Abstract
Müllerian mimicry describes the close resemblance between aposematic prey species; it is thought to be beneficial because sharing a warning signal decreases the mortality caused by sampling by inexperienced predators learning to avoid the signal. It has been hypothesized that selection for mimicry is strongest in multi-species prey communities where predators are more prone to misidentify the prey than in simple communities. In this study, wild great tits (Parus major) foraged from either simple (few prey appearances) or complex (several prey appearances) artificial prey communities where a specific model prey was always present. Owing to slower learning, the model did suffer higher mortality in complex communities when the birds were inexperienced. However, in a subsequent generalization test to potential mimics of the model prey (a continuum of signal accuracy), only birds that had foraged from simple communities selected against inaccurate mimics. Therefore, accurate mimicry is more likely to evolve in simple communities even though predator avoidance learning is slower in complex communities. For mimicry to evolve, prey species must have a common predator; the effective community consists of the predator's diet. In diverse environments, the limited diets of specialist predators could create ‘simple community pockets’ where accurate mimicry is selected for.
Keywords: aposematism, avoidance learning, Batesian mimicry, generalization
1. Introduction
With his original equations of mimicry between defended prey, Müller [1] assumed that naive predators sample a fixed amount of unpalatable prey individuals with a certain signal until they learn avoidance of that signal, and showed that if prey species shared a warning signal, they would spread out the mortality costs of predator learning over a greater number of individuals. Despite theoretical [2–6], genetic [7,8], phylogenetic [9–11] and experimental studies [12–17] of Müllerian mimicry, the general conditions that select for it remain unclear. Specifically, there is continuing discussion about the importance of naive versus experienced predators [16–19], and the importance of prey community structure in selection for mimicry [2,3,20–22]. It has been argued that mimicry between defended prey is strongly favoured in complex communities because where prey types are numerous, predators learn more slowly to avoid aposematic prey and make more discrimination errors, resulting in more attacks [2]. By contrast, in simple prey communities where the foraging task is less cognitively challenging, selection for mimicry would be weaker [2]. A study with humans as predators of virtual prey supported this hypothesis: Beatty et al. [21] concluded that pressure for mimicry is higher when there are multiple species in the prey community. Beatty et al. focused on the learning phase of naive predators, and the benefit of mimicry in their experiment was owing to a shared distinctive trait (crude resemblance) between the co-mimics.
Whether warning signal similarity is acquired gradually or in two distinctive phases is an open empirical question, but both scenarios assume that signal similarity starts as crude resemblance (or no resemblance) that is further refined [4–6,23,24]. Because predators should generalize widely between defended prey [14,25,26], it is unclear what conditions select for precise resemblance (especially if there are costs of mimicry [24]); consequently, it is unclear why Müllerian co-mimics in nature can resemble each other with astonishing accuracy [12,27].
We studied whether accurate Müllerian mimicry could evolve more readily in a complex prey community rather than a simple one. We defined complexity of a prey community as the number of edible and aposematic prey appearances the predators meet, and used great tits (Parus major) as predators of artificial prey in a laboratory. We first examined whether, when foraging from the prey community, complexity affects predator learning so that there would be differential mortality for aposematic prey depending on community structure. Naive birds were presented with individual prey items in a continuous randomized sequence where 50 per cent of the prey were edible and 50 per cent were aposematic. Among the aposematic prey was a specific ‘model species’ the birds encountered at the same rate in all treatments; the model was always present at a constant frequency but was surrounded by either a simple or a complex prey community (see §2; figure 1).
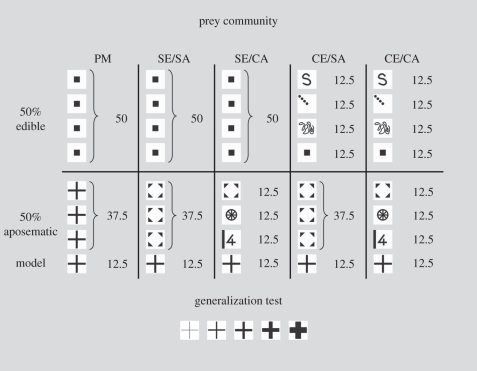
Figure 1.
The experimental set-up and the symbols used as prey appearances. Numbers indicate the frequency (percentage of all prey items presented in the ‘prey community’ sequence) in which the prey types appeared in the treatments. The signals used as imperfect mimics in the generalization test were modifications (thinner and bolder) of the cross signal of the model prey; the cross signal in the middle is a perfect mimic of the model prey. PM, ‘perfect mimicry’; SE/SA, ‘simple edible/simple aposematic’; SE/CA, ‘simple edible/complex aposematic’; CE/SA, ‘complex edible/simple aposematic’; CE/CA, ‘complex edible/complex aposematic’.
We subsequently tested whether the community structure affects the extent to which predators select for signal accuracy in a generalization test. We presented the birds with continuum of signal accuracy consisting of a perfect mimic, two alternative forms of moderately imperfect mimics and two inaccurate mimics of the model prey (see §2; figure 1). We ranked the survival of the mimics according to the order in which they were attacked. If the birds attacked the most inaccurate mimics first and avoided the perfect mimics, they would select for accurate mimicry. We can thus combine the foraging behaviour of naive and experienced predators and evaluate how it is linked to prey mortality and consequently the selection for mimicry in the different communities.
