Abstract
Females of internally fertilizing species can significantly extend sperm lifespan and functionality during sperm storage. The mechanisms for such delayed cellular senescence remain unknown. Here, we apply current hypotheses of cellular senescence developed for diploid cells to sperm cells, and empirically test opposing predictions on the relationship between sperm metabolic rate and oxygen radical production in an insect model, the cricket Gryllus bimaculatus. Using time-resolved microfluorimetry, we found a negative correlation between metabolic rate (proportion of protein-bound NAD[P]H) and in situ intracellular oxygen radicals production in freshly ejaculated sperm. In contrast, sperm stored by females for periods of 1 h to 26 days showed a positive correlation between metabolic rate and oxygen radicals production. At the same time, stored sperm showed a 37 per cent reduced metabolic rate, and 42 per cent reduced reactive oxygen species (ROS) production, compared with freshly ejaculated sperm. Rank differences between males in ROS production and metabolic rate observed in ejaculated sperm did not predict rank differences in stored sperm. Our method of simultaneously measuring ROS production and metabolic rate of the same sample has the advantage of providing data that are independent of sperm density and any extracellular antioxidants that are proteins. Our method also excludes effects owing to accumulated hydrogen peroxide. Our results unify aspects of competing theories of cellular ageing and suggest that reducing metabolic rate may be an important means of extending stored sperm lifespan and functionality in crickets. Our data also provide a possible explanation for why traits of ejaculates sampled from the male may be rather poor predictors of paternity in sexual selection studies and likelihood of pregnancy in reproductive medicine.
Keywords: sperm senescence, sexual selection, reactive oxygen species, sperm longevity
1. Introduction
Like all other cells, sperm cells age [1], but cellular senescence of sperm is significantly delayed during female sperm storage in internally fertilizing species [1–5]. While sperm live for only a few seconds to hours after ejaculation in external fertilizers [4], they can remain alive and functionally intact for days in the sperm storage organs of most mammals, or even for weeks to years in birds, reptiles and arthropods [2,3,5–7]; ant queens can fertilize eggs with sperm they received up to 30 years ago [5]. The physiological mechanisms underlying the reduction in sperm ageing have largely remained unexplored [1–3], but sperm function is known to deteriorate considerably with oxidative damage [8–10].
Current theories of cellular senescence, developed for diploid somatic cells, link cellular metabolic rate to cellular senescence, often via oxidative stress. Oxidative stress is an imbalance of the production and removal of reactive oxygen species (ROS) and antioxidants and can, therefore, be reduced in two main ways in sperm cells. Either ROS are scavenged as they are produced in and around sperm in the sperm stores, called passive ROS removal [1–3,6,7], or intra-sperm production of ROS can be reduced by altering sperm metabolism [2]. However, it is currently debated how cell metabolic rate should be altered to reduce ROS production and for most cells, including sperm, empirical data are lacking.
We identified two broad categories of hypotheses that can be used to predict the relationship between sperm metabolic rate and sperm ROS production. In most cells, 90 per cent of cellular ROS are produced by the mitochondria [11]. Mitochondrial biologists have specified that metabolically active mitochondria have high ATP production rates and low membrane potential and, therefore, produce very few ROS [11–15]. This hypothesis predicts that fewer ROS are produced when sperm metabolism is increased. It may be consistent with the long and widely held belief that sperm are nourished by the female during storage [2,3]. If additional nourishment maintains a high metabolic rate under constant oxygen pressure, ROS production is predicted to be reduced during female sperm storage.
In contrast, high membrane potentials are observed and high rates of ROS production are expected if metabolic rate increases with increasing tissue oxygen pressure, with more mitochondria or when more mitochondria are in a resting stage [11–15]. This hypothesis is in agreement with the well-known caloric restriction theory [16], the increasingly challenged [11–15] oxygen free radical theory of ageing [17] and the observation that mitochondrial ROS can negatively affect sperm function [8]. Caloric restriction and the oxygen free radical theories of ageing both predict that decreasing, rather than increasing, the metabolic rate reduces cellular ROS production and senescence. For sperm cells, proposed mechanisms include the reduction of metabolic rate by binding sperm to epithelia and thereby increasing local sperm density or reducing the activity of mitochondria in sperm [1–3,6,9].
While a change in sperm ROS production during sperm storage may indicate female interference with sperm metabolism, it may also be male-driven. For example, sperm of some species switch to a glycolytic pathway in vitro [10,18,19]. From such metabolic changes in the absence of female influences, one might infer the existence of a sperm trigger to reduce ROS production. The existence of a sperm trigger predicts that sperm stored in vitro show reduced ROS production.
In summary, predictions about the relationship between metabolic rate and ROS production derived from diploid cell metabolism theory are partially contrary. Applied to a wide range of sperm physiologies [2–4,8,10,18,19], hypotheses have mainly focused on reducing ROS by reducing sperm metabolism but empirical data on sperm metabolism in the female reproductive tract are lacking, even for the basic prediction that during storage sperm ROS production should be reduced. Here, we address this fundamental prediction by introducing fluorescence-lifetime technology to sperm analysis. Our technology uniquely monitors cell metabolic rate and ROS production simultaneously. It also allows us to measure the in situ intra-sperm ROS production, rather than the concentration of accumulated ROS, enabling us to quantify active female antioxidant processes independently of any proposed passive ones [2,3,7]. We chose the cricket Gryllus bimaculatus as a model species because in this species, as in humans, ejaculated sperm can be sampled non-destructively and from the male without prior contact with female substances. Females can store sperm for several weeks before fertilizing the eggs [20]. From the same male, we compared sperm when freshly ejaculated and when stored by the female. This allowed us to examine the assumption in sexual selection studies and reproductive medicine assessments that sperm traits of freshly ejaculated sperm predict pregnancy or paternity.
