Abstract
Determining how prey learn the identity of predators and match their vigilance with current levels of threat is central to understanding the dynamics of predator–prey systems and the determinants of fitness. Our study explores how feeding history influences the relative importance of olfactory and visual sensory modes of learning, and how the experience gained through these sensory modes influences behaviour and survival in the field for a juvenile coral reef damselfish. We collected young fish immediately prior to their settlement to benthic habitats. In the laboratory, these predator-naïve fish were exposed to a high- or low-food ration and then conditioned to recognize the olfactory cues (odours) and/or visual cues from two common benthic predators. Fish were then allowed to settle on reefs in the field, and their behaviour and survival over 70 h were recorded. Feeding history strongly influenced their willingness to take risks in the natural environment. Conditioning in the laboratory with visual, olfactory or both cues from predators led fish in the field to display risk-averse behaviour compared with fish conditioned with sea water alone. Well-fed fish that were conditioned with visual, chemical or a combination of predator cues survived eight times better over the first 48 h on reefs than those with no experience of benthic predator cues. This experiment highlights the importance of a flexible and rapid mechanism of learning the identity of predators for survival of young fish during the critical life-history transition between pelagic and benthic habitats.
Keywords: learned predator recognition, chemical alarm cue, visual cues, olfactory cues, prey feeding history, coral reef fish
1. Introduction
Many organisms live under the constant threat of predation, but for most of these, the magnitude of threats changes through time. Organisms are most vulnerable when they are small or young, or when they undergo ontogenetic shifts in habitat and encounter a new suite of predators. A lack of familiarity with local predators greatly hinders the assessment of risk, particularly when there is a high diversity of potential predators. For this reason, individuals should develop mechanisms that promote the rapid and efficient identification of novel predators to enhance risk assessment and promote survival. Indeed, only prey that recognize the risk associated with specific predators and locations have the ability to fine-tune their behaviour to optimize foraging and reproductive effort in the face of predation. Understanding how prey learn and modify their behaviour in relation to predators is not only fundamental to the dynamics of predator–prey interactions; it also has repercussions for conservation and management [1,2].
Olfaction and vision are the primary senses used by aquatic prey to assess predation risk. Olfactory cues are thought to be particularly important in aquatic systems because of the limited visibility owing to high topographic complexity, turbidity or low light levels at night. Moreover, many species learn the identity of predators through the coupling of damage-released chemical alarm cues and the odour of predators [3,4]. This information can then be quickly disseminated to local groups by social learning [5].
For a variety of vertebrate and invertebrate prey, the magnitude of antipredator behaviours elicited by a threat is dependent on the feeding history of the individual [6,7]. Hungry individuals, or those in poor body condition, take more risks to gain their next meal compared with individuals that have fed recently [8–10]. Classic examples of this behaviour are shown by ants and sticklebacks that prefer a profitable but more risky food patch when hungry and a less profitable but safe patch when satiated [11,12]. Feeding history also influences memory of a predator encounter [13] and a prey's ability to evade a predatory attack [14].
While an individual's history of risk and feeding is predicted to influence fitness and survival, there are few demonstrations of how the two interact to produce a behavioural response. Prey that have experienced predation threats early in their development may have a different response to risky situations compared with those exposed to predators later in life. Similarly, individuals that learn that an animal represents a threat through one sensory mode (e.g. vision) may respond differently to that threat compared with a prey that has learned the threat through a different sensory mode (e.g. olfaction), or through the stimulation of multiple sensory systems. The degree of the antipredator response elicited by a threat should be commensurate to an individual's relative level of experience as well as its body condition. To date, however, no studies have tried to determine the relative importance of feeding history and visual and/or olfactory experience with predators for survival.
Organisms with complex life cycles, such as many insects, amphibians and fishes, are ideal models for the study of learning as they undergo a series of rapid habitat shifts and multiple life-history stages, each exposed to a different set of predators. Decoupling of adult and juvenile life stages through larval dispersal means that adults are often unable to predict the predatory assemblage that newly settling juveniles will experience. For this reason, innate knowledge, or experiences during the larval phase, will be of limited use in risk assessment in the juvenile habitat. Fishes on coral reefs are particularly good models because they live in a food-limited system [15] and settle with a broad range of body conditions [16] into habitat patches that contain a high diversity of potential predators.
Our study explored how feeding history influenced the relative importance of olfactory and visual sensory modes of learning, and how the experience gained through these sensory modes influenced behaviour and survival in the field of the juvenile damselfish, Pomacentrus wardi. The experiment manipulated the feeding history of predator-naïve juvenile fish, exposed them to visual and/or olfactory cues of two common predators, and then examined the behaviour and survival of these prey fish in the field. Many studies have shown that fishes, and a wide range of other aquatic organisms, can learn danger through the coupling of chemical or visual cues from a predator with cues from a damaged conspecific [4]. Fewer have shown how feeding history influences the propensity to take risk [4]. Ours is the first to examine the interactive effects of learning and diet to determine the real-world consequences for fitness. We highlight the critical importance of experience with a predator in influencing survival in the field at this key early life-history stage, and the interactive role that motivation to feed has in modifying behaviour and risk.
2. Methods
(a). Study species and sampling
Pomacentrus wardi (Pomacentridae) is a site-faithful damselfish that is common on the shallow reefs of the Indo-Pacific. Adults and juveniles occur in shallow lagoons, where they inhabit the reef edge or reef top associated with rubble. Larval duration is 16–21 days, with fish reaching 13–14 mm standard length (SL) at the end of the larval stage [17]. Fish can potentially disperse hundreds of kilometres from their natal location [18], and newly metamorphosed fish settle as solitary individuals into habitats with conspecific adults and sub-adults.
