Abstract
The rich diversity of primate faces has interested naturalists for over a century. Researchers have long proposed that social behaviours have shaped the evolution of primate facial diversity. However, the primate face constitutes a unique structure where the diverse and potentially competing functions of communication, ecology and physiology intersect, and the major determinants of facial diversity remain poorly understood. Here, we provide the first evidence for an adaptive role of facial colour patterns and pigmentation within Neotropical primates. Consistent with the hypothesis that facial patterns function in communication and species recognition, we find that species living in smaller groups and in sympatry with a higher number of congener species have evolved more complex patterns of facial colour. The evolution of facial pigmentation and hair length is linked to ecological factors, and ecogeographical rules related to UV radiation and thermoregulation are met by some facial regions. Our results demonstrate the interaction of behavioural and ecological factors in shaping one of the most outstanding facial diversities of any mammalian lineage.
Keywords: sociality, coloration, species recognition, facial diversity, mammals
1. Introduction
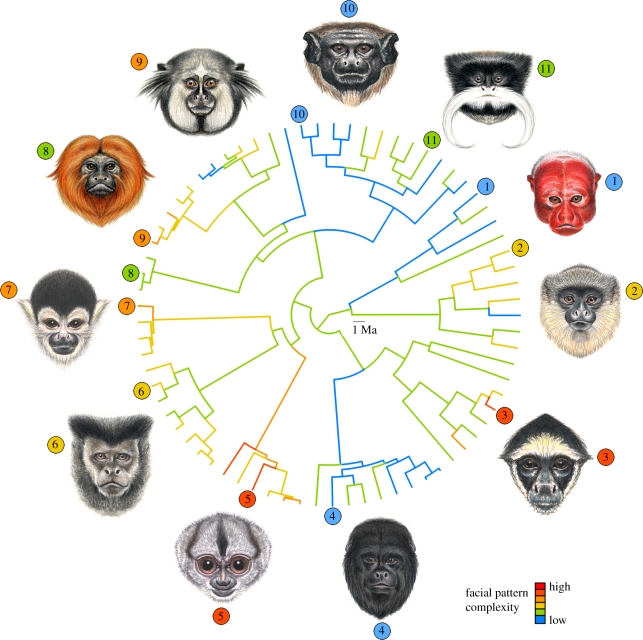
Faces have evolved in many social lineages as complex and variable structures that must satisfy multiple functional demands [1–3]. Faces constitute complex phenotypes because they integrate numerous parts (eyes, nose, ears, patches of skin and hair, etc.) that differ in characteristics such as embryological origin, function, location, size, colour and shape. These elements may all contribute to the striking diversity of facial colour patterns found in living primates. In Neotropical primates, faces range from having very simple colour patterns such as those of uakaris (Cacajao calvus; figure 1, number 1), where all parts of the face are virtually the same colour, to faces with a high colour pattern complexity such as those of white-bellied spider monkeys (Ateles belzebuth; figure 1, number 3) where facial parts contain various colours and there is a combination of furred areas and bare skin. While there is striking colour pattern diversity across species, inter-individual facial coloration within species can also exhibit continuous and often more subtle variation in luminance, hue and saturation across some or all facial parts [4–6].
Figure 1.
Maximum-likelihood ancestral state reconstruction of facial colour pattern complexity across the New World primate species studied. Warmer colours indicate higher complexity in facial colour patterns (i.e. faces with higher number of areas, as illustrated in figure 2b, that are uniquely different from other facial areas based on hair and skin colour). Species shown in illustrations are (1) Cacajao calvus, (2) Callicebus hoffmansi, (3) Ateles belzebuth, (4) Alouatta caraya, (5) Aotus trivirgatus, (6) Cebus nigritus, (7) Saimiri boliviensis, (8) Leontopithecus rosalia, (9) Callithrix kuhli, (10) Saguinus martinsi and (11) Saguinus imperator. Illustrations by Stephen Nash.
For many social animals, including paper wasps, birds and primates, facial colour patterns provide rich sources of information that are essential for social interactions [3,7–10]. In primates, facial cues are used to convey signals about identity, behaviour and condition [4,11–14]. Facial colour patterns are hypothesized to be primarily used by primates to assess species identity [9,15,16], while intraspecific variation and polymorphisms in the coloration and shape of facial features appear most important for assessment of individual identity and condition [4–6,12–14]. Thus, faces are hypothesized to fulfil two functional roles with regard to recognition [3]: class-level recognition, where receivers match the signaller's phenotype to an internal template associated with different classes (in this case, species); and ‘true’ individual recognition, where the receiver learns the signaller’s individually distinctive characteristics and associates them with individual-specific information about the signaller.
Given the importance of faces for communication in social species such as primates, it seems reasonable to assume that selection has played a role in shaping both interspecific and intraspecific distinctiveness in facial colour patterns, although the drivers of interspecific diversity and intraspecific variation in facial coloration may differ. For example, the relative abundance of conspecifics or the presence of heterospecifics may create pressures on distinctiveness in facial colour patterns among species to facilitate species recognition and avoid interbreeding. Other characteristics of the social system (e.g. migration pattern, hierarchy, sex ratios, etc.) may create pressures for variation in coloration within a species, as long as individuals benefit from being identified and having their behavioural tendencies and personal history known by others [3,17].