2. Material and methods
(a). Measuring metabolic rate by NAD(P)H fluorescence
NAD(P)H is found either free in solution or bound to protein. Total NAD(P)H decreases with increasing ATP production, but free NAD(P)H is used up first [21]. Consequently, the proportion of bound-NAD(P)H relative to total NAD(P)H increases with increasing metabolic rate. Free NAD(P)H is autofluorescent with an emission peak at 460 nm and a lifetime τ1 of less than 1 ns, whereas the emission maximum of protein-bound NAD(P)H is blue shifted and its lifetime τ2 several-fold longer (2–10 ns) [21–26]. If the relative contributions of the amplitudes of τ1 and τ2 are a1 for free NAD(P)H and a2 for bound-NAD(P)H, then a2/(a1 + a2) gives an estimate of the metabolic rate [22–26].
(b). Reactive oxygen species measurement by 1-pyrene butyric acid fluorescence
1-Pyrene butyric acid (PBA) is a well-characterized, cell permeable oxygen probe used for free radical measurements in solution [27] and in living cells [28,29]. Upon collision with PBA, oxygen free radicals change PBA fluorescence intensity and decrease fluorescence lifetime. The method is particularly sensitive to small, mobile radical molecules such as superoxide radical (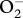 ) and nitric oxide (NO°) [27–29], but does not respond to the hydroxyl radical (which has too short a half-life). Non-radical ROS, such as hydrogen peroxide and peroxynitrite, do not affect PBA lifetime either [28]. Therefore, by measuring the concentration of free radicals with short half-life (less than 20 min) while simultaneously disregarding the concentration of the stable (and hence accumulating) hydrogen peroxide to which these radicals dismute, we can obtain an estimate of the in situ (i.e. less than 20 min half-life) production of ROS. Importantly, because membrane crossing by external oxidants or antioxidants is several orders of magnitude slower than PBA fluorescence changes, the occurrence of chemical reactions between oxygen radicals and antioxidants is unlikely to affect the results. In addition, PBA preferentially binds to dissolved proteins [28] rather than entering cellular membranes, so that a PBA signal obtained from sperm indicates that extracellular antioxidant proteins are absent.
) and nitric oxide (NO°) [27–29], but does not respond to the hydroxyl radical (which has too short a half-life). Non-radical ROS, such as hydrogen peroxide and peroxynitrite, do not affect PBA lifetime either [28]. Therefore, by measuring the concentration of free radicals with short half-life (less than 20 min) while simultaneously disregarding the concentration of the stable (and hence accumulating) hydrogen peroxide to which these radicals dismute, we can obtain an estimate of the in situ (i.e. less than 20 min half-life) production of ROS. Importantly, because membrane crossing by external oxidants or antioxidants is several orders of magnitude slower than PBA fluorescence changes, the occurrence of chemical reactions between oxygen radicals and antioxidants is unlikely to affect the results. In addition, PBA preferentially binds to dissolved proteins [28] rather than entering cellular membranes, so that a PBA signal obtained from sperm indicates that extracellular antioxidant proteins are absent.
A decrease in PBA fluorescence lifetime is related to an increase in concentrations of oxygen free radicals (see §2e). Measuring ROS production by PBA fluorescence changes has a number of additional advantages over other approaches. Fluorescence lifetime is independent of (i) intracellular probe concentration [28] and (ii) of the cell density in the sample. Furthermore, unlike the lucigenin-based ROS measurements routinely used in clinical settings that have been criticized because this method produces ROS itself [30], our method does not generate ROS. Finally, PBA is insensitive to alterations in the lipid concentration of the membrane [31] suggesting that even if such changes do occur (for example, during mammalian sperm capacitation [32]), they would not affect the fluorescence lifetime of PBA.
(c). Relevant aspects of the sperm biology of Gryllus bimaculatus
Cricket sperm are ca 1 mm long, of needle-like shape and have two long mitochondria, called mitochondrial derivatives, alongside the axoneme (sperm ‘tail’) [33]. In G. bimaculatus, spermatozoa are moved from the testes into a container called the spermatophore, the placement of which in an abdominal pouch triggers male calling to attract females [34]. The male attaches the spermatophore to the genital opening of the female, after which sperm migrate through a tube into the female sperm storage organ, the spermatheca, situated at the end of the genital tract [35]. Sperm are stored in the spermatheca and used for fertilization at the moment of egg-laying. Single-mated G. bimaculatus females can lay eggs from stored sperm for a period of ca five weeks [20].
(d). Sample preparation and staining procedure
The sperm-containing spermatophore was removed from the male using forceps and placed into a drop of 40 µl buffer (see electronic supplementary material, methods) on a microscope slide and broken at the nexus. Sperm that had been pumped out from the spermatophore (the majority within 10 min) were collected using a pipette before seminal proteins started to flow out of the spermatophore [36,37] because seminal fluids have been shown to affect sperm mortality [37,38] and so possibly metabolism in some insect species. Sperm were placed into a 20 µl drop of 1 µM PBA, incubated for 4 min and washed three times in three different drops of 20 µl of buffer on a microscope slide by drawing the sperm solution ten times up and down a pipette. The sperm were then pipetted into 10 µl buffer in the centre of a Sykes-Moore chamber. ROS concentration was measured in this chamber (see §2e).
To collect sperm from the female storage organ, female crickets were decapitated, cut ventrally and placed in a dissection bowl filled with buffer, after which the spermatheca was removed, rinsed on the outside in 20 µl buffer and placed into a new 20 µl drop. The spermatheca was opened by tearing its ends apart carefully, which released a single clump of sperm into buffer that was then transferred into 20 µl 1 µM PBA. Sperm volume was estimated based on the filling stage of the spermatheca. It ranged from very low (1), to normal (5) or large volume (10), a scale that was based on previous experience of one of the authors (K.R.). PBA incubation and the washing procedure were identical to that described for males.
