Abstract
We provide here unique data on elephant skeletal ontogeny. We focus on the sequence of cranial and post-cranial ossification events during growth in the African elephant (Loxodonta africana). Previous analyses on ossification sequences in mammals have focused on monotremes, marsupials, boreoeutherian and xenarthran placentals. Here, we add data on ossification sequences in an afrotherian. We use two different methods to quantify sequence heterochrony: the sequence method and event-paring/Parsimov. Compared with other placentals, elephants show late ossifications of the basicranium, manual and pedal phalanges, and early ossifications of the ischium and metacarpals. Moreover, ossification in elephants starts very early and progresses rapidly. Specifically, the elephant exhibits the same percentage of bones showing an ossification centre at the end of the first third of its gestation period as the mouse and hamster have close to birth. Elephants show a number of features of their ossification patterns that differ from those of other placental mammals. The pattern of the initiation of the ossification evident in the African elephant underscores a possible correlation between the timing of ossification onset and gestation time throughout mammals.
Keywords: elephant, development, skeletogenesis, heterochrony, ossification timing
1. Introduction
Elephants are among the most iconic and unique vertebrates, for example, by showing the longest gestation time among mammals, with 623–729 days for Elephas and 640–673 days for Loxodonta [1,2]. While the order Proboscidea has been heavily studied, and regularly topped the list of the number of articles per annum for a given species [3], few studies have focused on their development. Previous workers have described isolated foetuses [4–7] or focused on placentation [8–10], teeth [11,12], the skull [13] or the reproductive and respiratory system [14]. However, these do not detail development of the skeleton or provide a comparative basis upon which to determine if/how elephants depart osteologically from other mammalian groups.
Hildebrandt et al. [15] have recently produced accurate growth curves for early ontogenetic stages in elephants that provide reliable estimates of their gestational age. Here, we provide novel data on elephant skeletal ontogeny focusing on cranial and post-cranial ossification events during growth; these data constitute, to our knowledge, the first afrotherian ossification sequence studied to date. We employ techniques for quantifying sequence heterochrony [16–18] in order to test the hypothesis that they show developmental distinctiveness compared with other placentals, especially xenarthrans. By combining our data with the criteria of Hildebrandt et al. [15], we have been able to define major ossification events in the light of the absolute timing of development in elephants. We used these data to determine the extent to which the longest mammalian pregnancy departs from what is observed in other placentals as well as to reveal general patterns of ossification across mammals.
2. Material and methods
(a). Data collection
We sampled material from collections of the Paul Mellon Laboratory of Equine Reproduction in Newmarket (UK), the Elephant Research Unit in Zimbabwe, the Natural History Museum London, the Muséum National d'Histoire Naturelle in Paris and the Institut Royal des Sciences Naturelles de Belgiques in Brussels (IRSNB), including specimens from Kruger National Park in South Africa and the Savé Valley Conservancy in Southern Zimbabwe. The specimen studied by Eales [7] was also considered in the analyses. A total of 17 unsexed foetuses of Loxodonta africana were studied (electronic supplementary material, S1 and figure 1). They range in size from 34.7 to 280 mm crown rump length (CRL), measured from the vertex of the skull to the base of the tail. Collections of such non-model organisms often include specimens collected decades ago and invariably lack data on individual age. The CRL was used to estimate the age of the foetuses following the formula of Hildebrandt et al. [15]: age = 35.14 + 10.80 × CRL1/2, r2 = 0.98. Forty-one ossification events were recorded and compared (electronic supplementary material, S2) with the ossification sequences of other placental mammals and marsupials from Hautier et al. ([19] and references therein). We obtained ossification sequences for Ovis and Bos from Harris [20], Lindsay [21] and Soana et al. [22].
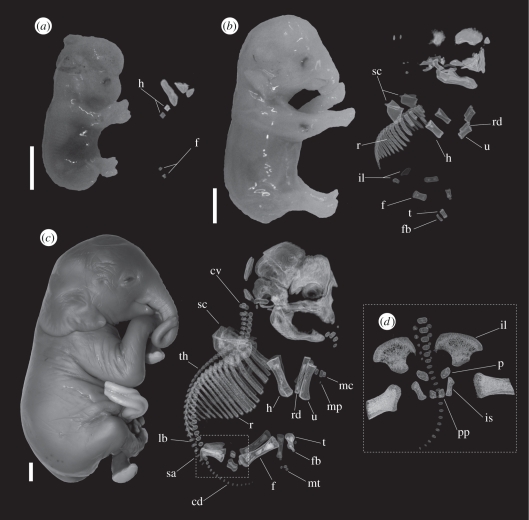
Figure 1.
Representative ontogenetic stages of elephants. Lateral view of specimens (left) and three-dimensional reconstruction of computerized tomography (CT) scans of skeleton (right) in (a) a 99 day-old specimen (age reconstructed from CRL as described in the text and Hildebrandt et al. [15]), PMLER 1, CRL = 34.7 mm; (b) a 118 day-old specimen, PMLER 2, CRL = 59.3 mm; and (c) a 176 day-old specimen, PMLER 8, CRL = 171.4 mm. (d) Close-up of the pelvic girdle showing a fourth ossification centre. cd, caudal vertebrae; cv, cervical vertebrae; f, femur; fb, fibula; h, humerus; il, ilium; is, ischium; lb, lumbar vertebrae; mc, metacarpals; mp, manual phalanges; mt, metatarsals; p, pubis; pp, post-pubic bones; r, ribs; rd, radius; sa, sacral vertebrae; sc, scapula; t, tibia; th, thoracic vertebrae; u, ulna. Scale bars, 1 cm.