What characteristics provide distinct facial signals that allow for species or individual recognition? Facial distinctiveness may evolve through selection on contrasting colour patterns that maximize the potential for facial differences across species and high levels of variation within species. Distinct faces may be also be achieved through the evolution of other morphological traits, including variation in the size and shape of hair tufts, switches in colour and intensity, etc. Facial colour pattern complexity, however, combines many discrete features that describe facial coloration as a whole, and thus integrates multiple signals that could function in conveying species identity. Biologists have proposed that social functions are the primary drivers of the evolution of primate facial colour patterns across species [1,18], and that variation within species is consistent with a behavioural drive model of evolution. This model predicts that behaviours initiate evolutionary shifts in morphology, physiology or ecology [19–21]. Behavioural pressures have been invoked to explain the evolution of the facial musculature [22] and red skin [4,23] in non-human primates. Along with behavioural pressures, ecological pressures that drive adaptive evolution in mammalian coat colour, such as regulation of physiological processes and concealment from predators [1,2,24], may also drive the evolution of facial differences across species.
Despite these predictions, little is known about how behavioural and ecological factors interact to shape the diversity in colour pattern complexity of primate faces (figure 1). Here, for the first time, we investigate the long-standing paradigm [1,18] that sociality explains interspecific differences in primate external facial traits (facial colour pattern). We focus on the highly diverse and geographically restricted radiation of Neotropical primates (Platyrrhini; 199 species [25]) and predict that facial colour pattern complexity evolves in response to selective pressures for either species recognition or individual recognition. We consider two contrasting scenarios for this relationship. (i) Individual recognition hypothesis: first, it is possible that facial colour patterns evolved to be more complex in species with larger groups, as more complex faces would provide a template in which parts can vary and evolve independently, facilitating individual recognition. Socially relevant variation in the colour of individual facial parts has been reported in Old World primates, such as mandrills and drills [5,26]. (ii) Species recognition hypothesis: alternatively, species living in small groups or that are solitary may have evolved more complex facial colour patterns to produce contrasting coloration that would facilitate targeting and identifying conspecifics. By being more conspicuous, these complex facial patterns would facilitate species recognition, especially for species in which individuals meet sporadically or only for mating, and are thus more dispersed across their habitat [1]. Complementing this scenario, species living in large groups would be expected to experience either relaxed selection on facial colour pattern complexity or selection for reduced pattern complexity, as simple facial patterns may be advantageous for more effective communication through facial expressions at close range [27]. Recent findings of computer simulation studies [28] highlight this possibility.
If differences in complexity in facial colour patterns allow for distinctiveness and species recognition, then we also expect high complexity to evolve in species at high risk of interbreeding with closely related species owing to sympatry. More complex faces in these species would present more parts, or modules [29], that could evolve independently to generate facial differences among sympatric species to aid in selecting a conspecific mate from an environment full of heterospecific distracters. Alternately, the selective pressures on facial recognition may be broader in nature, and sympatry with an increased number of any primate species within the same family may be enough to drive facial colour pattern diversity.
Ecological factors might also explain patterns of facial colour pattern complexity. In this case, facial colour patterns would be the result of selective pressures on functions such as thermoregulation, reduction of solar glare and concealment from predators [2]. We test two ecogeographical rules that may influence facial colour pattern. First, we test Gloger's rule, which predicts that darker colours will be associated with forested habitats where light conditions are low and humidity is high [30]. Second, we explore the idea that dark regions around the eyes, or ‘eye masks’, are adaptive in species exposed to high solar glare [31]. Additionally, we test another aspect of interspecific facial variation by assessing the ‘hair rule’, which predicts that longer and thicker hair will be associated with colder areas [32].
Another hypothesis proposed to explain the evolution of primate coloration is the metachromism hypothesis, suggested by Hershkovitz [33] in 1968 based on his studies of body coloration in saddle-back tamarins. This hypothesis presents another alternative, non-adaptive explanation for primate facial coloration where lineages are expected to exhibit a predictable, linear and irreversible sequence of colour changes through time and across species, although the exact evolutionary mechanism for this change is not explained. Starting with the ancestral condition of agouti banding, the hair may progressively saturate following one of the two pathways: (i) eumelanic saturation leading to black hair or (ii) phaeomelanic saturation leading to red hair. Upon saturation, each pathway is followed by a bleaching process that ultimately results in hair totally devoid of pigment (electronic supplementary material, figure S1). We evaluate the metachromism hypothesis for the first time at a broad phylogenetic scale.
2. Methods
(a). Facial morphology
External facial morphology of Neotropical primate species (n = 129) was quantified from photographs of adult males gathered from the All the World's Primates database (http://www.awpdb.com), ARKive (http://www.arkive.org) and Primate Info Net (http://pin.primate.wisc.edu/). We limited the photographs to adult males in order to standardize across species in terms of sex and age class. With a few exceptions, adult male and female Neotropical primates have the same facial coloration patterns [34]. Samples included two to ten photographs per species, and only high-resolution close-ups taken under good light conditions. Photographs of individuals facing the camera were preferred, but profile shots were also used when frontal shots were not sufficient to fully assess facial characteristics. For species that presented polymorphisms, one of the morphs was chosen at random. Our results did not differ significantly when other morphs within a species were used for analyses, and facial pattern complexity values did not vary significantly across alternative pictures of species within a subset of 10 randomly selected species (n = 10 different pictures per species, average coefficient of variation = 0.096, min = 0, max = 0.28). All categorizations were made by one observer (S.E.S.), and these were not significantly different from those assigned by naïve observers (e.g. facial colour pattern complexity: paired t-test: t = 0.232, p = 0.826, n = 6 observers). Facial colour patterns were quantified in following ways.