Dead sperm with intact membranes were needed to calculate lifetime τ0 (see §2e), which is terminated when ROS production stops. These sperm were handled as described above, but after rinsing in buffer they were fixed in Baker solution (10% paraformaldehyde in 1% aqueous calcium chloride) for 15–20 min [28].
(e). Measurement of metabolic rate and reactive oxygen species production
Using time-resolved microfluorimetry, we recorded the fluorescence decay of sperm cells loaded with PBA [28] (electronic supplementary material, methods). Fluorescence decay was partitioned into three exponentials and their lifetime and amplitude obtained as described previously [27–29]. The shortest two decays τ1 and τ2 correspond to the autofluorescence of the free and protein-bound forms of NAD(P)H. In order to relate their amplitudes a1 and a2 directly to changes in the sperm metabolic rate, we calculated the relative change in metabolic rate as [a2/(a1 + a2)]/[(a2reference/(a1reference + a2reference)], where a1reference and a2reference are the mean fluorescence amplitudes of freshly ejaculated sperm for free NAD(P)H (a1) and bound-NAD(P)H (a2), respectively. The third time constant (τ3 > 100 ns) is characteristic of pyrene derivatives.
Absolute radical concentrations cannot be obtained with our method but the change in fluorescence lifetime is proportional to a change in ROS production and can be calculated applying the Stern–Volmer equation [39] to the fluorescence-lifetime data, τ3:
where τsample was the fluorescence lifetime measured in the sample, τreference the mean fluorescence lifetime of all samples of ejaculated sperm and τ0 (155 ns) the fluorescence lifetime measured in the absence of ROS production (cells dead, fixed with Baker solution). The mean of all samples of ejaculated sperm was set at 100 per cent, so that plots give ejaculate-specific values relative to that mean.
(f). Study design and statistical analysis
Virgin female crickets 6–12 days post-eclosion were kept for 2 h with a previously sexually isolated male, providing sufficient time for one or two matings. After this, males and females were kept individually. Sperm was sampled from the male as described in §2d. Females were kept until their storage organs were examined at a predetermined date (see §2d). In females, the time between mating and examination was 1 h to 28 days, which represents the normal post-ejaculation sperm lifespan in the female body [20]. Using the statistical software R v. 2.13.1. [40], we combined cross-sectional and longitudinal (repeated measures) data in a mixed-model approach [41]. PBA fluorescence lifetime [PBA] and sperm metabolic rate were modelled as a function of whether sperm was ejaculated or female-stored [storage], its handling time, and the interaction between storage and handling times. All effects were nested within male identity (i.e. sperm genotype) as a random effect [42] (electronic supplementary material). If ROS production (or metabolic rate) in stored sperm can be predicted from ROS production (or metabolic rate) in ejaculated sperm, a positive correlation between ejaculated and stored sperm is necessary. We calculated the intraclass correlation coefficient and 95% CI of ROS production and metabolic rate between ejaculated sperm across to stored sperm, using ICC.lme in the psychometric package in R [40] (electronic supplementary material).
3. Results
Sperm metabolism differed fundamentally between freshly ejaculated and stored sperm (interaction storage × PBA: P < 0.0001; electronic supplementary material, table S2). This interaction was probably driven by a positive relationship between metabolic rate and ROS production in sperm stored by females and no, or a negative, relationship in males (figure 1).
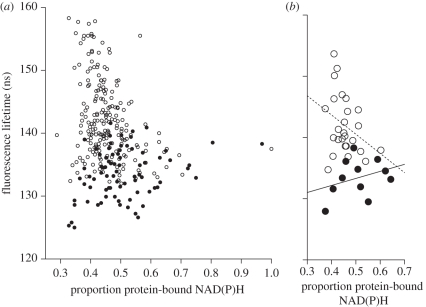
Figure 1.
The relationship between the proportion of NAD(P)H bound to protein and the fluorescence lifetime of 1-pyrene butyric acid (PBA) in sperm of the cricket Gryllus bimaculatus. The proportion of NAD(P)H bound to protein is a measure of sperm metabolic rate whereas PBA fluorescence lifetime is inversely related to the production of ROS (see §2). (a) Individual samples obtained from freshly ejaculated sperm (solid circles) and stored sperm (open circles). (b) The same data shown for male means (solid circles) or female means (open circles). Sperm of some males were obtained after ejaculation as well as from storage (paired design; see figure 2 and electronic supplementary material, figure S1 and table S2 for paired comparisons). The reversal of the relationship between metabolic rate and oxygen radicals production effect in the female is significant (t = 5.617, P < 0.001; electronic supplementary material, table S2). Linear regressions carried out separately for ejaculated and stored sperm show the non-significant individual trends for stored sperm (broken line) and ejaculated sperm (solid line; electronic supplementary material, tables S3 and S4).
