Abstract
The fitness consequences of animal personalities (also known as behavioural syndromes) have recently been studied in several solitary species. However, the adaptive significance of collective personalities in social insects and especially of behavioural variation among group members remains largely unexplored. Although intracolonial behavioural variation is an important component of division of labour, and as such a key feature for the success of societies, empirical links between behavioural variation and fitness are scarce. We investigated aggression, exploration and brood care behaviour in Temnothorax longispinosus ant colonies. We focused on two distinct aspects: intercolonial variability and its consistency across time and contexts, and intracolonial variability and its influence on productivity. Aggressiveness was consistent over four to five months with a new generation of workers emerging in between trial series. Other behaviours were not consistent over time. Exploration of novel environments responded to the sequence of assays: colonies were faster in discovering when workers previously encountered opponents in aggression experiments. Suites of correlated behaviours (e.g. aggression–exploration syndrome) present in the first series did not persist over time. Finally, colonies with more intracolonial behavioural variation in brood care and exploration of novel objects were more productive under standardized conditions than colonies with less variation.
Keywords: personality, behavioural syndromes, division of labour, fitness, social insects, aggression
1. Introduction
The concept of personality or behavioural syndromes has re-emerged as a way to study the association of consistent behavioural variants with the reproductive success of animals [1–3]. Behavioural syndromes involve: (i) behavioural consistency across time, (ii) consistency of the same behaviour across different contexts, or (iii) correlations of different behaviours [4]. Among the well-studied behavioural traits are aggression, exploration and boldness, which have been found to influence the survival and fitness of individuals in a variety of animal species [5]. The effect of a behavioural trait on its owner's fitness is generally context-dependent. While it may be beneficial to be exploratory or bold in a predator-free environment, these behaviours are clearly costly under high predation pressure. The study of suites of correlated behaviours that are consistent across situations or contexts, can help to understand why and how a behavioural type evolved, even if it appears non-adaptive at first sight [6]. For example, high aggressiveness in the North American fishing spider was found to decrease the reproductive success of females as it led to pre-copulatory sexual cannibalism, but aggression was beneficial in the context of foraging, resulting in higher feeding rates and increased fecundity [7]. Consequently, behavioural traits that are frequently associated should be studied together, rather than in isolation [8].
Behavioural syndromes are generally used to describe differences among individuals, i.e. single organisms. In the case of social insects, each colony can be viewed as a superorganism, with a reproductive queen and non-reproductive workers as her extended phenotype [9]. Indeed, natural selection in social insects does not only act on the individual level, but also on the level of the colony [10]. This colony level selection can lead to the evolution of colony behavioural variants, for example, honeybee colonies were shown to exhibit a collective personality [11]. Behavioural traits like foraging activity and defensive response were not only part of a behavioural syndrome in honeybees, but also correlated with colony productivity. Consequently, personalities can also be studied on the colony level in social insects.
A first goal of this study was to investigate intercolonial behavioural consistency across time (also known as differential consistency [12]) in the ant Temnothorax longispinosus in aggressiveness, exploration and brood care. We also explored suites of correlated behaviours, between aggression, exploration and brood care. Furthermore, we tested whether these suites of correlated behaviours were consistent over time and contexts (defined as structural consistency [12]). Some colonies might be very aggressive and exploratory (proactive), while others could be more shy and cautious (reactive). Similar to solitary animals, proactive colonies are expected to be very competitive and flourish in stable environments, while reactive ones are better in adapting to changes in the environment [8].
Our second goal was to investigate whether intracolonial variability in behaviour influences colony productivity. Animal groups may not only differ in their average collective personality, e.g. mean aggressiveness, but also in the behavioural variability of their members. While two groups may spend the same total amount of time on brood care, aggressive interactions or foraging, the allocation of tasks among group members could be quite different. In one group, specialists primarily perform a single task, whereas in other groups generalists with a uniform task threshold take over all tasks with the same likelihood. Further, individual task specialization may increase task efficiency. That this is not necessarily the case has been shown recently in Temnothorax ants, in which specialists were not more efficient in performing their tasks [13]. Nevertheless, colonies with a higher behavioural variation among workers can in theory react faster and more appropriately to changing colony needs. As a result, these colonies should show a more efficient task allocation and higher colony fitness than colonies with less behavioural variability [14].
The ecological success of social insects can be mainly attributed to their division of labour [15,16]. Surprisingly, empirical data linking behavioural variation among group members to fitness are still scarce (but see [17]). We recently demonstrated a positive relationship between intracolonial behavioural variation in aggression and colony productivity in Temnothorax ants in the field [18]. However, our study also revealed that environmental factors, i.e. habitat quality and population density, can be associated with behavioural variation and could have created the positive correlation between variation in aggression and productivity. High habitat quality could have led to higher productivity and higher ant densities. The latter could have not only increased mean colony aggression but also its variation. Presumably, aggression in ants is generated through a combination of inherited, age-related or environmental factors similar to the honeybees [19].
The present study had three main objectives. First, we asked whether ant colonies differ in behaviour even when maintained for several months in the laboratory under the same conditions. We designed three standardized experiments that measured aggression, brood care and exploration behaviour of individual ants. In each trial series, 10 workers per colony were tested, so that we could also study the intracolonial behavioural variation in ant colonies. Second, we were interested in the consistency of behavioural variation between colonies over longer time periods (i.e. several months). We tested 10 workers per colony at two points in time, with the development of a new worker generation in between the two testing periods. Finally, we were interested in whether there is a relationship between behavioural variation among group members and colony productivity under standardized conditions.