2. Methods
The experiment was run from October 2008 to March 2009 at the Konnevesi Research Station in Central Finland. Great tits were trapped from feeding sites, ringed and released at their capture sites after they had completed the experiment. The birds were kept individually in illuminated, ventilated plywood cages (64 × 46 × 77 cm) indoors in a daily light period of 11 h and 30 min. Sunflower seeds, tallow and fresh water were available ad libitum except prior to the trials when the birds were food deprived for ca 2 h to ensure motivation to forage on artificial prey. In total, 12 birds were used for testing the signals of the prey (see later text) and 87 for the actual experiment.
(a). Prey and experimental cages
The prey items were pieces of almond (approx. 0.01 g) within a paper shell (two 8 × 8 mm pieces of white paper glued with non-toxic glue UHU Stic). We pre-trained the birds in a stepwise manner in their home cages to handle the artificial prey [26]. Pre-training prey was made of blank brown paper.
Experimental prey items were white with a black symbol printed on both sides of the paper shell. The symbols for the prey community were designed to be distinctive (figure 1), so that adding prey appearances would create real visual complexity and the birds could not treat any signal combinations as perceptual categories [28]. The signal of the model prey was always a cross symbol and the imperfect mimics used in the generalization test (see later text) were modified thinner and bolder crosses (figure 1). Almond for the aposematic prey was made unpalatable by soaking it for an hour in a solution of 30 ml of water and 2 g of chloroquine phosphate (malaria drug Heliopar).
The experiment was run in illuminated cages (plywood, sized 50 × 50 × 70 cm) that had a perch and a water bowl inside. Prey items were presented on a Petri dish, with brown paper on the bottom. The dish was slid through a hatch into the cage where it remained hidden behind a visual barrier. This allowed us to determine exactly when the bird detected the prey item because they had to fly on top of the barrier or go around it to see the prey. Prior to the experiment, the birds were familiarized with the experimental cages for at least an hour during which they had to eat six sunflower seeds from a Petri dish. This was to train the birds to expect food behind the barrier. The birds were observed through a small mesh-covered window, and the cages were placed in a dark room so that the birds would be less aware of the observer.
(b). Testing the imperfect mimics
We verified our mimic signal continuum (the modified crosses used in the generalization test, see later in the text) in a series of tests that confirmed that the birds did not have initial preferences for any of the cross symbols, and that they saw the difference between the symbols and treated them as a continuum—as we intended. We used only the cross signal continuum because of the difficulty in designing continuous signal variation that is neutral in this way (with wild predators, the signals should also be novel enough to exclude effects of previous experience) and because of the need to test the signals prior to the experiment.
We first tested 12 birds for possible signal preferences by presenting them with the five cross symbols (as edible prey, figure 1) simultaneously for five rounds so that the prey items were arranged in a circle on the Petri dish and their position was randomized for each round. There was no time limit for attacking, and the birds were allowed to eat all prey items. Generally, all prey items were eaten in less than 5 min. We ranked the birds’ signal preferences according to the order in which they ate the prey items (1–5); the rank analysed was the mean over the five presentations. There were no preferences for any of the cross symbols per se (no difference in mean ranks, Friedman's test 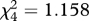 , p = 0.885).
, p = 0.885).
We subsequently trained the same birds to avoid one extreme of the signal continuum as unpalatable and accept the other extreme as edible prey. Six birds were trained to accept the thinnest; and six birds, the boldest cross. We trained the birds by presenting them with a semi-randomized sequence of unpalatable and edible crosses (no more than three items of each type in a row) until they had ‘killed’ (opened the paper shell and tasted or eaten the almond inside) 50 prey items in a single trial. The birds were allowed 1 min to attack the prey item, after which it was removed and classed as rejected. The birds learned to avoid the signal of the unpalatable prey: we divided the 50 attacks into five sets of 10 attacks and recorded the number of unpalatable crosses killed within the sets of 10. The numbers decreased during the trial (repeated measures ANOVA F4,40 = 6.026; p = 0.001) at the same rate for both the thin and the bold cross (interaction term F4,40 = 1.938; p = 0.123). Finally, we presented the birds again with the whole continuum of cross signals (as edible prey) simultaneously for five rounds. The birds had 1 min to attack the first prey item. After handling the first prey item, they had another minute to attack the next one and so on until all five were attacked or the bird refused to take more prey items. We ranked the survival as described earlier; only now the rejected prey items were given rank 6. The mean ranks of the cross signals over the five rounds differed (Friedman test 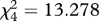 , p = 0.010 when the thinnest cross had been unpalatable and
, p = 0.010 when the thinnest cross had been unpalatable and 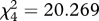 p < 0.001 when the boldest cross had been unpalatable) and the birds’ reluctance to attack the cross signals generally increased with increasing similarity to the trained signal (see the electronic supplementary material, figure S1). Therefore, we can be more confident that in the main experiment, the results of the generalization test are not biased because the birds perceive signal similarity differently to how humans do.
p < 0.001 when the boldest cross had been unpalatable) and the birds’ reluctance to attack the cross signals generally increased with increasing similarity to the trained signal (see the electronic supplementary material, figure S1). Therefore, we can be more confident that in the main experiment, the results of the generalization test are not biased because the birds perceive signal similarity differently to how humans do.