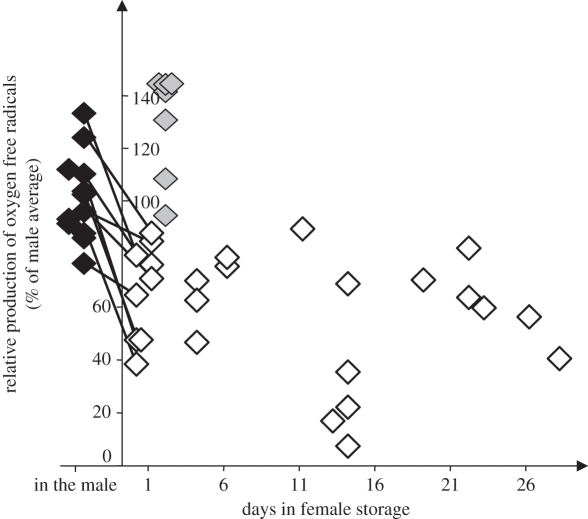
Freshly ejaculated, semen-free sperm showed mean intracellular PBA lifetimes of τ3 = 133.5 ± 3.4 (s.d.) nanoseconds (n = 12 males), based on 6.6 ± 1.4 (s.d.) fluorescence decay samples per male. Sperm in the female storage organ showed significantly lower ROS production, i.e. longer fluorescence lifetimes of 141.3 ± 4.2 (s.d.) nanoseconds (n = 26 females), based on n = 8.2 ± 3.0 (s.d.) fluorescence decay samples per female. The difference in fluorescence lifetime was statistically significant for sperm from the same male either in female storage or when freshly ejaculated (P < 0.0001; electronic supplementary material, table S1). The mean difference of 7.8 ns translates into a 41.9 per cent lower ROS production by stored than freshly ejaculated sperm (figure 2). ROS reduction was observed in stored sperm as early as 1 h after mating (figure 2), but not in sperm kept in vitro without exposure to female substances (figure 2).
Figure 2.
Reduced production of oxygen free radicals in stored sperm by females of the cricket, Gryllus bimaculatus. Sperm stored by females (open diamonds; all are independent data points) show substantially reduced oxygen radical production compared with freshly ejaculated sperm (black diamonds; male sample mean 100%) and freshly ejaculated sperm kept alive in vitro in saline buffer (grey diamonds). Lines connecting male and female samples denote paired measurements of sperm from the same male, when freshly ejaculated and after being in storage for less than 1 day.
On average, the sperm metabolic rate was 37.2 per cent lower in stored sperm when compared with freshly ejaculated sperm from the same male (electronic supplementary material, figure S1, storage: P = 0.0001; electronic supplementary material, table S2). Both metabolic rate and ROS production in stored sperm stayed at a low level for the entire experimental period (1 h to 28 days of sperm storage; ROS: Pearson's r26 = 0.140, P = 0.495; controlling for sperm volume in the storage organ: partial r26 = 0.045, P > 0.5; metabolic rate: r26 = −0.259, P = 0.192; figure 2 and electronic supplementary material, figure S1).
Examining the subset of males in which sperm were measured immediately after ejaculation and within 24 h of storage (n = 9 paired samples) showed that the mean difference was 6.9 ns, i.e. 27.7 per cent lower ROS in the female, or 32.9 per cent lower metabolism. However, ROS production in the male did not significantly predict ROS production in the female, because there was no significant repeatability of ROS production between sperm from the same male that was ejaculated and sperm that was stored: the 95% CI of the intraclass correlation coefficient (lower CI, mean, upper CI) included zero when considering all individual measurements (−0.0105, 0.1027, 0.473), male means only (−0.795, −0.361, 0.343) or male ranks (−0.238, 0.432, 0.833; see the crossing lines in figure 2 and electronic supplementary material, S1). Metabolic rates in ejaculated sperm also relatively poorly predicted metabolic rates in stored sperm, being significantly repeatable only when considering all individual measurements (0.0489, 0.214, 0.628) but not when using male means (−0.406, 0.269, 0.766) or male ranks (−0.140, 0.511, 0.862).
4. Discussion
Our study shows that NAD(P)H fluorescence measurements to characterize metabolic rate, mainly used in cancer and stem cell research, can solve questions in reproductive and evolutionary biology. To the best of our knowledge, this is among the first studies that simultaneously examine cell metabolic rate and ROS production. Our study had three important outcomes. First, we report for the first time that sperm undergo a reversal of the relationship between ROS production and metabolic rate after contact with the female reproductive tract. Second, because we measured the production of ROS (rather than their accumulation), our results are in agreement with the fundamental, but previously untested assumption that ROS reduction during sperm storage is based on an interference with sperm metabolism [3] and that females contribute to this interference. Third, in our limited sample, ranks of between-male differences in metabolic rate were not maintained in the female, suggesting that metabolism of ejaculated sperm is not a good predictor of metabolism in stored sperm. Below, we discuss these three outcomes further after critically examining the effect of sampling bias.
(a). Is reactive oxygen species reduction caused by sampling bias?
In a related cricket species, more recently produced sperm cells were over-represented in the female storage organ compared with the male reproductive duct [43]. This raises the possibility that we have not demonstrated a reduction in ROS production in stored sperm but merely sampled later-produced (younger), low ROS-producing sperm cohorts that might have been preferentially stored by the female. This argument predicts that ROS variation in stored sperm is part of the variation in ejaculated sperm. Because figure 1 shows that there is little overlap in PBA lifetime between male and female sperm samples (variation along the y-axis), ROS reduction in stored sperm is either not, or only partly, caused by biased sampling of sperm age cohorts.
(b). The relationship between metabolic rate and reactive oxygen species production
Increased cellular metabolic rate lowers the membrane potential and decreases the production of ROS when the tissue oxygen pressure remains constant [11–15, §1]. Applied to our data of ejaculated sperm, this suggests that the surrounding oxygen pressure must have been constant (and the effect sustained until we measured the sperm). In contrast, if increased ROS production is caused by increased metabolic rate under higher tissue oxygen pressure [11–15], the sperm metabolic rate may have been reduced by decreasing the oxygen pressure in the spermatheca. It is also possible that a hypoxic pathway of sperm metabolism is induced in the female. However, there are at least two arguments that call for caution in accepting the latter hypothesis as a general explanation: (i) cell metabolism under hypoxia commonly results in significant ROS production [42,44] and (ii) hypoxic/anaerobic glycolysis may be limited as it mainly occurs in the cytoplasm, which is barely present in sperm cells.