(b). Three-dimensional data acquisition
Skeletons were imaged using high-resolution X-ray microtomography (μCT; figures 1 and 2) at the engineering department of the University of Cambridge (Cambridge, UK), at the Natural History Museum (London, UK) and at VISCOM SARL (Saint Ouen l'Aumône, France). Threshold values between ossified parts and soft tissues were substantial and easily allowed osteological reconstructions. Three-dimensional rendering and visualization were performed using Drishti v. 1.0 (Drishti Paint and Render, [23]). Following the protocol of Mitgusch et al. [24], observed ossified elements were tabulated for each specimen (electronic supplementary material, S1). Following Weisbecker [25], we distinguished clearly ossified bones (‘B’) from elements displaying barely detectable ossification (‘m’). Pooled elements (e.g. carpals, metacarpals and phalanges) were considered to be ossified when at least one of the constituting elements has started its ossification. The list of ossification ranks is given in table 1 and the electronic supplementary material, S2.
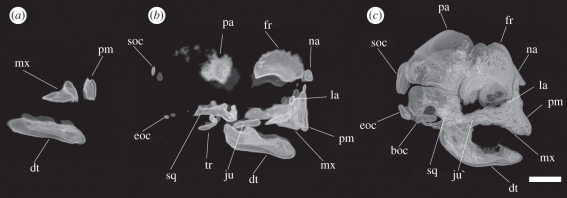
Figure 2.
Lateral view of three-dimensional reconstructions of cranial CT scans in (a) a 99 day-old specimen (age reconstructed from CRL as described in the text and Hildebrandt et al. [15]), PMLER 1, CRL = 34.7 mm; (b) a 118 day-old specimen, PMLER 2, CRL = 59.3 mm; and (c) a 176 day-old specimen, PMLER 8, CRL = 171.4 mm. boc, basioccipital; dt, dentary; eoc, exoccipital; fr, frontal; ju, jugal; la, lacrimal; mx, maxillary; na, nasal; pa, parietal; pm, premaxillary; soc, supraoccipital; sq, squamosal; tr, tympanic ring. Scale bar, 1 cm.
Table 1.
Relative timing of onset of ossification (ranks) in pooled cranial and post-cranial elements for Loxodonta africana.
| ranks | elements |
|---|---|
| 1 | premaxilla |
| 1 | maxilla |
| 3 | palatine |
| 1 | dentary |
| 3 | frontal |
| 3 | parietal |
| 3 | squamosal |
| 5 | basioccipital |
| 3 | nasal |
| 3 | pterygoid |
| 3 | exoccipital |
| 12 | basisphenoid |
| 3 | jugal |
| 3 | lacrimal |
| 3 | alisphenoid |
| 10 | orbitosphenoid |
| 16 | periotic |
| 1 | humerus |
| 3 | ribs |
| 2 | femur |
| 3 | radius |
| 3 | ulna |
| 3 | scapula |
| 4 | cervicals |
| 3 | thoracics |
| 3 | tibia |
| 3 | fibula |
| 6 | lumbar |
| 8 | sacral |
| 9 | caudal |
| 3 | ilium |
| 13 | manual phalanges |
| 14 | pedal phalanges |
| 7 | ischium |
| 13 | pubis |
| 7 | metacarpals |
| 11 | metatarsals |
| 15 | tarsals |
| 16 | carpals |
| 15 | sternum |
(c). Quantification of developmental trajectories
To maximize compatibility with previous studies [17,19], cranial and post-cranial elements of the skeleton were treated separately in the analyses (electronic supplementary material, S2). Following Hautier et al. [19], we used two methods to quantify sequence heterochrony: the sequence method [16,26,27] and Parsimov [28]. The sequence method of Smith [16] requires that every species be sampled for the same series of elements. Thus, several species (post-cranial: Meriones, Ovis, Bos and Sus; cranial: Tarsius, Rattus, Meriones, Mesocricetus, Felis, Sus, Ovis, Bos and Manis) could not be included. For our remaining sample, the first step of the sequence method of Smith [16] constructs the developmental sequence by ordering the events by their relative stage for each taxon. This method uses standard non-parametric ranking procedures to deal with tied ossification events. The sequence method is less explicitly phylogenetic than Parsimov. Nevertheless, it can effectively illuminate the pattern of change of different skeletal elements of elephants relative to the mean developmental trajectory of other placental mammals. In the case of ties, we used the average rank for the tied events, as described in Hautier et al. [19]. The dataset is then converted into transformed ranks (electronic supplementary material, S2b,d). The ranked dataset is then plotted graphically (figure 3). Smith [16] used ANOVA to recognize elements that show significantly more differences in rank position between- than within-groups (see also [26]). Here, we could not statistically compare the results obtained for elephants to the data on ossification in placentals because ANOVA requires more than two sequences in a group to be known. However, the variability in the mean placental ranks was graphically displayed with error bars of ±1 s.d. in order to show the extent to which elephant sequences depart from the range of variation observed in other mammals.
