Abstract
Quantifying the mechanistic links between carbon fluxes and forest canopy attributes will advance understanding of leaf-to-ecosystem scaling and its potential application to assessing terrestrial ecosystem metabolism. Important advances have been made, but prior studies that related carbon fluxes to multiple canopy traits are scarce. Herein, presenting data for 128 cold temperate and boreal forests across a regional gradient of 600 km and 5.4°C (from 2.4°C to 7.8°C) in mean annual temperature, I show that stand-scale productivity is a function of the capacity to harvest light (represented by leaf area index, LAI), and to biochemically fix carbon (represented by canopy nitrogen concentration, %N). In combination, LAI and canopy %N explain greater than 75 per cent of variation in above-ground net primary productivity among forests, expressed per year or per day of growing season. After accounting for growing season length and climate effects, less than 10 per cent of the variance remained unexplained. These results mirror similar relations of leaf-scale and canopy-scale (eddy covariance) maximum photosynthetic rates to LAI and %N. Collectively, these findings indicate that canopy structure and chemistry translate from instantaneous physiology to annual carbon fluxes. Given the increasing capacity to remotely sense canopy LAI, %N and phenology, these results support the idea that physiologically based scaling relations can be useful tools for global modelling.
Keywords: leaf area index, phenology, scaling, nitrogen, climate
1. Introduction
The search for effective means of scaling from tissue traits to ecosystem processes has been a key activity bridging the plant ecophysiology, ecosystem ecology, atmosphere–biosphere flux and biogeochemical modelling communities for several decades [1–22]. General trait-scaling relations have been discovered at the leaf scale [12,13], but not yet at the ecosystem scale. However, the possibility of general relations between carbon fluxes and simple canopy attributes has been offered as a potential way to understand fundamental ecosystem scaling [2–5,8,9,14,17–21] and as a path towards tools useful in remotely measuring the metabolism of terrestrial ecosystems [1,3,8,9,11,19,21]. Such tools are critically needed, as challenges in assessing photosynthetic productivity, along with difficulties in characterizing the extent of terrestrial vegetation, contribute to the enormous range of uncertainty of annual terrestrial gross primary production (GPP) that exists even given state-of-the-art technologies, models and conceptual understanding [23].
As expected from first principles [18], it has long been known that forest productivity is related to LAI at time scales ranging from the second to the year [1,3–6,8,9]. This is logical as LAI is a surrogate for both the fraction of intercepted light and for the size of the foliar canopy capable of exchanging gases with the atmosphere. However, it is also known that LAI alone is not always diagnostic of productivity (and thus not necessarily sufficient for modelling) [9]. Instead, the productivity of leaves and canopies is influenced also by the photosynthetic chemistry of the foliage and the duration of leaf display [9–12,21,24,25], as well by a variety of environmental factors (e.g. temperature and soil water availability). Several analyses and models [5,10,17,20] have been built on these premises, with some success, although comprehensive tests that are not confounded by other factors are rare (but see [5]). Herein, I show for diverse forests at a regional domain, that both their annual and mean daily (growing season) productivity are predictable functions of the canopy elements LAI and foliage %N, especially when regional climate variation is also accounted for. I then show that these scaling relationships mirror those that occur at short time scales (i.e. near instantaneously) for individual leaves and for entire ecosystems.
A number of early studies showed correlations of annual above-ground productivity with leaf area index (LAI) especially across large gradients of climate and vegetation types. The fraction of variance in productivity explained solely by LAI ranges from modest to substantial, depending on the particulars of the study in question [1,3,5–9,19,26]. Pooling of different forest types and/or biomes and climate zones can result in better or worse fits of productivity–LAI regressions compared with within-forest type relations, and the slopes and intercepts of the overall relations vary within and among studies and forest types. Differences in the strength of relations, and the slopes and intercepts, have been attributed to a wide range of factors, including soil fertility, climate, forest functional type, canopy chemistry and others.
