Abstract
Recent advances in root biology are making it possible to genetically design root systems with enhanced soil exploration and resource capture. These cultivars would have substantial value for improving food security in developing nations, where yields are limited by drought and low soil fertility, and would enhance the sustainability of intensive agriculture. Many of the phenes controlling soil resource capture are related to root architecture. We propose that a better understanding of the root phenome is needed to effectively translate genetic advances into improved crop cultivars. Elementary, unique root phenes need to be identified. We need to understand the ‘fitness landscape’ for these phenes: how they affect crop performance in an array of environments and phenotypes. Finally, we need to develop methods to measure phene expression rapidly and economically without artefacts. These challenges, especially mapping the fitness landscape, are non-trivial, and may warrant new research and training modalities.
Keywords: root architecture, phenome, plant breeding
1. The challenge
The writing of this paper coincides with the ominous milestone of the human population reaching 7 billion. The population is projected to reach 9–10 billion in the next several decades [1]. This will present an unprecedented challenge to global agriculture. Food security in developing nations, which are experiencing the greatest population growth, is already a crisis. Approximately one billion people are undernourished, the greatest number of undernourished in the history of our species. Although there are diverse factors associated with global food insecurity, a primary cause is the low crop yields, often less than 10 per cent of potential yields, obtained in the low-input agricultural production systems characteristic of many poor nations [2,3]. Drought and low soil fertility, aggravated by ongoing soil degradation, are primary yield constraints for smallholder farmers with limited access to fertilizers and irrigation. In rich nations, crop yields are sustained with intensive use of fertilizers and irrigation at the cost of significant environmental degradation owing to aquifer depletion, salinization, effluents of N and P as well as the energy costs of producing and using these inputs. Global climate change is likely to exacerbate plant abiotic stress in coming decades by increasing temperatures and water stress, and by accelerating soil fertility degradation [4,5]. To respond to this set of challenges, we need to develop agricultural systems with significantly greater productivity and resilience that at the same time use limited natural resources more efficiently.
2. The opportunity
Since water and nutrient availability are primary limitations to productivity in low-input agriculture, and fertilizers and irrigation are primary resource inputs in intensive agriculture, a critical feature of future agricultural systems will be new crop cultivars with improved conversion of soil resources (including inputs) to yield. These new cultivars would have improved productivity in low-input systems and decreased input requirements in high-input systems. There are two distinct avenues to improve crop resource-use efficiency: (i) improved resource acquisition, and (ii) improved physiological utilization of acquired resources. While both avenues merit exploration, we have argued that improving resource acquisition represents the greatest opportunity for crop breeding [3,6]. For example, over half of N fertilizer applied in intensive agriculture is not taken up by the crop. Improved N recovery by crops would represent substantial economic savings, along with reduced production of greenhouse gases from denitrification and fertilizer production, and reduced water pollution from leaching and runoff. Crops typically acquire only 5 per cent of applied P fertilizer during the current season. A relatively small gain in P recovery would translate into significant economic and environmental benefits, since P entering surface waters from runoff and erosion is a significant pollutant, and high-grade P ore deposits are a limited, non-renewable resource [7,8]. In many agricultural soils, an increase in crop rooting depth results in increased water acquisition [9]. Genotypic variation for soil resource acquisition may be largely untapped in crop breeding programmes which have historically focused on adaptation to high-input systems, and which have rarely used root traits as selection criteria. Root biology is, therefore, a central component of efforts to develop crops with improved soil resource acquisition.
Several classes of root traits have the potential to be deployed in crop breeding programmes to improve soil resource acquisition, including rhizosphere modification to mobilize nutrients or detoxify ions [10,11], anatomical traits that can reduce the metabolic cost of soil exploration [12,13]; enhanced symbioses with nitrogen-fixing bacteria or mycorrhizal fungi [11,14]; longer, denser root hairs to enhance the acquisition of P and other immobile resources [11,15] and root architectural traits capable of optimizing soil exploration in time and space [3,15]. Root system architecture (RSA) is particularly important for soil resource acquisition by allocating root foraging to soil domains with the greatest resource availability. RSA is important given the large spatial variation in soil properties and the yield penalty associated with sustaining massive root systems that indiscriminately explore the soil profile. Genotypic variation for RSA has been associated with substantial variation in the acquisition of P [3], N [16], and water [17,18]. Several RSA traits are being used as selection criteria in bean breeding programmes in Africa and Latin America. RSA traits may have synergies with organ- and tissue-level traits, such as root hairs, by positioning those traits in soil domains where they are most effective (see §8). Utilization of RSA traits in crop breeding programmes would be greatly facilitated by a better understanding of the genetic, physiological and environmental regulation of the elements of RSA, namely root elongation, growth angles, lateral branching and longevity.