(c). Foraging from the prey community
In the first part of the experiment, the birds were foraging from ‘prey communities’ where 50 per cent of the prey were edible and 50 per cent aposematic in all treatments. We manipulated where complexity (i.e. higher number of prey appearances) occurred in the community: nowhere; among the edible prey only; among the aposematic prey only; or in both parts of the community (see later text). The prey items were presented in a semi-randomized sequence where no more than three items of one symbol (and therefore also either edible or aposematic prey) appeared in a row. Randomization was done within blocks of 40 prey items. Each prey item was presented for a minute, starting from when the bird saw the item behind the barrier. If a prey item was attacked within a minute, the bird was allowed to finish handling and eating it before removing the shell. A new item was slid in after a 30 s interval. The trial was terminated when 50 prey items were attacked. The birds completed four trials that were run on consecutive days and thus killed 200 prey items in total. Therefore, all birds gained the same amount of experience of the prey and had a chance to learn the discrimination between edible and unpalatable prey; their experience differed qualitatively. Using the number of killed prey as an ending criterion to the trials ensured that the birds did not enter the generalization test after having learned nothing because they, for example, refused prey items if they were not hungry. On average, it took 83 (s.e. 2.5) presentations to complete the first trial, 76 (s.e. 2.2) to complete the second, 83 (s.e. 2.2) to complete the third and 89 (s.e. 1.9) to complete the fourth trial, although the numbers varied somewhat between treatments. We recorded the number of each prey type (edible/aposematic/model) killed.
The aposematic prey included the model that appeared in a constant frequency of 12.5 per cent. In the first treatment, ‘simple edible/simple aposematic’ all edible prey were of the same symbol and the aposematic prey were either models (12.5%) or of one other symbol (37.5%). In the second treatment, ‘simple edible/complex aposematic’ all edible prey were of one symbol but the aposematic prey had four different symbols (including the model)—all appearing in 12.5 per cent frequencies. In ‘complex edible/simple aposematic’, there were four types of edible prey but the aposematic part of the community consisted only of models and one other symbol (12.5% and 37.5%, respectively). In ‘complex edible/complex aposematic’, both the edible and the aposematic prey had four different symbols (figure 1). For comparison, we included a ‘perfect mimicry’ treatment where the edible prey were uniform and the model was surrounded by identical aposematic prey, increasing the frequency of the cross symbol in the community to 50 per cent. The symbol of the model prey was always the cross, but other symbols were circulated so that they appeared in different combinations for each individual bird (except in ‘perfect mimicry’ where two to three birds had the same combination) and alternated as edible and aposematic prey. There were 18 birds in ‘simple edible/simple aposematic’ and ‘simple edible/complex aposematic’ and 17 in the other treatments.
We manipulated the number of appearances of both the edible and the aposematic prey because if only the number of aposematic prey types is increased and the edible prey remains uniform in appearance, the foraging task does not necessarily become more difficult: the predators may use a rule of thumb by attacking the abundant, uniform edible prey and ignoring the rest without having to learn their signals specifically [26].
Note that the design is not fully symmetrical because the simple edible side consisted of only one symbol, but the simple aposematic side had the model and another aposematic prey. Consequently, ‘simple edible/complex aposematic’ has a total of five symbols, whereas ‘complex edible/simple aposematic’ has six. This was because we wanted to keep the frequency of the model constant across all treatments (except in ‘perfect mimicry’) and the random probability of the prey being edible or unpalatable constant at 50 per cent, while keeping the number of symbols in the simple treatment as low as possible (i.e. no additional edible symbol in ‘simple edible/simple aposematic’).
(d). Generalization test
On the fifth day, after the birds had foraged from the community, we tested how the birds generalized their avoidance of the model prey to a continuum of imperfect mimics. We presented the birds simultaneously with a perfect mimic of the original model cross and the four modified crosses (figure 1) for five rounds. All prey items including the perfect mimic were edible (so the birds had no opportunity for further avoidance learning), and they were arranged in a circle on the Petri dish so that the positions of the symbols were randomized for each round. The birds were allowed to attack as many prey items as they chose. They had 1 min to attack the first prey item. If no prey items were attacked during that time, all were considered rejected. If a bird attacked a prey item, it had another minute (starting when it stopped handling and eating the prey) to attack another one. We ranked the survival of the mimics according to the order in which they were attacked: survival rank was 1 if the symbol was attacked first and 5 if it was attacked last; all rejected symbols were ranked as sixth. If the birds attacked the most inaccurate mimics first and avoided the perfect mimics, they would select for accurate mimicry, creating a (roughly) bell-shaped generalization curve where the perfect mimic with the highest survival rank would occupy the peak and the most inaccurate mimics would form the tails of the curve. The key question is whether the simple or the complex community treatments result in these steep generalization curves that indicate selection towards better mimicry.