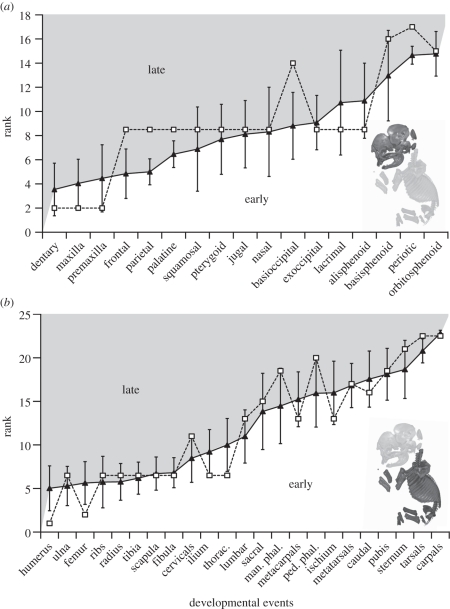
Figure 3.
Ossification sequence of (a) cranial and (b) post-cranial elements in elephants (unfilled squares) relative to the mean rank of placentals (solid triangles) with error bars of ±1 s.d.
We constructed event-pair matrices of ossification sequences in which the ossification onset of 17 cranial elements and 24 post-cranial elements were compared with every other element. Following previous studies [17,19], we considered cranial and post-cranial events in two separate data matrices: one with one-half (172 – 17) = 136 events for cranial elements and the other with one-half (242 – 24) = 276 event pairs for the post-cranial elements. Three character states were used to represent the relative timing of one event relative to another: 0, 1 and 2, corresponding to prior, simultaneous or subsequent ossification of one element relative to another (respectively). Then, we ran the Parsimov analyses using both ACCTRAN and DELTRAN optimizations as recommended by Jeffery et al. [26]. Parsimov is phylogenetic and allows us to test whether the branch leading to elephant is characterized by more sequence heterochronies than other branches leading to marsupials and placentals. While previous studies have interpreted heterochrony only when events are reported using both optimization approaches, we have previously shown that Parsimov is overly conservative, and using either ACCTRAN or DELTRAN (not both) may be justified in some cases [19]. Furthermore, following Hautier et al. [19] and Sánchez-Villagra et al. [29], we performed additional Parsimov analyses with all ties converted to missing data (electronic supplementary material, S3 and S4) to control for the artefactual interpretation of heterochrony owing to accumulation of ties across events. We did not use the Pgi heterochrony search algorithm by Harrison & Larsson [30], as it is currently not programmed to analyse datasets with ties excluded [19].
Because of the uncertain phylogenetic position of the placental root, the event-pair analyses were performed in two phylogenetic contexts. The African elephant was considered either as the sister clade of xenarthrans (i.e. Atlantogenata, [31,32]), or as the sister clade of all other placental mammals (i.e. Exafroplacentalia, [33]; alternate phylogenetic topologies are given in the electronic supplementary material, S5).
3. Results
(a). Ossification of the skull
Cranially, Loxodonta africana resembles the ossification sequence of other placental mammals in that bones of the rostrum (i.e. dentary, maxilla and premaxilla) ossify very early, before those of the basicranium and posterior skull (electronic supplementary material, S2). In the youngest specimen, the dentary forms a slender bar corresponding to the horizontal ramus (PMLER1, figure 2a). The maxilla forms a small subtriangular plate-like element with a tiny pars palatina extending medially. The paired premaxillae are present as very small bar-like elements that extend anteriorly to the maxilla. The dentary shows coronoid and condylar processes by the following stage (PMLER2, figure 2b). By then, the palatine, frontal, parietal, squamosal, nasal, pterygoid, exoccipital, supraoccipital, jugal, lacrimal and alisphenoid have also started to ossify. The posterior elements of the skull show only minute ossifications, whereas facial bones are all well ossified (figure 2b). The orbit is recognizable and the alveolus of the tusk is already visible. The ossification of these bones is followed by the ossification of the basioccipital and orbitosphenoid (PMLER8, figure 2c). The basisphenoid is the penultimate bone to ossify, followed by the periotic, similar to the pattern observed in most other mammals considered here. Mineralization of the cheek teeth was not detected in any of the specimens studied. Relative to certain late-ossifying post-cranial elements, only the orbitosphenoid showed a variable timing of ossification among the cranial dataset (electronic supplementary material, S1).