(i). Colour pattern complexity
Faces were divided into 14 areas, spanning the diversity in colour patterns across New World primate species (figure 2b). For each area, we recorded (i) its hair colour: bleached (hair with no pigment), agouti (banded hair), black/brown or red/yellow, each of which could be light, medium or dark; and (ii) its skin colour: depigmented (skin without pigment), hypervascularized (red skin), mottled (depigmented skin with very small pigmented patches) and hyperpigmented (dark skin). Reference colours for this scale are shown in the electronic supplementary material, table S1. This scale comprises the variation in facial colours observed across all the species studied. From these data, we produced a measure of facial colour pattern complexity for each species (i.e. the total number of uniquely different colour areas on the face; data available upon request). For example, Cebus capucinus (shown in figure 2) has a facial colour pattern complexity score of 3; the colour categories found across the 14 facial regions are black hair, light yellow hair and depigmented skin.
Figure 2.
(a) The face of a white-faced capuchin monkey (Cebus capucinus) illustrating the procedure used to characterize facial colour pattern complexity. (b) Faces were subdivided into 14 areas which were used to record traits describing the hair and skin colour, and hair length. (c) These areas were then grouped into regions that covaried across species (A, crown; B, forehead; C, eye mask; D, nose–mouth; E, face margin). Bilaterally symmetrical areas or regions are shown for only one side of the face.
(ii). Pigmentation of skin and hair, and hair length across facial regions
Areas defined in figure 2b that covaried in colour in more than 50 per cent of species were grouped into larger regions (figure 2c). Each region was coded according to the predominant (greater than 90% of the region) pigmentation of the hair (1 = bleached, 2 = cream/light yellow, 3 = light agouti, 4 = light brown/grey, 5 = medium yellow/reddish, 6 = medium agouti, 7 = medium brown/grey, 8 = dark yellow/red, 9 = black-saturated agouti, 10 = dark brown/black), skin (1 = depigmented, 2 = hypervascularized, 3 = mottled, 4 = hyperpigmented) and hair length (1 = depilated: no hair, 2 = short, 3 = medium, 4 = long, 5 = hypertrychy: very long hair; regions with hairs of variable length were scored by the greatest hair length in that region). Hair and skin coloration, and hair length in each facial region and species, were coded using the continuous scale shown in the electronic supplementary material, table S2. The continuous scale for facial pigmentation was built by arranging most of the colours sampled in step (i) in the order of decreasing brightness. This allowed us generate a continuous metric that approximated the degree of pigmentation of facial regions and could be related to continuous ecological factors.
(iii). Metachromic pathway
The metachromic sequence of hair-coloration change [33] was also used to describe trends in facial coloration and test the null hypothesis of non-adaptive change. For this purpose, facial regions (same as shown in figure 2b) were scored following Hershkovitz's scale [33] (electronic supplementary material, figure S1; 0 = agouti, 1 = agouti with eumelanin saturation, 2 = black, 3 = brown, 4 = drab/grey, 5 = agouti with phaeomelanin saturation, 6 = red, 7 = orange, 8 = yellow/cream, 9 = white, 10 = depilated).
(b). Social and ecological variables
Data on average group size were collected from the literature (electronic supplementary material, table S3; n = 135). Mean community size was used for species presenting fission–fusion societies. Species distribution and sympatry data (n = 125; electronic supplementary material, table S4) were generated from geographical distribution shape files, downloaded from NatureServe's InfoNatura database [35] (http://www.natureserve.org/infonatura). These shape files contain the known range of each species, depicted as polygons where a species is widespread, or as points where there are isolated records. For each species, we determined the number of sympatric species at the genus, family and parvorder levels (electronic supplementary material, table S4) using tools in ArcView (ESRI, USA) that counted the number of overlapping distributions. This geographical information systems software was also used to generate data summarizing the species’ geographical ranges by calculating the centroid of each polygon and noting its latitude and longitude. Absolute values of latitudes were used in our analyses to describe trends associated with tropical versus subtropical environments.
(c). Phylogenetic analyses
Variables were tested for phylogenetic signal (lambda [36]) and phylogenetic autocorrelation (Moran's I [37]). Results from these tests indicated the need to account for evolutionary relationships in our analyses (electronic supplementary material, table S5). Phylogenetic generalized least-squares (PGLS) regressions, using a Brownian motion model and a pruned version of a recent species-level phylogeny [38], were used to test for evolutionary relationships among facial, social and ecological variables. Facial traits (colour pattern complexity, pigmentation and hair length) across facial regions were used as response variables, whereas social (group size) and ecological variables (species sympatry and geographical distribution) were the predictor variables. Both social and ecological predictor variables were introduced in the model simultaneously. For variables that were significantly correlated with latitude and longitude, we used their residuals from a regression on latitude and/or longitude.
To test the hypothesis that evolutionary changes in the coloration of facial regions are not adaptive but follow the metachromic progression proposed by Hershkovitz [33], we conducted maximum-likelihood ancestral state reconstructions (ASRs) of colour traits for each facial region (figure 2c). These ASRs were performed using a Brownian motion model with equal transition rates across all characters (i.e. all changes across character states equally likely), and compared with an ASR where the transition rates were restricted to the plausible changes according to Hershkovitz's model. These ASRs used a matrix that enforced changes from the ‘ancestral’ agouti coloration to white colour through either the eumelanic or phaeomelanic pathways, with no reversals allowed (electronic supplementary material, figure S1). Log-likelihoods between the two ASR were compared using a likelihood-ratio test. Phylogenetic analyses were conducted using functions in the APE, GEIGER and NMLE packages in R (R Core Development Team).