3. Results and discussion
We found that the structure of the community surrounding the model prey affected how well the predators learned to avoid the model, which in turn affected the extent to which they selected for signal accuracy in potential mimics (i.e. how broadly they generalized their avoidance to similar-looking prey).
(a). Foraging from the prey community
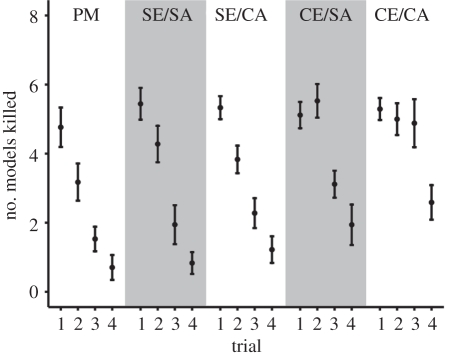
In general, when foraging from the community, the birds learned to avoid the model prey: the number of models killed decreased from trial 1 through to trial 4 in all treatments (repeated measures ANOVA, Greenhouse–Geisser corrected for sphericity F2.705,221.774 = 82.302; p < 0.001), but not at the same rate (learning by treatment interaction F10.818,221.774 = 2.179; p = 0.017; figure 2). We assumed model learning to be most efficient in the ‘perfect mimicry’ treatment because the discrimination task on the whole involved only two prey appearances (figure 1). Therefore, to analyse model learning rates further, we compared all other treatments with the ‘perfect mimicry’ treatment. We found that birds in ‘simple edible/simple aposematic’ and ‘simple edible/complex aposematic’ learned to avoid the model at the same rate as in the ‘perfect mimicry’ treatment (simple contrasts p = 0.169 and p = 0.141, respectively). Thus, as long as the edible prey remained uniform in appearance, learning about the model was efficient even if the number of aposematic prey types increased in the community. Only when the edible part of the community was complex (at the same time, the total number of prey appearances reached six or eight) did the birds learn to avoid the model more slowly than in ‘perfect mimicry’ (‘perfect mimicry’ versus ‘complex edible/simple aposematic’ p = 0.002; ‘perfect mimicry’ versus ‘complex edible/complex aposematic’ p < 0.001; figure 2).
Figure 2.
The number of models killed in trials 1–4. Results are shown for each treatment as mean ± s.e.m.; n = 18 in ‘simple edible/simple aposematic’ (SE/SA) and ‘simple edible/complex aposematic’ (SE/CA), and 17 in the other treatments. PM, ‘perfect mimicry’; CE/SA, ‘complex edible/simple aposematic’; CE/CA, ‘complex edible/complex aposematic’.
Because model avoidance learning was slower in these two more complex treatments (‘complex edible/simple aposematic’ and ‘complex edible/complex aposematic’) and it resulted in higher per capita mortality of models (compared to ‘perfect mimicry’; see Mortality of the model prey in the electronic supplementary material), it would—at first glance—seem that mimicry should evolve more readily in multi-species communities. We cannot conclude specifically whether learning rates slowed and mortality increased because the total number of prey types in the treatment increased above a critical threshold (six or eight signals as opposed to two in ‘perfect mimicry’ or because the edible part of the community, rather than the aposematic part, was complex. When analysing per capita mortality of models during learning excluding ‘perfect mimicry’ (square root transformed to meet the requirements of the test), complexity of the edible part of the community increases model mortality (ANOVA F1,66 = 12.28, p = 0.001) but complexity of the aposematic part does not (F1,66 = 1.431, p = 0.236; there is also no edible complexity × aposematic complexity interaction F1,66 = 0.694, p = 0.408). However, the effect of edible prey on model mortality must be interpreted with caution because changing the edible prey from simple to complex increases the total number of prey types in the set-up by three, whereas changing the aposematic prey increases it by two (figure 1). Similarly, if we look at the combined per capita mortality of all aposematic prey rather than that of an individual prey type (the model), the benefits of mimicry in complex communities seem less certain: there is a possibility that being mimetic (reducing the number of warning signals) would not help the defended prey when predators are learning to avoid them because the complexity of the edible prey assemblage has an overriding effect on their mortality (see Mortality of all aposematic prey in the electronic supplementary material, and figure S2). Furthermore, for signal similarity to evolve, predators should actively select for good mimics and against poor ones; in the present complex communities, they did not. Only the birds that had foraged in communities with uniform edible prey selected against any of the inaccurate mimics (figure 1) in the following generalization test. These treatments (‘simple edible/simple aposematic’, ‘simple edible/complex aposematic’ and also ‘perfect mimicry’) also had the lowest total numbers of symbols present (figure 1).
(b). Generalization test
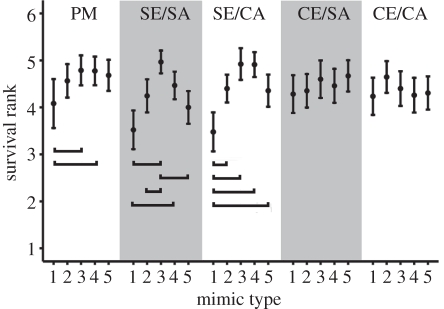
To investigate whether the generalization curves from the mimic survival ranks (figure 3) indicated selection, we tested whether the mean survival ranks of the mimic types differed. We used mean ranks because there was no effect of the round of presentation on rank, indicating the birds’ attack behaviour did not change as the trial progressed (see Selection for accurate mimicry via generalization: parametric tests in the electronic supplementary material). We tested all treatments separately.