(b). Ossification pattern in the post-cranial skeleton
The youngest specimen (PMLER1, figure 1a) shows a well-ossified centre in the humerus and a minute ossification of the femur. In the second youngest specimen (PMLER2, figure 1b), the ribs, tibia, fibula, radius, ulna, ilium and scapula are well ossified, as are the centra of the thoracic vertebrae. In older specimens (e.g. PMLER8, figure 1c), the cervical vertebrae start to ossify their neural arches, followed by the centra of lumbar vertebrae, the ischium, metacarpals, sacral vertebrae, caudal vertebrae and metatarsals (electronic supplementary material, S2). They are followed by the manual phalanges and the pubis that start their ossification simultaneously, just before the ossification of the pedal phalanges. The ossification of the sternum is next, followed by the tarsals and carpals (electronic supplementary material, S2). It is worth mentioning here that we observed a fourth centre of ossification in the pelvic girdle in our oldest samples (figure 1d). This fourth centre has previously been described as a ‘post-pubic’ bone [7]. We also observed intraspecific variability in the ossification of the metacarpals and metatarsals.
The ossification of the periotic, carpals and tarsals was not observed in the oldest specimen. These bones were scored as occurring last in order to compare the elephant sequence with those of other placentals. In addition, we observed no indication of an ossification of the clavicle. This bone, which appears very early in other placentals and is never present in adult elephants, was excluded from the following analyses. However, we cannot rule out a possible clavicular ossification in a stage not represented in our sample. Presence of a rudimentary clavicle followed by resorption has been observed in sheep [20], cows [21] and tree sloths [34].
(c). Sequence heterochrony in elephants
Our growth series exhibits a concentration of ossification events within the first two stages with three out of 17 elements (17%) ossifying first, and 10 out of 17 elements ossifying second (59%). By discounting the ‘significance’ of heterochronies resulting from the accumulation of events tied at the beginning of a given ontogenetic series [19], the late ossification of the basioccipital and periotic distinguishes elephants from other mammals using the sequence method of Smith [16] (figure 3a). When ties are treated as missing data, Parsimov does not identify any heterochronic shift for cranial elements, similar to the results of the sequence method of Smith [16] (electronic supplementary material, S3). Otherwise, it detects only shifts involving bones that ossify at the same rank (electronic supplementary material, S3) that should be considered as a methodological artefact.
The post-cranial sequence is much more resolved than that for the cranium, and we retrieved a concentration of relative simultaneity only for the events of rank 3 (ribs, radius, ulna, scapula, thoracic vertebrae, tibia, fibula and ilium; see the electronic supplementary material, S2). Using the sequence method of Smith [16], elephants differ from other placentals in showing a late ossification of the phalanges and cervical vertebrae, and an early ossification of the humerus, femur, ilium, ischium, metacarpals and thoracic vertebrae (figure 3b). With ties coded as present, Parsimov identifies only seven of the heterochronic shifts, depending on the position of the placental root (i.e. Atlantogenata versus Exafroplacentalia; electronic supplementary material, S4): early ossification of the femur, humerus, ilium, ischium and thoracic vertebrae, and late ossification of the manual and pedal phalanges. When ties are treated as missing data, Parsimov detects only a late ossification of the manual phalanges (electronic supplementary material, S4).
The shifts involving the humerus and femur may be owing to artefacts of sampling in the earliest stages of other placental mammals. Because the early ossification of the ilium and thoracic vertebrae are among several events tied at number 3, their apparent shifts are probably a methodological artefact.
(d). Foetal age versus ossification
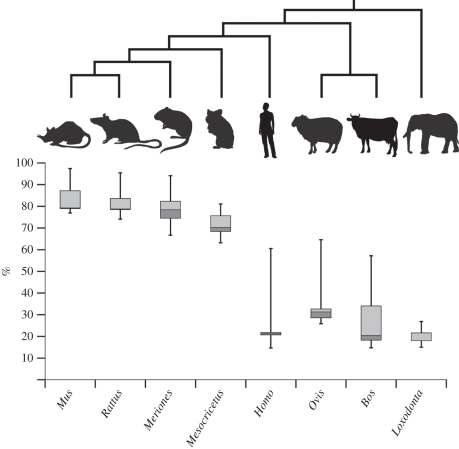
All elements of skull except the basisphenoid, orbitosphenoid and periotic ossify very early relative to the post-cranial skeleton (table 1). By using the precise formula of Hildebrandt et al. [15], we determined that our ontogenetic series of elephants ranges from 99 to 216 days of gestation (electronic supplementary material, S1), casting some light on early skeletal development in proboscideans. The first centres of ossification (mandible, premaxillary, maxillary, humerus and femur) were detected in the youngest specimen, so the skeleton should start ossifying slightly before day 99 of gestation (i.e. ca 15% into total gestation time; see the electronic supplementary material, S6; and figure 4). The appearance of the first centres of ossification follows the initiation of cardiac activity around day 80 [15]. This early ossification involves the whole skeleton; 90 per cent of all cranial and post-cranial elements have started ossifying before the end of the first third of the gestation period (i.e. 27% into total gestation time; electronic supplementary material, S6; and figure 4).
Figure 4.
Box plots representing the cumulative percentage of bones present in the adult showing an ossification centre in the cranial and post-cranial skeleton of the elephant, human, cow, sheep, hamster, mouse, rat, and gerbil relative to gestation length.
