Abstract
While chance events, oceanography and selective pressures inject stochasticity into the replenishment of marine populations with dispersing life stages, some determinism may arise as a result of characteristics of breeding individuals. It is well known that larger females have higher fecundity, and recent laboratory studies have shown that maternal traits such as age and size can be positively associated with offspring growth, size and survival. Whether such fecundity and maternal effects translate into higher recruitment in marine populations remains largely unanswered. We studied a population of Amphiprion chrysopterus (orange-fin anemonefish) in Moorea, French Polynesia, to test whether maternal size influenced the degree of self-recruitment on the island through body size–fecundity and/or additional size-related maternal effects of offspring. We non-lethally sampled 378 adult and young juveniles at Moorea, and, through parentage analysis, identified the mothers of 27 self-recruits (SRs) out of 101 recruits sampled. We also identified the sites occupied by each mother of an SR and, taking into account variation in maternal size among sites, we found that females that produced SRs were significantly larger than those that did not (approx. 7% greater total length, approx. 20% greater biomass). Our analyses further reveal that the contribution of larger females to self-recruitment was significantly greater than expected on the basis of the relationship between body size and fecundity, indicating that there were important maternal effects of female size on traits of their offspring. These results show, for the first time in a natural population, that larger female fish contribute more to local replenishment (self-recruitment) and, more importantly, that size-specific fecundity alone could not explain the disparity.
Keywords: maternal size, maternal effects, fecundity, Amphiprion chrysopterus, self-recruitment, population replenishment
1. Introduction
Population replenishment of most benthic marine animals requires the arrival of larvae that have dispersed in the water column some distance from their natal reef. The timing and rate of arrival of larvae are influenced by several phenomena, including oceanographic conditions, patchy environmental selection and sweepstakes reproductive success, which together can generate high stochasticity in recruitment in time and space [1–4]. Significant changes in allele frequencies of larvae or recruits at marker loci provide evidence for such stochasticity at small to moderate temporal and spatial scales [5–9]. In wild populations, not all individuals contribute equally to replenishment and, in some cases, a very small percentage of adults may be responsible for the replenishment of entire areas [6,10,11]. Which individuals among a breeding population contribute disproportionately to population replenishment remains an important but largely unanswered question in ecology.
Possible mechanisms that could allow enhanced contributions by some individuals to population replenishment include increased fecundity of larger females [12,13], as well as the influence of maternal phenotypic traits on the phenotype of offspring (maternal effects). There is mounting experimental evidence that maternal traits influence larval quality [14–16]. In particular, it has been shown across a wide range of fish and invertebrates that larger and older females often produce higher-quality offspring that grow faster and are more resistant to starvation [15,17]. However, a variety of natural sources of selection may prevent a clear relationship between maternal characteristics and population replenishment in the wild, and such variation is not replicable/mirrored in experimental manipulations [18].
It is clear that field studies of natural populations are needed to explore whether maternal attributes translate into higher recruitment success. We studied a coral reef fish, the orange-fin anemonefish (Amphiprion chrysopterus), whose recruit and adult stages are easily sampled and whose high self-recruitment rates make it a good candidate to test the importance of larger female fish to population replenishment. In many marine systems, self-recruitment may be tightly connected to sustainability and resilience potential of populations [19]. Since 2005, genetic fingerprinting coupled with parentage analysis estimates have successfully been used to estimate self-recruitment levels [20,21]. Identifying the parents of self-recruits provides a means to test for the existence of individual maternal traits (beyond size-specific fecundity) to local population replenishment. In this study, we provide the first empirical evidence from a field study that larger females contribute disproportionately more to self-recruitment of a coral reef fish than would be expected from the positive relationship between body size and fecundity.