3. Lateral roots and root system architecture
Research on Arabidopsis and a few model crops has led to substantial knowledge of the molecular and cell biology of lateral root initiation and growth [19,20] (see also [21–24]). Since lateral roots represent the most common type of root branching and, in most cases, the majority of root length, these advances portend applied approaches to harness these pathways to develop improved cultivars. Many of these advances are described in detail in this issue.
Despite this progress, we know very little about the genetic basis of natural variation in lateral root development. There have been a number of studies describing quantitative trait loci (QTL) for lateral root number and density in Arabidopsis and crops, e.g. [25–30]. Understanding the inheritance of these and other root traits is an important step towards breeding for RSA. Such efforts are complicated by the considerable plasticity of RSA. For example, mineral nutrients such as nitrate and phosphorus alter lateral root branching patterns [31]. Individual nutrients vary in their effects on lateral branching, even in a simple root system like Arabidopsis [32]. Root systems respond to heterogenous distribution of nutrients and other resources by changing their branching patterns [33–35], the mechanisms of which have not been studied extensively in crops despite their importance for agriculture. Likewise, other abiotic and biotic factors can modify root branching patterns, and this plasticity is a challenge to those attempting to modify RSA via plant breeding. Knowing how the various environmental cues are integrated, and how they interact when multiple stresses occur, would be a step towards identifying genetic factors that would provide a stable phenotype across environments.
Understanding root branching requires that we understand not only lateral root development, but also the development of the other root classes from which the laterals arise. Initiation and growth of these root classes may be distinct from, yet share signalling elements with, lateral root development. In legumes, basal roots and the tap (primary) root create the scaffolding for RSA. The number of basal roots has a genetic basis, but the genetic determinants are not known (see further discussion of basal roots in §8). Many species form shoot-borne (adventitious) roots, which in turn can branch extensively to comprise a significant part of the root system, especially if some or all of the primary root system is lost or compromised. In cereals, nodal roots, which are a particular type of shoot-borne root, represent most of the root system after the first few weeks. Although there is genetic variation for nodal root development and branching, we know little about the genetic basis of this variation. Since these roots do not arise from the pericycle, their initiation must be different from that of lateral roots. Maize mutants have been found that affect initiation of specific root classes, indicating that there are distinct pathways of genetic control for each class [36]. Maize mutants were identified with reduced lateral root elongation on the seminal root system but not the crown root system (slr1 and slr2) [37]. It should, therefore, be possible to select for various types of RSA independently, including branching of the various root classes.
Since roots represent a substantial and ongoing metabolic cost to the plant, it is important to understand how compensation mechanisms are integrated with external signals to develop one root type versus another. Increases in allocation to one root class often come at the expense of another, i.e. compensation mechanisms are in place to control the total cost of the root system [38]. In addition, the extent of adjustment of root to shoot ratios varies among stresses, with results that may or may not be advantageous for yield.
4. The phenome bottleneck
The advent of ever more powerful tools for genomics research is speeding the discovery of the genetic and cellular networks underlying the primary components of root growth and development. Understanding these processes will be a significant breakthrough for plant biology, and will be an invaluable tool in the effort to develop new crops with superior soil resource acquisition. However, informed deployment of such information in crop improvement also requires detailed understanding of the root phenome. The root phenome is the set of phenes manifested by roots of a plant or plant taxa, where phenes have the same relationship to phenotype as genes have to genotype. Phenes, not genes, determine crop performance. Indeed, crop domestication and traditional crop breeding relied on phenotypic selection. Genomic information is useful for crop breeding only in the context of expressed traits that influence crop performance, i.e. the phenome. Unfortunately, the root phenome is poorly understood, and as our understanding of the plant genome (and other molecular ‘omes’ such as the transcriptome, proteome, metabolome, etc.) rapidly evolve, our ignorance of the root phenome is increasingly a bottleneck to the utilization of these advances in crop breeding.