3. Results
(a). Facial colour pattern complexity, sociality and species recognition
We found evidence for an evolutionary relationship between sociality and facial colour pattern complexity across Neotropical primates. Species living in smaller groups tended to have faces with more complex patterns than species living in large groups (PGLS, β = −0.0312 ± 0.0147, t = −2.129, d.f. = 54, p = 0.038). Our results further supported the prediction that facial colour pattern complexity was related to species recognition: facial patterns were more complex in species experiencing a higher number of sympatric congener species, independent of group size (PGLS, β = 0.1344 ± 0.0632, t = 2.126, d.f. = 54, p = 0.038). In contrast, we found no significant relationship between facial colour pattern complexity and the number of sympatric species at the family or parvorder levels. The number of sympatric species was well predicted by latitude at the parvorder level (Platyrrhini), reflecting the pattern of increasing biodiversity in the tropics [39], but this trend was not observed at the genus level (p = 0.609). Colour pattern complexity of faces was not related to the geographical distribution of the species (PGLS, Platitude = 0.909, Plongitude = 0.319).
(b). Facial pigmentation and ecology
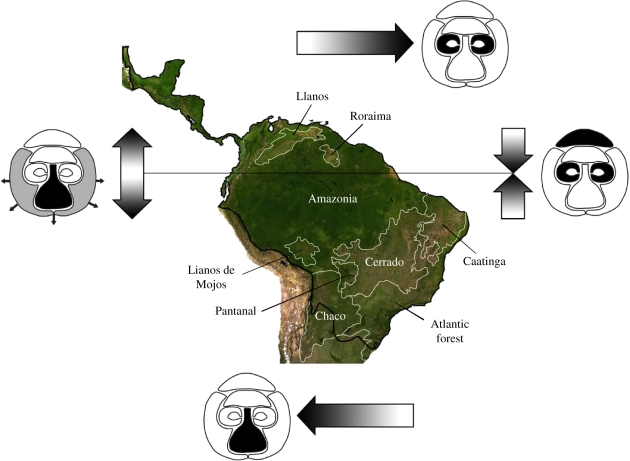
Interspecific differences in pigmentation and hair length across facial regions were poorly explained by sociality. However, we found strong evidence that ecological factors have shaped the evolution of these traits. Continuous descriptors of facial coloration and hair length across several facial regions (figure 2c) were strongly predicted by variables describing the geographical distribution of species. The pigmentation of the hair in the crown (PGLS, β = −0.6636 ± 0.3221, t = −2.060, d.f. = 61, p = 0.044) and eye mask (PGLS, β = −1.2914 ± 0.5576, t = −2.316, d.f. = 25, p = 0.031) was negatively related to the latitude of species ranges, but the pigmentation of the nose and mouth (PGLS, β = 0.8199 ± 0.2141, t = 3.829, d.f. = 18, p = 0.002) and the hair length around the face margin (PGLS, β = 0.1132 ± 0.0395, t = 2.866, d.f. = 62, p = 0.006) were positively related to latitude. Both the pigmentation of the hair in the eye mask (PGLS, β = −0.5406 ± 0.1732, t = −3.121, d.f. = 21, p = 0.006), and nose and mouth (PGLS, β = −0.2426 ± 0.0819, t = −2.963, d.f. = 18, p = 0.010) were negatively related to the longitude of species’ distributions. Combining these results, as species’ ranges approach the Equator, and go from dry, open regions such as the Caatinga and the Cerrado into Amazonian forested habitats, animals tend to have darker crowns, darker regions around the eyes, lighter nose and mouth, and shorter hairs around the face (figure 3). As species move westward, they tend to have darker nose and mouth, and lighter eye masks.
Figure 3.
Geographical trends in pigmentation and hair length across facial regions. Arrows indicate the direction towards which facial parts become darker (regions highlighted in black) or hair becomes longer (region highlighted in grey). The distribution of Neotropical primates (black outline), the Equator and major lowland tropical biomes (white outlines) are indicated in the map.
(c). Metachromism
Our data did not support the metachromism hypothesis (electronic supplementary material, figure S1) as a general explanation of facial coloration in Neotropical primates. Using ASRs, we found much higher support for a model assuming no constraints in colour change across all facial regions (crown, forehead, eye mask, nose–mouth and face margin, all p < 0.0001; electronic supplementary material, table S6) over the metachromic model assuming a predictable sequence of pigment saturation and desaturation [33].
4. Discussion
The evolution of complex phenotypes, such as the colour patterns in the primate face, is the result of the interplay among functional, developmental and genetic factors acting on the different parts [40]. Sociality is widely assumed to be one of the primary functional drivers of colour pattern complexity in primate faces, although the link has never been explicitly tested through comparative studies. Our results indicate a strong evolutionary relationship between facial colour pattern complexity and group size, and between facial pigmentation and ecology in Neotropical primates. Species living in small groups have more complex colour patterns in their faces than those living in large groups. This result highlights the possibility that living in small groups promotes the evolution of complex colour patterns via selection for conspicuousness to conspecifics. Because social interactions are less frequent in species living in small groups or that are solitary [41], highly complex colour patterns could provide visual cues that would allow individuals to more easily target and identify conspecifics in their habitat.