Newly settled P. wardi are subject to an array of resident and transient predators. The most common predators at our study site on the shallow (2–3 m depth) coral reefs of Lizard Island, northern Great Barrier Reef, Australia (14°41′ S, 145°27′ E) are the moon wrasse (Thalassoma lunare) a lizardfish (Synodus dermatogenys [19,20]) and an array of flatfishes (family Pleuronectidae). All predators can be seen striking at and occasionally capturing recently settled and juvenile reef fishes during the summer recruitment period. Studies that have monitored newly settled damselfish that were individually identified through tagging have found high levels of mortality in the first few days after settlement, with high levels of variability among sites located hundreds of metres apart [17,19,21].
During October 2010, light traps (figure 1; see [22] for design) were used to collect P. wardi at the end of their larval phase. Traps were moored at least 100 m away from the reef edge overnight and catches were brought back to the Lizard Island research station just after dawn. Fish were placed into 40 l aquaria with aerated flowing sea water for 24 h (density: approx. 50–100 per 40 l), where they were fed Artemia twice per day. Research over the last two decades suggests that fishes collected in light traps are intercepted as they come into the vicinity of the reef to settle, and most are in the process of metamorphosis to their juvenile form [23–25]. At this stage, they also appear to display active and consistent choices of habitat [26,27], and have little post-settlement movement over the initial juvenile phase [28], suggesting that they do not attempt to return to the plankton.
Figure 1.
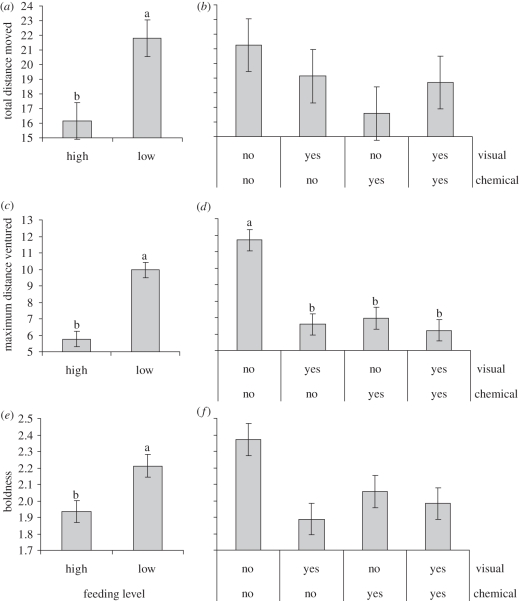
Comparison of behaviour in the field of poorly fed or well-fed Pomacentrus wardi (a,c,e) that had been exposed to the odour (plus a conspecific skin extract; chemical) or the visual presence of two common predator species (visual), or a combination of both (b,d,f). Controls were not pre-exposed to predator cues. Behaviours are (a,b) total distance moved in 3 min, (c,d) maximum distance ventured from the coral patch in 3 min and (e,f) boldness (recorded on a 3-point continuous scale, where 0 is shy and 3 is bold). Error bars are s.e. Letters above the bars represent unequal-sample Tukey's HSD groupings. Number of replicates between feeding levels is 106–107 fish; number of replicates for treatments pooled over feeding levels is 50–52 fish.
Studies of coral reef fishes have found that the pairing of skin extract from prey with a novel predator odour results in an antipredator response in conspecific prey upon exposure to the novel odour alone [29,30]. Fish can also learn the visual identity of a predator by the pairing of a conspecific skin extract with the sight of the predator [4,31].
(b). Experimental protocol
After initial acclimation, fish were randomly transferred into 60 l aquaria (50 fish per tank, four tanks per treatment) and feeding trials were commenced as per Lönnstedt & McCormick [32] (table 1 for protocol summary). Fish were pre-conditioned with one of two feeding treatments for 6–8 days (well- and poorly fed fish, 2500 and 320 Artemia per litre in the rearing tanks three times daily, respectively). An airstone within each tank kept the Artemia in suspension and distributed throughout the tank, so all fish had similar access to food. Prior to being released in the field, fish from both feeding treatments were conditioned to the sight or smell of a predator in a 2 × 2 design, where fish were exposed to (i) a control that received neither visual nor olfactory stimuli from predators (i.e. no visual or chemical learning), (ii) the sight of two key predators (S. dermatogenys and T. lunare placed in a plastic bag within the conditioning aquarium; the predators often attempted to strike at the prey through the bag; visual learning only), (iii) the odour of the two predators together with skin extracts from P. wardi (i.e. an alarm cue; chemical learning only, PO), or (iv) a combination of predator odour (and P. wardi skin extracts) and the presentation of predators (both visual and chemical learning). This resulted in eight treatments in total (two feeding levels × four predator cues). Conditioning was undertaken in 15 l aquaria on random samples of three to five fish per treatment 2 h prior to those fish being released on the reef. Group size was random across treatments based on fish availability on the day of conditioning. Olfactory cues were collected from 30 l tanks of aerated sea water containing each of the two predators that had been fed P. wardi, with no flow-through water for at least 12 h. Chemical alarm cues were collected from the skin extracts of a total of 12 P. wardi for each treatment tank by making six superficial cuts along the flank of each freshly euthanized donor fish with a clean scalpel and rinsing the fish with 60 ml of sea water. Five millilitres of this chemical alarm cue was injected into the conditioning tanks every 5 min for 30 min for both the olfactory and combined visual and olfactory treatments.