2. Material and methods
(a). Field sampling of Amphiprion chrysopterus
In 2007 and 2010, we sampled the orange-fin anemonefish Amphiprion chrysopterus around the island of Moorea (French Polynesia; figure 1). Individuals live in close association with host anemones (Heteractis magnifica) [22]. Each fish was captured with a handnet by a scuba diver, measured for total length (TL) to the nearest 0.1 mm and fin-clipped for genetic analyses. Specimens up to 60 mm in TL were considered recruits, corresponding to the size of A. chrysopterus after 1 year [23]. Even though growth may depend on population density, predation pressure, and so on [24], this length range probably encompasses several cohorts, as successive recruitment events occur throughout the year. For each sampled individual, the position of its associated host anemone was recorded. An estimate of the total population size of A. chrysopterus around the island of Moorea was based on 20 randomly placed visual transects covering both the lagoon and the outer reef, together with 50 manta tow transects, which allowed us to cover approximately one-fifth of the reef area of the island.
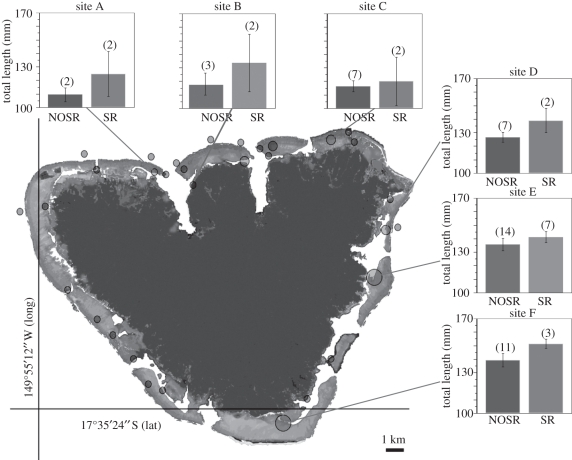
Figure 1.
Location of A. chrysopterus females in couples at 32 sites in Moorea (French Polynesia). Size of sites (circles) is proportional to female abundance: small circles 1–5, medium circles 6–11 and large circles >11 females. For marked circles, histograms represent variation in total length (mm; average and SE) for females that produced self-recruits (SR) and those that did not (NOSR). At each site, the number of females in each category (SR and NOSR) is shown in parentheses above bars.
(b). DNA extraction and microsatellite testing
Immediately after collection, fin clips were placed in 95 per cent ethanol. Total genomic DNA was prepared from 20 mg of fin tissue by proteinase K digestion in lysis buffer (10 mM Tris, 400 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 1% sodium dodecyl sulphate) overnight at 55°C. This was followed by purification using phenol/chloroform extractions and alcohol precipitation [25]. We amplified a total of 11 microsatellites (as described by Beldade et al. [26] and Quenouille et al. [27]). Scoring of peaks was performed manually using Genemapper v. 3.7 (Applied Biosystems). Using Arlequin [28] and Micro-checker [29], we did not find deviations from Hardy–Weinberg equilibrium owing to the presence of null alleles, nor linkage disequilibrium for nine variable microsatellites (table 1).
Table 1.
Unlinked microsatellite markers in Hardy–Weinberg equilibrium; number of alleles (Na); and observed and expected heterozygocity (Ho (He)) for the whole population sampled in Moorea (n = 378) in both years.
| locus | Na | Ho (He) |
|---|---|---|
| A130 | 14 | 0.75 (0.82) |
| D108 | 20 | 0.78 (0.88) |
| D103 | 6 | 0.54 (0.63) |
| 10TCTA | 20 | 0.84 (0.89) |
| 44 | 9 | 0.52 (0.53) |
| 61 | 21 | 0.79 (0.85) |
| CF11 | 11 | 0.63 (0.79) |
| A115 | 14 | 0.72 (0.86) |
| D114 | 13 | 0.76 (0.87) |
(c). Parentage analysis
In this study, all sampled breeding pairs and mature single individuals were considered putative parents (breeders). We used a maximum-likelihood approach for parentage reconstruction using the software FAMOZ [30] to identify putative parents for each captured recruit. We considered self-recruits (SRs) to be all sampled individuals whose parents were found among the sampled adults. In FAMOZ, statistical confidence in ancestry assignment was based on offspring simulations from allele frequencies and genotyped parents. To account for possible genotyping errors, we considered an error level of 0.001 [31]. There were no missing data in our datasets. Type II error (i.e. assign as true parent when true parent was not sampled) was further reduced by re-extracting and rescoring all the positive parent–offspring matches. This step allowed us to exclude genotyping errors in all parent–offspring assignments.