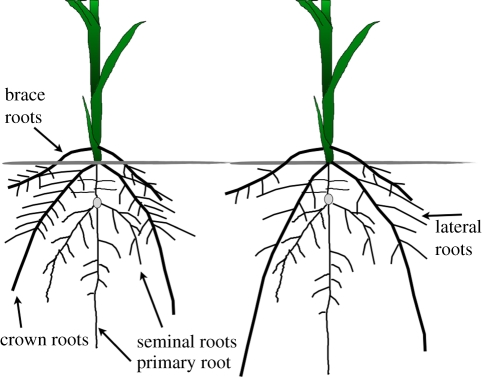
Consider, for example, the need to develop maize genotypes with greater acquisition of soil N. Since nitrate is highly mobile in soil and is readily lost to leaching, it is likely that RSA traits influence N capture. Assuming we have complete knowledge of the genetic and cellular processes controlling lateral root formation, an important component of RSA, how can this information be harnessed to develop maize lines with improved N capture? Should we develop plants with greater lateral root production, and consequently greater exploitation of soil domains in the vicinity of the axial root at the expense of axial elongation, or should we develop plants with reduced lateral root formation, and consequently, less exploration of shallow soil horizons, but greater axial elongation and therefore rooting depth (figure 1)? The utility of lateral rooting will depend on various environmental and endogenous factors. In coarse-textured soils with high precipitation, nitrate leaching will be rapid and therefore deeper axial roots with reduced lateral branching may be useful, whereas in environments with slower nitrate leaching, increased lateral branching may be advantageous. Maize is often grown in high density stands where below-ground competition for nitrate may occur, so the optimal RSA for plant N capture may also depend on the root phenotype of neighbouring plants. Lateral rooting in some taxa can respond to local N availability, and it has been proposed that this response could be useful in breeding crops with greater N capture [16,39]. Indeed, since the response (or ‘plasticity’) of lateral rooting to nutrient availability in maize is under genetic control [30], plasticity per se can be considered a distinct phene. However, the utility of this phene is unknown: proliferation of lateral roots in response to a local nitrate patch may be counterproductive by diverting plant resources to an ephemeral resource, in effect by growing to where the nitrate is today rather than to where it is going to be tomorrow. Altered lateral root production could affect the overall production of roots by the plant via, for example, carbohydrate competition, which would depend on the expression of several other phenes such as the total number of axial roots and their carbohydrate requirements [40]. As this brief example illustrates, our understanding of the utility of lateral rooting for N capture is quite limited, but it is likely to be complex and governed by nonlinear interactions with a number of endogenous and environmental factors. In this example, and in many cases of RSA phenes related to soil resource capture, intelligent deployment of mechanistic information at the genetic and cellular scale in crop breeding programmes is limited by our ignorance of the functional utility of specific phenes in specific environmental and phenotypic contexts—the ‘fitness landscape’.
Figure 1.
Maize root system showing lateral root development. The root system on the right has greater axial extension at the expense of lateral root branching, which may be useful for capturing nitrogen as it moves deeper through the soil.
An alternative to the informed deployment of root phenes for crop improvement is a ‘black box’ or ‘brute force’ approach consisting of measuring the yield of many genotypes in many environments, possibly augmented by massive sets of genomic data, to identify genotypes and genetic patterns that are correlated with performance without mechanistic information. Although a comparative analysis of this approach is beyond the scope of this paper, it has several limitations in developing crops with improved soil resource acquisition, including the difficulty inherent in identifying cause–effect relationships between DNA information and crop yield when yield is responsive to interactions among many distinct root phenes, between root phenes and variable environmental factors, and to numerous factors unrelated to soil resource acquisition. These approaches also require significant investment. It is unclear how effective they may be for low-input agroecosystems, which generally are highly variable and are served by crop improvement programmes with limited resources. In any case, mechanistic information regarding the expression and utility of specific root phenes for soil resource capture would be useful in reducing the uncertainty associated with statistical models of the genetic determinants of yield.
5. Defining root phenes
The concept of a phene as any observable characteristic of an organism is less well defined than the analogous concept of a gene, encompassing everything from gene products to yield. We propose that the most useful root phenes for crop improvement will be elementary and unique; elementary meaning that they are elementary units or building blocks rather than aggregate components of the root phenotype, i.e. they cannot be decomposed to more elementary phenes at the same scale of organization; unique meaning that they are the product of a only one set of genes and processes; that there is only one genetic pathway to this phenotype.