4. Discussion
(a). Sequence heterochrony in elephants
The sequence method of Smith [16] showed that the elephant sequence appears to be within the range of variation observed in other placentals with the exception of shifts affecting the basioccipital, periotic, phalanges, ischium, metacarpals and cervical vertebrae. Even if Parsimov is commonly viewed as overly conservative [18,19,35], it retrieved similar shifts when either ACCTRAN or DELTRAN analyses were used alone, and to a lesser extent when the strict consensus of the two was used (electronic supplementary material, S3 and S4). Both methods are subject to type II errors owing to the accumulation of ties at early events, which artefactually elevate the ‘significance’ of early shifts owing to low resolution of the earliest developmental events [17–19,36]. Using the Parsimov analysis, it appears that the branch leading to elephant is characterized by a large number of sequence heterochronies, but this abundance of shifts could simply result from the isolation of elephants (as the sole afrotherian) within the presented phylogenetic trees. Despite these qualifications, our results show that heterochrony still has played a role in the early skeletal development of elephants. Although they show similarities with other placentals [17–19,36–38], there are some non-artefactual differences in their developmental trajectory: a late ossification of the basioccipital, periotic and phalanges, and an early ossification of the ischium and metacarpals (figure 3).
It has been proposed that the time of initiation of an anatomical structure is related to its adult size [17,39]. Elephants are characterized by large hips and small manual and pedal phalanges, leading one to expect early ossification of the pelvis and late ossification of the phalanges, which is indeed supported by our data. In addition, the early ossification of the metacarpals could be linked to the unique, subcutaneous cushions that are functionally linked to the skeletal architecture of their graviportal stance [40]. Interestingly, the late ossification of the phalanges in elephants clearly departs from what is observed for xenarthrans, a group which exhibits a unique early ossification of the phalanges [19]. If the Atlantogenata hypothesis is correct (i.e. afrotherians and xenarthrans as sister groups at the base of Placentalia), then this result indicates some homoplasy in the evolution of the proboscidean manus and pes.
(b). Timing of ossification in placental mammals
Ossification of the skeleton in elephants starts very early during development, and appears to progress rapidly throughout the body (figure 4). This result distinguishes elephants from other placental mammals for which the relative percentage of bones showing an ossification centre and the order of ossification are known [20–22,41–46]. From the comparison of the percentages of bones that have began to ossify in the cranial and post-cranial skeleton, elephants attain the same percentage of ossification by the end of the first third of the gestation period as the mouse, hamster and other rodents attain in the last (figure 4). This could explain why the prenatal growth of the golden hamster has previously been described as ‘explosive’ [46]; most of the ossification centres appear in an extremely brief time (i.e. a few hours) by the end of the gestation period.
In fact, with the exception of the pubis (which ossifies late in humans, 60.5% of the gestation period, and in sheep 62.5%), ossification seems to progress rapidly once started, whatever the group considered (electronic supplementary material, S6). This reflects the discussion of Palmer [47] who considered that ossification occurs at a nearly constant rate whatever the species and regardless the growth rate. In the elephant, human and cow, most of the ossification centres appear during the second half of the first third of gestation. The initiation of ossification is delayed in sheep compared with elephants; the first bones ossify in sheep (28%) when the last bones ossify in elephants (27%). The last bone to ossify in sheep does so roughly at the same time as in humans (figure 4). This shows that the pattern of ossification in placental mammals is not simply linked to adult size.
Early onset of ossification has already been observed in slow-growing species [48,49] and may in fact be functionally related to gestation time. Interestingly, these results corroborate Bruce's [50] observations on the cranial development of rabbits. He noted that the longer the gestation period, the earlier the centres of ossification appear during gestation. This is also corroborated by the intermediate status of sheep in our dataset. Johnson [41] reached the same conclusion by comparing the percentage of bones showing an ossification centre in the mouse and human. However, these studies did not take phylogenetic non-independence into account, and we cannot discard that the late ossification only characterizes rodents as a whole. An exception to this pattern is evident by comparing humans with cows. Humans start to ossify our skeleton slightly earlier than the cow, despite our shorter gestation period. In mammals, the gestation period seems to be highly correlated with the size of the neonate and the mode of development (i.e. altricial versus precocial, [51]). With the exception of humans, all species of our dataset that show a long gestation period are precocial while human and rodents are altricial. The fact that the skeletons of elephants and humans start to ossify roughly at the same gestational time complicates the interpretation of a link between the timing of ossification and the mode of development. In general, the results of the analysis presented here suggest a partial correspondence between the length of gestation and the timing of ossification; mammals with a long gestation period begin ossification earlier than mammals with a short gestation, but this conclusion remains tentative considering the limited taxon sample. Future studies would benefit from linking the timing of ossification to the gestation period, and not simply to ossification ranks, in order to increase the resolution of the sequences.
Since the nineteenth century, biologists have been fascinated by the morphological peculiarities of the elephant and have made some intriguing observations on their development. Gray [4] noted that ‘they are remarkable […] for the different parts of the animal [are] much more nearly of the proportions of those of the adult than they generally are in foetuses of such a small size’ (p. 491). This developmental study shows that Loxodonta africana exhibits some interesting and unique developmental features in terms of both relative and absolute timing of skeletal ossification. Further investigations using more developmental stages and species are desirable to test the developmental novelties in Loxodonta, the extent to which they characterize other afrotherians, and the correlation between gestation time and ossification onset we have identified in our dataset.