(d). Maternal size and self-recruitment
Anemonefish live in small groups consisting of an adult breeding pair and a varying number of subadults where the largest individual is the female [32,33]. We observed six egg clutches including both large (TL = 158 mm) and small (TL = 104 mm) breeding females (i.e. living with at least one other adult conspecific) throughout the year, and thus do not expect a correlation between maternal size and the period of active spawning. To assess the importance of female body size on local population replenishment, we used a randomized block design. The block design strategy was chosen because localized phenomena, such as particular water circulation patterns, may influence self-recruitment. All sites where more than one female contributed an SR and more than one female did not produce self-recruits (NOSR) were included in the analysis (highlighted by circles in figure 1). Size (TL) of females that produced SRs was compared with those that did not (NOSR), taking into account the variance in sizes among sites. Assumptions of normality and homogeneity of variances for the ANOVA were met. All statistical analyses were run in SAS v. 9. The percentage difference in mean TL between SR and NOSR females was translated into percentage difference in biomass using the following relationship between weight (W) and length (L) for A. chrysopterus on Moorea: W = 0.0145 L3 [34].
(e). Fecundity and self-recruitment
To assess the importance of maternal fecundity to self-recruitment, we first estimated the relationship between female body size and fecundity (number of eggs), which we did by estimating in situ the number of eggs in the clutches of six different-sized females. In each clutch, three 1 cm2 replicates were sampled by scuba divers to calculate egg density, which was then multiplied by the total area of the clutch, calculated by πr2 given that clutches essentially were circular (following Robertson et al. [35]). On the basis of the TL of six females (range of female TL: 104.4–158.0 mm) and number of eggs in the respective egg clusters (fecundity range: 338.91–711.71 eggs), fecundity was extrapolated for all mature female sizes in the population using the equation fecundity = −313.4762 + 5.9333007 TL (r2 = 0.96; electronic supplementary material). Expected values of the mean size of females producing SRs based on the number of eggs they potentially contribute were calculated at each of the six sites included in the analysis (figure 1). This was achieved by weighting the size of each individual by its estimated fecundity (calculated using the equation provided earlier). The weighted mean of SR females (i.e. the sum of the products of the size of each SR female multiplied by its estimated fecundity divided by the sum of the expected fecundity values) was compared with the weighted mean for all females at that site (those who produced SRs and those who did not) using a paired t-test. A significant difference in this comparison would indicate that the incidence of self-recruitment among the offspring of larger females is higher than would be expected based on average fecundity alone. All statistical analyses were run in SAS v. 9.
3. Results
A total of 378 specimens was sampled, comprising 118 females, 159 males and 101 recruits (table 2). Anemonefish were patchily distributed around the island in low population densities (32 sites; circles in figure 1). Anemones harbouring fish were found between 0.5 m and 30 m depth, which matches the depth range of the orange-fin anemonefish, with the vast majority of fish occurring at depths of less than 15 m. Nests of Amphiprion chrysopterus were observed throughout the year. On the basis of the estimates of the total population size for the entire island from underwater visual counts, which we estimate to be on the order of 500 individuals, we sampled approximately 75 per cent of the population of A. chrysopterus in this study.
Table 2.
Number of Amphiprion chrysopterus females, males and juveniles sampled in Moorea (French Polynesia) in each year.
| 2007 | 2010 | |
|---|---|---|
| females | 43 | 75 |
| males | 59 | 100 |
| recruits | 50 | 51 |
| total | 152 | 226 |
Parentage analysis allowed us to identify 27 SRs (14 and 13 in 2007 and 2010, respectively). The lengths of SRs ranged from 26 to 59 mm, implying that this group was probably composed of individuals from several cohorts. Parentage analysis established the location of the mothers that produced SRs.
Taking into account the variation of female size among sites, we found that females that produced SRs were significantly larger (3–12.4% TL, 8–32% biomass) than those that did not (p = 0.006; figure 1 and table 3). With two exceptions, all the sites where SRs were produced had medium to high densities of females, and in all cases except one females that did not produce SRs were more abundant than those that did (figure 1).