(c). Sperm storage, reactive oxygen species reduction and sperm lifespan
Several general mechanisms have been proposed that reduce the fertilization ability of oxidative stress-damaged, or otherwise aged, sperm [1–3,6,7,10]. For example, den Boer et al. [45] have recently shown that towards the end of the sperm storage period ant queens use more sperm per egg, and have proposed that this is a safeguard mechanism against fertilization with aged sperm. Here, we add that female effects alter sperm metabolism in such a way that ROS production is reduced. This begs the question of what contribution a ca 40 per cent reduction in ROS production would make. The honeybee is one of the few species where cellular lifespan has been estimated for both male-collected and female-stored sperm. Sperm can be quite robust even if collected from the male, it took 1 year for most sperm to die in vitro under room temperature [46], i.e. without any female influence. Assuming that oxidative damage is linearly proportional to ROS production, and that oxidative damage at a certain threshold leads to cell death, a 40 per cent lower rate translates into a predicted 0.7-year increase in sperm lifespan in the honeybee queen. This compares favourably with the mean reproductive lifespan of 1–2 years reported for honeybee queens [47]. It is, therefore, possible that the change in metabolic rate during sperm storage may by itself explain a substantial part of sperm lifespan extension during storage, even without invoking antioxidant scavenging.
The rapid and extended reduction in ROS production in crickets provides a clue as to a likely mechanism for reducing ROS production in stored sperm. Previous research has shown that female moths and grasshoppers can alter sperm morphologically by building an extra membrane around sperm [48,49]. However, such changes occurred only after 2 days of sperm storage [48,49] rather than as rapidly as we measured the metabolic changes (within 1 h after contact with the female). This suggests a physiological alteration of sperm metabolism rather than a morphological one. We note that ROS reduction was of similar magnitude to metabolic reduction, suggesting that the two might be linearly related over the sperm lifespan that we studied.
We found ROS reduction over an extended period of sperm storage. Similar to tumor cells whose experimentally altered metabolic rate is maintained in culture when the cells are returned to normoxic conditions [50], it is possible that a single female signal on sperm metabolism has a sustained effect for the entire period of sperm storage [20]. Alternatively, female effects may continuously interfere with sperm ROS production and metabolism. In two insect species, sperm storage has been shown to be costly to females [51,52]. Given that in one of them, the costs were found late in the storage period [51] but not early (as expected under a on–off manipulation upon receipt of sperm), we currently consider a continuous interference more likely.
There is another issue that possibly connects ROS, sperm storage and sperm lifespan. It is generally assumed that the superoxide anion and other ROS cause cellular damage and senescence, but some recent studies have suggested that ROS may be the molecules used by damaged cells to elicit repair mechanisms [14,15,53,54]. Sperm may represent an interesting model system to further test this idea as the sperm nucleus is transcriptionally silent because sperm lack many repair mechanisms [8–10,55] and because the small volume of cytoplasm in sperm cells provides only limited space to induce repair. If major repair mechanisms are lacking, then one may also predict a reduced need to induce them. For our system, the proposed inverse causation of ROS being the consequence, not the cause, of cellular damage [53] would predict that the reduced production of sperm ROS (including the superoxide anion) leads to reduced sperm repair during storage.
(d). Sperm metabolism and sperm function tests in sexual selection and reproductive medicine
While freeze storage probably shuts down sperm metabolism completely, our data show that sperm in females maintain a basic metabolic rate and some constant rate of ROS production. Testing whether constant ROS production translates into a continuous functional decay by oxidative damage, or results in sudden cell death without a preceding reduction in cell functionality, is decisive for predicting male and female strategies to circumvent sperm ageing [1]. If sperm functionality decreases, then the interaction between sperm genotype and the sperm environment, i.e. the sperm phenotype, will probably be a better predictor of paternity than male or female genotype in isolation [1].
If sperm functionality does not decline during storage or during the sperm's lifetime, then variation in sperm metabolic rate during sperm storage may qualify as the currently unknown [56] physiological mechanism that connects variation in sperm traits with variation in male reproductive success [57]. For example, it is currently unknown how differences in sperm length cause variation in male reproductive success (see Gage & Morrow [58] for a cricket example). It seems possible that these differences may be related to sperm of different lengths having different metabolic rates. However, the latter study also included female effects of postcopulatory sexual selection [56], which might be consistent with our observation that differences in sperm ROS production and metabolism between males changed after storage by the female. The notion that a considerable part of sperm performance in the female cannot be predicted from sperm function tests of freshly ejaculated sperm runs counter to current practice in clinical and veterinary reproductive biology [8,59] and sexual selection research [56] that seek to predict the likelihood of conception or paternity from function tests of ejaculated sperm. Clinical and evolutionary studies have noted that sperm functions have considerably improved predictability when measured after sperm had contact with the female reproductive tract [60] (but see other studies [59,61]). In these, as in our study, it is possible that (i) male×female genotype interactions for sperm metabolism and ROS production are strong [62], (ii) natural selection for reduced ROS sperm damage in females [1–3] may override sexually selected differences in sperm function or (iii) the sperm metabolic phenotype is better explained by the sperm environment, including the female reproductive tract, than by the sperm genotype [1,3].
5. Conclusion
We introduced technology that simultaneously measures sperm metabolic rate and ROS production. We demonstrated a marked difference in metabolic rate between ejaculated and spermatheca-stored sperm, which is consistent with the notion that sperm metabolism and ROS production are reduced in unison. This suggests that future comparative studies testing the relationship between female-mediated sperm lifespan extension and metabolic rate might be fruitful. We also found that differences in sperm metabolism and ROS production between males were not consistently upheld across females that stored ejaculates. Future studies should, therefore, address the repeatability across ejaculates within males and the genetic basis of between-male and between-female (spermathecal) variation in sperm metabolism. Our results make a potentially interesting connection between the seemingly contradictory views of metabolic rate and ROS production (see §1) and may have implications for future research and methodology in evolutionary and senescence biology, as well as reproductive medicine.