Table 1.
Sequence of methods for the field assessment of the influence of feeding history and predatory experience on the survival of Pomacentrus wardi.
| step 1 | 2 | 3 |
4 |
5 | 6 | ||
|---|---|---|---|---|---|---|---|
| collect fish | acclimate | pre-conditioning feeding | predator experience conditioning | field behaviour assessment | monitoring survival (72 h) | ||
| well-fed | poorly fed | olfactory predator cues (yes/no) | visual predator cues (yes/no) | ||||
After the 30 min predator conditioning, fish were placed into a labelled plastic bag containing sea water and photographed against a 1 cm grid for the measurement of body size. Fish were then released onto individual patch reefs (25 × 20 × 15 cm) positioned 3 m from the reef edge (2 m apart) within 2 h of conditioning. Patch reefs were composed of a combination of live and dead Pocillopora damicornis, a bushy hard coral. A fine mesh cage was placed over the patch reef for 40–60 min to prevent predation during acclimation to their new habitat.
(c). Behavioural assessment
Following acclimation, the behaviour of fish was quantified for 3 min following previously developed protocols [17,28,33,34]. Briefly, the behaviour of each fish was assessed by a scuba diver positioned approximately 1 m away from the patch. A magnifying glass (4×) aided the assessment of bite rates and space use over the 3 min focal animal sampling period for each fish. Four aspects of activity and behaviour were assessed: (i) bite rate; (ii) total distance moved (cm); (iii) maximum distance ventured from the habitat patch (cm); and (iv) boldness (recorded as a variable on a continuous scale from 0 to 3, where 0 was hiding in hole and seldom emerging; 1 was retreating to a hole when scared and taking more than 5 s to re-emerge, weakly or tentatively striking at food; 2 was shying to shelter when scared but quickly emerging, with purposeful strikes at food; and 3 was not hiding when scared, exploring around the coral patch and striking aggressively at food). At the end of the 3 min observation period, the fish were approached with a pencil, and the fish's reaction and latency to emerge from shelter was taken into account in the assessment of boldness. This measure of boldness has been found to be repeatable between observers, consistent in the short term for newly settled damselfish and related to survival in the field ([17]; M. I. McCormick 2009, unpublished data). The number of replicate fish for each of the 8 factor combinations (2 food levels × 2 visual levels × 2 olfactory levels) ranged from 23 to 27 depending upon availability of fish in light trap catches.
(d). Survival
Fish were released onto the reef between 10.00 and 12.00 hrs. Survival was monitored twice a day for 70 to 96 h after release. Previous tagging studies suggest that migration between patches or to the main reef is negligible [17,21,28]. In this study, there were no instances where two fish were found on a single patch reef.
(e). Statistical analyses
The standard lengths of fish from the two feeding levels (high and low) were compared using an independent-sample t-test. A three-factor MANOVA tested whether the behaviour of fish differed between the two feeding levels (well-fed versus poorly fed), whether fish had experienced visual (visual predator, or none) or chemical information (predator odour + conspecific skin extract, or sea water), or whether behaviour was affected by the interaction between the three factors. The variables included in the analysis were bite rate, boldness, total distance moved and maximum distance ventured. The last variable was log10(x + 1) transformed to meet assumptions of normality. Three-factor ANOVAs (type III sums of squares) were employed to examine the nature of the significant difference found by MANOVA. Significant effects in ANOVAs were further explored using unequal-sample Tukey's HSD tests. A Bonferroni-corrected significance level of 0.0125 was used to account for the possible error inflation caused by non-independent variables, and univariate analyses were interpreted in relation to this more conservative alpha level. To further describe how boldness was affected by feeding history and experience with predators, and to examine the potential mechanism underlying the patterns found in mortality (below), two planned comparisons were used: the first examined whether the poorly fed predator-odour-exposed fish differed from the sea water controls (high- and low-fed); the second, whether these three means (pooled) differed from the remaining five treatments.
Multi-sample survival analysis using a Cox's proportional hazard model compared the survival of fish in the eight treatments through the 3–4-day census period. In total, there were 213 valid observations, involving 66 censored and 147 complete observations. A Kaplan–Meier survival plot was used to illustrate mortality trajectories. Two further survival analyses were used to determine the nature of the significant difference found among treatments by the first analysis. These determined whether there was any difference in survival among the treatments that fell into two groups that were evident from the Kaplan–Meier plot. The software Statistica v. 9.0 was used for all analyses.
3. Results
Fish used in the field trials from the well-fed treatment were slightly larger than fish from the poorly fed treatment (13.9 mm SL ± 0.05 s.e. and 13.7 mm SL ± 0.05 s.e., respectively; t0.05,211 = 3.917, p = 0.0001). There were no significant correlations between standard length of fish and any behavioural variable for either well-fed or poorly fed fish (Pearson's correlations, p > 0.05, n = 102).