Root depth may be an important factor in soil exploration but in itself is not an elementary component of the root phenotype, nor is it ontogenetically unique, since there are several independent ways in which a plant may develop deep roots including steeper root growth angles, production of fewer axial roots, greater ability to penetrate hard subsoil, etc. Each of these mechanisms to achieve deep rooting may have distinct costs and benefits for the plant, and are likely to be under distinct genetic control. A crop improvement programme targeting specific components of rooting depth such as root growth angle is therefore likely to be more efficient than one that targets rooting depth itself.
The extreme example of an aggregate phene is yield itself, which is the result of the entire plant phenotype, composed of many elementary phenes, interacting with many environmental factors. Metrics related to the size of root systems often aggregate phenes associated with overall plant vigour in a specific growth environment with several elementary phenes controlling root elongation, branching, resource capture, etc. While vigour in a given environment may be a useful selection target to enhance soil resource acquisition in that environment, it may not be generally useful in other environments. For this reason, size-independent metrics such as root growth angle may be more informative than metrics that are allometrically related to plant size, such as the length of lateral branches.
In the context of RSA, there are many potential metrics of the overall size, shape and complexity of a root system, most of which do not meet the criteria of being elementary and unique. Variation in the expression of a relatively small number of architectural traits can result in a large number of diverse root shapes over time. These elementary or shaping phenes are more useful for crop breeding than the aggregate shape phenes they regulate, being under more simple genetic control, and permitting more precise control over RSA.
6. Understanding the fitness landscape
Informed deployment of a phene for crop improvement requires an understanding of its utility in production environments, including crop management, agroecology and potential interactions with other phenes in the target phenotypes. In ecological terms, we need to map the fitness landscape of crop performance against a multi-dimensional array of interacting environmental and internal factors. In the case of root phenes for soil resource acquisition, these factors include high-input and low-input agroecosystems, a wide range of soil environments, contrasting water and nutrient regimes, complexes of co-occuring stresses, tradeoffs for contrasting soil resources, interactions with other root phenes, phene plasticity to environmental conditions and the importance of diverse polyculture systems in low-input agriculture. This is a challenging task even for high-input monocultures such as US maize production; for the diverse, stressful environments typical of low-input agriculture the task is even more daunting. We are aware of no cases where the fitness landscape for a root phene related to soil resource acquisition is understood beyond a rudimentary level of resolution. This is a major challenge facing root phenomics.
7. Phenotyping
In order to use a phene as a selection criterion for crop improvement, either through direct phenotypic selection or through molecular assisted or genomic selection, it is necessary to develop protocols to accurately measure phenotypes. In programmes employing direct phenotypic selection, the phenotype of a large number of genotypes must be measured on an ongoing basis. In marker-assisted or genomic selection programmes, thousands of individuals need to be accurately measured to establish reliable links between genetic markers and phene expression. The need to evaluate a large number of individuals with the smallest possible time and effort has led to the concept of ‘high-throughput phenotyping’, analogous to high-throughput sequencing.
Unfortunately, there are very few protocols for phenotyping roots that qualify as ‘high-throughput’. Roots are highly complex objects whose natural environment, soil, is diverse, complex, non-uniform, and opaque. Non-invasive techniques to visualize roots in soil continue to advance as research tools but do not have the economy of cost for a crop improvement programme, and require effort, resources, and expertise beyond that normally available in breeding programmes, especially in poor nations. The majority of putative ‘high-throughput’ phenotyping protocols for root phenes therefore employ artificial media that facilitate root observation. There are obvious tradeoffs between convenience and realism in many of these systems, some of which, like nutrient solutions, gels, aeroponics, and growth pouches, bear little resemblance to soil. A general concern is that any method that requires roots to grow into or against a physical barrier such as the side of a growth container or transparent observation plate will dramatically change RSA and growth dynamics. For RSA phenes in large crops such as maize, large container volumes are required to avoid artefacts arising from root growth in interfaces.
In the context of phenes for soil resource acquisition, these concerns are especially problematic. It is challenging to develop artificial media that mimic the spatiotemporal dynamics of limiting resource regimes in soil. For this reason, environments employed for phenotyping should be confirmed against phenotypes obtained in natural soil. Since soils are quite diverse, such validation will require analysis of root phenotypes in the range of soil types found in the targeted production environments. An alternative is to simply conduct root phenotyping in the field, which can provide robust and rapid evaluation of many RSA phenes [41].