Acknowledgements
We thank J. Abbott, T. Birkhead, R. Jones, E. de Lamirande, A. Pacey, T. Pizzari and J. Rolff for comments on previous drafts, and R. Dobler for help with figure 1. This work was supported by an advanced postdoctoral fellowship from the VolkswagenStiftung and a fellowship from Natural Environment Research Council (UK) (NE/D009634/1) to K.R.
References
- 1.Reinhardt K. 2007. Evolutionary consequences of sperm cell aging. Q. Rev. Biol. 82, 375–393 10.1086/522811 (doi:10.1086/522811) [DOI] [PubMed] [Google Scholar]
- 2.Heifetz Y., Rivlin P. K. 2010. Beyond the mouse model: using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology 73, 723–739 10.1016/j.theriogenology.2009.11.001 (doi:10.1016/j.theriogenology.2009.11.001) [DOI] [PubMed] [Google Scholar]
- 3.Holt W. V., Lloyd R. E. 2010. Sperm storage in the vertebrate female reproductive tract: how does it work so well? Theriogenology 73, 713–722 10.1016/j.theriogenology.2009.07.002 (doi:10.1016/j.theriogenology.2009.07.002) [DOI] [PubMed] [Google Scholar]
- 4.Cosson J., Groison A. L., Suquet M., Fauvel C., Dreanno C., Billard R. 2008. Marine fish spermatozoa: racing ephemeral swimmers. Reproduction 136, 277–294 10.1530/rep-07-0522 (doi:10.1530/rep-07-0522) [DOI] [PubMed] [Google Scholar]
- 5.Keller L. 1998. Queen lifespan and colony characteristics in ants and termites. Insectes Soc. 45, 235–246 10.1007/s000400050084 (doi:10.1007/s000400050084) [DOI] [Google Scholar]
- 6.Suarez S. S. 2008. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 52, 455–462 10.1387/ijdb.072527ss (doi:10.1387/ijdb.072527ss) [DOI] [PubMed] [Google Scholar]
- 7.Breque C., Surai P., Brillard J. P. 2003. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Dev. 66, 314–323 10.1002/mrd.10347 (doi:10.1002/mrd.10347) [DOI] [PubMed] [Google Scholar]
- 8.Koppers A. J., De Iuliis G. N., Finnie J. M., McLaughlin E. A., Aitken R. J. 2008. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 93, 3199–3207 10.1210/jc.2007-2616 (doi:10.1210/jc.2007-2616) [DOI] [PubMed] [Google Scholar]
- 9.Ramalho-Santos J., Varum S., Amaral S., Mota P. C., Sousa A. P., Amaral A. 2009. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 15, 553–572 10.1093/humupd/dmp016 (doi:10.1093/humupd/dmp016) [DOI] [PubMed] [Google Scholar]
- 10.Storey B. T. 2008. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int. J. Dev. Biol. 52, 427–437 10.1387/ijdb.072522bs (doi:10.1387/ijdb.072522bs) [DOI] [PubMed] [Google Scholar]
- 11.Balaban R. S., Nemoto S., Finkel T. 2005. Mitochondria, oxidants, and aging. Cell 120, 483–495 10.1016/j.cell.2005.02.001 (doi:10.1016/j.cell.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 12.St-Pierre J., Buckingham J. A., Roebuck S. J., Brand M. D. 2002. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277, 44 784–44 790 10.1074/jbc.M207217200 (doi:10.1074/jbc.M207217200) [DOI] [PubMed] [Google Scholar]
- 13.Van Voorhies W. A. 2004. Live fast: live long? A commentary on a recent paper by Speakman et al. Aging Cell 3, 327–330 10.1111/j.1474-9728.2004.00113.x (doi:10.1111/j.1474-9728.2004.00113.x) [DOI] [PubMed] [Google Scholar]
- 14.Murphy M. P. 2009. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 10.1042/BJ20081386 (doi:10.1042/BJ20081386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapointe J., Hekimi S. 2010. When a theory of aging ages badly. Cell. Mol. Life Sci. 67, 1–8 10.1007/s00018-009-0138-8 (doi:10.1007/s00018-009-0138-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson R. M., Weindruch R. 2010. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol. Metab. 21, 134–141 10.1016/j.tem.2009.11.005 (doi:10.1016/j.tem.2009.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harman D. 2001. Aging: overview. Ann. N.Y. Acad. Sci. 928, 1–21 10.1111/j.1749-6632.2001.tb05631.x (doi:10.1111/j.1749-6632.2001.tb05631.x) [DOI] [PubMed] [Google Scholar]
- 18.Werner M., Simmons L. W. 2008. Insect sperm motility. Biol. Rev. 83, 191–208 10.1111/j.1469-185X.2008.00039.x (doi:10.1111/j.1469-185X.2008.00039.x) [DOI] [PubMed] [Google Scholar]
- 19.Anilkumar G., Sudha K., Anitha E., Subramoniam T. 1996. Aspects of sperm metabolism in the spermatheca of the brachyuran crab Metopograpsus messor (Forskal). J. Crust. Biol. 16, 310–314 10.2307/1548888 (doi:10.2307/1548888) [DOI] [Google Scholar]
- 20.Simmons L. W. 1988. The contribution of multiple mating and spermatophore consumption to the lifetime reproductive success of female field crickets (Gryllus bimaculatus). Ecol. Entomol. 13, 57–69 10.1111/j.1365-2311.1988.tb00333.x (doi:10.1111/j.1365-2311.1988.tb00333.x) [DOI] [Google Scholar]