There was a strong influence of prior feeding history on the behaviour of P. wardi on isolated patch reefs in the field (Pillai's trace: F4,194 = 0.173, p < 0.0001). ANOVA on the four variables showed that there were significant differences in total distance moved in 3 min, maximum distance ventured and boldness between well-fed and poorly fed fish (p < 0.004; figure 1a,c,e). Poorly fed fish were more active, ventured further from shelter and were bolder than well-fed fish. While there was a trend towards poorly fed fish having a higher bite rate, this was not significant at the adjusted alpha level (F1,198 = 4.10, p = 0.044). There were no significant interactions between food levels and exposure to chemical or visual information either in the overall analysis of behaviour (MANOVA, p ≥ 0.28) or in the univariate analyses on individual variables (p ≥ 0.07). Despite this overall non-significant three-way interaction, univariate contrasts on boldness indicated that poorly fed fish exposed to predator odour displayed a high level of boldness similar to the sea water controls, regardless of prior feeding history (contrasts between sea water controls versus poorly fed predator odour: F1,196 = 0.088, p = 0.768), and that these three treatments exhibited higher boldness than the five remaining treatments (F1,196 = 20.516, p < 0.0001; electronic supplementary material, figure S1).
There was an interaction between the effects of chemical and visual cues on fish behaviour (Pillai's trace: F4,194 = 0.075, p = 0.004; figure 1b,d,f). This was driven by a strong interaction in maximum distance ventured (F1,197 = 11.597, p = 0.0008) that was caused by the sea water control differing from all three other treatment levels, which did not differ from each other (figure 1d). This suggested that pre-exposure to visual or olfactory cues of key predators cause prey fish to act conservatively once in the field, although one source of information did not appear to outweigh the importance of the other. The same trend was displayed in boldness, with fish pre-exposed to sea water (i.e. controls) being the boldest and fish exposed to chemical, visual or a combination of cues being equally shy; however, this was not significant at the adjusted alpha level (F1,196 = 4.540, p = 0.034; figure 1e). There was no suggestion that chemical and visual cues had additive effects on behaviour (figure 1).
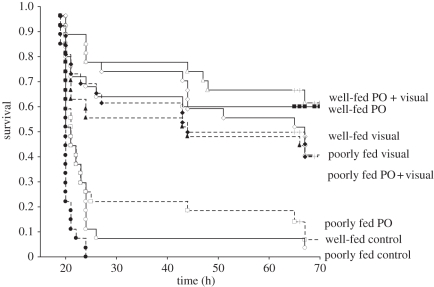
Survival of young fish was affected by predator experience and feeding history (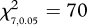 , p < 0.0001; figure 2). Patterns of survival among treatments were established within the first 24 h from release. Initial survival analyses found that two groupings of treatments and further survival analyses did not find any significant differences among treatments within these two groups (p > 0.05). A group of three treatments had the lowest and similar survival, and comprised both sea water controls (i.e. either well-fed or poorly fed fish that had received no predator conditioning) and the poorly fed–predator-odour treatment (figure 2). The second group contained the remaining five treatments. These analyses suggested that feeding history and predator experience interacted to influence survival of young fish.
, p < 0.0001; figure 2). Patterns of survival among treatments were established within the first 24 h from release. Initial survival analyses found that two groupings of treatments and further survival analyses did not find any significant differences among treatments within these two groups (p > 0.05). A group of three treatments had the lowest and similar survival, and comprised both sea water controls (i.e. either well-fed or poorly fed fish that had received no predator conditioning) and the poorly fed–predator-odour treatment (figure 2). The second group contained the remaining five treatments. These analyses suggested that feeding history and predator experience interacted to influence survival of young fish.
Figure 2.
Survival curves (Kaplan–Meier plot) of poorly fed or well-fed Pomacentrus wardi that had been exposed to the odour (plus a conspecific skin extract; PO) or the visual presence (visual) of two common predator species, or a combination of both. Controls were not pre-exposed to predator cues (control). Fish were placed on small patch reefs along the edge of a shallow reef and survivorship monitored over 3–4 days. The time variable represents hours from release (12.00–14.00 hrs). n = 25–27.
4. Discussion
Experience with a predator threat, whether olfactory or visual, was found to increase the likelihood of surviving in the natural environment. As predicted, feeding history influenced the likelihood that fish would take risks, especially when they had no visual experience of common predators in the local area. The mechanism underlying survivorship appeared to be behavioural; experience and a better history of feeding reduced the propensity to take risk, as shown by reduced activity levels and reluctance to venture far from shelter. While other studies have shown that exposure to chemical cues of predators coupled with chemicals released from damaged conspecifics leads to risk-averse behaviour by prey, this is the first study to demonstrate that both olfactory and visual cues can play an equally important role in affecting survival of prey in the field.
Our study expands the understanding of the significance of feeding history and experience for the survival of fish at the critical life-history transition between pelagic and benthic habitats. Fish that had experience with the two common predators, whether through olfactory cues or visual presence, exhibited less risky behaviours when in the field than fish without this experience. Earlier work has shown that the survival of another tropical damselfish, Pomacentrus amboinensis, in size-matched trials was influenced by experience with two common predators [35]. However, the relative importance of experience from visual, olfactory or mechanical (e.g. vibration or impact) cues could not be determined. Moreover, Holmes & McCormick [19] found that only experience influenced survival at the one of two study sites where mortality (and selection) was highest. Combined with our results, such observations stress the importance of having fast and efficient ways of learning the identity of predators, and the context-specific nature of this information.
Laboratory studies have found that chemical information about the identity of predators may increase the survival of prey in predator encounters. Experiments with northern pike (Esox lucius) have shown that conditioning with a skin extract from conspecifics and predator odour increased the survival time of juvenile rainbow trout (Oncorhynchus mykiss) when placed together with the pike predator in an aquarium [36]. Zhao et al. [37] found that goldfish (Carassius auratus) responded in a threat-sensitive way to varying concentrations of odour from a pike that had previously consumed goldfish. Those that had been exposed to high concentrations of the odour prior to interaction with predators in staged interactions within a tank had higher survival than those fish exposed to lower concentrations.