2. Material and methods
(a). Study system
Temnothorax longispinosus ant colonies were collected in late July 2009 at the Watoga State Park, Pocahontas County, WV, USA. They were transported to our laboratory at the Ludwig Maximilian University Munich, and kept under identical conditions for eight months until the start of the experiment in April 2010. In aggression experiments, ant workers were confronted with a dead conspecific opponent. Temnothorax longispinosus colonies from which these opponents were taken were collected at the Huyck Preserve, Albany County, NY, USA, in March–April 2009. Ant colonies were kept in artificial nests in three-chambered plastic boxes (9.5 × 9.5 × 2.7 cm) with a moistened plaster floor in a climate chamber. From July 2009 to the end of September 2009 and again from the end of January 2010, the ant colonies were kept at 20°C : 15°C in a 12 L : 12 D cycle. In-between, ant colonies were kept on a lower temperature cycle to simulate winter conditions (10°C to −5°C). During their active period, ants were fed weekly with honey and pieces of dead crickets. We used 27 monogynous T. longispinosus colonies with 23.11 ± 7.33 (mean ± s.d.) workers.
(b). Behavioural experiments
Ten workers per colony were randomly selected and subsequently used for the brood care, exploration and aggression experiments. Each worker was separately tested and the mean behaviour and standard deviation over the 10 workers from a single colony used as a measure of colony behaviour and its variation. Brood care was measured first, followed by exploration and finally aggression. Workers from the same colony were always tested in all behaviours on the same day, with about 1 h in between assays. All arenas were wiped with ethanol after each test to remove residual odours and the ethanol was allowed to evaporate for at least 30 s. All experiments were conducted at room temperature (22–25°C).
We conducted two trial series, during each of which 10 workers per colony were tested. The first trial series was conducted between the 5 April and the 2 July 2010 by the second author. The second series, to test for intercolonial behavioural consistency over time was performed between the 8 November and the 5 December 2010 by the first author.
Near the end of the first series of experiments some of the worker pupae began to hatch. We decided not to include freshly emerged callow workers into our experiments as they are known to be mostly inactive during their first days as an adult. Freshly eclosed callow workers are easy to identify because their cuticle is lighter in colour. Within two weeks, the cuticle darkens and workers are no longer distinguishable from older workers. Hence, we cannot exclude that some of the workers that were tested close to the end of the trial series eclosed within the last few weeks.
(i). Brood care behaviour
Each worker was confronted with a worker pupa from its own colony in a small circular arena (diameter 12 mm, height 3 mm) for 5 min. We used pupae instead of larvae for these experiments because the behaviour of a larva could influence worker behaviour. For example, hungry larvae might beg for food, and variation in begging behaviour might thus lead to differences between experiments. Worker behaviour was recorded via scan sampling every 20 s. Frequency of pupa grooming was used as a measure of brood care behaviour, which is a good measure for overall brood care, because earlier work showed that there were no differences in the tendencies to care for eggs, larvae or pupae among the workers performing brood care [20]. One worker pupa was randomly selected from the workers’ colony and re-used for all 10 workers of this colony. We would have preferred to use a new pupa for every worker, but we did not have enough pupae in all colonies. To avoid treating colonies differently, all workers from a single colony were tested with the same pupa.
(ii). Exploration of novel objects and environments
Single ants were observed in 5 min trials in a multi-chamber set-up consisting of a central circular chamber (diameter 29 mm, height 3 mm) that was connected to eight equally-sized side chambers through eight corridors (length 32 mm, width 7 mm). Each of the eight chambers contained an unfamiliar chemically distinct object: dried pieces of oregano, thyme, rosemary, spruce needles, sage, savoury, chamomile and caraway. All objects were exchanged in between tests. As one measure of exploration, we counted how often each worker antennated these objects. This exploration measure is identical to the one used in an earlier study and can be described as the exploration of novel objects [18]. We analysed a second behavioural aspect of these trials that reflects the exploration of novel environments by the focal ants. This exploration measure was calculated for each ant as the proportion of side chambers that an ant entered (n of chambers entered/total number of chambers).
(iii). Aggression
Aggression was measured by confronting each worker with a freshly defrosted dead non-nest-mate worker from one of 13 opponent colonies from a different population in New York in a small circular arena (diameter 12 mm, height 3 mm). In the aggression experiments, we used dead non-nest-mate conspecifics as opponents, because therefore we can exclude an influence of the opponent's behaviour on the outcome of the trial. Moreover, we could show earlier that aggression against dead opponents reflects aggression against live opponents [18]. Opponents were killed by freezing. All aggressive interactions (mandible spreading, biting, dragging, carrying and stinging) were recorded every 20 s for 5 min. The frequency of aggressive interactions was calculated by dividing the number of aggressive acts by the number of observations. Opponents were replaced after each encounter.
(c). Behavioural consistency on the colony level
In order to test for consistency in aggressive and exploratory behaviour on the colony level, 20 randomly chosen colonies were re-tested after four to five months. We examined only 20 colonies because some of the colonies had died, were too small (contained less than 10 workers) or had lost their queen before the start of the second trial series. Importantly, we were interested in colony personality and therefore killed all tested workers after the first trial series. Consequently, all workers included in the second series were naïve to the behavioural experiments. Moreover, a new generation of workers had emerged in between the two trial series, so that focal workers of the first and second trial series often belonged to two different worker generations. Measuring the colony size before the second trial series allowed us to calculate the percentage of newly hatched workers for every colony. On average, 53.6 per cent of all workers in the second trial had emerged in between the two trial series.
We were unable to re-test brood care behaviour during the second trial series in the autumn because during this time of year T. longispinosus nests did not contain pupae. Behavioural experiments were identical to the first series with the exception that we evaluated possible order effects of the behavioural assays. Carryover effects from previous assays cannot only bias results, but also decrease the statistical power and should therefore always be dealt with if possible [21,22]. To estimate potential order effects, we split the colonies in two treatment groups of 10 colonies each with either aggression tested before exploration (AE) or exploration tested before aggression (EA).