The selection of an appropriate phenotyping environment is greatly simplified by a clear definition of the phene or phenes of interest. An environment that is artefactual for one phene may be fine for other root phenes.
8. Basal root traits in bean
The case of basal root phenes in common bean illustrates several of the concepts outlined above. Bean genotypes with shallow basal root growth angle (BRGA) have superior P acquisition from low P soil, since P is concentrated in the topsoil, whereas genotypes with steep BRGA have superior water acquisition under terminal drought [42,43]. An unrelated phene, root hair length, is much more beneficial for P acquisition in shallow-rooted genotypes than in deep-rooted genotypes, since shallow roots position root hairs in P-rich topsoil (M. Miguel & J. P. Lynch 2011, unpublished data). The functional trade-off between acquisition of water and P is problematic for bean breeding since both stresses are important yield limitations in tropical bean production.
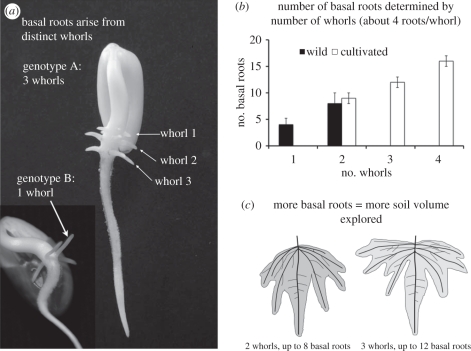
Closer analysis of BRGA revealed that basal roots appear in distinct positions or ‘whorls’ that vary among genotypes from one to four (figure 2) [44,45]. Basal roots arising from distinct whorls have distinct BRGA, so that genotypes with greater basal root whorl number (BRWN) have a greater vertical range of soil exploration. Structural–functional modelling indicates that this should translate into greater acquisition of both P and water (H. Rangarajan and J. P. Lynch 2012, unpublished data), a prediction supported by recent field studies showing that high BRWN genotypes have greater biomass under either drought or P stress (J. Burridge & J. P. Lynch 2011, 2012, unpublished data). BRWN can be assessed in seedlings 3 days after germination, and is not affected by nutrient supply during that period, so it can be readily evaluated in paper ‘roll-ups’ in the laboratory. Screening of bean lines from southern Africa identified several landraces with BRWN of four and five; these are now being used as parents to introgress this trait into elite lines in Mozambique, using direct phenotypic selection.
Figure 2.
(a) Basal root whorls in common bean seedlings. (b) Basal roots at the base of the hypocotyl. When aggregated radially, they can be evaluated as basal root whorl number (BRWN). (c) Greater BRWN is associated with more basal roots, a greater range of basal root growth angles and greater yield under low phosphorus and drought stress. From Lynch [6] (www.plantphysiol.org, © American Society of Plant Biologists).
BRWN is an elementary trait influencing BRGA, which in turn is an important driver of RSA and soil resource acquisition. As an elementary trait, it is genetically simpler than BRGA (M. Miguel & J. P. Lynch 2012, unpublished data) and as an early ‘shaping’ phene is readily and rapidly measurable in young plants. At present, we do not have enough genetic information about this root phene or any other RSA phene to determine if it is unique. This case illustrates how clear definition of an elementary phene greatly simplified evaluation and breeding, and opened avenues for avoiding an important production trade-off of BRGA.
9. Future prospects
To realize the potential of harnessing root phenes to develop future crops with greater soil resource acquisition, we will need a better understanding of the root phenome. What are the most useful root phenes? How do they affect performance in production systems? How can they be deployed in breeding programmes in both rich and poor nations? Of these tasks, mapping the fitness landscape is the most challenging. There are a limited number of root phenes to be defined, and once they are defined and understood, we can develop platforms to rapidly measure them. However, the sheer complexity of the fitness landscape is daunting. The dominant paradigm in plant biology is a linear, deterministic assumption that genes are causes and organismic fitness is an effect. This paradigm does not fully appreciate that genes only affect fitness via the phenome, and the phenome, especially the root phenome, is governed by multiple nonlinear interactions among phenes and multiple environmental variables. Indeed, such systems are characterized by mathematicians as ‘chaotic’. To understand the phenome will require collaboration among researchers at multiple scales of biological organization; no amount of genomic information alone will be sufficient. Heuristic modelling may be particularly useful in this endeavour, to evaluate hypotheses and scenarios across the myriad of potential present and future phenotypes and environments [46,47]. Training of young scientists may have to evolve beyond narrow specialization. The research community may have to move beyond the gene-centric paradigm. The opportunity costs of not doing so are great.