- 21.Blinova K., Levine R. L., Boja E. S., Griffiths G. L., Shi Z. D., Rubby B., Balaban R. S. 2008. Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry 47, 9636–9645 10.1021/bi800307y (doi:10.1021/bi800307y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakowicz J. R., Szmacinski H., Nowaczyk K., Johnson M. L. 1992. Fluorescence lifetime imaging of free and protein-bound NADH. Proc. Natl Acad. Sci. USA 89, 1271–1275 10.1073/pnas.89.4.1271 (doi:10.1073/pnas.89.4.1271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chorvat D., Jr, Chorvatova A. 2009. Multi-wavelength fluorescence lifetime spectroscopy: a new approach to the study of endogenous fluorescence in living cells and tissues. Laser Phys. Lett. 6, 175–193 10.1002/lapl.200810132 (doi:10.1002/lapl.200810132) [DOI] [Google Scholar]
- 24.Schneckenburger H., Wagner M., Weber P., Strauss W. S., Sailer R. 2004. Autofluorescence lifetime imaging of cultivated cells using a UV picosecond laser diode. J. Fluoresc. 14, 649–654 10.1023/B:JOFL.0000039351.09916.cc (doi:10.1023/B:JOFL.0000039351.09916.cc) [DOI] [PubMed] [Google Scholar]
- 25.Skala M. C., Riching K. M., Gendron-Fitzpatrick A., Eickhoff J., Eliceiri K. W., White J. G., Ramanujam N. 2007. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl Acad. Sci. USA 104, 19 494–19 499 10.1073/pnas.0708425104 (doi:10.1073/pnas.0708425104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghukasyan V. V., Kao F.-J. 2009. Monitoring cellular metabolism with fluorescence lifetime of reduced nicotinamide adenine dinucleotide. J. Phys. Chem. C 113, 11 532–11 540 10.1021/jp810931u (doi:10.1021/jp810931u) [DOI] [Google Scholar]
- 27.Oter O., Ribou A.-C. 2009. Quenching of long lifetime emitting fluorophores with paramagnetic molecules. J. Fluoresc. 19, 389–397 10.1007/s10895-008-0425-z (doi:10.1007/s10895-008-0425-z) [DOI] [PubMed] [Google Scholar]
- 28.Ribou A.-C., Vigo J., Salmon J. M. 2004. Lifetime of fluorescent pyrene butyric acid probe in single living cells for measurement of oxygen fluctuation. Photochem. Photobiol. 80, 274–280 10.1562/2004-03-11-RA-109.1 (doi:10.1562/2004-03-11-RA-109.1) [DOI] [PubMed] [Google Scholar]
- 29.Rharass T., Vigo T., Salmon J. M., Ribou A. C. 2006. Variation of 1-pyrenebutyric acid fluorescence lifetime in single living cells treated with molecules increasing or decreasing reactive oxygen species levels. Anal. Biochem. 357, 1–8 10.1016/j.ab.2006.07.009 (doi:10.1016/j.ab.2006.07.009) [DOI] [PubMed] [Google Scholar]
-
30.Fridovich I.
1997.
Superoxide anion radical (
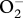 ), superoxide dismutases, and related matters. J. Biol. Chem.
272, 18 515–18 517 10.1074/jbc.272.30.18515 (doi:10.1074/jbc.272.30.18515) [DOI] [PubMed] [Google Scholar]
), superoxide dismutases, and related matters. J. Biol. Chem.
272, 18 515–18 517 10.1074/jbc.272.30.18515 (doi:10.1074/jbc.272.30.18515) [DOI] [PubMed] [Google Scholar] - 31.Dumas D., Muller S., Gouin F., Baros F., Viriot M. L., Stoltz J. F. 1997. Membrane fluidity and oxygen diffusion in cholesterol-enriched erythrocyte membrane. Arch. Biochem. Biophys. 341, 34–39 10.1006/abbi.1997.9936 (doi:10.1006/abbi.1997.9936) [DOI] [PubMed] [Google Scholar]
- 32.de Lamirande E., Lamothe G. 2009. Reactive oxygen-induced reactive oxygen formation during human sperm activation. Free Radic. Biol. Med. 46, 502–510 10.1016/j.freeradbiomed.2008.11.004 (doi:10.1016/j.freeradbiomed.2008.11.004) [DOI] [PubMed] [Google Scholar]
- 33.Baccetti B. 1970. Evolutionary biology of orthopteroid sperm cells. Proc. Int. Study Conf. Curr. Future Probl. Acridol. (Lond.) 149–158 [Google Scholar]
- 34.Huber F., Moore T. E., Loher W. 1989. Cricket neurobiology and behavior. Ithaca, NY: Cornell University Press [Google Scholar]
- 35.Hall M. D., Beck R., Greenwood M. 2000. Detailed developmental morphology of the spermatophore of the Mediterranean field cricket, Gryllus bimaculatus (De Geer) (Orthoptera: Gryllidae). Arthropod Struct. Dev. 29, 23–32 10.1016/S1467-8039(00)00010-4 (doi:10.1016/S1467-8039(00)00010-4) [DOI] [PubMed] [Google Scholar]
- 36.Khalifa A. 1949. The mechanism of insemination and the mode of action of the spermatophore in Gryllus domesticus. Q. J. Microsc. Sci. 90, 281–292 [Google Scholar]
- 37.Simmons L. W., Beveridge M. 2011. Seminal fluid affects sperm viability in a cricket. PLoS ONE 6, e17975. 10.1371/journal.pone.0017975 (doi:10.1371/journal.pone.0017975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holman L. 2009. Sperm viability staining in ecology and evolution: potential pitfalls. Behav. Ecol. Sociobiol. 63, 1679–1688 10.1007/s00265-009-0816-4 (doi:10.1007/s00265-009-0816-4) [DOI] [Google Scholar]
- 39.Rharass T., Vigo J., Salmon J. M., Ribou A.-C. 2008. New method for the detection of reactive oxygen species in anti-tumoral activity of adriamycin: a comparison between hypoxic and normoxic cells. Free Radic. Res. 42, 124–134 10.1080/10715760701834552 (doi:10.1080/10715760701834552) [DOI] [PubMed] [Google Scholar]