(a). The role of facial colour patterns in sociality and species recognition
Primates often use colours and patterns instead of information directly derived from social interactions to assess the identity, status, dominance rank and sexual condition of individuals [4,26,42]. Lower facial colour pattern complexity in species living in larger groups might be the product of relaxed selection on this trait, high reliance on facial expressions, or both. In larger groups, more subtle intraspecific variation in facial traits (e.g. variation in size of and distances among facial structures, or colour intensity of patches) could be of importance for recognizing individuals within groups. In non-human anthropoids, the variety of facial movements species produce is positively correlated with group size [27]. The more simple faces found in platyrrhines living in larger groups may also be a consequence of selection for traits that allow for more effective communication using facial movements, including facial expressions during conflicts and other interactions at close range. Although quantitative data on facial movements in New World primates are scarce, species with relatively low facial colour pattern complexity in our dataset (e.g. Alouatta caraya; figure 1, number 4) have been reported to have high scores in metrics of facial mobility [27]. Because sociality is associated with increases in the number of facial muscles [22,43], our results highlight the possibility of an inverse relationship between colour pattern complexity and muscular facial complexity in response to social pressures. Comparative and experimental studies are needed to verify if and how external facial morphology relates to communication through facial expressions, and the potential trade-offs between these two traits across primates.
The positive relationship found between an increased number of species in congeneric sympatry and the complexity of facial colour patterns further indicates selection for species-specific colour patterns that would facilitate species recognition. In many ecological communities, character displacement allows species recognition and prevents hybridization [44,45]. This process could be a mechanism underlying the changes within individual facial regions, ultimately leading to differences in colour pattern complexity among sympatric species. We propose that, within the highly diverse social communities of platyrrhines, the evolution of higher colour pattern complexity may be enabled by an increase in the number of underlying evolutionary modules [29]. Higher levels of modularity may provide more parts that can evolve independently to differentiate species, reduce interbreeding and promote higher degrees of diversity [29,46]. Studies of the integration among these modules could provide further insights about their functional roles and their relation with underlying gene networks.
The radiation of platyrrhines seems to have been accompanied by multiple transitions in the complexity of facial colour patterns (figure 1). The function of facial colour pattern complexity in social interactions is particularly relevant for species that rely primarily on visual communication. Variation in the ability to perceive colours, as well as the use of other types of communication, may confound the relationship between sociality and facial colour pattern complexity. Platyrrhines are diverse in their ability to perceive colours; owl monkeys (Aotidae) have monochromatic vision, howler monkeys (Alouatta spp.) have routinely trichromatic vision, and most of the rest of the genera are known to be polymorphic for trichromatism, with males being dichromatic and females di- or trichromatic [23]. Although we have not found significant relationships between these broad vision types and facial colour pattern complexity (results not shown), two main types of information are critically needed to incorporate the potential role of visual systems in the evolution of facial colour patterns. The first is the integration of detailed information on the visual system of Neotropical primates [47–50] with metrics of the visual environment experienced by species. These data may help elucidate if other environmental factors not measured here have also influenced the evolution of facial colour pattern complexity. For example, some environments that favour large group sizes or high sympatry may also favour the ability of individuals to perceive facial patterns, hence causing a correlation. The second type of information is the use of systematic approaches to data collection on variation in primate facial coloration in the field, captivity and museum collections, especially because species descriptions do not always reflect the true colour variation observed within or among taxa [1]. Our colour pattern classifications provide a first approach to quantifying the complex morphology of primate faces and set the stage for future work incorporating information about the primate visual system and their visual environment.
(b). Facial diversity and ecology
Many-to-one mapping of form to function is an emergent property of complex systems that promotes the evolution of diversity [51]. Because similar levels of colour pattern complexity can be achieved through different colour combinations across facial regions, the primate face may be an example of many-to-one mapping of facial morphology, social and ecological functions. This could explain why colour pattern complexity was not related to the geographical distribution of species. Nevertheless, ecogeographical rules that may reflect adaptations to local conditions were validated by our results for several facial regions. Darker eye masks have evolved towards the Equator, and these might function in glare reduction in habitats with higher UV incidence, a phenomenon that has also been observed in carnivorans [31] and birds [52]. Darker facial regions in warmer and more humid areas support Gloger's rule [30], in which darker, melanin-based colours may reduce predation pressure by making individuals more cryptic [53], protect against UV radiation [2], facilitate thermoregulation [54] and increase resistance against pathogens [55]. A recent study by Kamilar & Bradley [56] has also found support for Gloger's rule for the dorsal body pelage in tropical primates, with increasing pelage darkness being significantly related to increasing levels of evapotranspiration. Finally, increases in facial hair length in temperate regions provide support for another, less-studied ecogeographical rule: the ‘hair rule’, which predicts that mammals will have longer and thicker hair as thermoregulation requirements increase in colder areas [32]. Because the ecogeographical rules described above are met by some but not all facial regions, the trends observed in primate faces potentially reflect conflicts in selective pressures among physiological, ecological and social functions.
(c). Re-evaluating the metachromism and behavioural drive hypotheses
Studies of the genetics underlying colour changes across mammals [57] indicate that point mutations can lead to changes in the distribution of eumelanin and pheomelanin, and dramatic differences in hair coloration. This current understanding of the genetics of pigmentation, along with the results presented here and by other genus-level studies [58,59], sharply contrasts with the irreversible pathways proposed by the metachromism hypothesis. Although the agouti banding pattern seems to be the primitive condition for mammalian hair [2], and the evolutionary sequence of colour changes fits the metrachromism model in the Neotropical genus Saguinus [60], this non-adaptive hypothesis does not constitute a general principle for Neotropical primates.