Acknowledgements
We are grateful to M. Herbin, C. Bens, F. Renoult, C. Denys and J. Cuisin (Museum National d'Histoire Naturelle, Paris), Paula Jenkins and Roberto Portela Miguez (Natural History Museum, London), Georges Lenglet (IRSNB) and their colleagues for access to comparative material. A. Heaver (University of Cambridge), R. Abel, L. Howard, R. Garwood (Natural History Museum), F. Landru, C. Morlier, G. Guillemain and all the staff from Viscom SARL (St Ouen l'Aumône, France) provided generous help and advice with acquisition of CT scans. We thank Eudes Thouand for providing living accommodation in Paris. For support of the project as a whole, we acknowledge a research grant from the Leverhulme Trust, UK.
References
- 1.Sukumar R. 2003. The living elephants: evolutionary ecology, behaviour and conservation, p. 478 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Meyer J. M., Walker S., Freeman E. W., Steinetz B. G., Brown J. L. 2004. Species and fetal gender effects on endocrinology of pregnancy in elephants. Gen. Comp. Endocrinol. 138, 263–270 10.1016/j.ygcen.2004.06.010 (doi:10.1016/j.ygcen.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 3.May R. M. 1988. How many species are there on Earth? Science 247, 1441–1449 10.1126/science.241.4872.1441 (doi:10.1126/science.241.4872.1441) [DOI] [PubMed] [Google Scholar]
- 4.Gray J. E. 1868. Notes on the fetus of an elephant and of a hippopotamus in the collection of the British Museum. Proc. Zool. Soc. London 1, 491–492 [Google Scholar]
- 5.Eales N. B. 1925. External characters, skin and temporal gland of a foetal African elephant. Proc. Zool. Soc. Lond. 2, 445–456 [Google Scholar]
- 6.Eales N. B. 1926. The anatomy of the head of a foetal African elephant, Elephas africanus (Loxodonta africana). Trans. R. Soc. Edin. 11, 491–551 [Google Scholar]
- 7.Eales N. B. 1929. The anatomy of a foetal African elephant, Elephas africanus (Loxodonta africana). Part III. The contents of the thorax and abdomen, and the skeleton. Trans. R. Soc. Edin. 56, 203–246 [Google Scholar]
- 8.Allen W. R., Mathias S., Wooding F. B. P., Van Aarde R. J. 2003. Placentation in the African elephant (Loxodonta africana). II. Morphological changes in the uterus and placenta throughout gestation. Placenta 24, 598–617 10.1016/S0143-4004(03)00102-4 (doi:10.1016/S0143-4004(03)00102-4) [DOI] [PubMed] [Google Scholar]
- 9.Allen W. R. 2006. Ovulation, pregnancy, placentation and husbandry in the African elephant (Loxodonta africana). Phil. Trans. R. Soc. B 361, 821–834 10.1098/rstb.2006.1831 (doi:10.1098/rstb.2006.1831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto Y., Yamamoto T., Taya K., Watanabe G., Stansfield F. J., Allen W. R. 2011. Placentation in the African elephant (Loxodonta africana). V. The trophoblast secretes placental lactogen. Placenta 32, 506–510 10.1016/j.placenta.2011.04.012 (doi:10.1016/j.placenta.2011.04.012) [DOI] [PubMed] [Google Scholar]
- 11.Luckett W. P. 1996. Ontogenetic evidence for incisor homologies in proboscideans. In The proboscidea: evolution and palaeocology of elephants and their relatives (eds Shoshani J., Tassy P.), pp. 26–31 Oxford, UK: Oxford University Press [Google Scholar]
- 12.Raubenheimer E. J. 2000. Development of the tush and tusk and tusklessness in African elephant (Loxodonta africana). Koedoe 43, 57–64 [DOI] [PubMed] [Google Scholar]
- 13.Eales N. B. 1931. The development of the mandible in the elephant. Proc. Zool. Soc. Lond. 101, 115–127 10.1111/j.1469-7998.1931.tb06189.x (doi:10.1111/j.1469-7998.1931.tb06189.x) [DOI] [Google Scholar]
- 14.Gaeth A. P., Short R. V., Renfree M. B. 1999. The developing renal, reproductive, and respiratory systems of the African elephant suggest an aquatic ancestry. Proc. Natl Acad. Sci. USA 96, 5555–5558 10.1073/pnas.96.10.5555 (doi:10.1073/pnas.96.10.5555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrandt T., et al. 2007. Foetal age determination and development in elephants. Proc. R. Soc. B 274, 323–331 10.1098/rspb.2006.3738 (doi:10.1098/rspb.2006.3738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith K. K. 2001. Heterochrony revisited: the evolution of developmental sequences. Biol. J. Linn. Soc. 73, 169–186 10.1111/j.1095-8312.2001.tb01355.x (doi:10.1111/j.1095-8312.2001.tb01355.x) [DOI] [Google Scholar]