Table 3.
Randomized block ANOVA for the effect of female size on the production of self-recruits in a population of orange-fin anemonefish (Amphiprion chrysopterus) in Moorea, French Polynesia. Site was considered a random factor and SR/NOSR (which distinguishes females that produced self-recruits and those that did not) was treated as fixed (i.e. F-value calculated using type III MS for site × SR/NOSR as an error term).
| source | d.f. | type III SS | mean square | F-value | p > F |
|---|---|---|---|---|---|
| year | 1 | 59.071 | 59.071 | 0.26 | 0.610 |
| site | 5 | 4636.677 | 927.335 | 4.13 | 0.003 |
| site × SR/NOSR | 5 | 224.191 | 44.838 | 0.20 | 0.961 |
| SR/NOSR | 1 | 965.656 | 965.656 | 21.54 | 0.006 |
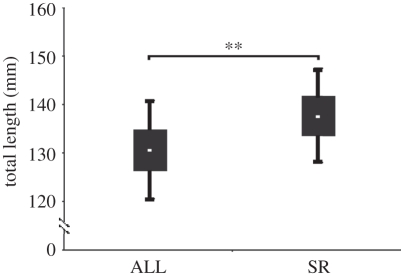
The expected value of the mean size based on fecundity was significantly larger for females that produced SRs than the expected value of female size in the population as a whole (t = 5.82, d.f. = 5, p = 0.0011; figure 2). Indeed, these expected values were higher at every site than the expected value for all females at that site (table 4). These results show that fecundity alone cannot account for the higher number of SRs contributed by females that produced SRs.
Figure 2.
Expected mean total length (mm) based on fecundity for females that produced self-recruits (SR) and all females (ALL) in the six sites identified in figure 1 were significantly different (**p < 0.01). Box plots denote mean (white dot), standard error (box) and standard deviation (whiskers) for each of the female groups.
Table 4.
Number of Amphiprion chrysopterus females in couples (n), fecundity (average number of eggs per breeding event ± s.e.) and TL (estimated average total length in mm based on fecundity ± s.e.) for females that produced self-recruits (SR) and all females (ALL) at each of the sites detailed in figure 1.
| site | female type | n | fecundity | TL (estimated) |
|---|---|---|---|---|
| A | SR | 2 | 426.1 ± 98.8 | 128.51 |
| ALL | 4 | 380.3 ± 99.5 | 120.21 | |
| B | SR | 2 | 483.5 ± 123.1 | 139.54 |
| ALL | 5 | 425.1 ± 118.3 | 129.92 | |
| C | SR | 2 | 398.5 ± 106.8 | 124.82 |
| ALL | 9 | 381.9 ± 77.2 | 119.54 | |
| D | SR | 2 | 511.3 ± 53.4 | 139.94 |
| ALL | 9 | 454.0 ± 65.5 | 130.79 | |
| E | SR | 7 | 524.0 ± 24 | 142.25 |
| ALL | 21 | 502.2 ± 89.5 | 140.01 | |
| F | SR | 3 | 585.0 ± 20.8 | 151.68 |
| ALL | 14 | 528.7 ± 90.7 | 144.37 |
4. Discussion
The relatively high number of anemonefish recruits we sampled that were produced by adults on Moorea enabled us to assess the relationship between maternal size and self-recruitment. The results show, for the first time in a natural marine population, that larger female fish contributed disproportionately to local replenishment (self-recruitment).