- 40.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 41.Crawley M. J. 2002. Statistical computing. Chichester, UK: Wiley [Google Scholar]
- 42.Clanton T. L. 2007. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. 102, 2379–2388 10.1152/japplphysiol.01298.2006 (doi:10.1152/japplphysiol.01298.2006) [DOI] [PubMed] [Google Scholar]
- 43.Reinhardt K., Siva-Jothy M. T. 2005. An advantage for young sperm in the house cricket Acheta domesticus. Am. Nat. 165, 718–723 10.1086/430010 (doi:10.1086/430010) [DOI] [PubMed] [Google Scholar]
- 44.Bailey D. M., et al. 2009. Increased cerebral output of free radicals during hypoxia: implications for acute mountain sickness? Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1283–R1292 10.1152/ajpregu.00366.2009 (doi:10.1152/ajpregu.00366.2009) [DOI] [PubMed] [Google Scholar]
- 45.den Boer S. P. A., Baer B., Dreier S., Aron S., Nash D. R., Boomsma J. J. 2009. Prudent sperm use by leaf-cutter ant queens. Proc. R. Soc. B 276, 3945–3953 10.1098/rspb.2009.1184 (doi:10.1098/rspb.2009.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins A. M. 2000. Survival of honey bee (Hymenoptera: Apidae) spermatozoa stored at above-freezing temperatures. J. Econ. Entomol. 93, 568–571 10.1603/0022-0493-93.3.568 (doi:10.1603/0022-0493-93.3.568) [DOI] [PubMed] [Google Scholar]
- 47.Winston M. L. 1987. The biology of the honey bee. Cambridge, MA: Harvard University Press [Google Scholar]
- 48.Renieri T., Vegni Talluri M. 1974. Sperm modification in the female ducts of a grasshopper. Monit. Zool. Ital. 8, 1–9 [Google Scholar]
- 49.Riemann J. G., Thorson B. J. 1971. Sperm maturation in the male and female genital tracts of Anagasta kühniella (Lepidoptera: Pyralidae). Int. J. Insect Morphol. Embryol. 1, 1–19 10.1016/0020-7322(71)90002-X (doi:10.1016/0020-7322(71)90002-X) [DOI] [Google Scholar]
- 50.Gatenby R. A., Gillies R. J. 2004. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 10.1038/nrc1478 (doi:10.1038/nrc1478) [DOI] [PubMed] [Google Scholar]
- 51.Roth S., Reinhardt K. 2003. Facultative sperm storage in response to nutritional status in a female insect. Proc. R. Soc. Lond. B 270, S54–S56 10.1098/rsbl.2003.0008 (doi:10.1098/rsbl.2003.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baer B., Armitage S. A. O., Boomsma J. J. 2006. Sperm storage induces an immunity cost in ants. Nature 441, 872–875 10.1038/nature04698 (doi:10.1038/nature04698) [DOI] [PubMed] [Google Scholar]
- 53.Yang W., Hekimi S. A. 2010. Mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 8, e1000556. 10.1371/journal.pbio.1000556 (doi:10.1371/journal.pbio.1000556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cocheme H. M., et al. 2011. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350 10.1016/j.cmet.2011.02.003 (doi:10.1016/j.cmet.2011.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker M. A., Aitken R. J. 2004. The importance of redox regulated pathways in sperm cell biology. Mol. Cell. Endocrinol. 216, 47–54 10.1016/j.mce.2003.10.068 (doi:10.1016/j.mce.2003.10.068) [DOI] [PubMed] [Google Scholar]
- 56.Birkhead T. R., Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Gen. 3, 262–273 10.1038/nrg774 (doi:10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 57.Ball M. A., Parker G. A. 1996. Sperm competition games: external fertilization and ‘adaptive infertility’. J. Theor. Biol. 180, 141–150 10.1006/jtbi.1996.0090 (doi:10.1006/jtbi.1996.0090) [DOI] [PubMed] [Google Scholar]
- 58.Gage M. J. G., Morrow E. H. 2003. Experimental evidence for the evolution of numerous, tiny sperm via sperm competition. Curr. Biol. 9, 754–757 10.1186/1471-2148-8-319 (doi:10.1186/1471-2148-8-319) [DOI] [PubMed] [Google Scholar]
- 59.Aitken R. J. 2006. Sperm function tests and fertility. Int. J. Androl. 29, 69–75 10.1111/j.1365-2605.2005.00630.x (doi:10.1111/j.1365-2605.2005.00630.x) [DOI] [PubMed] [Google Scholar]
- 60.Glazener C. M., Ford W. C., Hull M. G. 2000. The prognostic power of the post-coital test for natural conception depends on duration of infertility. Hum. Reprod. 15, 1953–1957 10.1093/humrep/15.9.1953 (doi:10.1093/humrep/15.9.1953) [DOI] [PubMed] [Google Scholar]
- 61.Birkhead T. R., Martinez J. G., Burke T., Froman D. P. 1999. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. Lond. B 266, 1759–1764 10.1098/rspb.1999.0843 (doi:10.1098/rspb.1999.0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark A. G., Begun D. J., Prout T. 1999. Female × male interactions in Drosophila sperm competition. Science 283, 217–220 10.1126/science.283.5399.217 (doi:10.1126/science.283.5399.217) [DOI] [PubMed] [Google Scholar]