Acknowledgements
The authors acknowledge useful comments from Shawn Kaeppler. This research was supported by the National Science Foundation PGRP (grant DBI 0820624) and National Science Foundation/Basic Research to Enhance Agricultural Development (grant no.4184–UM–NSF–5380).
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division 2010. World population prospects: the 2010 revision. New York, NY: United Nations [Google Scholar]
- 2.World Bank 2007. Agriculture for development. Washington, DC: World Bank [Google Scholar]
- 3.Lynch J. P. 2007. Roots of the second green revolution. Aust. J. Bot. 55, 493–512 10.1071/BT06118 (doi:10.1071/BT06118) [DOI] [Google Scholar]
- 4.St Clair S. B., Lynch J. P. 2010. The opening of Pandora's Box: climate change impacts on soil fertility and crop nutrition in developing countries. Plant Soil 335, 101–115 10.1007/s11104-010-0328-z (doi:10.1007/s11104-010-0328-z) [DOI] [Google Scholar]
- 5.Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. 2007. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Lynch J. P. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol. 156, 1041–1049 10.1104/pp.111.175414 (doi:10.1104/pp.111.175414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordell D., Drangert J.-O., White S. 2009. The story of phosphorus: global food security and food for thought. Global Environ. Change 19, 292–305 10.1016/j.gloenvcha.2008.10.009 (doi:10.1016/j.gloenvcha.2008.10.009) [DOI] [Google Scholar]
- 8.FAO 2010. Current world fertilizer trends and outlook to 2014. Rome, Italy: Food and Agriculture Organization of the United Nations [Google Scholar]
- 9.Richards R. A., Watt M., Rebetzke G. J. 2007. Physiological traits and cereal germplasm for sustainable agricultural systems. Euphytica 154, 409–425 10.1007/s10681-006-9286-1 (doi:10.1007/s10681-006-9286-1) [DOI] [Google Scholar]
- 10.Ryan P. R., Delhaize E., Jones D. L. 2001. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 527–560 10.1146/annurev.arplant.52.1.527 (doi:10.1146/annurev.arplant.52.1.527) [DOI] [PubMed] [Google Scholar]
- 11.Richardson A. E., et al. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil. 349, 121–156(doi:10.1007/s11104-011-0950-4) [Google Scholar]
- 12.Postma J. A., Lynch J. P. 2011. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol. 156, 1190–1201 10.1104/pp.111.175489 (doi:10.1104/pp.111.175489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan M. S., Zhu J. M., Richards C., Brown K. M., Lynch J. P. 2003. Physiological roles for aerenchyma in phosphorus-stressed roots. Funct. Plant Biol. 30, 493–506 10.1071/FP03046 (doi:10.1071/FP03046) [DOI] [PubMed] [Google Scholar]
- 14.Graham P. H., Rosas J. C., de Jensen C. E., Peralta E., Tlusty B., Acosta-Gallegos J., Pereira P. A. A. 2003. Addressing edaphic constraints to bean production: the Bean/Cowpea CRSP project in perspective. Field Crops Res. 82, 179–192 10.1016/S0378-4290(03)00037-6 (doi:10.1016/S0378-4290(03)00037-6) [DOI] [Google Scholar]
- 15.Lynch J. P., Brown K. B. 2008. Root strategies for phosphorus acquisition. In The ecophysiology of plant–phosphorus interaction (eds White P. J., Hammond J. P.), pp. 83–116 The Netherlands: Springer [Google Scholar]
- 16.Hirel B., Le Gouis J., Ney B., Gallais A. 2007. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 58, 2369–2387 10.1093/jxb/erm097 (doi:10.1093/jxb/erm097) [DOI] [PubMed] [Google Scholar]
- 17.Manschadi A. M., Hammer G. L., Christopher J. T., deVoil P. 2008. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303, 115–129 10.1007/s11104-007-9492-1 (doi:10.1007/s11104-007-9492-1) [DOI] [Google Scholar]