There are several mechanisms that could have produced the results we report here, including the underlying relationship between body size and fecundity of a female. As demonstrated for other anemonefish such as Amphiprion melanops [36], maternal size is correlated with the number of eggs in a clutch. Female fecundity of A. chrysopterus in Moorea (Society Islands) was similar to that reported for Eniwetok Atoll (Marshall Islands) by Allen [23] and the number of eggs in a clutch scaled positively with female body size. However, the fundamental size–fecundity relationship for A. chrysopterus alone could not explain the higher contribution of larger females to self-recruitment we measured on Moorea. This indicates that there were probably maternal size effects on phenotypic traits of offspring. These potentially could have involved faster growth and/or earlier competency of larvae from larger mothers, either of which could enhance survivorship through the larval dispersal stage. Larger mothers potentially could have produced larvae that hatch at a larger body size, which has been observed for other species of coral reef fish [37]. Larval size is highly correlated with swimming performance in teleost fishes [38], and can increase survival rates through enhanced acquisition of food and predator avoidance [39]. By contrast, varying selective pressures in the wild should contribute to maintenance of phenotypic variation in growth rate and size of recruits [37,40], which in turn could be related to variation in maternal size.
Larger and presumably older females in an anemonefish population are also more likely to contribute to multiple recruitment cohorts. Size and growth in anemonefish depend on the social rank of each individual [41]; thus larger females are not, in all cases, the oldest individuals in a population. Given that we do not know which females contributed to the larval pool prior to our sampling, we do not know whether the participation of larger and presumably older individuals in multiple breeding events can be sufficient to explain their increased contribution to population replenishment. The results presented here may also be the consequence of natural phenomena, including oceanographic conditions and patchy environmental selection, that are capable of influencing recruitment dynamics.
In this study, we sampled neither larvae that recruited to other islands, nor parents in potential source populations outside Moorea; so it was not possible to determine whether recruits dispersing to, or arriving from, other populations were produced by larger or smaller females. However, a few recent studies have been able to identify recruits dispersing to other populations [21,42], which may permit identifying prevalent maternal traits of the successful exogenous recruits. In this study, we were not able to sample 100 per cent of the population of Amphiprion chrysopterus and thus it is possible that large females also produced a disproportionate number of offspring that settled to locations (on Moorea and other islands) in which we did not sample. It is also possible that self-recruits from large females identified as NOSR may not have been sampled, thus rendering our findings conservative.
The results presented here show a clear link between female size and population replenishment for a marine fish, which has substantial implications for fisheries management and conservation. For example, arguments have been made that maximum catch size restrictions need to be incorporated into fisheries management considerations for several commercially important species of fish; yet these arguments have been based solely on experimental data [43–45] and not on any measured effect on the replenishment of a natural population. Our findings bridge the gap between the importance of maternal size, fecundity and population replenishment, and, by extension, recruitment dynamics, to reinforce the notion that size truncation, commonly induced by fisheries, may have greater consequences for the sustainability of fish populations than previously thought.
Acknowledgements
We thank personnel at the University of California Berkeley Gump Research Station and Le Centre de Recherches Insulaires et Observatoire de l'Environnement de Polynésie Française (CRIOBE) for field assistance, P. Raimondi for statistical advice, and S. C. Mills and two anonymous reviewers for comments on the manuscript. This research was supported by grants OCE 04-17412 and OCE 1026851 from the National Science Foundation (the Moorea Coral Reef LTER) and an award from the Gordon and Betty Moore Foundation. R.B. was funded by Fundação para a Ciência e Tecnologia (SFRH/BPD/26901/2006).