Given that the concentration of enzymes involved in photosynthesis is strongly related with leaf %N and that leaf %N influences the physiological capacity for photosynthetic C uptake [12,13], it should not be surprising that %N of canopies can also influence their productivity on instantaneous to annual time frames [4,5,8,9,19,21]. As a result, an approach was developed that combined absorbed photosynthetically active radiation, and the so-called light-use efficiency (in some cases as influenced by canopy nutrient status) to empirically predict or to model productivity [3,4,8,10,20] . However, while some eddy covariance-based examinations of scaling of instantaneous maximum GPP or light-use efficiency to canopy N have shown strong correlations with N [8,9], others have not [6]. Given that canopy N is important to photosynthesis and light-use efficiency, which in turn is key to being able to predict carbon gain from remotely sensed LAI patterns, better understanding of the combined impacts of LAI and %N is critical. In regions where soil P is co-limiting or the main limiting plant nutrient, canopy %P should also influence canopy productivity and light-use efficiency across multiple time scales [25,27,28].
Because of the extensive prior work on canopy scaling [1–21], many readers (including as it turns out, reviewers of this paper) might have thought that quantitative relationships between forest C flux and multiple canopy properties (in particular LAI and %N) were well established based on comprehensive datasets. This is not the case however. A number of important papers focused on the relationships between forest productivity and LAI alone [1,3,17,18,26]). Two temperate forest studies had some data available on forest C flux, LAI and canopy %N, but these studies focused on characterizing the relationship of C flux to %N [9,21], perhaps because relatively few stands in each study had all data necessary to examine the relation of productivity to multiple canopy traits. Two recent tropical studies compared forest production with foliar nutrients, but not LAI [25,28]. And yet two other studies standardized productivity per unit light absorption to examine relations of light-use efficiency with canopy %N across broad gradients of vegetation and climate [4,8]. Light-use efficiency is of course important and potentially useful for C flux modelling, but as it integrates LAI (specifically light interception or absorption) and productivity, it obscures the role that variation in LAI plays in conjunction with %N in influencing C fluxes at instantaneous to annual time frames within and across local to global scales. Thus, our quantitative basis for the direct relationship of productivity to LAI and canopy N is surprisingly limited.
Additionally, those two latter comprehensive studies that combined stand-scale C flux with absorbed light data to quantify light-use efficiency, and related that to canopy %N, included a very wide variation in vegetation types and in site climate [4,8]. These analyses used datasets that included different vegetation types in extremely different biomes and climates (ranging by as much as 28°C in mean annual temperature (MAT)), and each study included species and ecosystem types (e.g. agricultural crop, bog, chaparral, fen, forest, prairie and tundra) that vary tremendously in leaf structure, chemistry, physiology and allocation. Examining broad-scale correlations among productivity and canopy attributes is of course valuable. However, interpreting such correlations is difficult and potentially prone to confounding, such as in Simpson's paradox [29], as across strong gradients (such as across a 28°C inter-biome gradient) canopy traits themselves can strongly co-vary with climate, such that relations of productivity or light-use efficiency to LAI may to some degree be due to this covariation. As an illustration of this problem, one can ask whether very low productivity of arctic tundra compared with warm temperate forest is due to their large differences in LAI or to large differences in growing season conditions and length, or to all of these? Teasing apart the role of climate from canopy traits may not be possible in such cases owing to the strong statistical and mechanistic covariation of climate and canopy traits.
Comparisons across narrower gradients (i.e. where climate has a smaller influence and covaries less tightly with canopy traits such as LAI) are thus needed to separate climate from canopy controls on production, i.e. to ensure we have the physiological underpinnings right. However, whether variation in productivity within a relatively homogenous biome and climate zone can be well explained by canopy properties is still unclear, as comprehensive assessment of productivity-LAI-%N scaling at such scales is rare. To address this information gap I assess relations between above-ground net primary production ANPP, LAI, canopy %N and climate for 128 forest stands (61 angiosperm-dominated and 67 gymnosperm-dominated) in Minnesota and Wisconsin, USA (table 1).
Table 1.
Summary of forest stands by type. Includes number of stands, and mean values (± one standard deviation) of mean annual temperature (MAT, °C), aboveground net primary production (ANPP, Mg ha−1 yr−1), leaf area index (LAI, m2 m−2) and mean canopy foliage %N.