Researchers have long suspected that social traits are key drivers of the high morphological diversity of primate faces [1,18]. Although frequently invoked, behavioural and ecological explanations for complex phenotypes are seldom tested fully. We have provided support for a long-standing adaptationist paradigm by identifying a significant evolutionary relationship among sociality, ecology and morphological traits in the context of the Neotropical primate radiation. Understanding whether there are reverse evolutionary trends between external and muscular facial complexity, and whether similar social and ecological factors have served to foster the facial diversity of other mammals, await the collection and analysis of additional quantitative social, ecological and morphological data. We propose, however, that applying similar approaches to those outlined here has the potential to elucidate the drivers of facial diversity across other species that rely on facial cues for communication.
Acknowledgements
We thank Noel Rowe for access to the All the World Primates Database; Jonathan Chang, Joseph Smith and Graham Slater for assistance during data collection and analyses; Stephen Nash for illustrations of primate faces; Amisha Gadani, Princess Gilbert, Tina Marcroft, Graham Slater and Laurie Sorenson for re-scoring faces; and Susan Perry, Joan Silk, Jenn Smith and three anonymous reviewers for useful suggestions on the manuscript. This study was conducted under a postdoctoral fellowship from the UCLA Center for Society and Genetics to S.E.S. and partially supported by NSF BCS 0918748 and DEB 6861953.
References
- 1.Bradley B., Mundy N. 2008. The primate palette: the evolution of primate coloration. Evol. Anthropol. 17, 97–111 10.1002/evan.20164 (doi:10.1002/evan.20164) [DOI] [Google Scholar]
- 2.Caro T. 2005. The adaptive significance of coloration in mammals. Bioscience 55, 125–136 10.1641/0006-3568(2005)055[0125:TASOCI]2.0.CO;2 (doi:10.1641/0006-3568(2005)055[0125:TASOCI]2.0.CO;2) [DOI] [Google Scholar]
- 3.Tibbetts E. A., Dale J. 2007. Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537 10.1016/j.tree.2007.09.001 (doi:10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 4.Setchell J. M. 2005. Do female mandrills prefer brightly coloured males? Int. J. Primatol. 26, 715–735 10.1007/s10764-005-5305-7 (doi:10.1007/s10764-005-5305-7) [DOI] [Google Scholar]
- 5.Setchell J. M., Jean Wickings E., Knapp L. A. 2006. Signal content of red facial coloration in female mandrills (Mandrillus sphinx). Proc. R. Soc. B 273, 2395–2400 10.1098/rspb.2006.3573 (doi:10.1098/rspb.2006.3573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clough D., Heistermann M., Kappeler P. M. 2009. Individual facial coloration in male Eulemur fulvus rufus: a condition-dependent ornament? Int. J. Primatol. 30, 859–875 10.1007/s10764-009-9379-5 (doi:10.1007/s10764-009-9379-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronenberg W., Ash L. E., Tibbetts E. A. 2007. Correlation between facial pattern recognition and brain composition in paper wasps. Brain Behav. Evol. 71, 1–14 10.1159/000108607 (doi:10.1159/000108607) [DOI] [PubMed] [Google Scholar]
- 8.Dale J., Lank D. B., Reeve H. K. 2001. Signaling individual identity versus quality: a model and case studies with ruffs, queleas, and house finches. Am. Nat. 158, 75–86 10.1086/320861 (doi:10.1086/320861) [DOI] [PubMed] [Google Scholar]
- 9.Pascalis O., Bachevalier J. 1998. Face recognition in primates: a cross-species study. Behav. Process. 43, 87–96 10.1016/S0376-6357(97)00090-9 (doi:10.1016/S0376-6357(97)00090-9) [DOI] [PubMed] [Google Scholar]
- 10.Gauthier I., Logothetis N. K. 2000. Is face recognition not so unique after all? Cogn. Neuropsychol. 17, 125–142 10.1080/026432900380535 (doi:10.1080/026432900380535) [DOI] [PubMed] [Google Scholar]
- 11.Schmidt K. L., Cohn J. F. 2001. Human facial expressions as adaptations: evolutionary questions in facial expression research. Am. J. Phys. Anthropol. 116, 3–24 10.1002/ajpa.20001 (doi:10.1002/ajpa.20001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calder A., Young A. 2005. Understanding the recognition of facial identity and facial expression. Nat. Rev. Neurosci. 6, 641–651 10.1038/nrn1724 (doi:10.1038/nrn1724) [DOI] [PubMed] [Google Scholar]
- 13.Parr L., Heintz M., Akamagwuna U. 2006. Three studies on configural face processing by chimpanzees. Brain Cogn. 62, 30–42 10.1016/j.bandc.2006.03.006 (doi:10.1016/j.bandc.2006.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvergne A., et al. 2009. Human ability to recognize kin visually within primates. Int. J. Primatol. 30, 199–210 10.1007/s10764-009-9339-0 (doi:10.1007/s10764-009-9339-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufour V., Pascalis O., Petit O. 2006. Face processing limitation to own species in primates: a comparative study in brown capuchins, Tonkean macaques and humans. Behav. Process. 73, 107–113 10.1016/j.beproc.2006.04.006 (doi:10.1016/j.beproc.2006.04.006) [DOI] [PubMed] [Google Scholar]