(d). Influence of behavioural variation on colony productivity
As a measure of fitness, we estimated per capita productivity (total production divided by colony size), according to a standardized protocol [18]. Total biomass production was calculated by counting all male, queen and worker pupae in a colony and subsequently multiplying their number with the average dry weights of the respective castes [23]. As the caste of pre-pupae could not be reliably determined we included them in the calculation as worker pupae. Relative productivity was subsequently calculated for every colony by dividing the total biomass production by the total number of adult workers. The census for the biomass calculation (including the count of colony size) was performed on the 1 April, 4 days before the first series of experiments started. Temnothorax ants have a highly synchronized brood production [24], with an emergence of new workers, queens and males in July in the field. However, as our laboratory colonies experienced warmer summer conditions starting already from end of January onwards, brood development was much progressed in April. Most larvae had developed into pupae, which can be easily identified by caste. We only analysed the association between colony behaviour and its variation on productivity during the first trial series.
(e). Statistical analyses
Behavioural data on the individual worker level were not normally distributed, so we used Kruskal–Wallis tests to check for behavioural differences between colonies. By contrast, all data on the colony level were normally distributed (Kolmogorov–Smirnov tests, p > 0.2), allowing us to use parametric analyses.
We used factor analysis to investigate suites of correlated behaviours. Principal component or factor analysis are often used to describe behavioural syndromes because they do not require hypothesis testing [25,26]. Data for the factor analysis were standardized (Z-score) by subtracting the sample mean from the colony behaviour value, the difference was finally divided by the sample standard deviation [27]. All colony behaviours were included in the analysis, but only factors with an eigenvalue greater than one were extracted. Factors were rotated using varimax rotation to facilitate interpretation. We used Pearson correlation to examine the relationships between individual pairs of behaviours on the colony level. To examine whether behavioural syndromes on the colony level were owing to syndromes on the worker level, we used Spearman rank correlation, because individual data were not normally distributed.
We investigated consistency of colony behaviour using analysis of covariance (ANCOVA) with the order of experiments (AE or EA) as a categorical factor, and colony size and original behaviour (in the first series) as a continuous predictor. In order to test for consistency of intracolonial variation, we repeated this approach for the standard deviation of each behaviour (s.d. aggression, s.d. exploration (object) and s.d. exploration (environment)). To analyse potential order effects on the structure of behavioural syndromes, we performed a factor analysis for each treatment separately.
We used multiple regressions to analyse if behaviours or variation in behaviours influence the fitness measure, i.e. relative productivity. In addition, colony size was added as an explanatory variable to the mean colony behaviours (aggression, exploration (object), exploration (environment) and brood care) and s.d. as a measure of their intracolonial variation. As an alternative measure of intracolonial variation, we performed the same multiple regression with the intracolonial range of behaviours instead of s.d.
To further investigate which pattern of behavioural variation is particularly beneficial at the colony level, we calculated moments of distribution, i.e. skewness and kurtosis, for every colony. We performed linear regressions with relative productivity as the dependent and intracolonial skewness or kurtosis of the focal behaviour as an explanatory variable. These additional analyses were only conducted for those behaviours for which the multiple regression uncovered an association between behavioural variation and productivity. As an alternative to the separate analysis of skewness (g1) and kurtosis (g2), we also calculated degrees (arctan (g1/g2)) for all behaviours according to Gilboa & Nonacs [28] and added degrees into the multiple regression model.
3. Results
(a). Intercolonial behavioural differences
We found strong differences among ant colonies maintained under standardized laboratory conditions for over eight months in brood care, exploration (object), exploration (environment) and aggression (Kruskal–Wallis tests; brood care: H26,270 = 67.56, p < 0.0001; exploration (object): H26,270 = 51.74, p < 0.002; exploration (environment): H26,270 = 42.54, p < 0.03; aggression: H26,270 = 67.94, p < 0.0001).
(b). Suites of correlated behaviours
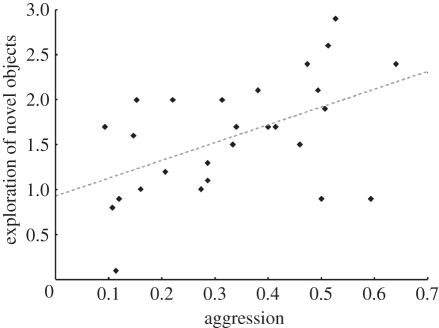
Factor analysis revealed a behavioural syndrome on the colony level described by two different factors that, respectively, accounted for 38.5 per cent and 34.5 per cent or a total of 73 per cent of the observed intercolonial behavioural variation in brood care, exploration (object), exploration (environment) and aggression (table 1). The first factor was described by a positive influence of both aggression and exploration (object). The second factor contained heavy loadings from brood care and exploration (environment). Pearson correlation showed a positive relationship between aggression and exploration (object: r = 0.507, p < 0.01, n = 27; figure 1) on the colony level. By contrast, brood care and exploration (environment) were not significantly correlated (p = 0.12). No other pairs of behavioural traits were correlated on the colony level (p > 0.16). On the individual worker level, we also found the aggression–exploration (object) syndrome (Spearman's r = 0.257, p < 0.0001, n = 270). In addition, brood care and exploration (environment) were positively correlated (Spearman's r = 0.144, p = 0.02, n = 267).
Table 1.