References
- 1.Schmitt R. J., Holbrook S. J. 2002. Spatial variation in concurrent settlement of three damselfishes: relationships with near-field current flow. Oecologia 131, 391–401 10.1007/s00442-002-0893-9 (doi:10.1007/s00442-002-0893-9) [DOI] [PubMed] [Google Scholar]
- 2.Eckert G. L. 2003. Effects of the planktonic period on marine population fluctuations. Ecology 84, 372–383 10.1890/0012-9658(2003)084[0372:EOTPPO]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0372:EOTPPO]2.0.CO;2) [DOI] [Google Scholar]
- 3.Beldade R., Erzini K., Gonçalves E. J. 2006. Temporal dynamics of a temperate cryptobenthic rocky fish assemblage. J. Mar. Biol. Assoc. UK 86, 1221–1228 10.1017/S0025315406014226 (doi:10.1017/S0025315406014226) [DOI] [Google Scholar]
- 4.Hedgecock D., Barber P. H., Edmands S. 2007. Genetic approaches to measuring connectivity. Oceanography 20, 70–79 10.5670/oceanog.2007.30 (doi:10.5670/oceanog.2007.30) [DOI] [Google Scholar]
- 5.Ruzzante D. E., Taggart C. T., Cook D. 1996. Spatial and temporal variation in the genetic composition of a larval cod (Gadus morhua) aggregation: cohort contribution and genetic stability. Can. J. Fish. Aquat. Sci. 53, 2695–2705 10.1139/cjfas-53-12-2695 (doi:10.1139/cjfas-53-12-2695) [DOI] [Google Scholar]
- 6.Li G., Hedgecock D. 1998. Genetic heterogeneity, detected by PCR-SSCP, among samples of larval Pacific oysters (Crassostrea gigas) supports the hypothesis of large variance in reproductive success. Can. J. Fish. Aquat. Sci. 55, 1025–1033 10.1139/cjfas-55-4-1025 (doi:10.1139/cjfas-55-4-1025) [DOI] [Google Scholar]
- 7.Moberg P. E., Burton R. S. 2000. Genetic heterogeneity among adult and recruit red sea urchins, Strongylocentrotus franciscanus. Mar. Biol. 136, 773–784 10.1007/s002270000281 (doi:10.1007/s002270000281) [DOI] [Google Scholar]
- 8.Planes S., Lenfant P. 2002. Temporal change in the genetic structure between and within cohorts of a marine fish, Diplodus sargus, induced by a large variance in individual reproductive success. Mol. Ecol. 11, 1515–1524 10.1046/j.1365-294X.2002.01521.x (doi:10.1046/j.1365-294X.2002.01521.x) [DOI] [PubMed] [Google Scholar]
- 9.Selkoe K. A., Gaines S. D., Caselle J. E., Warner R. R. 2006. Current shifts and kin aggregation explain genetic patchiness in fish recruits. Ecology 87, 3082–3094 10.1890/0012-9658(2006)87[3082:CSAKAE]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[3082:CSAKAE]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 10.Hedgecock D., Sly F. 1990. Genetic drift and effective population sizes of hatchery-propagated stocks of the Pacific oyster, Crassostrea gigas. Aquaculture 88, 21–38 10.1016/0044-8486(90)90316-F (doi:10.1016/0044-8486(90)90316-F) [DOI] [Google Scholar]
- 11.Hedgecock D., Launey S., Pudovkin A.I., Naciri Y., Lapegue S., Bonhomme F. 2007. Small effective number of parents (N-b) inferred for a naturally spawned cohort of juvenile European flat oysters Ostrea edulis. Mar. Biol. 150, 1173–1182 10.1007/s00227-006-0441-y (doi:10.1007/s00227-006-0441-y) [DOI] [Google Scholar]
- 12.Hislop J. R. G. 1988. The influence of maternal length and age on the size and weight of the eggs and the relative fecundity of the haddock, Melanogrammus aeglefinus, in British waters. J. Fish Biol. 32, 923–930 (doi:10.1111/j.1095–8649.1988.tb05435.x) [DOI] [Google Scholar]
- 13.Jennings S., Kaiser M. J., Reynolds J. D. 2005. Marine fisheries ecology. Oxford, UK: Blackwell Science Ltd [Google Scholar]
- 14.Heath D. D., Blouw D. M. 1998. Are maternal effects in fish adaptive or merely physiological side effects? In Maternal effects as adaptations (eds Mousseau T. A., Fox C. W.), pp. 178–201 New York, NY: Oxford University Press [Google Scholar]