- 16.Tsao D. Y., Livingstone M. S. 2008. Mechanisms of face perception. Annu. Rev. Neurosci. 31, 411–437 10.1146/annurev.neuro.30.051606.094238 (doi:10.1146/annurev.neuro.30.051606.094238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale J. 2006. Intraspecific variation in coloration. In Bird coloration: function and evolution (eds Hill G. E., McGraw K. J.), pp. 36–86 Boston, MA: Harvard University Press [Google Scholar]
- 18.Darwin C. 1872. The expression of the emotions in man and animals. New York, NY: Oxford University Press [Google Scholar]
- 19.Wcislo W. 1989. Behavioural environments and evolutionary change. Annu. Rev. Ecol. Syst. 20, 137–169 10.1146/annurev.es.20.110189.001033 (doi:10.1146/annurev.es.20.110189.001033) [DOI] [Google Scholar]
- 20.Schmalhausen I. I. 1949. Factors of evolution: the theory of stabilizing selection. Philadephia, PA: Blakeston [Google Scholar]
- 21.Wyles J. S., Kunkel J. G., Wilson A. C. 1983. Birds, behaviour, and anatomical evolution. Proc. Natl Acad. Sci. USA 80, 4394–4397 10.1073/pnas.80.14.4394 (doi:10.1073/pnas.80.14.4394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burrows A. 2008. The facial expression musculature in primates and its evolutionary significance. BioEssays 30, 212–225 10.1002/bies.20719 (doi:10.1002/bies.20719) [DOI] [PubMed] [Google Scholar]
- 23.Surridge A. K., Osorio D., Mundy N. I. 2003. Evolution and selection of trichromatic vision in primates. Trends Ecol. Evol. 18, 198–205 10.1016/S0169-5347(03)00012-0 (doi:10.1016/S0169-5347(03)00012-0) [DOI] [Google Scholar]
- 24.Kamilar J. M., Bradley B. J. 2010. Countershading is related to positional behaviour in primates. J. Zool. 283, 227–233 10.1111/j.1469-7998.2010.00765.x (doi:10.1111/j.1469-7998.2010.00765.x) [DOI] [Google Scholar]
- 25.Rylands A. B., Mittermeier R. A. 2009. The diversity of the New World primates (Platyrrhini): an annotated taxonomy. In South American primates (eds Garber P. A., Estrada A., Bicca-Marques J. C., Heymann E. W., Strier K. B.), pp. 23–54 New York, NY: Springer [Google Scholar]
- 26.Marty J. S., Higham J. P., Gadsby E. L., Ross C. 2009. Dominance, coloration, and social and sexual behaviour in male drills Mandrillus leucophaeus. Int. J. Primatol. 30, 807–823 10.1007/s10764-009-9382-x (doi:10.1007/s10764-009-9382-x) [DOI] [Google Scholar]
- 27.Dobson S. D. 2009. Socioecological correlates of facial mobility in nonhuman anthropoids. Am. J. Phys. Anthropol. 139, 413–420 10.1002/ajpa.21007 (doi:10.1002/ajpa.21007) [DOI] [PubMed] [Google Scholar]
- 28.Vick S. J., Waller B. M., Parr L. A., Smith Pasqualini M. C., Bard K. A. 2007. A cross-species comparison of facial morphology and movement in humans and chimpanzees using the facial action coding system (FACS). J. Nonverbal Behav. 31, 1–20 10.1007/s10919-006-0017-z (doi:10.1007/s10919-006-0017-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner G., Pavlicev M., Cheverud J. 2007. The road to modularity. Nat. Rev. Genet. 8, 921–931 10.1038/nrg2267 (doi:10.1038/nrg2267) [DOI] [PubMed] [Google Scholar]
- 30.Gloger C. L. 1833. Das abändern der vögel durch einfluss des klimas. Breslau, Poland: Schulz [Google Scholar]
- 31.Ortolani A. 1999. Spots, stripes, tail tips and dark eyes: predicting the function of carnivore colour patterns using the comparative method. Biol. J. Linn. Soc. 67, 433–476 10.1111/j.1095-8312.1999.tb01942.x (doi:10.1111/j.1095-8312.1999.tb01942.x) [DOI] [Google Scholar]
- 32.Rensch B. 1938. Some problems of geographical variation and species-formation. Proc. Linn. Soc. Lond. 150, 275–285 10.1111/j.1095-8312.1938.tb00182k.x (doi:10.1111/j.1095-8312.1938.tb00182k.x) [DOI] [Google Scholar]
- 33.Hershkovitz P. 1968. Metachromism or the principle of evolutionary change in mammalian tegumentary colours. Evolution 22, 556–575 10.2307/2406880 (doi:10.2307/2406880) [DOI] [PubMed] [Google Scholar]
- 34.Rowe N. 1996. The pictorial guide to the living primates. New York, NY: Pogonias Press [Google Scholar]
- 35.Patterson B., Ceballos G., Sechrest W., Tognelli M., Brooks T., Luna L., Ortega P., Salazar I., Young B. 2003. Digital distribution maps of the mammals of the Western Hemisphere. Arlington, VA: NatureServe; CD-ROM compilation. [Google Scholar]
- 36.Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zool. Scr. 26, 331–348 10.1111/j.1463-6409.1997.tb00423.x (doi:10.1111/j.1463-6409.1997.tb00423.x) [DOI] [Google Scholar]