Our study strongly suggests that associative learning was the mechanism underlying the survival benefit of the olfactory treatment, although this result must be treated with caution, because logistical constraints prevented the implementation of all possible treatment combinations in our study that would have conclusively demonstrated the survival benefits of associative learning of predator smells. An increase in antipredator behaviour after exposure to skin extracts from a damaged conspecific is an innate response common to many freshwater and marine fishes, as well as invertebrates [3,29,38,39]. The fish used in our field experiment were the sole occupants of patch reefs and could only have been potentially exposed to dilute alarm cues from predatory activity within the general area. Moreover, laboratory experiments have shown that prey do not increase their antipredator behaviour in response to the odour of unknown predators [30]. Thus, the important difference between the sea water control and olfactory treatment in our study was the co-occurrence of the alarm cue with odours from key predators prior to release of fish in the treatment. Although further experiments are required, our study strongly suggests that cataloguing predators through smell benefits survival of young fish at a key life-history stage.
Visual presence of a predator in the absence of damage-released chemicals from prey also enhanced survival of young fish by making their behaviours more risk-averse. In nature, this may occur through social learning, whereby one individual learns a behavioural response to a predator through watching the antipredator behaviour of another and mimicking that behaviour in subsequent independent encounters with the predator. Although there are as yet few marine examples, social learning has been shown to occur in a diverse range of animals, from tammar wallabies (Macropus eugenii [40]) to freshwater fishes [5] and insects [41]. The ability to associate an image with danger may also occur through the co-occurrence of damage-released chemicals with the visual presence of the animal [8], or through direct experience with a predator strike or unsuccessful capture attempt [35]. Mechanisms to learn the identity of potential predators that do not involve olfactory cues should be expected to evolve in most aquatic systems because, simply due to chance, 50 per cent of all first encounters by prey with novel predators will occur from a down-current direction, where chemical cues will not be available to prey.
Prey conditioned with either visual or olfactory predator cues in isolation displayed levels of risk-taking behaviour in the field that did not differ from prey that had been conditioned with olfactory and visual cues in combination. This similarity between the effects of visual and olfactory experience may be because predators seldom occurred close to the experimental reefs when divers were in the vicinity for behavioural observations, so visual cues of predators for the focal damselfish would have been minimal. The risk-averse behaviour of visually experienced damselfish suggests that conditioning led to behaviour that was inherently conservative. This appears counterintuitive because it would lead to reduced feeding opportunities, access to a smaller variety of food items and a lower energy intake. A possible reason for this finding is that, at this vulnerable life stage, experienced prey generalize any visual presence with danger, and the human observer was therefore seen as a threat. Regardless of the underlying reason, our study suggests that visual experience with two key predators enhanced survival of young fish.
Feeding history had a dramatic influence on behaviour and survival in a predator-rich environment. We showed that fish from the well-fed treatment were more risk-averse in their behaviours than poorly fed fish, a result that parallels a recent finding that naïve recruit fish that were in lower body condition exhibited riskier behaviour in the laboratory than fish in good body condition [32]. In our study, poorly fed fish that had just been exposed to the olfactory cues of predators, coupled with damage-released skin extracts of conspecifics, were bolder and had a lower survival than those exposed to visual cues or a combination of visual and chemical experience. This reinforces the idea, suggested by others [42,43], that olfactory cues forewarn fish of potential danger and this reaction can be moderated by visual information, which acts as a more direct indication of impending threat. Interestingly, it was experience with a predator (regardless of the cue) rather than feeding history that dominated the differences in survival among groups, suggesting that mortality is not simply explained by behavioural vulnerability alone.
Mortality of newly settled coral reef fishes tends to be size-selective, with positive and negative selection for a particular size range dependent upon predator selection profiles in the vicinity of the settlement site [19,30,44]. However, in our study, size differences between well-fed and poorly fed treatments were very small (0.2 mm for an approx. 14 mm SL fish), and much less than the size differences on which selection has typically acted in field experiments. Various aspects of body morphology and body condition covary with feeding history [45], and have been shown to be selected by predators. For instance, Holmes & McCormick [46] found selection by a common predator, Pseudochromis fuscus, acted on variation in body weight of a damselfish of a standardized size. Moreover, Gagliano et al. [47] found that there were marked carry-over effects associated with previous growth history that influenced survival later in life, but the present-day traits under actual selection (which covaried with previous growth history) could not be identified [17]. In our study, it appears that size plays a more minor role than experience in influencing survival, at least during the first few days after settlement.
Early experience of a predation threat appears to be crucial in determining the survival of juveniles during the transition between pelagic and settled life stages. Although feeding history influenced space use and activity, it did not appear to affect survival as much as whether individuals had been forewarned of the identity of common predators or not. Many fishes undergo ontogenetic habitat shifts; for example, juveniles moving from nursery grounds to adult habitats [48–50]. Retention of predator learning and recognition mechanisms, such as visual and olfactory labelling [51], will be critical for the rapid cataloguing of novel predators. Given the commonality of the bipartite life cycle in reef fishes, it is likely that our findings of the importance of predator recognition systems are general to all coral reef fishes. Indeed, previous laboratory studies suggest that it is a mechanism that occurs among diverse taxa [4]. With increasing pressures on coral reefs from harvest [52], some species are being restocked onto reefs in the hope of supplementing depleted populations [53,54]. Teaching fishes key predators prior to juvenile release may greatly enhance the early survival of stocked fish and improve stocking efficacy.