- 18.Henry A., Gowda V. R. P., Torres R. O., McNally K. L., Serraj R. 2011. Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Res. 120, 205–214 10.1016/j.fcr.2010.10.003 (doi:10.1016/j.fcr.2010.10.003) [DOI] [Google Scholar]
- 19.Nibau C., Gibbs D. J., Coates J. C. 2008. Branching out in new directions: the control of root architecture by lateral root formation. New Phytol. 179, 595–614 10.1111/j.1469-8137.2008.02472.x (doi:10.1111/j.1469-8137.2008.02472.x) [DOI] [PubMed] [Google Scholar]
- 20.Peret B., Larrieu A., Bennett M. J. 2009. Lateral root emergence: a difficult birth. J. Exp. Bot. 60, 3637–3643 10.1093/jxb/erp232 (doi:10.1093/jxb/erp232) [DOI] [PubMed] [Google Scholar]
- 21.Smith S., De Smet I. 2012. Root system architecture: insights from Arabidopsis and cereal crops. Phil. Trans. R. Soc. B 367, 1441–1452 10.1098/rstb.2011.0234 (doi:10.1098/rstb.2011.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goh T., Kasahara H., Mimura T., Kamiya T., Fukaki H. 2012. Multiple Aux/IAA–ARF modules regulate lateral root formation: the role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Phil. Trans. R. Soc. B 367, 1461–1468 10.1098/rstb.2011.0232 (doi:10.1098/rstb.2011.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen L., Roberts I., De Rycke R., Beeckman T. 2012. Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Phil. Trans. R. Soc. B 367, 1525–1533 10.1098/rstb.2011.0239 (doi:10.1098/rstb.2011.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babé A., Lavigne J. T., Séverin J.-P., Nagel K., Walter A., Chaumont F., Batoko H., Beeckman T., Draye X. 2012. Repression of early lateral root initiation events by transient water deficit in barley and maize. Phil. Trans. R. Soc. B 367, 1534–1541 10.1098/rstb.2011.0240 (doi:10.1098/rstb.2011.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loudet O., Gaudon V., Trubuil A., Daniel-Vedele F. 2005. Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theor. Appl. Genet. 110, 742–753 10.1007/s00122-004-1900-9 (doi:10.1007/s00122-004-1900-9) [DOI] [PubMed] [Google Scholar]
- 26.Ruta N., Liedgens M., Fracheboud Y., Stamp P., Hund A. 2010. QTLs for the elongation of axile and lateral roots of maize in response to low water potential. Theor. Appl. Genet. 120, 621–631 10.1007/s00122-009-1180-5 (doi:10.1007/s00122-009-1180-5) [DOI] [PubMed] [Google Scholar]
- 27.Hund A., Fracheboud Y., Soldati A., Frascaroli E., Salvi S., Stamp P. 2004. QTL controlling root and shoot traits of maize seedlings under cold stress. Theor. Appl. Genet. 109, 618–629 10.1007/s00122-004-1665-1 (doi:10.1007/s00122-004-1665-1) [DOI] [PubMed] [Google Scholar]
- 28.Liu J. C., Li J. S., Chen F. J., Zhang F. S., Ren T. H., Zhuang Z. J., Mi G. H. 2008. Mapping QTLs for root traits under different nitrate levels at the seedling stage in maize (Zea mays L.). Plant Soil 305, 253–265 10.1007/s11104-008-9562-z (doi:10.1007/s11104-008-9562-z) [DOI] [Google Scholar]
- 29.Zheng B. S., Yang L., Zhang W. P., Mao C. Z., Wu Y. R., Yi K. K., Liu F. Y., Wu P. 2003. Mapping QTLs and candidate genes for rice root traits under different water-supply conditions and comparative analysis across three populations. Theor. Appl. Genet. 107, 1505–1515 [DOI] [PubMed] [Google Scholar]
- 30.Zhu J., Kaeppler S. M., Lynch J. P. 2005. Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor. Appl. Genet. 111, 688–695 10.1007/s00122-005-2051-3 (doi:10.1007/s00122-005-2051-3) [DOI] [PubMed] [Google Scholar]