Figure 3.
Mean survival ranks of the mimics in the generalization test. Mimic type 1 denotes the thinnest cross, 2 the second thinnest cross, 3 the perfect mimic, 4 the second boldest cross and 5 denotes the boldest cross. Brackets denote Friedman's pairwise comparisons that are significant at the 0.05 level. Results are shown for each treatment as mean ± s.e.m.; n = 18 in ‘simple edible/simple aposematic’ (SE/SA) and ‘simple edible/complex aposematic’ (SE/CA), 17 in the other treatments. PM, ‘perfect mimicry’; CE/SA, ‘complex edible/simple aposematic’; CE/CA, ‘complex edible/complex aposematic’.
Selection for accurate mimicry was clearest in the ‘simple edible/simple aposematic’ treatment: the perfect mimic survived better than the two most inaccurate mimics and the second thinnest cross (a moderately imperfect mimic; table 1 and figure 3). In the ‘simple edible/complex aposematic’ treatment, the birds only selected against the thinnest inaccurate mimic that had a lower mean survival rank than all the other mimics, including the boldest inaccurate mimic (table 1 and figure 3). This difference in the survival of the two most imperfect mimics is probably owing to a peak-shift-like tendency [29] to perceive the boldest cross as a stronger signal than the thinnest cross. In ‘perfect mimicry’, the thinnest cross ranked lower than the perfect mimic and the bolder moderately imperfect mimic (table 1 and figure 3). When the edible prey had been diverse (‘complex edible/simple aposematic’ and ‘complex edible/complex aposematic’), the birds either rejected all mimics or attacked them randomly not selecting for mimicry (no overall difference in mean survival ranks, Friedman's test 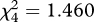 ; p = 0.834 and
; p = 0.834 and 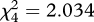 ; p = 0.729, respectively; figure 3). Thus, the moderately complex treatments ‘simple edible/complex aposematic’ and ‘complex edible/simple aposematic’ again yielded different results (see also figure 2): there was some—although modest—selection for accurate mimicry in the former but none in the latter. Note that although the focus is in whether survival differences between the mimics indicate selection for signal accuracy in the different treatments, the mean survival ranks for all mimics are generally high (above 3, see figure 3): in all treatments, some birds refused all or most mimics, increasing the mean ranks.
; p = 0.729, respectively; figure 3). Thus, the moderately complex treatments ‘simple edible/complex aposematic’ and ‘complex edible/simple aposematic’ again yielded different results (see also figure 2): there was some—although modest—selection for accurate mimicry in the former but none in the latter. Note that although the focus is in whether survival differences between the mimics indicate selection for signal accuracy in the different treatments, the mean survival ranks for all mimics are generally high (above 3, see figure 3): in all treatments, some birds refused all or most mimics, increasing the mean ranks.
Table 1.
Friedman's test for the mean ranks of the mimics in the generalization test. Results of the overall test and pairwise comparisons are shown for ‘perfect mimicry’ (PM) ‘simple edible/simple aposematic’ (SE/SA) and ‘simple edible/complex aposematic’ (SE/CA) treatments where the survival ranks differed. Significant (sig.) differences in the 0.05 level are highlighted and shown also in figure 3.
| PM |
SE/SA |
SE/CA |
||||
|---|---|---|---|---|---|---|
overall test |
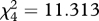 ; sig. =0.023 ; sig. =0.023 |
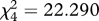 ; sig. < 0.001 ; sig. < 0.001 |
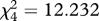 ; sig. = 0.004 ; sig. = 0.004 |
|||
| pairwise comparison | test value | sig. | test value | sig. | test value | sig. |
| −1.088 | 0.045 | −2.000 | <0.001 | −1.444 | 0.006 | |
| −0.529 | 0.329 | −1.056 | 0.045 | −0.361 | 0.493 | |
| −0.118 | 0.828 | 0.611 | 0.246 | −0.083 | 0.874 | |
| 0.118 | 0.828 | 1.427 | 0.005 | 0.361 | 0.493 | |
| −0.559 | 0.303 | −0.944 | 0.073 | −1.083 | 0.040 | |
| −1.206 | 0.026 | −1.389 | 0.008 | −1.528 | 0.004 | |
| −0.971 | 0.735 | −0.528 | 0.317 | −1.083 | 0.040 | |
| −0.647 | 0.233 | −0.444 | 0.399 | −0.444 | 0.399 | |
| −0.412 | 0.448 | 0.417 | 0.429 | 0 | 1.000 | |
| 0.235 | 0.664 | 0.861 | 0.102 | 0.444 | 0.399 | |
The result that only birds from ‘perfect mimicry’, ‘simple edible/simple aposematic’ and ‘simple edible/complex aposematic’ selected against any imperfect mimics mirrors how well they had learned to avoid the model when foraging from the prey community (figure 2). The birds’ avoidance of the model does not completely explain their generalization behaviour however, because birds from the ‘perfect mimicry’ treatment (that had encountered more unpalatable prey with the cross signal than birds in any other treatment and learned them well) only selected against the thinnest inaccurate mimic (table 1 and figure 3). Selection for accurate mimicry was clearest in the ‘simple edible/simple aposematic’ treatment where the community had been very simple, but the model had appeared in lower frequency than the other two prey types (figure 1). It is possible that even though avoidance learning was equally efficient in ‘perfect mimicry’ as in ‘simple edible/simple aposematic’, being the only rare signal in the latter treatment made the model prey ‘stand out’ and the birds were consequently more selective in their approach to imperfect mimics.