Acknowledgements
This research was funded by the ARC Centre of Excellence for Coral Reef Studies and undertaken under JCU animal ethics guidelines.
References
- 1.Mirza R., Chivers D. 2000. Predator-recognition training enhances survival of brook trout: evidence from laboratory and field-enclosure studies. Can. J. Zool. 78, 2198–2208 10.1139/z00-164 (doi:10.1139/z00-164) [DOI] [Google Scholar]
- 2.Bischof R., Zedrosser A. 2009. The educated prey: consequences for exploitation and control. Behav. Ecol. 20, 1228–1235 10.1093/beheco/arp124 (doi:10.1093/beheco/arp124) [DOI] [Google Scholar]
- 3.Chivers D. P., Smith R. J. F. 1998. Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5, 338–352 [Google Scholar]
- 4.Ferrari M. C. O., Wisenden B. D., Chivers D. P. 2010. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. 88, 698–724 10.1139/Z10-029 (doi:10.1139/Z10-029) [DOI] [Google Scholar]
- 5.Brown C., Laland K. N. 2003. Social learning in fishes: a review. Fish Fish. 4, 280–288 10.1046/j.1467-2979.2003.00122.x (doi:10.1046/j.1467-2979.2003.00122.x) [DOI] [Google Scholar]
- 6.Lima S. L., Dill L. M. 1990. Behavioural decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 (doi:10.1139/z90-092) [DOI] [Google Scholar]
- 7.Lima S. L. 1998. Stress and decision making under the risk of predation: recent developments from behavioural, reproductive and ecological perspectives. Adv. Study Behav. 27, 215–290 10.1016/S0065-3454(08)60366-6 (doi:10.1016/S0065-3454(08)60366-6) [DOI] [Google Scholar]
- 8.Brown G. E., Smith R. J. F. 1996. Foraging trade-offs in fathead minnows (Pimephales promelas, Osteichthyes, Cyprinidae): acquired predator recognition in the absence of an alarm response. Ethology 102, 776–785 10.1111/j.1439-0310.1996.tb01166.x (doi:10.1111/j.1439-0310.1996.tb01166.x) [DOI] [Google Scholar]
- 9.Chivers D. P., Puttlitz M. H., Blaustein A. R. 2000. Chemical alarm signaling by reticulate sculpins, Cottus perplexus. Environ. Biol. Fish. 57, 347–352 10.1023/A:1007616212592 (doi:10.1023/A:1007616212592) [DOI] [Google Scholar]
- 10.Krause E. T., Steinfartz S., Caspers B. A. 2011. Poor nutritional conditions during the early larval stage reduce risk-taking activities of fire salamander larvae (Salamandra salamandra). Ethology 117, 416–421 10.1111/j.1439-0310.2011.01886.x (doi:10.1111/j.1439-0310.2011.01886.x) [DOI] [Google Scholar]
- 11.Heller R., Milinski M. 1979. Optimal foraging of sticklebacks on swarming prey. Anim. Behav. 27, 1127–1141 10.1016/0003-3472(79)90061-7 (doi:10.1016/0003-3472(79)90061-7) [DOI] [Google Scholar]
- 12.Nonacs P., Dill L. M. 1990. Mortality risk versus food quality trade-offs in a common currency: patch preferences in ants. Ecology 71, 1886–1892 10.2307/1937596 (doi:10.2307/1937596) [DOI] [Google Scholar]
- 13.Brown G. E., Ferrari M. C. O., Malka P. H., Oligny M., Romano M., Chivers D. P. 2011. Growth rate and retention of learned predator cues by juvenile rainbow trout: faster-growing fish forget sooner. Behav. Ecol. Sociobiol. 65, 1267–1276 10.1007/s00265-011-1140-3 (doi:10.1007/s00265-011-1140-3) [DOI] [Google Scholar]
- 14.McCormick M. I., Molony B. W. 1993. Quality of the reef fish Upeneus tragula (Mullidae) at settlement: is size a good indicator of condition? Mar. Ecol. Prog. Ser. 98, 45–54 10.3354/meps098045 (doi:10.3354/meps098045) [DOI] [Google Scholar]
- 15.Jones G. P., McCormick M. I. 2002. Numerical and energetic processes in the ecology of coral reef fishes. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P. F.), pp. 221–238 London, UK: Academic Press [Google Scholar]
- 16.Hoey A., McCormick M. I. 2004. Selective predation for low body condition at the larval–juvenile transition of a coral reef fish. Oecologia 139, 23–29 10.1007/s00442-004-1489-3 (doi:10.1007/s00442-004-1489-3) [DOI] [PubMed] [Google Scholar]
- 17.McCormick M. I., Meekan M. G. 2010. The importance of attitude: the influence of behaviour on survival at an ontogenetic boundary. Mar. Ecol. Prog. Ser. 407, 173–185 10.3354/meps08583 (doi:10.3354/meps08583) [DOI] [Google Scholar]
- 18.Stobutzki I. C., Bellwood D. R. 1997. Sustained swimming abilities of the late pelagic stages of coral reef fishes. Mar. Ecol. Prog. Ser. 149, 35–41 10.3354/meps149035 (doi:10.3354/meps149035) [DOI] [Google Scholar]