| forest type | no. stands | MAT | ANPP | LAI | %N |
|---|---|---|---|---|---|
| conifer plantation | 4 | 7.8 (0) | 9.11 (1.66) | 7.22 (2.19) | 1.55 (0.47) |
| northern hardwood | 31 | 6.7 (0.6) | 6.86 (3.20) | 4.11 (1.43) | 2.15 (0.37) |
| aspen-dominated | 30 | 3.1 (0.3) | 4.61 (1.19) | 4.02 (0.95) | 2.25 (0.48) |
| jack pine-dominated | 44 | 3.1 (0.3) | 3.91 (1.07) | 3.49 (0.92) | 1.29 (0.18) |
| black spruce-dominated | 19 | 3.1 (0.3) | 1.98 (1.49) | 2.12 (1.50) | 0.83 (0.16) |
2. Results and discussion
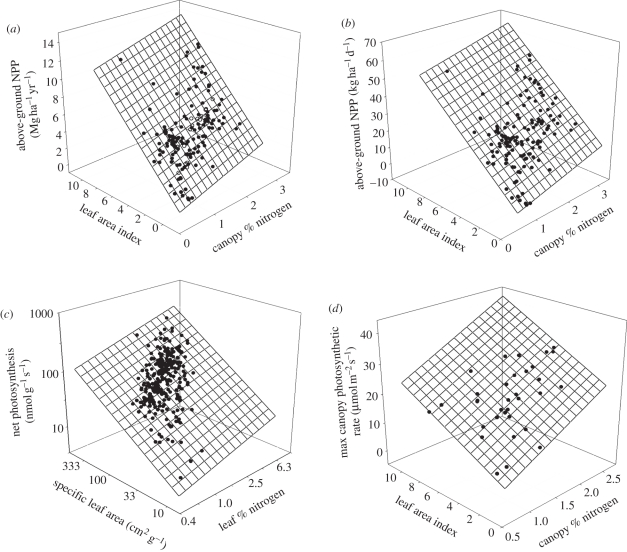
The canopy traits LAI and %N explained most of the observed variation in forest productivity. Across the 128 stands, annual ANPP (hereafter ANPP, for brevity) was positively related to LAI and %N (model A in table 2 and figure 1a), with 72 per cent of total variance explained by the main effects of the two canopy traits, and a modest but significant synergistic interaction explained an additional 4 per cent of the variance (model B). A single model was suitable for evergreen conifer and broadleaved deciduous species, suggesting a common functional basis. Further, 18 forest stands where ANPP, LAI and %N had been measured in New Hampshire, USA [21], are situated well on the plane for this relationship, as shown in figure 1a.
Table 2.
Summary of analyses of above-ground net primary productivity in relation to canopy traits. Total annual above-ground net primary productivity (ANPP, Mg ha−1 yr−1) or mean daily above-ground net primary productivity (ANPP per day, growing season only; kg ha−1 d−1) in relation to canopy traits LAI and %N alone or with additional climate metrics. For models with more than two terms, the minimum-corrected Akaike Information Criterion was used to choose the best model, but results were nearly identical when the minimum Bayesian Information Criterion was used to choose the best model. Each final model included the terms and interactions of the variables shown (bold if p < 0.001; p > 0.05 if in italics; p < 0.05 otherwise), except for models D and G where, for brevity, non-significant terms included in the final model are not shown. Growing season length, GSL; mean annual temperature, MAT; summer (three summer months) mean maximum temperature, summerC; summer (three summer months); precipitation, summerRain. Full models available from the author.
| variable | model | no. terms | model | r2 |
|---|---|---|---|---|
| ANPP | A | 2 | LAI, N | 0.72 |
| ANPP | B | 2 | LAI, N, LAI × N | 0.76 |
| ANPP | C | 3 | LAI, N, GSL, LAI × GSL, %N × GSL | 0.88 |
| ANPP | D | 3 | LAI, N, MAT, LAI × MAT, %N × MAT | 0.88 |
| ANPP | E | 5 | LAI, N, N × GSL, N × summerRain, N × summerC × summerRain, LAI × GSL × summerRain, N × GSL × summerRain, N × GSL × summerC × summerRain | 0.92 |
| ANPP per day | F | 2 | LAI, N | 0.76 |
| ANPP per day | G | 2 | LAI, N, LAI × N | 0.78 |
| ANPP per day | H | 3 | LAI, N,LAI×N,summerC, LAI × summerC, N × summerC | 0.83 |
| ANPP per day | I | 4 | LAI, N, summerC, LAI × summerC, N × summerC, summerC × summerRain, LAI × summerC × summerRain, N × summerC × summerRain | 0.87 |
Figure 1.