- 15.Berkeley S., Chapman C., Sogard S. M. 2004. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology 85, 1258–1264 10.1890/03-0706 (doi:10.1890/03-0706) [DOI] [Google Scholar]
- 16.Sato T., Suzuki N. 2010. Female size as a determinant of larval size, weight and survival period in the coconut crab, Birgus latro. J. Crust. Biol. 30, 624–628 10.1651/10-3279.1 (doi:10.1651/10-3279.1) [DOI] [Google Scholar]
- 17.Green B. S. 2008. Maternal effects in fish populations. Adv. Mar. Biol. 54, 1–105 10.1016/S0065-2881(08)00001-1 (doi:10.1016/S0065-2881(08)00001-1) [DOI] [PubMed] [Google Scholar]
- 18.Marshall D. J., Heppel S. S., Munch S. B., Warner R. R. 2010. The relationship between maternal phenotype and offspring quality: do older mothers really produce the best offspring? Ecology 91, 2862–2873 10.1890/09-0156.1 (doi:10.1890/09-0156.1) [DOI] [PubMed] [Google Scholar]
- 19.Planes S., Jones G. P., Thorrold S. M. 2009. Larval dispersal connects fish populations in a network of marine protected areas. Proc. Natl Acad. Sci. USA 106, 5693–5697 10.1073/pnas.0808007106 (doi:10.1073/pnas.0808007106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones G. P., Planes S., Thorrold S. R. 2005. Coral reef fish larvae settle close to home. Curr. Biol. 15, 1314–1318 10.1016/j.cub.2005.06.061 (doi:10.1016/j.cub.2005.06.061) [DOI] [PubMed] [Google Scholar]
- 21.Saenz-Agudelo P., Jones G. P., Thorrold S. R., Planes S. 2011. Connectivity dominates larval replenishment in a coastal reef fish metapopulation. Proc. R. Soc. B 278, 2954–2961 10.1098/rspb.2010.2780 (doi:10.1098/rspb.2010.2780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holbrook S. J., Schmitt R. J. 2005. Growth, reproduction and survival of a tropical sea anemone (Actiniaria): benefits of hosting anemonefish. Coral Reefs 24, 67–73 10.1007/s00338-004-0432-8 (doi:10.1007/s00338-004-0432-8) [DOI] [Google Scholar]
- 23.Allen G. R. 1972. The anemonefishes: their classification and biology. Neptune City, NJ: TFH Publications, Inc [Google Scholar]
- 24.Ochi C. H. 1986. Growth of the anemonefish Amphiprion clarkii in temperate waters, with special reference to the influence of settling time on the growth of 0-year olds. Mar. Biol. 92, 223–229 10.1007/BF00392839 (doi:10.1007/BF00392839) [DOI] [Google Scholar]
- 25.Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory [Google Scholar]
- 26.Beldade R., Holbrook S. J., Schmitt R. J., Planes S., Bernardi G. 2009. Isolation and characterization of eight polymorphic microsatellite markers from the orange-fin anemonefish, Amphiprion chrysopterus. Conserv. Genet. Resour. 1, 333–335 10.1007/s12686-009-9077-9 (doi:10.1007/s12686-009-9077-9) [DOI] [Google Scholar]
- 27.Quenouille B., Bouchenak-Khelladi Y., Hervet C., Planes S. 2004. Eleven microsatellite loci for the saddleback clownfish Amphiprion polymnus. Mol. Ecol. Notes 4, 291–293 10.1111/j.1471-8286.2004.00646.x (doi:10.1111/j.1471-8286.2004.00646.x) [DOI] [Google Scholar]
- 28.Excoffier L., Laval G., Schneider S. 2005. Arlequin version 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1, 7–50 [PMC free article] [PubMed] [Google Scholar]
- 29.van Oosterhout C. D., Weetman D., Hutchinson W. F. 2006. Estimation and adjustment of microsatellite null alleles in non-equilibrium populations. Mol. Ecol. Notes 6, 255–256 10.1111/j.1471-8286.2005.01082.x (doi:10.1111/j.1471-8286.2005.01082.x) [DOI] [Google Scholar]
- 30.Gerber S., Mariette S., Streiff R., Bodenes C., Kremer A. 2000. Comparison of microsatellites and amplified fragment length polymorphism markers for parentage analysis. Mol. Ecol. 9, 1037–1048 10.1046/j.1365-294x.2000.00961.x (doi:10.1046/j.1365-294x.2000.00961.x) [DOI] [PubMed] [Google Scholar]
