 10.1093/jexbot/53.369.689 (doi:10.1093/jexbot/53.369.689) [DOI] [PubMed] [Google Scholar]
- 17.Nagel K. A., Schurr U., Walter A. 2006. Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity. Plant Cell Environ. 29, 1936–1945 10.1111/j.1365-3040.2006.01569.x (doi:10.1111/j.1365-3040.2006.01569.x) [DOI] [PubMed] [Google Scholar]
- 18.Schmundt D., Stitt M., Jähne B., Schurr U. 1998. Quantitative analysis of the local rates of growth of dicot leaves at a high temporal and spatial resolution, using image sequence analysis. Plant J. 16, 505–514 10.1046/j.1365-313x.1998.00314.x (doi:10.1046/j.1365-313x.1998.00314.x) [DOI] [Google Scholar]
- 19.Pagès L., Serra V., Draye X., Doussan C., Pierret A. 2010. Estimating root elongation rates from morphological measurements of the root tip . Plant Soil 328, 35–44 10.1007/s11104-009-0079-x (doi:10.1007/s11104-009-0079-x) [DOI] [Google Scholar]
- 20.Zolman B. K., Yoder A., Bartel B. 2000. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156, 1323–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casero P. J., Casimiro I., Lloret P. G. 1995. Lateral root initiation by asymmetrical transverse divisions of pericycle cells in four plant species: Raphanus sativus, Helianthus annuus, Zea mays, and Daucus carota. Protoplasma 188, 49–58 10.1007/BF01276795 (doi:10.1007/BF01276795) [DOI] [Google Scholar]
- 22.MacLeod R. D. 1990. Lateral root primordium inception in Zea Mays L. Environ. Exp. Bot. 30, 225–234 10.1016/0098-8472(90)90068-F (doi:10.1016/0098-8472(90)90068-F) [DOI] [Google Scholar]
- 23.Draye X., Kim Y., Lobet G., Javaux M. 2010. Model-assisted integration of physiological and environmental constraints affecting the dynamic and spatial patterns of root water uptake from soils. J. Exp. Bot. 61, 2145–2155 10.1093/jxb/erq077 (doi:10.1093/jxb/erq077) [DOI] [PubMed] [Google Scholar]
- 24.Williamson L. C., Ribrioux S. P. C. P., Fitter A. H., Leyser H. M. O. 2001. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 126, 875–882 10.1104/pp.126.2.875 (doi:10.1104/pp.126.2.875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beemster G. T. S., Baskin T. I. 1998. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 116, 1515–1526 10.1104/pp.116.4.1515 (doi:10.1104/pp.116.4.1515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Der Weele C. M., Spollen W. G., Sharp R. E., Baskin T. I. 2000. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 51, 1555–1562 10.1093/jexbot/51.350.1555 (doi:10.1093/jexbot/51.350.1555) [DOI] [PubMed] [Google Scholar]
- 27.Deak K. I., Malamy J. 2005. Osmotic regulation of root system architecture. Plant J. 43, 17–28 10.1111/j.1365-313X.2005.02425.x (doi:10.1111/j.1365-313X.2005.02425.x) [DOI] [PubMed] [Google Scholar]
- 28.Stasovski E., Peterson C. A. 1991. The effects of drought and subsequent rehydration on the structure and vitality of Zea mays seedling roots. Can. J. Bot. 69, 1170–1178 10.1139/b91-150 (doi:10.1139/b91-150) [DOI] [Google Scholar]
- 29.Ito K., Tanakamaru K., Morita S., Abe J., Inanaga S. 2006. Lateral root development, including responses to soil drying, of maize (Zea mays) and wheat (Triticum aestivum) seminal roots. Physiol. Plant 127, 260–267 10.1111/j.1399-3054.2006.00657.x (doi:10.1111/j.1399-3054.2006.00657.x) [DOI] [Google Scholar]
- 30.Benkova E., Hejatko J. 2009. Hormone interactions at the root apical meristem. Plant Mol. Biol. 69, 383–396 10.1007/s11103-008-9393-6 (doi:10.1007/s11103-008-9393-6) [DOI] [PubMed] [Google Scholar]
- 31.De Smet I., Signora L., Beeckman T., Inze D., Foyer C. H., Zhang H. M. 2003. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 33, 543–555 10.1046/j.1365-313X.2003.01652.x (doi:10.1046/j.1365-313X.2003.01652.x) [DOI] [PubMed] [Google Scholar]
- 32.Shkolnik-Inbar D., Bar-Zvi D. 2010. Abi4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22, 3560–3573 10.1105/tpc.110.074641 (doi:10.1105/tpc.110.074641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casimiro I., et al. 2001. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13, 843–852 10.1105/tpc.13.4.843 (doi:10.1105/tpc.13.4.843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laplaze L., et al. 2007. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19, 3889–3900 10.1105/tpc.107.055863 (doi:10.1105/tpc.107.055863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friml J., Wisniewska J., Benkova E., Mendgen K., Palme K. 2002. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809 10.1038/415806a (doi:10.1038/415806a) [DOI] [PubMed] [Google Scholar]
- 36.Perez-Torres C. A., Lopez-Bucio J., Cruz-Ramirez A., Ibarra-Laclette E., Dharmasiri S., Estelle M., Herrera-Estrella L. 2008. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20, 3258–3272 10.1105/tpc.108.058719 (doi:10.1105/tpc.108.058719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas M., Guedon Y., Jay-Allemand C., Godin C., Laplaze L. 2008. An auxin transport-based model of root branching in Arabidopsis thaliana. PLoS ONE 3, e3673. 10.1371/journal.pone.0003673 (doi:10.1371/journal.pone.0003673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hackett C. 1972. A method of applying nutrients locally to roots under controlled conditions, and some morphological effects of locally applied nitrate on the branching of wheat roots. Aust. J. Biol. Sci. 25, 1169–1180 [Google Scholar]