- 17.Sánchez-Villagra M. R., Goswami A., Weisbecker V., Mock O., Kuratani S. 2008. Conserved relative timing of cranial ossification patterns in early mammalian evolution. Evol. Dev. 10, 519–530 10.1111/j.1525-142X.2008.00267.x (doi:10.1111/j.1525-142X.2008.00267.x) [DOI] [PubMed] [Google Scholar]
- 18.Weisbecker V., Goswami A., Wroe S., Sànchez-Villagra M. R. 2008. Ossification heterochrony in the therian postcranial skeleton and the marsupial-placental dichotomy. Evolution 62, 2027–2041 10.1111/j.1558-5646.2008.00424.x (doi:10.1111/j.1558-5646.2008.00424.x) [DOI] [PubMed] [Google Scholar]
- 19.Hautier L., Weisbecker V., Goswami A., Knight F., Kardjilov N., Asher R. 2011. Skeletal ossification and sequence heterochrony in xenarthran evolution. Evol. Dev. 13, 460–476 10.1111/j.1525-142X.2011.00503.x (doi:10.1111/j.1525-142X.2011.00503.x) [DOI] [PubMed] [Google Scholar]
- 20.Harris H. A. 1937. The foetal growth of the sheep. J. Anat. 71, 516–527 [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay F. E. F. 1969. Observations on the loci of ossification in the prenatal and neonatal bovine skeleton. I. The appendicular skeleton. Br. Vet. J. 125, 101–111 [DOI] [PubMed] [Google Scholar]
- 22.Soana S., Bertoni G., Gnudi G., Botti P. 1996. Osteogenesis of the fetal bovine skull. Anat. Histol. Embryol. 25, 167–173 10.1111/j.1439-0264.1996.tb00078.x (doi:10.1111/j.1439-0264.1996.tb00078.x) [DOI] [PubMed] [Google Scholar]
- 23.Limaye A.2006. Drishti: volume exploration and presentation tool, Poster presentation, Vis Baltimore.
- 24.Mitgutsch C., Wimmer C., Sánchez-Villagra M. R., Hahnloser R., Schneider R. A. 2011. Timing of ossification in duck, quail, zebra finch: intraspecific variation, heterochronies, and life history evolution. Zool. Sci. 28, 491–500 10.2108/zsj.28.491 (doi:10.2108/zsj.28.491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisbecker V. 2011. Monotreme ossification sequences and the riddle of mammalian skeletal development. Evolution 65, 1323–1335 10.1111/j.1558-5646.2011.01234.x (doi:10.1111/j.1558-5646.2011.01234.x) [DOI] [PubMed] [Google Scholar]
- 26.Nunn C. L., Smith K. K. 1998. Statistical analyses of developmental sequences: the craniofacial region in marsupial and placental mammals. Am. Nat. 152, 82–101 10.1086/286151 (doi:10.1086/286151) [DOI] [PubMed] [Google Scholar]
- 27.Keyte A. L., Smith K. K. 2010. Developmental origins of precocial forelimbs in marsupial neonates. Development 137, 4283–4294 10.1242/dev.049445 (doi:10.1242/dev.049445) [DOI] [PubMed] [Google Scholar]
- 28.Jeffery J. E., Bininda-Emonds O. R. P., Coates M. I., Richardson M. K. 2005. A new technique for identifying sequence heterochrony. Syst. Biol. 54, 230–240 10.1080/10635150590923227 (doi:10.1080/10635150590923227) [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Villagra M. R., Müller H., Sheil C. A., Scheyer T. M., Nagashima H., Kuratani S. 2009. Skeletal development in the Chinese soft shelled turtle Pelodiscus sinensis (Testudines : Trionychidae). J. Morphol. 270, 1381–1399 10.1002/jmor.10766 (doi:10.1002/jmor.10766) [DOI] [PubMed] [Google Scholar]
- 30.Harrison L. B., Larsson H. C. E. 2008. Estimating evolution of temporal sequence changes: a practical approach to inferring ancestral developmental sequences and sequence heterochrony. Syst. Biol. 57, 378–387 10.1080/10635150802164421 (doi:10.1080/10635150802164421) [DOI] [PubMed] [Google Scholar]
- 31.Hallström B. M., Kullberg M., Nilsson M. A., Janke A. 2007. Phylogenomic data analyses provide evidence that Xenarthra and Afrotheria are sister groups. Mol. Biol. Evol. 24, 2059–2068 10.1093/molbev/msm136 (doi:10.1093/molbev/msm136) [DOI] [PubMed] [Google Scholar]
- 32.Wildman D. E., et al. 2007. Genomics, biogeography and the diversification of placental mammals. Proc. Natl Acad. Sci. USA 104, 14 395–14 400 10.1073/pnas.0704342104 (doi:10.1073/pnas.0704342104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallström B. M., Janke A. 2010. Mammalian evolution may not be strictly bifurcating. Mol. Biol. Evol. 27, 2804–2816 10.1093/molbev/msq166 (doi:10.1093/molbev/msq166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hautier L., Weisbecker V., Sánchez-Villagra M. R., Goswami A., Asher R. 2010. Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proc. Natl Acad. Sci. USA 107, 18 903–18 908 10.1073/pnas.1010335107 (doi:10.1073/pnas.1010335107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maxwell E. E., Larsson H. C. E., Harrison L. B. 2010. Assessing the phylogenetic utility of sequence heterochrony: evolution of avian ossification sequences as a case study. Zoology 113, 57–66 10.1016/j.zool.2009.06.002 (doi:10.1016/j.zool.2009.06.002) [DOI] [PubMed] [Google Scholar]