- 37.Paradis E., Claude J., Strimmer K. 2004. APE: analyses of phylogenetics and evolution in r language. Bioinformatics 20, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee H., Ho S., Barnes I., Groves C. 2009. Estimating the phylogeny and divergence times of primates using a supermatrix approach. BMC Evol. Biol. 9, 259. 10.1186/1471-2148-9-259 (doi:10.1186/1471-2148-9-259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jablonski D., Roy K., Valentine J. W. 2006. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106 10.1126/science.1130880 (doi:10.1126/science.1130880) [DOI] [PubMed] [Google Scholar]
- 40.Pigliucci M. 2003. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Ecol. Lett. 6, 265–272 10.1046/j.1461-0248.2003.00428.x (doi:10.1046/j.1461-0248.2003.00428.x) [DOI] [Google Scholar]
- 41.Smuts B. B., Cheney D. L., Seyfarth R. M., Wrangham R. W., Struhsaker T. T. 1987. Primate societies. Chicago, IL: University of Chicago Press [Google Scholar]
- 42.Setchell J. M., Kappeler P. M. 2003. Selection in relation to sex in primates. Adv. Stud. Behav. 33, 87–173 10.1016/S0065-3454(03)33003-7 (doi:10.1016/S0065-3454(03)33003-7) [DOI] [Google Scholar]
- 43.Burrows A. M., Diogo R., Waller B. M., Bonar C. J., Liebal K. 2011. Evolution of the muscles of facial expression in a monogamous ape: evaluating the relative influences of ecological and phylogenetic factors in hylobatids. Anat. Rec. 294, 645–663 10.1002/ar.21355 (doi:10.1002/ar.21355) [DOI] [PubMed] [Google Scholar]
- 44.Brown W. L., Wilson E. O. 1956. Character displacement. Syst. Biol. 5, 49–65 [Google Scholar]
- 45.Dayan T., Simberloff D. 2005. Ecological and community wide character displacement: the next generation. Ecol. Lett. 8, 875–894 10.1111/j.1461-0248.2005.00791.x (doi:10.1111/j.1461-0248.2005.00791.x) [DOI] [Google Scholar]
- 46.Yang A. 2001. Modularity, evolvability, and adaptive radiations: a comparison of the hemi- and holometabolous insects. Evol. Dev. 3, 59–72 10.1046/j.1525-142x.2001.003002059.x (doi:10.1046/j.1525-142x.2001.003002059.x) [DOI] [PubMed] [Google Scholar]
- 47.Jacobs G. H., Deegan J. F., II 2003. Cone pigment variations in four genera of New World monkeys. Vision Res. 43, 227–236 10.1016/S0042-6989(02)00565-5 (doi:10.1016/S0042-6989(02)00565-5) [DOI] [PubMed] [Google Scholar]
- 48.Jacobs G. H., Williams G. A. 2006. L and M cone proportions in polymorphic New World monkeys. Vis. Neurosci. 23, 365–370 10.1017/S0952523806233066 (doi:10.1017/S0952523806233066) [DOI] [PubMed] [Google Scholar]
- 49.Osorio D., Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B 272, 1745–1752 10.1098/rspb.2005.3156 (doi:10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osorio D., Smith A., Vorobyev M., Buchanan-Smith H. 2004. Detection of fruit and the selection of primate visual pigments for colour vision. Am. Nat. 164, 696–708 10.1086/425332 (doi:10.1086/425332) [DOI] [PubMed] [Google Scholar]
- 51.Wainwright P. C., Alfaro M. E., Bolnick D. I., Hulsey C. D. 2005. Many-to-one mapping of form to function: a general principle in organismal design? Integr. Comp. Biol. 45, 256–262 10.1093/icb/45.2.256 (doi:10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]
- 52.Burtt E. H. 1986. An analysis of physical, physiological, and optical aspects of avian coloration with emphasis on wood-warblers. Ornithol. Monogr. 38, 1–126 [Google Scholar]
- 53.Zink R., Remsen J., Jr 1986. Evolutionary processes and patterns of geographic variation in birds. Curr. Ornithol. 4, 1–69 [Google Scholar]
- 54.Burtt E. H. 1981. The adaptiveness of animal colours. Bioscience 31, 723–729 10.2307/1308778 (doi:10.2307/1308778) [DOI] [Google Scholar]
- 55.Burtt E. H., Jr, Ichida J. M. 2004. Gloger's rule, feather-degrading bacteria, and colour variation among song sparrows. Condor 106, 681–686 10.1650/7383 (doi:10.1650/7383) [DOI] [Google Scholar]
- 56.Kamilar J. M., Bradley B. J. 2011. Interspecific variation in primate coat colour supports Gloger's rule. J. Biogeogr. 38, 2270–2277 10.1111/j.1365-2699.2011.02587.x (doi:10.1111/j.1365-2699.2011.02587.x) [DOI] [Google Scholar]
- 57.Hoekstra H. 2006. Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity 97, 222–234 10.1038/sj.hdy.6800861 (doi:10.1038/sj.hdy.6800861) [DOI] [PubMed] [Google Scholar]
- 58.Shapley R. An analysis of metachromism within the phylogeny of the Neotropical primate genus Alouatta, the howler monkeys. University of California Berkeley, Berkeley, CA. [Google Scholar]
- 59.Mundy N. I., Kelly J. 2001. Phylogeny of lion tamarins (Leontopithecus spp.) based on interphotoreceptor retinol binding protein intron sequences. Am. J. Primatol. 54, 33–40 10.1002/ajp.1010 (doi:10.1002/ajp.1010) [DOI] [PubMed] [Google Scholar]
- 60.Jacobs S. C., Larson A., Cheverud J. M. 1995. Phylogenetic relationships and orthogenetic evolution of coat colour among tamarins (genus Saguinus). Syst. Biol. 44, 515. 10.1093/sysbio/44.4.515 (doi:10.1093/sysbio/44.4.515) [DOI] [Google Scholar]