Relationship of productivity to leaf area and nitrogen concentration. Simple additive relationships shown: (a) ANPP per year (Mg ha−1 yr−1) in relation to leaf area index (LAI) and canopy % nitrogen (ANPP = 1.174 × LAI + 1.242 × canopy%N – 1.715; p < 0.0001, R2 = 0.72, n = 128). Data for 18 (of 66 stands in [21]) forests in New Hampshire with all three kinds of data are shown superimposed on the response surface (open circles). (b) ANPP per day (kg ha−1 d−1) in relation to LAI and canopy % nitrogen (ANPP per day = 5.329 × LAI + 5.548 × canopy%N – 5.356; p < 0.0001, R2 = 0.76, n = 128). (c) Instantaneous net photosynthetic capacity in relation to specific leaf area and leaf %nitrogen for trees worldwide (data from [13]) (log net photosynthesis = 0.761 × logSLA + 0.476 × log%N + 0.33, R2 = 0.62, n = 296). (d) Relationship of maximum instantaneous ecosystem C flux to LAI and nitrogen concentration. Data from 23 forest stands from Kergoat et al. [8] and 10 stands from Ollinger [9] (see electronic supplementary material). Maximum ecosystem net photosynthesis (µmol m−2 s−1) in relation to LAI and canopy % nitrogen (maximum canopy photosynthetic rate = 1.933 × LAI + 9.58 × canopy%N + 0.365; p < 0.0001, R2 = 0.70, n = 33).
The 128 stands from Minnesota and Wisconsin span a MAT and growing season length (GSL) gradient associated with the approximately 600 km north–south range among sites, so it is worth addressing whether this might contribute to some of the unexplained variance. For simplicity, GSL was defined as the number of days each year when mean daily temperature is greater than 5°C; results were similar if GSL was defined in other ways (see electronic supplementary material). Adding GSL to the model, productivity was significantly related to the combination of LAI, %N, GSL and their interactions (model C, table 2), with 88 per cent of variance explained. ANPP increased with LAI, %N and GSL; and ANPP increased more steeply for a given increase in LAI or %N at sites with increasing GSL. As expected, similar results (model D, table 2) were obtained replacing GSL with MAT in the model, as given the temperate zone location and north–south orientation of the stands, GSL and MAT were highly correlated.
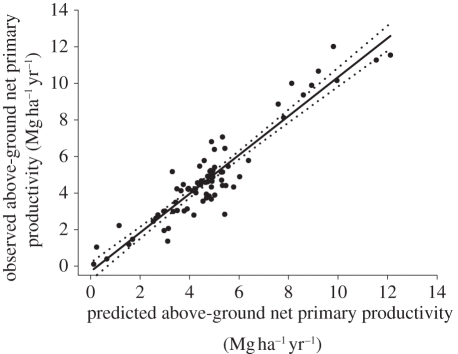
These results suggest a strong concordance between forest productivity and two key elements of forest canopies, especially once climate is accounted for. The canopy factors LAI and %N only weakly covaried with each other (R2 = 0.14), with GSL (R2 = 0.19 and 0.15, respectively) and MAT (R2 = 0.19 and 0.14, respectively). Therefore, for these 128 stands, LAI, %N and GSL (or MAT) represent largely distinct information. Roughly two-thirds of the explained variance (model C) was due to LAI and its interaction with GSL, with almost one-third owing to canopy %N and its interaction with GSL. GSL by itself (as a main effect) explained only approximately 2 per cent of the variance in ANPP, which is much less than would be likely across large continental and cross-biome scales. A model of ANPP in relation to LAI, N and GSL was developed from a random selection of 40 per cent of stands and used to predict ANPP for the remaining 60 per cent of sites. The modelled ANPP was a good fit to the observed ANPP (R2 = 0.88) with a slope near to the 1 : 1 line (figure 2).
Figure 2.
Observed forest productivity versus predicted forest productivity. Predicted productivity for randomly selected 40% of the stands, based on a model obtained using the other 60% of the stands. Observed productivity = 1.064 × predicted ANPP – 0.30, R2 = 0.88 (standard errors, slope = 0.046; intercept = 0.24).
As GSL and MAT were strongly related, it is difficult to tell whether it is the longer growing season or the warmer conditions during the growing season, or both, that contribute to the statistical effects on ANPP of either GSL or MAT. To further address this, I evaluated how productivity per day (constrained to the growing season, i.e. ANPP/GSL) relates to LAI, N and growing season climate. Average productivity per day during the growing season was positively and linearly related to main effects of LAI and %N, with 76 per cent of mean daily productivity explained by these two canopy traits (model F, table 2 and figure 1b). A significant interaction between the two traits explained an additional 2 per cent of the variance in daily productivity (model G, table 2), and including site summer temperatures in the model explained an additional 5 per cent of the variance (model H, table 2), owing to interactions of both %N and LAI with summer temperatures (increase in productivity with rising %N or rising LAI is enhanced at warmer temperatures). The model with most explanatory power for daily mean productivity also included summer rainfall and explained 87 per cent of variance in ANPP per day (model I, table 2). Using different indices of GSL minimally altered the results of any of these analyses.