- 19.Holmes T. H., McCormick M. I. 2006. Location influences size-selective predation on newly-settled reef fish. Mar. Ecol. Prog. Ser. 317, 203–209 10.3354/meps317203 (doi:10.3354/meps317203) [DOI] [Google Scholar]
- 20.Holmes T., McCormick M. I. 2010. Size-selectivity of predatory reef fish on juvenile prey. Mar. Ecol. Prog. Ser. 399, 273–283 10.3354/meps08337 (doi:10.3354/meps08337) [DOI] [Google Scholar]
- 21.McCormick M. I., Hoey A. S. 2004. Larval growth history determines juvenile growth and survival in a tropical marine fish. Oikos 106, 225–242 10.1111/j.0030-1299.2004.13131.x (doi:10.1111/j.0030-1299.2004.13131.x) [DOI] [Google Scholar]
- 22.Meekan M. G., Wilson S. G., Halford A., Retzel A. 2001. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar. Biol. 139, 373–381 10.1007/s002270100577 (doi:10.1007/s002270100577) [DOI] [Google Scholar]
- 23.Milicich M. J., Meekan M. G., Doherty P. J. 1992. Larval supply: a good predictor of the recruitment of three species of reef fish (Pomacentridae). Mar. Ecol. Prog. Ser. 86, 153–166 10.3354/meps086153 (doi:10.3354/meps086153) [DOI] [Google Scholar]
- 24.Wilson D. T., McCormick M. I. 1997. Spatial and temporal validation of settlement-marks in the otoliths of tropical reef fishes. Mar. Ecol. Prog. Ser. 153, 259–271 10.3354/meps153259 (doi:10.3354/meps153259) [DOI] [Google Scholar]
- 25.McCormick M. I., Makey L., Dufour V. 2002. Comparative study of metamorphosis in tropical reef fishes. Mar. Biol. 141, 841–853 10.1007/s00227-002-0883-9 (doi:10.1007/s00227-002-0883-9) [DOI] [Google Scholar]
- 26.Öhman M. C., Munday P. L., Jones G. P., Caley M. J. 1998. Settlement strategies and distribution patterns of coral-reef fishes. J. Exp. Mar. Biol. Ecol. 225, 219–238 10.1016/S0022-0981(97)00224-4 (doi:10.1016/S0022-0981(97)00224-4) [DOI] [Google Scholar]
- 27.McCormick M. I., Moore J. A. Y., Munday P. L. 2010. Influence of habitat degradation on fish replenishment. Coral Reefs 29, 537–546 10.1007/s00338-010-0620-7 (doi:10.1007/s00338-010-0620-7) [DOI] [Google Scholar]
- 28.McCormick M. I. 2009. Behaviourally mediated phenotypic selection in a disturbed coral reef environment. PLoS ONE 4, e7096. 10.1371/journal.pone.0007096 (doi:10.1371/journal.pone.0007096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson J. K., McCormick M. I. 2005. The role of chemical alarm signals in facilitating learned recognition of novel chemical cues in a coral reef fish. Anim. Behav. 69, 51–57 10.1016/j.anbehav.2004.04.005 (doi:10.1016/j.anbehav.2004.04.005) [DOI] [Google Scholar]
- 30.Holmes T., McCormick M. I. 2010. Smell, learn and live: the role of chemical alarm cues in predator learning during early life history in a marine fish. Behav. Process. 83, 299–305 10.1016/j.beproc.2010.01.013 (doi:10.1016/j.beproc.2010.01.013) [DOI] [PubMed] [Google Scholar]
- 31.Chivers D. P., Smith R. J. F. 1994. Fathead minnows (Pimephales promelas) acquire predator recognition when alarm substance is associated with the sight of an unfamiliar fish. Anim. Behav. 48, 597–605 10.1006/anbe.1994.1279 (doi:10.1006/anbe.1994.1279) [DOI] [Google Scholar]
- 32.Lönnstedt O. M., McCormick M. I. 2011. Growth history and intrinsic factors influence risk assessment at a critical life transition for a fish. Coral Reefs 30, 805–812 10.1007/s00338-011-0768-9 (doi:10.1007/s00338-011-0768-9) [DOI] [Google Scholar]
- 33.Fuiman L. A., Meekan M. G., McCormick M. I. 2010. Maladaptive behavior reinforces a recruitment bottleneck in newly settled reef fishes. Oecologia 164, 99–108 10.1007/s00442-010-1712-3 (doi:10.1007/s00442-010-1712-3) [DOI] [PubMed] [Google Scholar]
- 34.Meekan M. G., von Kuerthy C., McCormick M. I., Radford B. 2010. Behavioural mediation of the costs and benefits of fast growth in a marine fish. Anim. Behav. 79, 803–809 10.1016/j.anbehav.2009.12.002 (doi:10.1016/j.anbehav.2009.12.002) [DOI] [Google Scholar]
- 35.McCormick M. I., Holmes T. H. 2006. Prey experience of predation influences mortality rates at settlement in a coral reef fish, Pomacentrus amboinensis. J. Fish Biol. 68, 969–974 10.1111/j.0022-1112.2006.00982.x (doi:10.1111/j.0022-1112.2006.00982.x) [DOI] [Google Scholar]
- 36.Mirza R. S., Chivers D. P. 2003. Response of juvenile rainbow trout to varying concentrations of chemical alarm cue: response thresholds and survival during encounters with predators. Can. J. Zool. 81, 88–95 10.1139/Z02-216 (doi:10.1139/Z02-216) [DOI] [Google Scholar]