- 31.Hirel B., et al. 2001. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 125, 1258–1270 10.1104/pp.125.3.1258 (doi:10.1104/pp.125.3.1258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linkohr B. I., Williamson L. C., Fitter A. H., Leyser H. M. O. 2002. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 29, 751–760 10.1046/j.1365-313X.2002.01251.x (doi:10.1046/j.1365-313X.2002.01251.x) [DOI] [PubMed] [Google Scholar]
- 33.Drew M. C., Saker L. R. 1975. Nutrient supply and the growth of the seminal root system in barley. II. Localized, compensatory increases in lateral root growth and rates of nitrate uptake when nitrate supply is restricted to only part of the root system. J. Exp. Bot. 26, 79–90 10.1093/jxb/26.1.79 (doi:10.1093/jxb/26.1.79) [DOI] [Google Scholar]
- 34.Robinson D. 1994. The responses of plants to nonuniform supplies of nutrients. New Phytol. 127, 635–674 10.1111/j.1469-8137.1994.tb02969.x (doi:10.1111/j.1469-8137.1994.tb02969.x) [DOI] [PubMed] [Google Scholar]
- 35.Hodge A. 2006. Plastic plants and patchy soils. J. Exp. Bot. 57, 401–411 10.1093/jxb/eri280 (doi:10.1093/jxb/eri280) [DOI] [PubMed] [Google Scholar]
- 36.Hochholdinger F. 2009. The maize root system: morphology, anatomy, and genetics. In Handbook of maize: its biology (eds Bennetzen J. L., Hake S. C.), pp. 1160–1445 Berlin, Germany: Springer [Google Scholar]
- 37.Hochholdinger F., Park W. J., Feix G. H. 2001. Cooperative action of SLR1 and SLR2 is required for lateral root-specific cell elongation in maize. Plant Physiol. 125, 1529–1539 10.1104/pp.125.3.1529 (doi:10.1104/pp.125.3.1529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubio G., Lynch J. P. 2007. Compensation among root classes in Phaseolus vulgaris L. Plant Soil 290, 307–321 10.1007/s11104-006-9163-7 (doi:10.1007/s11104-006-9163-7) [DOI] [Google Scholar]
- 39.Mi G., Chen F., Wu Q., Lai N., Yuan L., Zhang F. 2010. Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. Science China Life Sci. 53, 1369–1373 10.1007/s11427-010-4097-y (doi:10.1007/s11427-010-4097-y) [DOI] [PubMed] [Google Scholar]
- 40.Walk T. C., Jaramillo R., Lynch J. P. 2006. Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 279, 347–366 10.1007/s11104-005-0389-6 (doi:10.1007/s11104-005-0389-6) [DOI] [Google Scholar]
- 41.Trachsel S., Kaeppler S., Brown K., Lynch J. 2011. Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341, 75–87 10.1007/s11104-010-0623-8 (doi:10.1007/s11104-010-0623-8) [DOI] [Google Scholar]
- 42.Ho M. D., Rosas J. C., Brown K. M., Lynch J. P. 2005. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 32, 737–748 10.1071/FP05043 (doi:10.1071/FP05043) [DOI] [PubMed] [Google Scholar]
- 43.Rubio G., Liao H., Yan X., Lynch J. P. 2003. Topsoil foraging and its role in plant competitiveness for phosphorus in common bean. Crop Sci. 43, 598–607 10.2135/cropsci2003.0598 (doi:10.2135/cropsci2003.0598) [DOI] [Google Scholar]
- 44.Basu P., Zhang Y. J., Lynch J. P., Brown K. M. 2007. Ethylene modulates genetic, positional, and nutritional regulation of root plagiogravitropism. Funct. Plant Biol. 34, 41–51 10.1071/FP06209 (doi:10.1071/FP06209) [DOI] [PubMed] [Google Scholar]
- 45.Widrig A. K. 2005. Genotypic variation in basal root number and phosphorus efficiency in common bean (Phaseolus vulgaris L.) MS thesis, Department of Horticulture, Pennsylvania State University. [Google Scholar]
- 46.Tardieu F., Tuberosa R. 2010. Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 13, 206–212 10.1016/j.pbi.2009.12.012 (doi:10.1016/j.pbi.2009.12.012) [DOI] [PubMed] [Google Scholar]
- 47.Manschadi A. M., Christopher J. T., Hammer G. L., Devoil P. 2010. Experimental and modelling studies of drought-adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosyst. 144, 458–462 10.1080/11263501003731805 (doi:10.1080/11263501003731805) [DOI] [Google Scholar]