Since standardizing productivity by GSL eliminates impacts of different lengths of the growing season on productivity, the positive effects of summer temperatures and precipitation in these ‘growing season only’ models indicate that both the higher growing season temperatures and rainfall, and the longer growing season, probably contribute to greater annual productivity at sites with higher MAT, higher mean annual precipitation and longer GSL. Given that both growing season climate and the length of the growing season appear to influence annual productivity, I also tested a model that included GSL and summer temperature and rainfall metrics, as well as the canopy traits LAI and %N. The best model explained 92 per cent of the variance in annual ANPP (model E, table 2).
These relations described in the above models (table 2) were also significant within the angiosperm and gymnosperm groups examined separately. Adding functional group (gymnosperm versus angiosperm) and all possible interactions to the ANPP models containing just LAI and %N (models A or B, table 2) did not increase the variance explained, and neither the main effect of functional group nor any of its interactions were significant. This indicates that the relationship between productivity and the combination of LAI and %N is similar for angiosperm and gymnosperm forests and that one predictive relation works for all forests in this region. In contrast, in models that include functional group and either LAI or %N (but not both) and their interaction, functional group and the interaction term were significant. The significant interaction indicates that the relationship of productivity to either LAI or %N differs for gymnosperm and angiosperm forests, so neither LAI nor %N alone would be particularly good as a predictor of ANPP across all forests in this region.
The influence of canopy LAI and %N on both annual and mean daily productivity (figure 1a,b) at the stand scale reflects how leaf area per unit mass and leaf %N influence instantaneous leaf-level photosynthetic capacity for trees worldwide (figure 1c), based on data from 264 species at 48 sites (data from Wright [13]). This suggests that the instantaneous leaf-level processes that regulate photosynthetic gain [12,13] translate to larger temporal (day and year) and spatial (whole ecosystem) scales for forests in this biome. Similarly, the maximum canopy photosynthetic rate (i.e. similar to maximum instantaneous gross primary production) is also a function of the joint effects of LAI and %N (figure 1d), based on data from 33 forest eddy covariance sites from a range of ecosystems and biomes [8,9]. Thus, at levels from the second to the year, and from the leaf to the canopy, these two key canopy properties, LAI and %N, drive most (greater than 70%) of the variation in forest net C gain. Adding site MAT to the model increases explained variance to 88 per cent for the 128 ground-based ANPP stands and 83 per cent for the 33 eddy flux stands.
It follows from fundamental ecophysiological theory that the larger the size of a canopy to intercept light and exchange gases with the atmosphere, coupled with the greater the biochemical capacity to fix carbon, the greater the resulting carbon uptake and production. This is consistent with long-held ideas in ecosystem physiology and is not new. Nonetheless, to my knowledge, the results from the 128 forests with annual ANPP presented herein, especially when coupled with the data from the 33 sites with instantaneous stand-scale maximum GPP, provide the first comprehensive demonstration that the combination of LAI and canopy %N can explain the vast majority of non-climate-related variation in net C flux among mature forest stands in a region. Moreover, these findings strongly suggest that both canopy traits are required to adequately explain, predict or model forest productivity across any or all temporal scales.
Although the findings support hypotheses based on mainstream concepts, there are several reasons why ANPP might not have been as well-related quantitatively to LAI and %N as was observed. These include that there is a unknown error in measuring any of the biological metrics examined herein (ANPP, LAI or %N), that only a single value of LAI and %N was determined for each stand, rather than a seasonal average, and that species differ in light interception per unit LAI, for instance, owing to difference in foliar clumping [3]. Additionally, forests vary in LAI and %N owing to both environmental heterogeneity and genotypic tendencies, and it is plausible that these might influence forest productivity in different ways (i.e. intraspecific and interspecific scaling might differ). Given these potentially confounding factors and sources of variation, the fact that consistent results with high explanatory power were obtained in this study strongly supports the idea that productivity of forest ecosystems should be well predicted by just a few key metrics that are influential with respect to light harvesting and biochemical aspects of photosynthesis.