(c). Conclusions
Our results predict that predators select for Müllerian mimicry in prey communities where learning about the individual model prey is efficient. These are more likely to be simple communities (figure 3), and judging by the difference between the moderately complex treatments (figures 2 and 3), they may be communities where especially the edible prey are easy to recognize. Thus, we conclude that mimicry should evolve more readily in simple communities. However, our results do not exclude the possibility that mimicry is advantageous in complex communities (figure 2) when predators are naive (unless the variability of the edible prey community has an overriding effect on the mortality of aposematic prey; see the electronic supplementary material).
Contrary to our findings, Beatty et al. [21] found that mimicry should evolve more readily in a complex community. One important difference is that the conclusion of Beatty et al. [21] was based on the benefits of coarse resemblance when predators are naive, whereas ours is based on generalization to a continuum on imperfect mimics when the predators already have experience on the model prey. Therefore, our results are not necessarily a direct contradiction: as discussed by Balogh & Leimar [4], broadly and narrowly generalizing predators might work in concert to refine mimicry. The experience of the predators (naive/experienced) or their diet (generalist/specialist) might amount to the same overall effect: even crude resemblance might be beneficial against the naive and widely generalizing predators [14,26], whereas the knowledgeable predators select for signal accuracy [14,16,19]. Interestingly, a study of peak shift with poultry chicks shows that individuals trained to make a fine discrimination between rewarded and unrewarded stimuli are less willing to accept novel prey types, i.e. they generalize narrowly compared to individuals trained to a coarse discrimination [30]. One could draw a speculative parallel with this study: perhaps specialist predators with a limited diet (simple prey community) are predators accustomed to ‘fine-tuned work’ in prey recognition and are consequently more selective for signal accuracy.
Our results could also be applied to Batesian mimicry because the imperfect mimics were all edible and we were thus measuring only the birds' willingness to sample prey with varying similarity to the model. It is assumed that Batesian mimics face a higher selection pressure for signal accuracy than Müllerian co-mimics because it is in the predators' interest to distinguish edible ‘cheats’ [23,25,31]. The puzzling existence of crude Batesian mimics has several possible explanations [32–36], and our results suggest that a complex community structure could also relax selection for accurate Batesian mimicry because it makes predators generalize more broadly.
Studies have found generalization behaviour to be similar across taxa and sensory modality, but it can vary depending on the details of experience and stimulus dimension [37]. Birds can have behavioural biases for signals, whether artificial symbols, colours or real prey [16,22,38,39]. When focusing on the effect of the surrounding prey community, signal efficacy of the models and mimic continuums is important: mimics that are all exceptionally efficient or poor warning signals might result in flat generalization curves irrespective of the prey community. While this was not the case in the present experiment, we did use only one model signal and a respective mimic continuum, and we hope the study will be repeated in other systems.
Even though we studied only one predator species and even though natural prey communities have important ‘complexity features’ beyond the number of prey appearances that vary (such as the ratio of edible and unpalatable prey, different levels of unpalatability and seasonality in prey availability), our study suggests that the question of what kind of ecological conditions (prey community structure in this case) promote Müllerian mimicry can be linked back to the questions of predator behaviour, cognition and diet: for selection to favour mimicry, prey species must have a common predator. Consequently, the effective prey community consists of the predator's diet. Therefore, specialist predators could create ‘pockets of simplicity’ even in most diverse communities such as the tropical or sub-tropical environments that are home to spectacular examples of Müllerian mimicry [12,27].
Acknowledgements
This study was granted a project license from the National Animal Experiment Board (ESLH-2007-09311/Ym-23) and the Central Finland Regional Environment Centre (KSU-2007-L-687/254).
We thank Helinä Nisu for taking care of the birds, Tom Hoogesteger for assisting with the experiment and the Konnevesi Research Station for facilities. E.I. and J.M. were funded by the Acadamy of Finland (projects 21000002461 and 21000004745); H.M.R., M.P.S. and G.D.R. were funded by NERC (NE/D010 667/1).