- 31.Gerber S., Chabrier P., Kremer A. 2003. FAMOZ: a software for parentage analysis using dominant, codominant and uniparentally inherited markers. Mol. Ecol. Notes 3, 479–481 10.1046/j.1471-8286.2003.00439.x (doi:10.1046/j.1471-8286.2003.00439.x) [DOI] [Google Scholar]
- 32.Godwin J. 1994. Histological aspects of protandrous sex change in the anemonefish Amphiprion melanopus, Pomacentridae, Teleostei. J. Zool. 232, 199–213 10.1111/j.1469-7998.1994.tb01569.x (doi:10.1111/j.1469-7998.1994.tb01569.x) [DOI] [PubMed] [Google Scholar]
- 33.Fautin D. J., Allen G. R. 1997. Field guide to anemone fishes and their host sea anemones. Perth, Australia: Western Australian Museum [Google Scholar]
- 34.Pauly D., Froese R., Albert J. S. 1998. The BRAINS table. In FishBase 98: concepts, design and data sources (eds Froese R., Pauly D.), pp. 195–198 Manila, Philippines: ICLARM [Google Scholar]
- 35.Robertson D. R., Green D. G., Victor B. C. 1988. Temporal coupling of production and recruitment of larvae of a Caribbean reef fish. Ecology 69, 370–381 10.2307/1940435 (doi:10.2307/1940435) [DOI] [Google Scholar]
- 36.Green B. S., McCormick M. I. 2005. Maternal and paternal effects determine size, growth and performance in larvae of a tropical reef fish. Mar. Ecol. Prog. Ser. 289, 263–272 10.3354/meps289263 (doi:10.3354/meps289263) [DOI] [Google Scholar]
- 37.Gagliano M., McCormick M. I., Meekan M. G. 2007. Survival against the odds: ontogenetic changes in selective pressure mediate growth-mortality trade-offs in a marine fish. Proc. R. Soc. B 274, 1575–1582 10.1098/rspb.2007.0242 (doi:10.1098/rspb.2007.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark D. L., Leis J. M., Hay A. C., Trnski T. 2005. Swimming ontogeny of larvae of four temperate marine fishes. Mar. Ecol. Prog. Ser. 292, 287–300 10.3354/meps292287 (doi:10.3354/meps292287) [DOI] [Google Scholar]
- 39.Sogard S. M. 1997. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull. Mar. Sci. 60, 1129–1157 [Google Scholar]
- 40.Donelson J., Munday P. L., McCormick M. I. 2009. Parental effects on offspring life histories: when are they important? Biol. Lett. 5, 262–265 10.1098/rsbl.2008.0642 (doi:10.1098/rsbl.2008.0642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buston P. M. 2003. Social hierarchies: size and growth modification in clownfish. Nature 424, 145–146 10.1038/424145a (doi:10.1038/424145a) [DOI] [PubMed] [Google Scholar]
- 42.Christie M. R., Tissot B. N., Albins M. A., Beets J. P., Jia Y., Ortiz D. M., Thompson S. E., Hixon M. A. 2010. Larval connectivity in an effective network of marine protected areas. PLoS ONE 5, e15715. 10.1371/journal.pone.0015715 (doi:10.1371/journal.pone.0015715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meekan M. G., Fortier L. 1996. Selection for fast growth during the larval life of Atlantic cod Gadus morhua on the Scotian Shelf. Mar. Ecol. Prog. Ser. 137, 25–37 10.3354/meps137025 (doi:10.3354/meps137025) [DOI] [Google Scholar]
- 44.Marteinsdottir G., Thorarinsson K. 1998. Improving the stock–recruitment relationship in Icelandic cod (Gadus morhua) by including age diversity of spawners. Can. J. Fish. Aquatic Sci. 55, 1372–1377 10.1139/cjfas-55-6-1372 (doi:10.1139/cjfas-55-6-1372) [DOI] [Google Scholar]
- 45.Marteinsdottir G., Steinarsson A. 1998. Maternal influence on the size and viability of Iceland cod Gadus morhua eggs and larvae. J. Fish Biol. 52, 1241–1258 10.1111/j.1095-8649.1998.tb00969.x (doi:10.1111/j.1095-8649.1998.tb00969.x) [DOI] [Google Scholar]