- 36.Wilson L. A. B., Schraden C., Mitgutsch C., Galliari F. C., Mess A., Sánchez-Villagra M. R. 2010. Skeletogenesis and sequence heterochrony in rodent evolution, with particular emphasis on the African striped mouse, Rhabdomys pumilio (Mammalia). Org. Div. Evol. 10, 243–258 10.1007/s13127-010-0020-4 (doi:10.1007/s13127-010-0020-4) [DOI] [Google Scholar]
- 37.Sánchez-Villagra M. R. 2002. Comparative patterns of postcranial ontogeny in therian mammals: an analysis of relative timing of ossification events. J. Exp. Zool. Mol. Dev. Evol. 294, 264–273 10.1002/jez.10147 (doi:10.1002/jez.10147) [DOI] [PubMed] [Google Scholar]
- 38.Bininda-Emonds O. R. P., Jeffery J. E., Richardson M. K. 2003. Inverting the hourglass: quantitative evidence against the phylotypic stage in vertebrate development. Proc. R. Soc. Lond. B 270, 341–346 10.1098/rspb.2002.2242 (doi:10.1098/rspb.2002.2242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maxwell E. E., Larsson H. C. E. 2009. Comparative ossification sequence and skeletal development of the postcranium of palaeognathous birds (Aves: Palaeognathae). Zool. J. Lin. Soc. 157, 169–196 10.1111/j.1096-3642.2009.00533.x (doi:10.1111/j.1096-3642.2009.00533.x) [DOI] [Google Scholar]
- 40.Weissengruber G. E., Egger G. F., Hutchinson J. R., Groenewald H. B., Elsässer L., Famini D., Forstenpointner G. 2006. The structure of the cushions in the feet of African elephants (Loxodonta africana). J. Anat. 209, 781–792 10.1111/j.1469-7580.2006.00648.x (doi:10.1111/j.1469-7580.2006.00648.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson M. L. 1933. The time and order of appearance of ossification centers in the albino mouse. Am. J. Anat. 52, 241–271 10.1002/aja.1000520203 (doi:10.1002/aja.1000520203) [DOI] [Google Scholar]
- 42.Mall F. P. 1906. On ossification centers in human embryos less than one hundred days old. Am. J. Anat. 5, 433–458 10.1002/aja.1000050403 (doi:10.1002/aja.1000050403) [DOI] [Google Scholar]
- 43.Beyerlein L., Hillemann H. H., Van Aradel I. W. 1951. Ossification and calcification from postnatal day eight to the adult condition in the golden hamster (Cricetus auratus). Anat. Rec. 111, 49–65 10.1002/ar.1091110104 (doi:10.1002/ar.1091110104) [DOI] [PubMed] [Google Scholar]
- 44.Strong R. M. 1925. The order, time, and rate of ossification of the albino rat (Mus norvegicus albinus) skeleton. Am. J. Anat. 36, 313–355 10.1002/aja.1000360206 (doi:10.1002/aja.1000360206) [DOI] [Google Scholar]
- 45.Yukawa M., Hayashi N., Takagi K., Mochizuki K. 1999. The normal development of the Mongolian gerbil foetuses and, in particular, the timing and sequence of the appearance of ossification centres. Anat. Histol. Embryol. 28, 319–324 10.1046/j.1439-0264.1999.00213.x (doi:10.1046/j.1439-0264.1999.00213.x) [DOI] [PubMed] [Google Scholar]
- 46.Kanazawa E., Mochizuki K. 1974. The time and order of appearance of ossification centers in the hamster before birth. Exp. Anim. 23, 113–122 [DOI] [PubMed] [Google Scholar]
- 47.Palmer A. R. 1981. Do carbonate skeletons limit the rate of body growth. Nature 292, 150–152 10.1038/292150a0 (doi:10.1038/292150a0) [DOI] [Google Scholar]
- 48.Starck J. M. 1994. Quantitative design of the skeleton in bird hatchlings: does tissue compartmentalization limit posthatching growth rates? J. Morphol. 222, 113–131 10.1002/jmor.1052220202 (doi:10.1002/jmor.1052220202) [DOI] [PubMed] [Google Scholar]
- 49.Arendt J. D., Wilson D. S. 2000. Population differences in the onset of cranial ossification in pumpkinseed (Lepomis gibbosus), a potential cost of rapid growth. Can. J. Fish. Aquat. Sci. 57, 351–356 10.1139/f99-250 (doi:10.1139/f99-250) [DOI] [Google Scholar]
- 50.Bruce J. A. 1941. Time and order of appearance of ossification centers and their development in the skull of the rabbit. Am. J. Anat. 68, 41–67 10.1002/aja.1000680103 (doi:10.1002/aja.1000680103) [DOI] [Google Scholar]
- 51.Starck J. M., Ricklefs R. E. 1998. Patterns of development: the altricial-precocial spectrum. In Avian growth and development. Evolution within the altricial precocial spectrum (eds Starck J. M., Ricklefs R. E.), pp. 3–30 New York, NY: Oxford University Press [Google Scholar]