Factor loadings from factor analysis. (Data of all colony behaviours were standardized (Z-score) and only eigenvalues greater than one were extracted. Factors were rotated using varimax rotation. Significant loadings are shown in bold.)
| colony behaviour | factor 1 | factor 2 |
|---|---|---|
| aggression | 0.861 | −0.250 |
| exploration (object) | 0.860 | 0.208 |
| exploration (environment) | 0.158 | 0.787 |
| brood care | −0.183 | 0.811 |
| % variation explained | 38.50 | 34.55 |
Figure 1.
Relationship between mean aggression and mean exploration of novel objects of 27 T. longispinosus colonies in the first trial series. Ten workers per colony were separately tested to measure mean colony behaviour. Pearson correlation gave the following result: r = 0.507, p < 0.01.
(c). Behavioural consistency on the colony level
Aggressive behaviour and intracolonial variation in aggressive behaviour were consistent on the colony level over a period of at least four to five months (ANCOVA: mean aggression: F1,16 = 8.55, p < 0.01; s.d. aggression: F1,16 = 7.33, p < 0.02; figure 2). The order of the experiments (p > 0.19) and colony size (p > 0.63) had no effect on aggression and its variation. Exploration (object) and its variation could not be explained by any variable (p > 0.28), indicating that it is inconsistent on the colony level and not influenced by order effects or colony size. Hence, intracolonial variation in both exploratory and aggressive behaviour was not correlated to colony size. Exploration (environment) was also inconsistent over time on the colony level (p > 0.99), but was influenced by the order of experiments (F1,16 = 13.47, p < 0.01) and by colony size (F1,16 = 9.68, p < 0.01). Colonies, whose workers participated in aggression trials before, showed higher exploration of environment scores. Smaller colonies also had higher exploration scores. Variance in exploration (environment) could not be explained by any variable (all p > 0.27).
Figure 2.
Consistency of aggressive behaviour of 20 T. longispinosus colonies. Ten workers per colony were separately tested to measure mean colony aggression. The second test was performed four to five months after the first test with the same colony but with different workers. In addition, a new generation of workers emerged in between experiments.
Factor analysis of the second trial series revealed low structural consistency, i.e. correlations between different behaviours from trial series one, were not maintained in the second series. Furthermore, the relationship among behaviours was different in the two treatment groups (table 2). Treatment EA, which is similar to the first trial series, yielded two factors that explained 51.1 per cent and 34.2 per cent, or a total of 85.3 per cent of the observed variation. Factor 1 showed significant negative loadings of exploration (object) and exploration (environment). Factor 2 consisted of one significant negative loading of aggression. Treatment AE generated two factors describing 37.4 per cent and 33.7 per cent, or a total of 71 per cent of the total variation. Aggression and exploration (object) had significant loadings in the first factor, however, in opposite directions. Exploration (environment) was the only significant loading in factor 2. The suggested relationships in treatment EA between exploration (object) and exploration (environment; p = 0.11, n = 10), and in treatment AE between aggression and exploration (object; p = 0.74) were not significant. We also performed correlations on the individual level for these relationships. Individual exploration scores of novel objects and environments were positively associated (Spearman's r = 0.224, p = 0.025, n = 100), whereas aggression and exploration (object) were not correlated on the individual level (p > 0.92).
Table 2.
Factor loadings from factor analysis of the second trial series. (Data for aggression, exploration (object) and exploration (environment) behaviours were standardized (Z-score) and only eigenvalues greater than one were extracted. Factors were rotated using varimax rotation. Significant loadings are shown in bold.)
| colony behaviour | factor 1 |
factor 2 |
factor 1 |
factor 2 |
|---|---|---|---|---|
| treatment EA |
treatment AE |
|||
| aggression | 0.020 | −0.990 | −0.741 | 0.195 |
| exploration (object) | −0.884 | −0.117 | 0.757 | 0.178 |
| exploration (environment) | −0.867 | 0.178 | 0.001 | −0.970 |
| % variation explained | 51.11 | 34.20 | 37.39 | 33.66 |
(d). Relative productivity
Colony productivity increased with higher among-worker variability in both brood care (β = 0.847, p < 0.001) and exploration (object) (β = 0.488, p = 0.007; table 3). Interestingly, colony productivity decreased with higher mean values of both brood care (β = −0.782, p < 0.001) and exploration (object) (β = −0.420, p = 0.04). Aggression, exploration (environment) and variation in those behaviours were not associated with colony productivity. Further, colony size did not correlate with productivity (p = 0.34). Using an intracolonial range of behaviours instead of s.d. yielded similar results. Both range in brood care behaviour (β = 0.760, p = 0.001) and in exploration (object; β = 0.496, p = 0.034) correlated positively with productivity (electronic supplementary material, table S1).
Table 3.
Results of the multiple regression on the relative productivity of 27 laboratory T. longispinosus colonies (F9,17 = 5.62; p < 0.001; r²adjusted = 0.615). (Colony behaviours were analysed by testing 10 workers per colony separately in standardized assays. Significant results are given in bold.)
| explanatory variable | β-value | p-value |
|---|---|---|
| aggression | 0.331 | 0.095 |
| s.d. aggression | −0.059 | 0.707 |
| exploration (object) | −0.420 | 0.040 |
| s.d. exploration (object) | 0.488 | 0.007 |
| exploration (environment) | −0.100 | 0.479 |
| s.d. exploration (environment) | −0.087 | 0.919 |
| brood care | −0.782 | 0.001 |
| s.d. brood care | 0.847 | 0.0002 |
| colony size | −0.166 | 0.340 |
The analysis of skewness and kurtosis revealed that intracolonial skewness of brood care behaviour was positively related to relative productivity (linear regression: r = 0.41, p = 0.036). Although intracolonial skewness and kurtosis scores were highly correlated (Pearson's r = 0.90, p < 0.001), intracolonial kurtosis values of brood care behaviour were not related to productivity (p = 0.18). Intracolonial skewness and kurtosis values for exploration (object) were not related to productivity (p > 0.16). The addition of degrees did not change the results of the original multiple regression model without degrees, and degrees themselves were not correlated with productivity (see the electronic supplementary material, table S2).