- 37.Zhao X., Ferrari M. C. O., Chivers D. P. 2006. Threat-sensitive learning of predator odours by a prey fish. Behaviour 143, 1103–1121 10.1163/156853906778607408 (doi:10.1163/156853906778607408) [DOI] [Google Scholar]
- 38.Ferrari M. C. O., Lysak K. R., Chivers D. P. 2010. Turbidity as an ecological constraint on learned predator recognition and generalization in a prey fish. Anim. Behav. 79, 515–519 10.1016/j.anbehav.2009.12.006 (doi:10.1016/j.anbehav.2009.12.006) [DOI] [Google Scholar]
- 39.Ferrari M. C. O., Dixson D. L., Munday P. L., McCormick M. I., Meekan M. G., Sih A., Chivers D. P. 2011. Intrageneric variation in tolerance of coral reef fishes to ocean acidification: implications for climate change projections on marine communities. Glob. Change Biol. 17, 2980–2986 10.1111/j.1365-2486.2011.02439.x (doi:10.1111/j.1365-2486.2011.02439.x) [DOI] [Google Scholar]
- 40.Griffin A. S., Evans C. S. 2003. Social learning of antipredator behaviour in a marsupial. Anim. Behav. 66, 485–492 10.1006/anbe.2003.2207 (doi:10.1006/anbe.2003.2207) [DOI] [Google Scholar]
- 41.Leadbeater E., Chittka L. 2006. Social learning in insects: from miniature brains to consensus building. Curr. Biol. 17, R703–R713 10.1016/j.cub.2007.06.012 (doi:10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- 42.Chivers D. P., Mirza R. S., Bryer P. J., Kiesecker J. M. 2001. Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can. J. Zool. 79, 867–873 10.1139/z01-049 (doi:10.1139/z01-049) [DOI] [Google Scholar]
- 43.Holmes T., McCormick M. I. 2011. Response across a gradient: behavioural reactions of newly settled fish to predation cues. Anim. Behav. 81, 543–550 10.1016/j.anbehav.2010.11.019 (doi:10.1016/j.anbehav.2010.11.019) [DOI] [Google Scholar]
- 44.McCormick M. I., Meekan M. G. 2007. Social facilitation of selective mortality. Ecology 88, 1562–1570 10.1890/06-0830 (doi:10.1890/06-0830) [DOI] [PubMed] [Google Scholar]
- 45.McCormick M. I., Molony B. W. 1992. Effects of feeding history on the growth characteristics of a reef fish at settlement. Mar. Biol. 114, 165–173 10.1007/BF00350866 (doi:10.1007/BF00350866) [DOI] [Google Scholar]
- 46.Holmes T. H., McCormick M. I. 2009. Influence of prey body characteristics and performance on predator selection. Oecologia 159, 401–413 10.1007/s00442-008-1220-x (doi:10.1007/s00442-008-1220-x) [DOI] [PubMed] [Google Scholar]
- 47.Gagliano M., McCormick M. I., Meekan M. G. 2007. Survival against the odds: ontogenetic changes in selective pressure mediate growth-mortality trade-offs in a marine fish. Proc. R. Soc. B 274, 1575–1582 10.1098/rspb.2007.0242 (doi:10.1098/rspb.2007.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helfman G. S., Meyer J. L., McFarland W. N. 1982. The ontogeny of twilight migration patterns in grunts (Pisces: Haemulidae). Anim. Behav. 30, 317–326 10.1016/S0003-3472(82)80042-0 (doi:10.1016/S0003-3472(82)80042-0) [DOI] [Google Scholar]
- 49.Werner E. E., Gilliam J. F. 1984. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 15, 393–425 10.1146/annurev.es.15.110184.002141 (doi:10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- 50.Sheaves M. 1995. Large lutjanid and serranid fishes in tropical estuaries: are they adults or juveniles? Mar. Ecol. Prog. Ser. 129, 31–40 10.3354/meps129031 (doi:10.3354/meps129031) [DOI] [Google Scholar]
- 51.Mitchell M. D., McCormick M. I., Ferrari M. C. O., Chivers D. P. 2011. Friend or foe? The role of latent inhibition in predator and non-predator labelling by coral reef fishes. Anim. Cogn. 14, 707–714 10.1007/s10071-011-0405-6 (doi:10.1007/s10071-011-0405-6) [DOI] [PubMed] [Google Scholar]
- 52.Hughes T. P., et al. 2007. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 17, 360–365 10.1016/j.cub.2006.12.049 (doi:10.1016/j.cub.2006.12.049) [DOI] [PubMed] [Google Scholar]
- 53.Bell J. D., Clua E., Hair C. A., Galzin R., Doherty P. J. 2009. The capture and culture of post-larval fish and invertebrates for the marine ornamental trade. Rev. Fish. Sci. 17, 223–240 10.1080/10641260802528541 (doi:10.1080/10641260802528541) [DOI] [Google Scholar]
- 54.Heenan A., Simpson S. D., Meekan M. G., Healy S. D., Braithwaite V. A. 2009. Restoring depleted coral-reef fish populations through recruitment enhancement: a proof of concept. J. Fish Biol. 75, 1857–1867 10.1111/j.1095-8649.2009.02401.x (doi:10.1111/j.1095-8649.2009.02401.x) [DOI] [PubMed] [Google Scholar]