As both LAI and %N vary throughout the growing season, models that are driven by time-integrated input values, or a model that runs on a short time step such as a day, using dynamic and varying LAI and N as inputs, should be able to do an even better job of explaining and predicting variation in forest productivity. Thus, though results herein can not be directly translated to detailed models that operate on second or hourly time steps, the close correspondence of forest C flux to LAI and canopy %N at instantaneous, daily and annual time steps suggest that models that capture short-term canopy dynamics should prove accurate in predicting productivity at ecologically relevant time steps from the day to the year. Thus, these results represent a ‘proof-of-concept’ that should help spur the carbon cycling community to test these findings in other biomes, and encourage the modelling community to develop increasingly sophisticated means of assessing LAI and canopy nutrient status from remote sensors, and developing predictive algorithms from these variables, as a means of driving coupled earth and climate systems models.
3. Material and methods
The study sites comprised 128 forest stands (61 angiosperm-dominated and 67 gymnosperm-dominated) in Minnesota and Wisconsin, USA (table 1) [30–36]. These include 123 mixed stands: 44 jack pine-dominated, 30 northern hardwood (oak-maple), 30 aspen-dominated and 19 black spruce-dominated; plus one oak and four conifer plantations. Although not randomly selected, the stands well represent the typical forest types in the regions sampled- with jack pine, aspen and black spruce stand sampled at the northern end and northern hardwoods sampled at the central and southern portion of the approximately 600 km gradient. Stands ranged in age (from approx. 30 and 120 years old) but were all closed canopy except for several semi-open oak woodland [36] and black spruce stands [35]. Standard methods were used to assess ANPP, whole canopy %N, LAI and light interception [30–36] and to compile climate data [37] such as MAT and GSL (see the electronic supplementary material for technical details). The data sources include published data for 114 stands [30–36] (the author (P.R.) was a collaborator in the research for all but 10 stands) and previously unpublished data by the author for 14 stands as an extension of Reich et al. [35].
Acknowledgements
I thank Jim Fownes for brainstorming about these issues many years ago, and the many researchers who participated in collecting the previously published and unpublished data used in this report. Special thanks go to Ray Dybzinski and Emily Peters for providing helpful critiques of earlier versions of this manuscript, and to Laurent Kergoat and Scott Ollinger for their assistance. Financial support was provided by the NSF LTER programme (DEB-0620652) and by the Wilderness Research Foundation.
References
- 1.Runyon J., Waring R. H., Goward S. N., Welles J. M. 1994. Environmental limits on net primary production and light-use efficiency across the Oregon transect. Ecol. Appl. 4, 226–237 10.2307/1941929 (doi:10.2307/1941929) [DOI] [Google Scholar]
- 2.Pierce L. L., Running S. W., Walker J. 1994. Regional-scale relationships of leaf area index to specific leaf area and leaf nitrogen content. Ecol. Appl. 4, 313–321 10.2307/1941936 (doi:10.2307/1941936) [DOI] [Google Scholar]
- 3.Gower S. T., Kucharik C. J., Norman J. M. 1999. Direct and indirect estimation of leaf area index, fAPAR, and net primary production of terrestrial ecosystems. Remote Sens. Environ. 70, 29–51 10.1016/S0034-4257(99)00056-5 (doi:10.1016/S0034-4257(99)00056-5) [DOI] [Google Scholar]
- 4.Green D. S., Erickson J. E., Kruger E. L. 2003. Foliar morphology and canopy nitrogen as predictors of light-use efficiency in terrestrial vegetation. Agric. For. Meteorol. 115, 163–171 10.1016/S0168-1923(02)00210-1 (doi:10.1016/S0168-1923(02)00210-1) [DOI] [Google Scholar]