References
- 1.Müller F. 1879. Ituna and Thyridia: a remarkable case of mimicry in butterflies. Proc. Entomol. Soc. Lond. 1879, 20–29 [Google Scholar]
- 2.MacDougall A., Dawkins M. S. 1998. Predator discrimination error and the benefits of Müllerian mimicry. Anim. Behav. 55, 1281–1288 10.1006/anbe.1997.0702 (doi:10.1006/anbe.1997.0702) [DOI] [PubMed] [Google Scholar]
- 3.Franks D. W., Noble J. 2004. Batesian mimics influence mimicry ring evolution. Proc. R. Soc. Lond. B 271, 191–196 10.1098/rspb.2003.2582 (doi:10.1098/rspb.2003.2582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balogh A. C. V., Leimar O. 2005. Müllerian mimicry: an examination of Fisher's theory of gradual evolutionary change. Proc. R. Soc. B 272, 2269–2275 10.1098/rspb.2005.3227 (doi:10.1098/rspb.2005.3227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franks D. W., Sherratt T. N. 2007. The evolution of multicomponent mimicry. J. Theor. Biol. 244, 631–639 10.1016/j.jtbi.2006.09.019 (doi:10.1016/j.jtbi.2006.09.019) [DOI] [PubMed] [Google Scholar]
- 6.Ruxton G. D., Franks D. W., Balogh A. C. V., Leimar O. 2008. Evolutionary implications of the form of predator generalization for aposematic signals and mimicry in prey. Evolution 62, 2913–2921 10.1111/j.1558-5646.2008.00485.x (doi:10.1111/j.1558-5646.2008.00485.x) [DOI] [PubMed] [Google Scholar]
- 7.Flanagan N. S., Tobler A., Davison A., Pybus O. G., Kapan D. D., Planas S., Linares M., Heckel D. 2004. Historical demography of Müllerian mimicry in the neotropical Heliconius butterflies. Proc. Natl Acad. Sci. USA 101, 9704–9709 10.1073/pnas.0306243101 (doi:10.1073/pnas.0306243101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kronforst M. R., Gilbert L. E. 2008. The population genetics of mimetic diversity in Heliconius butterflies. Proc. R. Soc. B 275, 493–500 10.1098/rspb.2007.1378 (doi:10.1098/rspb.2007.1378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Symula R., Schulte R., Summers K. 2001. Molecular phylogenetic evidence for a mimetic radiation in Peruvian poison frogs supports a Müllerian mimicry hyphothesis. Proc. R. Soc. Lond. B 268, 2415–2421 10.1098/rspb.2001.1812 (doi:10.1098/rspb.2001.1812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons R. B., Weller S. J. 2002. What kind of signals do mimetic tiger moths send? A phylogenetic test of wasp mimicry systems (Lepidoptera: Ardtiidae: Euchromiini). Proc. R. Soc. Lond. B 269, 983–990 10.1098/rspb.2002.1970 (doi:10.1098/rspb.2002.1970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marek P. E., Bond J. E. 2009. A Müllerian mimicry ring in Appalachian millipedes. Proc. Natl Acad. Sci. USA 106, 9755–9760 10.1073/pnas.0810408106 (doi:10.1073/pnas.0810408106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallet J. L. B., Barton N. H. 1989. Strong natural selection in a warning-color hybrid zone. Evolution 43, 421–431 10.2307/2409217 (doi:10.2307/2409217) [DOI] [PubMed] [Google Scholar]
- 13.Kapan D. D. 2001. Three-butterfly system provides a field test of Müllerian mimicry. Nature 409, 338–340 10.1038/35053066 (doi:10.1038/35053066) [DOI] [PubMed] [Google Scholar]
- 14.Rowe C., Lindström L., Lyytinen A. 2004. The importance of pattern similarity between Müllerian mimics in predator avoidance learning. Proc. R. Soc. Lond. B 271, 407–413 10.1098/rspb.2003.2615 (doi:10.1098/rspb.2003.2615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skelhorn J., Rowe C. 2005. Tasting the difference: do multiple defence chemicals interact in Müllerian mimicry? Proc. R. Soc. B 272, 339–345 10.1098/rspb.2004.2953 (doi:10.1098/rspb.2004.2953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ihalainen E., Lindström L., Mappes J., Puolakkainen S. 2008. Can experienced birds select for Müllerian mimicry? Behav. Ecol. 19, 362–368 10.1093/beheco/arm151 (doi:10.1093/beheco/arm151) [DOI] [Google Scholar]
- 17.Rowland H. M., Hoogesteger T., Ruxton G. D., Speed M. P., Mappes J. 2010. A tale of 2 signals: signal mimicry between aposematic species enhances predator avoidance learning. Behav. Ecol. 21, 851–860 10.1093/beheco/arq071 (doi:10.1093/beheco/arq071) [DOI] [Google Scholar]
- 18.Sherratt T. N., Speed M. P., Ruxton G. D. 2004. Natural selection on unpalatable species imposed by state-dependent foraging behaviour. J. Theor. Biol. 228, 217–226 10.1016/j.jtbi.2003.12.009 (doi:10.1016/j.jtbi.2003.12.009) [DOI] [PubMed] [Google Scholar]