4. Discussion
In the present study, we searched for personality differences in aggression, exploration and brood care in T. longispinosus ant colonies. Maintenance of ant colonies under common garden conditions over eight months did not reduce or eliminate differences in colony behaviour. Ant colonies differed in all analysed behaviours. We had already shown in an earlier study that colonies tested shortly after the collection in the field differed in aggression and exploration, but could not exclude that these differences were owing to environmental factors [18]. Moreover, our results of the second trial series demonstrate that at least in aggression these differences are retained over several months. This indicates that colony personalities are at least partially genetically determined. Alternatively or in addition, developmental processes or social environment could be responsible for the observed consistency. While only aggression remained consistent on the colony level, all behaviours were part of a behavioural syndrome. Most importantly, we found that colonies with higher behavioural variation also showed higher productivity under standardized conditions.
Seventy-three per cent of all behavioural variation in the first trial series could be explained by a behavioural syndrome with two distinct factors. One factor consisted of a positive association between aggression and exploration of novel objects, indicating that aggressive colonies were also more likely to inspect novel objects. This relationship existed on two scales: the colony and the individual level (see also [29]). Exploratory colonies were more aggressive because their workers also showed the exploration (object)–aggression syndrome and not because they contained two separate groups of exploratory and aggressive workers. We speculate that such ‘proactive’ colonies would be bold explorers and at the same time very competitive. This fits the general syndrome termed as the ‘proactive–reactive axis’ that has been found in a number of species [6]. The second factor showed a positive relationship between brood care behaviour and exploration of novel environments. This is a surprising result, because we did not expect that colonies which spend a lot of time on brood care would also be fast in discovering novel environments. A possible explanation would be that this is an artefact of our experimental design. We randomly chose 10 workers and tested them in situations that they would rarely experience in the field. Workers who rarely leave the nest, e.g. nurses, presumably want to return as fast as possible to their nest to continue with brood care. Thereby, they would discover many chambers without being really interested in exploration.
Aggressiveness and its intracolonial variation were consistent on the colony level and not influenced by the exact order of tests. Colonies remained consistent in their aggression for at least four to five months with a new generation of workers emerging in between. Consistency of aggression has already been shown in honeybees and other ant species [11,30]. However, we go one step further by examining consistency on the colony level: first, we removed tested individuals after the first experiment. Second, the subsequent experiment was performed after a new generation of workers had emerged. Around 54 per cent of all workers in the second trial series belonged to this new generation. Hence, our results suggest that intercolonial variation in aggressiveness was owing to genetic factors, developmental processes and/or social environment. By contrast, neither measure of exploration remained consistent on the colony level. While the positive relationship between aggression and exploration (object) suggests that the latter remains consistent on the individual worker level, its inconsistency on the colony level indicates a lower genetic influence. It seems that exploratory behaviour is more influenced by experience. The impact of early experience on ant behaviour was shown in an earlier study on the development of foraging behaviour [31]. Differences in exploratory behaviour could also be age-related. Age-related division of labour has been shown in a couple of social insects, e.g. honeybees and some ant species [32,33]. However, in our study species, division of labour is probably not age-related. Sendova-Franks & Franks [34] found a very weak association between age and task allocation in the closely related Temnothorax unifasciatus and further suggested that division of labour is based on workers ‘foraging for work’, i.e. workers look actively for work. Accordingly, even young workers could become foragers, while old workers remain nurses.
So why did we find the positive correlation between exploration (object) and aggression in the first trial series, but not in the second? There are three mechanisms that most probably influence behavioural syndromes: genetics, experience and neuroendocrine effects [6]. While the consistency of aggression indicates genetic influence, exploration of objects is probably more strongly affected by experiential factors. In a natural environment, aggressive workers may continue to be curious about new objects, while more peaceful individuals become shyer with every negative experience, i.e. fights with competitors. Therefore, colonies might not have shown a positive relationship between aggression and exploration of objects in the second trial, because they consisted of more workers raised in the laboratory which never lived in a competitive environment. An alternative explanation could be that we by chance tested different castes in the two trial series. Although we randomly picked 10 individuals per colony, we cannot exclude the possibility that we chose many patrollers/guards in the first trial and mainly nurses and foragers in the second trial series. A recent study in Myrmica ants showed that the aggression–boldness syndrome (similar to the aggression–exploration of objects syndrome in this study) is only present in the patroller caste and could not be found in the nurses [29].
We further demonstrated that the order of experiments, i.e. if exploration was tested before or after aggression, influenced exploration of novel environments. If workers were confronted with a dead conspecific, they were faster in the discovery of novel environments. After an encounter with a conspecific, workers could be alarmed and try to reach their nest as fast as possible. The different treatments generated two completely different sets of behaviours. The first set (EA treatment) led to a positive relationship between exploration of objects and of environments, while the second (AE treatment) suggested a negative relationship between aggression and exploration (object). This is an important point for future research in the context of behavioural syndromes. One experience alone, in this case an aggressive encounter with a conspecific, cannot only change a single behaviour, but moreover create a different relationship among all behaviours. Our result underlines the importance of controlling for order effects when studying behavioural syndromes [21,22]. Ignoring the sequence of behavioural assays could not only decrease statistical significance, but could also create relationships among behaviours that are owing to order effects. A possible explanation for the observed order effects and the inconsistency of exploration comes from a study on Argentine ants, showing that these ants can form expectations of their environment from previous experience that will subsequently change how they explore novel habitats [35]. The fact that ants are able to adjust their searching behaviour in response to food type [36], microclimatic factors [37] and group size [38] also point to a high flexibility of exploratory behaviour. Although plasticity in behaviour seems to be contradictory to the concept of personality, colonies of harvester ants displayed not only consistent colony-specific differences in foraging activity, but moreover the propensity to adjust to changing food availability [39,40].