- 5.Turner D. P., et al. 2005. Site-level evaluation of satellite-based global terrestrial gross primary production and net primary production monitoring. Global Change Biol. 11, 666–684 10.1111/j.1365-2486.2005.00936.x (doi:10.1111/j.1365-2486.2005.00936.x) [DOI] [Google Scholar]
- 6.Schwalm C. R., et al. 2006. Photosynthetic light use efficiency of three biomes across an east–west continental-scale transect in Canada. Agric. For. Meteor. 140, 269–286 10.1016/j.agrformet.2006.06.010 (doi:10.1016/j.agrformet.2006.06.010) [DOI] [Google Scholar]
- 7.Schwalm C. R., et al. 2010. A model-data intercomparison of CO2 exchange across North America: results from the North American carbon program site synthesis. J. Geophys. Res. Biogeochem. 115, G00H05. 10.1029/2009JG001229 (doi:10.1029/2009JG001229) [DOI] [Google Scholar]
- 8.Kergoat L., Lafont S., Arneth A., Le Dantec V., Saugier B. 2008. Nitrogen controls plant canopy light-use efficiency in temperate and boreal ecosystems. J. Geophys. Res. 113, G04017. 10.1029/2007JG000676 (doi:10.1029/2007JG000676) [DOI] [Google Scholar]
- 9.Ollinger S., et al. 2008. Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: Functional relations and potential climate feedbacks. Proc. Natl Acad. Sci. USA 105, 19 336–19 341 10.1073/pnas.0810021105 (doi:10.1073/pnas.0810021105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M., Running S. W. 2010. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329, 940–943 10.1126/science.1192666 (doi:10.1126/science.1192666) [DOI] [PubMed] [Google Scholar]
- 11.Richardson A. D., et al. 2010. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Phil. Trans. R. Soc. B 365, 3227–3246 10.1098/rstb.2010.0102 (doi:10.1098/rstb.2010.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reich P. B., Walters M. B., Ellsworth D. S. 1997. From tropics to tundra: global convergence in plant functioning. Proc. Natl Acad. Sci. USA 94, 13 730–13 734 10.1073/pnas.94.25.13730 (doi:10.1073/pnas.94.25.13730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright I. J., et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827 10.1038/nature02403 (doi:10.1038/nature02403) [DOI] [PubMed] [Google Scholar]
- 14.Spanner M. A., Pierce L. L., Peterson D. L., Running S. W. 1990. Remote sensing of temperate coniferous forest leaf area index. The influence of canopy closure, understory vegetation and background reflectance. Intl J. Remote Sens. 11, 95–111 10.1080/01431169008955002 (doi:10.1080/01431169008955002) [DOI] [Google Scholar]
- 15.White M. A., et al. 2009. Intercomparison, interpretation, and assessment of spring phenology in North America estimated from remote sensing for 1982–2006. Global Change Biol. 15, 2335–2359 10.1111/j.1365-2486.2009.01910.x (doi:10.1111/j.1365-2486.2009.01910.x) [DOI] [Google Scholar]
- 16.Richardson A. D., Braswell B. H., Hollinger D. Y., Jenkins J. P., Ollinger S. V. 2009. Near-surface remote sensing of spatial and temporal variation in canopy phenology. Ecol. Appl. 19, 1417–1428 10.1890/08-2022.1 (doi:10.1890/08-2022.1) [DOI] [PubMed] [Google Scholar]
- 17.Monteith J. L. 1977. Climate and the efficiency of crop production in Britain. Phil. Trans. R. Soc. Lond. B 281, 277–294 10.1098/rstb.1977.0140 (doi:10.1098/rstb.1977.0140) [DOI] [Google Scholar]
- 18.Monsi M., Saeki T. 2005. On the factor light in plant communities and its importance for matter production. Ann. Bot. 95, 549–567 10.1093/aob/mci052 (doi:10.1093/aob/mci052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner D. P., Urbanski S. P., Bremer D., Wofsy S. C., Meyers T., Gower S. T., Gregory M. 2003. A cross-biome comparison of daily light use efficiency for gross primary production. Global Change Biol. 9, 383–395 10.1046/j.1365-2486.2003.00573.x (doi:10.1046/j.1365-2486.2003.00573.x) [DOI] [Google Scholar]
- 20.Landsberg J. J., Waring R. H. 1997. A generalized model of forest productivity using simplified concepts of radiation-use efficiency, carbon balance and partitioning. For. Ecol. Manage. 95, 209–228 10.1016/S0378-1127(97)00026-1 (doi:10.1016/S0378-1127(97)00026-1) [DOI] [Google Scholar]