- 19.Langham G. M. 2006. Rufous-tailed jacamars and aposematic butterflies: do older birds attack novel prey? Behav. Ecol. 17, 285–290 10.1093/beheco/arj027 (doi:10.1093/beheco/arj027) [DOI] [Google Scholar]
- 20.Kokko H., Mappes J., Lindström L. 2003. Alternative prey can change model-mimic dynamics between parasitism and mutualism. Ecol. Lett. 6, 1068–1076 10.1046/j.1461-0248.2003.00532.x (doi:10.1046/j.1461-0248.2003.00532.x) [DOI] [Google Scholar]
- 21.Beatty C. D., Beirinckx K., Sherratt T. N. 2004. The evolution of Müllerian mimicry in multispecies communities. Nature 431, 63–67 10.1038/nature02818 (doi:10.1038/nature02818) [DOI] [PubMed] [Google Scholar]
- 22.Lindström L., Alatalo R. V., Lyytinen A., Mappes J. 2004. The effect of alternative prey on the dynamics of imperfect Batesian and Müllerian mimicries. Evolution 58, 1294–1302 10.1554/03-271 (doi:10.1554/03-271) [DOI] [PubMed] [Google Scholar]
- 23.Fisher R. A. 1958. The genetical theory of natural selection, 2nd revised edn New York, NY: Denver Publication [Google Scholar]
- 24.Turner J. R. G. 1977. Butterfly mimicry: the genetical evolution of an adaptation. Evol. Biol. 10, 163–206 [Google Scholar]
- 25.Huheey J. E. 1988. Mathematical models of mimicry. Am. Nat. 131, S22–S41 10.1086/284765 (doi:10.1086/284765) [DOI] [Google Scholar]
- 26.Ihalainen E., Lindström L., Mappes J. 2007. Investigating Müllerian mimicry: predator learning and variation in prey defences. J. Evol. Biol. 20, 780–791 10.1111/j.1420-9101.2006.01234.x (doi:10.1111/j.1420-9101.2006.01234.x) [DOI] [PubMed] [Google Scholar]
- 27.Mallet J. L. B., Gilbert L. E. 1995. Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies. Biol. J. Linn. Soc. 55, 159–180 10.1006/bijl.1995.0034 (doi:10.1006/bijl.1995.0034) [DOI] [Google Scholar]
- 28.Shettleworth S. J. 2010. Cognition, evolution and behavior, 2nd edn. New York, NY: Oxford University Press [Google Scholar]
- 29.Gamberale-Stille G., Tullberg B. S. 1999. Experienced chicks show biased avoidance of stronger signals: an experiment with natural colour variation in live aposematic prey. Evol. Ecol. 13, 579–589 10.1023/A:1006741626575 (doi:10.1023/A:1006741626575) [DOI] [Google Scholar]
- 30.Baddeley R. J., Osorio D., Jones C. D. 2007. Generalization of colors by chickens: experimental observations and a Bayesian model. Am. Nat. 169, S27–S41 10.1086/510142 (doi:10.1086/510142) [DOI] [PubMed] [Google Scholar]
- 31.Mappes J., Alatalo R. V. 1997. Batesian mimicry and signal accuracy. Evolution 51, 2050–2053 10.2307/2411028 (doi:10.2307/2411028) [DOI] [PubMed] [Google Scholar]
- 32.Goodale M. A., Sneddon I. 1977. The effect of distastefulness of the model on the predation of artificial Batesian mimics. Anim. Behav. 25, 660–665 10.1016/0003-3472(77)90117-8 (doi:10.1016/0003-3472(77)90117-8) [DOI] [Google Scholar]
- 33.Dittrich W., Gilbert F., Green P., McGregor P., Grewcock D. 1993. Imperfect mimicry: a pigeon's perspective. Proc. R. Soc. Lond. B 251, 195–200 10.1098/rspb.1993.0029 (doi:10.1098/rspb.1993.0029) [DOI] [Google Scholar]
- 34.Edmunds M. 2000. Why are there good and poor mimics? Biol. J. Linn. Soc. 70, 459–466 10.1111/j.1095-8312.2000.tb01234.x (doi:10.1111/j.1095-8312.2000.tb01234.x) [DOI] [Google Scholar]
- 35.Holloway G., Gilbert F., Brandt A. 2002. The relationship between mimetic imperfection and phenotypic variation in insect colour patterns. Proc. R. Soc. Lond. B 269, 411–416 10.1098/rspb.2001.1885 (doi:10.1098/rspb.2001.1885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnstone R. A. 2002. The evolution of inaccurate mimics. Nature 418, 524–526 10.1038/nature00845 (doi:10.1038/nature00845) [DOI] [PubMed] [Google Scholar]
- 37.Ghirlanda S., Enquist M. 2003. A century of generalization. Anim. Behav. 66, 15–36 10.1006/anbe.2003.2174 (doi:10.1006/anbe.2003.2174) [DOI] [Google Scholar]
- 38.Ham A. D., Osorio D. 2007. Colour preferences and colour vision in poultry chicks. Proc. R. Soc. B 274, 1941–1948 10.1098/rspb.2007.0538 (doi:10.1098/rspb.2007.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svádová K., Exnerová A., Štys P., Landová E., Valenta J., Fučíková A., Socha R. 2009. Role of different colours of aposematic insects in learning, memory and generalization of naïve bird predators. Anim. Behav. 77, 327–336 10.1016/j.anbehav.2008.09.034 (doi:10.1016/j.anbehav.2008.09.034) [DOI] [Google Scholar]