Colony size was not correlated to productivity in the laboratory (this study) and in the field [18]. However, our sample size may not be large enough to detect weak effects of colony size. S. Foitzik (2003, unpublished data, based on almost 500 colonies) showed that productivity decreases with colony size (termed as ‘Michener's paradox’ [41]). Wenzel & Pickering argued that this paradoxical inverse correlation can be explained with the central limit theorem. Accordingly, colony level variation in foraging success decreases with colony size. Small colonies may exhibit the highest productivity, but at the same time suffer more in periods of dearth [42].
Colony productivity increased with behavioural variation among workers in two behaviours: brood care behaviour and exploration of novel objects. This supports our hypothesis that behavioural variation increases colony fitness, presumably through a more efficient task allocation [14]. Similar to our earlier study [18], in which we studied productivity in the wild, variation in a behavioural trait increased with productivity. While aggression is probably the most important trait in the context of competition and nest defence, laboratory colonies do not have to compete for resources or defend their nest sites. Hence, laboratory productivity was best explained by two other behaviours, i.e. the among worker variation in exploration of novel objects and brood care. The more variation a colony had in these traits, the more brood they were able to produce. Uniform behaviour seems to be detrimental to fitness, especially when colonies reach high mean values in these behaviours. Colonies with many curious and brood caring workers were less productive than colonies with more variation in these behaviours. These results support part of the theoretical model of Myerscough & Oldroyd [14], i.e. colonies with high behavioural variation may perform better than colonies with uniform behaviour. While colonies with a uniform behaviour only have an all-or-nothing response to colony needs, colonies with higher variation show a more effective task allocation, in which an optimal number of workers is allocated to changing task needs. In solitary animals, selection acts on individual phenotypes, e.g. high aggression in Ural owls [43] or low boldness in swift foxes [44]. Social species, however, are under multi-level selection [10]. In a social group, it may not be optimal to reach a high or low score in a behaviour to reach the highest fitness, but instead have the perfect mix of behavioural types (e.g. [17,18]). The results of our study suggest that having individuals at the upper and lower bounds (i.e. a larger overall range) of behaviour makes the colony more productive. In case of brood care, a more right skewed distribution with many individuals in the lower bounds and a long tail of individuals in the upper bounds of behaviour (i.e. specialists or keystone individuals) seems to be the most beneficial pattern of intracolonial variation.
If the observed behavioural variation has a genetic basis, then genetic diversity itself could increase productivity in T. longispinosus. However, natural selection would probably reduce genetic diversity through directional selection and genetic drift. Nonacs & Kapheim introduced ‘social heterosis’ as a framework to explain why genetic diversity is not continuously reduced through natural selection. They demonstrated mathematically that differences across groups in productivity owing to genetic diversity could counteract both within-group selection and drift [45,46]. The results of our study that productivity increases with intracolonial variation in behaviour are supportive of social heterosis. Future studies on collective personalities and fitness that include intragroup variation could not only shed light on the evolution of division of labour, but moreover on the adaptive significance of group living in general.
In summary, we were able to show that colonies exhibit behavioural differences in aggression, exploration and brood care despite being held under standardized conditions in the laboratory for eight months. This indicates that the observed differences are not owing to short-term effects of the environment, but innate colony characteristics. All behaviours in the first series of experiments were part of a syndrome that also included an exploration of novel objects–aggression syndrome on two scales (i.e. colony and individual level). Similar to proactive individuals in solitary animals [8], curious colonies were also very aggressive. However, these syndromes did not persist over time. We were further able to demonstrate that colony aggression remains consistent over at least four months with a new generation of workers emerging in between. Therefore, we suggest that aggressiveness in Temnothorax ants is at least, in part, genetically and/or developmentally determined. Exploratory behaviour was inconsistent on the colony level indicating a stronger impact of environmental influences, experience or age. One of the observed exploratory behaviours (exploration of novel environments) was strongly influenced by the order in which behaviours were tested, suggesting a plastic response to stimuli such as aggressive encounters. Further, the order of behavioural assays influenced the relationship among all behaviours revealing different suites of correlated behaviours. Our study is therefore a good example for the influence of order effects on behavioural syndromes. Sequence of behavioural experiments should always be included as an explanatory variable if possible [21].
Finally, we were able to show that productivity of ant societies increased with intracolonial behavioural variation. In contrast to our earlier study [18], we were able to exclude environmental influences like density on productivity, because we held all colonies under standardized conditions in the laboratory for eight months. This result indicates that colonies with high behavioural variation outperform those with uniform behaviour, presumably through a more efficient task allocation. This study therefore suggests that ant societies which show a stronger division of labour are more productive, a fact that has been debated in human societies for centuries [47].
Acknowledgements
We thank Florian Menzel, Tobias Pamminger and Inon Scharf for helpful comments on the manuscript. We are particularly indebted to Deborah Gordon, Peter Nonacs and Stephen Pratt for their valuable comments during the review process. We are grateful to the Huyck Preserve, Albany County, NY, for access to facilities and support. This study was funded by the Deutsche Forschungsgemeinschaft (Fo 298/11). A.P.M. was supported by a graduate scholarship of the Universität Bayern e.v.