- 21.Smith M.-L., et al. 2002. Direct estimation of aboveground forest productivity through hyperspectral remote sensing of canopy nitrogen. Ecol. Appl. 12, 1286–1302 10.1890/1051-0761(2002)012[1286:DEOAFP]2.0.CO;2 (doi:10.1890/1051-0761(2002)012[1286:DEOAFP]2.0.CO;2) [DOI] [Google Scholar]
- 22.Muraoka H., Koizumi H. 2009. Satellite Ecology (SATECO)—linking ecology, remote sensing and micrometeorology, from plot to regional scale, for the study of ecosystem structure and function. J. Plant Res. 122, 3–20 10.1007/s10265-008-0188-2 (doi:10.1007/s10265-008-0188-2) [DOI] [PubMed] [Google Scholar]
- 23.Beer C., et al. 2010. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329, 834–838 10.1126/science.1184984 (doi:10.1126/science.1184984) [DOI] [PubMed] [Google Scholar]
- 24.Reich P. B., Ellsworth D. S., Walters M. B. 1998. Leaf structure (specific leaf area) regulates photosynthesis-nitrogen relations: evidence from within and across species and functional groups. Funct. Ecol. 12, 948–958 10.1046/j.1365-2435.1998.00274.x (doi:10.1046/j.1365-2435.1998.00274.x) [DOI] [Google Scholar]
- 25.Mercado L. M., et al. 2011. Variation in Amazon forest productivity correlated with foliar nutrients and modelled rates of photosynthetic carbon supply. Phil. Trans. R. Soc. B 366, 3316–3329 10.1098/rstb.2011.0045 (doi:10.1098/rstb.2011.0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fassnacht K. S., Gower S. T. 1997. Interrelationships among the edaphic and stand characteristics, leaf area index, and aboveground net primary production of upland forest ecosystems in north central Wisconsin. Can. J. For. Res. 27, 1058–1067 10.1139/x97-058 (doi:10.1139/x97-058) [DOI] [Google Scholar]
- 27.Reich P. B., Oleksyn J., Wright I. J. 2009. Leaf phosphorus influences the photosynthesis-nitrogen relation: a cross-biome analysis of 314 species. Oecologia 160, 207–212 10.1007/s00442-009-1291-3 (doi:10.1007/s00442-009-1291-3) [DOI] [PubMed] [Google Scholar]
- 28.Cleveland C. C., et al. 2011. Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol. Lett. 14, 939–947 10.1111/j.1461-0248.2011.01658.x (doi:10.1111/j.1461-0248.2011.01658.x) [DOI] [PubMed] [Google Scholar]
- 29.Blyth C. R. 1972. On Simpson's paradox and the sure-thing principle. J. Am. Stat. Assoc. 67, 364–366 10.2307/2284382 (doi:10.2307/2284382) [DOI] [Google Scholar]
- 30.Pastor J., Aber J. D., McClaugherty C. A., Melillo J. M. 1984. Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65, 256−268 10.2307/1939478 (doi:10.2307/1939478) [DOI] [Google Scholar]
- 31.Lennon J. M., Aber J. D., Melillo J. M. 1985. Primary production and nitrogen allocation of field grown sugar maples in relation to nitrogen availability. Biogeochemistry 1, 135–154 10.1007/BF02185038 (doi:10.1007/BF02185038) [DOI] [Google Scholar]
- 32.Nadelhoffer K. J., Aber J. D., Melillo J. M. 1985. Fine roots, net primary production, and soil nitrogen availability: a new hypothesis. Ecology 66, 1377–1390 10.2307/1939190 (doi:10.2307/1939190) [DOI] [Google Scholar]
- 33.Fownes J. 1985. Water use and primary production of Wisconsin hardwood forests 1987. Phd Thesis, 131 p. University of Wisconsin-Madison, Madison, USA [Google Scholar]
- 34.Gower S. T., Reich P. B., Son Y. 1993. Canopy dynamics and aboveground production of five tree species with different leaf longevities. Tree Physiol. 12, 327–345 [DOI] [PubMed] [Google Scholar]
- 35.Reich P. B., Bakken P., Carlson D., Frelich L., Friedman S., Grigal D. F. 2001. Influence of logging, fire, and forest type on biodiversity and productivity in southern boreal forests. Ecology 82, 2731–2748 10.2307/2679957 (doi:10.2307/2679957) [DOI] [Google Scholar]
- 36.Reich P. B., Peterson D. A., Wrage K., Wedin D. 2001. Fire and vegetation effects on productivity and nitrogen cycling across a forest-grassland continuum. Ecology 82, 1703–1719 10.2307/2679812 (doi:10.2307/2679812) [DOI] [Google Scholar]
- 37.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 10.1002/joc.1276 (doi:10.1002/joc.1276) [DOI] [Google Scholar]