References
- 1.Dall S. R. X., Houston A. I., McNamara J. M. 2004. The behavioral ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 2.Dingemanse N. J., Réale D. 2005. Natural selection and animal personality. Behaviour 142, 1165–1190 10.1163/156853905774539445 (doi:10.1163/156853905774539445) [DOI] [Google Scholar]
- 3.Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Phil. Soc. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 4.Sih A., Bell A., Johnson J. C. 2010. Behavioral syndromes. In Evolutionary behavioral ecology (eds Westneat D. F., Fox C. W.), pp. 516–530 New York, NY, Oxford University Press [Google Scholar]
- 5.Smith B. R., Blumstein D. T. 2008. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 10.1093/beheco/arm144 (doi:10.1093/beheco/arm144) [DOI] [Google Scholar]
- 6.Sih A., Bell A. M., Johnson J. C., Ziemba R. E. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 7.Johnson J. C., Sih A. 2005. Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behav. Ecol. Sociobiol. 58, 390–396 10.1007/s00265-005-0943-5 (doi:10.1007/s00265-005-0943-5) [DOI] [Google Scholar]
- 8.Sih A., Bell A., Johnson J. C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 9.Hölldobler B., Wilson E. O. 2009. The superorganism: the beauty, elegance and strangeness of insect societies. New York, NY: W. W. Norton and Co [Google Scholar]
- 10.Korb J., Heinze J. 2004. Multilevel selection and social evolution of insect societies. Naturwissenschaften 91, 291–304 10.1007/s00114-004-0529-5 (doi:10.1007/s00114-004-0529-5) [DOI] [PubMed] [Google Scholar]
- 11.Wray M. K., Mattila H. R., Seeley T. D. 2011. Collective personalities in honeybee colonies are linked to colony fitness. Anim. Behav. 81, 559–568 10.1016/j.anbehav.2010.11.027 (doi:10.1016/j.anbehav.2010.11.027) [DOI] [Google Scholar]
- 12.Stamps J., Groothuis T. G. G. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. Camb. Phil. Soc. 85, 301–325 10.1111/j.1469-185X.2009.00103.x (doi:10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 13.Dornhaus A. 2008. Specialisation does not predict individual efficiency in an ant. PLoS Biol. 6, 2368–2375 10.1371/journal.pbio.0060285 (doi:10.1371/journal.pbio.0060285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myerscough M. R., Oldroyd B. P. 2004. Simulation models of the role of genetic variability in social insect task allocation. Insect. Soc. 51, 146–152 10.1007/s00040-003-0713-1 (doi:10.1007/s00040-003-0713-1) [DOI] [Google Scholar]
- 15.Wilson E. O. 1987. Causes of ecological success: the case of the ants. J. Anim. Ecol. 56, 1–9 10.2307/4795 (doi:10.2307/4795) [DOI] [Google Scholar]
- 16.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Harvard University Press [Google Scholar]
- 17.Pruitt J. N., Riechert S. E. 2011. How within-group behavioural variation and task efficiency enhance fitness in a social group. Proc. R. Soc. B 278, 1209–1215 10.1098/rspb.2010.1700 (doi:10.1098/rspb.2010.1700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modlmeier A. P., Foitzik S. 2011. Productivity increases with variation in aggression among group members in Temnothorax ants. Behav. Ecol. 22, 1026–1032 10.1093/beheco/arr086 (doi:10.1093/beheco/arr086) [DOI] [Google Scholar]
- 19.Alaux C., et al. 2009. Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc. Natl Acad. Sci. USA 106, 15 400–15 405 10.1073/pnas.0907043106 (doi:10.1073/pnas.0907043106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbers J. M., Cunningham M. 1983. Social organization in Leptothorax longispinosus Mayr. Anim. Behav. 31, 759–771 10.1016/S0003-3472(83)80233-4 (doi:10.1016/S0003-3472(83)80233-4) [DOI] [Google Scholar]
- 21.Dochtermann N. A. 2010. Behavioral syndromes: carryover effects, false discovery rates, and a priori hypotheses. Behav. Ecol. 21, 437–439 10.1093/beheco/arq021 (doi:10.1093/beheco/arq021) [DOI] [Google Scholar]
- 22.Logue D. M., Mishra S., McCaffrey D., Ball D., Cade W. H. 2009. A behavioral syndrome linking courtship behavior toward males and females predicts reproductive success from a single mating in the hissing cockroach, Gromphadorhina portentosa. Behav. Ecol. 20, 781–788 10.1093/beheco/arp061 (doi:10.1093/beheco/arp061) [DOI] [Google Scholar]
- 23.Foitzik S., Backus V. L., Trindl A., Herbers J. M. 2004. Ecology of Leptothorax ants: impact of food, nest sites and social parasites. Behav. Ecol. Sociobiol. 55, 484–493 10.1007/s00265-003-0718-9 (doi:10.1007/s00265-003-0718-9) [DOI] [Google Scholar]
- 24.Headley A. E. 1943. Population studies of two species of ants, Leptothorax longispinosus Roger and Leptothorax curvispinosus Mayr. Ann. Entomol. Soc. Am. 36, 743–753 [Google Scholar]
- 25.Bell A. M. 2007. Future directions in behavioural syndromes research. Proc. R. Soc. B 274, 755–761 10.1098/rspb.2006.0199 (doi:10.1098/rspb.2006.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dingemanse N. J., Dochtermann N. A., Wright J. 2009. A method for exploring the structure of behavioural syndromes to allow formal comparison within and between data sets. Anim. Behav. 79, 439–450 10.1016/j.anbehav.2009.11.024 (doi:10.1016/j.anbehav.2009.11.024) [DOI] [Google Scholar]
- 27.Gotelli N. J., Ellison A. M. 2004. A primer of ecological statistics. Sunderland, MA: Sinauer [Google Scholar]
- 28.Gilboa S., Nonacs P. 2006. Testing models of parental investment strategy and offspring size in ants. Oecologia 146, 667–674 10.1007/s00442-005-0139-8 (doi:10.1007/s00442-005-0139-8) [DOI] [PubMed] [Google Scholar]
- 29.Chapman B. B., Thain H., Coughlin J., Hughes W. O. H. 2011. Behavioural syndromes at multiple scales in Myrmica ants. Anim. Behav. 82, 391–397 10.1016/j.anbehav.2011.05.019 (doi:10.1016/j.anbehav.2011.05.019) [DOI] [Google Scholar]
- 30.Crosland M. W. J. 1990. Variation in ant aggression and kin discrimination ability within and between colonies. J. Insect Behav. 3, 359–379 10.1007/BF01052114 (doi:10.1007/BF01052114) [DOI] [Google Scholar]
- 31.Ravary F., Lecoutey E., Kaminski G., Châline N., Jaisson P. 2007. Individual experience alone can generate lasting division of labor in ants. Curr. Biol. 17, 1308–1312 10.1016/j.cub.2007.06.047 (doi:10.1016/j.cub.2007.06.047) [DOI] [PubMed] [Google Scholar]
- 32.Seeley T. D. 1982. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav. Ecol. Sociobiol. 11, 287–293 10.1007/BF00299306 (doi:10.1007/BF00299306) [DOI] [Google Scholar]
- 33.Calabi P., Traniello J. F. A., Werner M. H. 1983. Age polyethism: its occurrence in the ant Pheidole hortensis, and some general considerations. Psyche 90, 395–412 10.1155/1983/57061 (doi:10.1155/1983/57061) [DOI] [Google Scholar]
- 34.Sendova-Franks A., Franks N. R. 1993. Task allocation in ant colonies within variable environments (a study of temporal polyethism, experimental). Bull. Math. Biol. 55, 75–96 10.1007/BF02460295 (doi:10.1007/BF02460295) [DOI] [Google Scholar]
- 35.Nonacs P., Soriano J. L. 1998. Patch sampling behaviour and future foraging expectations in Argentine ants, Linepithema humile. Anim. Behav. 55, 519–527 10.1006/anbe.1997.0615 (doi:10.1006/anbe.1997.0615) [DOI] [PubMed] [Google Scholar]
- 36.Traniello J. F. A., Kozol A. J., Fournier M. A. 1992. Resource-related spatial patterns of search in the ant Formica schaufussi: a field study. Psyche 99, 87–83 10.1155/1992/65423 (doi:10.1155/1992/65423) [DOI] [Google Scholar]
- 37.Azcárate F. M., Kovacs E., Peco B. 2007. Microclimatic conditions regulate surface activity in harvester ants Messor barbatus. J. Insect Behav. 20, 315–329 10.1007/s10905-007-9074-3 (doi:10.1007/s10905-007-9074-3) [DOI] [Google Scholar]
- 38.Gordon D. M. 1995. The expandable network of ant exploration. Anim. Behav. 50, 995–1007 10.1016/0003-3472(95)80100-6 (doi:10.1016/0003-3472(95)80100-6) [DOI] [Google Scholar]
- 39.Gordon D. M. 1991. Behavioral flexibility and the foraging ecology of seed-eating ants. Am. Nat. 138, 379–411 10.1086/285223 (doi:10.1086/285223) [DOI] [Google Scholar]
- 40.Gordon D. M., Guetz A., Greene M. J., Holmes S. 2011. Colony variation in the collective regulation of foraging by harvester ants. Behav. Ecol. 22, 429–435 10.1093/beheco/arq218 (doi:10.1093/beheco/arq218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michener C. D. 1964. Reproductive efficiency in relation to colony size in Hymenopterous societies. Insect Soc. 11, 317–342 10.1007/BF02227433 (doi:10.1007/BF02227433) [DOI] [Google Scholar]
- 42.Wenzel J. W., Pickering J. 1991. Cooperative foraging, productivity, and the central limit theorem. Proc. Natl Acad. Sci. USA 88, 33–38 10.1073/pnas.88.1.36 (doi:10.1073/pnas.88.1.36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kontiainen P., Pietäinen H., Huttunen K., Karell P., Kolunen H., Brommer J. E. 2009. Aggressive Ural owl mothers recruit more offspring. Behav. Ecol. 20, 789–796 10.1093/beheco/arp062 (doi:10.1093/beheco/arp062) [DOI] [Google Scholar]
- 44.Bremner-Harrison S., Prodohl P. A., Elwood R. W. 2004. Behavioural trait assessment as a release criterion: boldness predicts early death in a reintroduction programme of captive-bred swift fox (Vulpes velox). Anim. Conserv. 7, 313–320 10.1017/S1367943004001490 (doi:10.1017/S1367943004001490) [DOI] [Google Scholar]
- 45.Nonacs P., Kapheim K. M. 2007. Social heterosis and the maintenance of genetic diversity. J. Evol. Biol. 20, 2253–2265 10.1111/j.1420-9101.2007.01418.x (doi:10.1111/j.1420-9101.2007.01418.x) [DOI] [PubMed] [Google Scholar]
- 46.Nonacs P., Kapheim K. M. 2008. Social heterosis and the maintenance of genetic diversity at the genome level. J. Evol. Biol. 21, 631–635 10.1111/j.1420-9101.2007.01489.x (doi:10.1111/j.1420-9101.2007.01489.x) [DOI] [PubMed] [Google Scholar]
- 47.Smith A. 1776. An inquiry into the nature and causes of the wealth of nations. London, UK: W. Strahan and T. Cadell [Google Scholar]