Abstract
The macroscopic, three-dimensional surface layout geometry of an enclosure apparently provides a different contribution for spatial reorientation than the geometric cues associated with freestanding objects arranged in arrays with similar geometric shape. Here, we showed that a unitary spatial representation can account for the capability of animals to reorient both by extended surfaces and discrete objects in a small-scale spatial task. We trained domestic chicks to locate a food-reward from an opening on isolated cylinders arranged either in a geometrically uninformative (square-shaped) or informative (rectangular-shaped) arrays. The arrays were located centrally within a rectangular-shaped enclosure. Chicks trained to access the reward from a fixed position of openings proved able to reorient according to the geometric cues specified by the shape of the enclosure in all conditions. Chicks trained in a fixed position of opening with geometric cues provided both by the arena and the array proved able to reorient according to each shape separately. However, chicks trained to access the reward from a variable position of openings failed to reorient. The results suggest that the physical constrains associated with the presence of obstacles in a scene, rather than their apparent visual extension, are crucial for spatial reorientation.
Keywords: spatial reorientation, geometric module, view-based navigation, environmental axis, array of freestanding objects
1. Introduction
A large body of literature based on small-scale laboratory studies has shown that the macroscopic, three-dimensional surface layout of an enclosed space might provide a different and more effective contribution for spatial reorientation than that provided by freestanding objects arranged in an array of a shape similar to that of the enclosed space. In a seminal study by Ken Cheng [1], rats trained in a working memory task to locate a food-reward in a rectangular arena with distinctive coloured panels on its perimeter walls failed to discriminate the correct site from its geometrically equivalent site after disorientation, despite the fact that the panels provided unambiguous cues to reorient. These results have been interpreted according to the hypothesis of central modularity in the spatial domain, according to which vertebrate species would rely on a mental representation of the environmental geometry (metric and sense) for visuo-spatial reorientation [1–6].
Although vertebrate species reorient efficiently according to geometric cues provided by the shape of an arena, both humans [7–12] (but see [13]) and non-human animals [14–16] usually fail to reorient according to geometric cues provided by freestanding objects in an array of a similar geometric shape. It has been hypothesized that the macroscopic, three-dimensional surface layout of an environment geometry may have a primacy for visuo-spatial reorientation over other sources of geometric cues, including those provided by discrete objects in arrays, because the former cues are distinctive, stable and enduring over time in a natural environment [2,10].
According to the hypothesis, the analysis of the three-dimensional surface layout geometry would occur incidentally through an obligatory process [17], largely impervious to non-geometric information [3] and available at birth without any specific training experience [18–22]. Animals trained to search for a reward at locations marked with a local beacon, for instance in the vicinity of a coloured card at one of the corners of a rectangular enclosure [3,5] or at the centre of a symmetrical enclosure [23], retrieve the position of the reward even after removal of the beacon, thus showing that they have incidentally encoded the geometric shape of the enclosure (see also [24], but see [25]).
Environmental geometry would be represented in geocentric coordinates [26] through the activation of specific neuronal circuitries, hippocampal- and parahippocampal-dependent (mammals [27–29]; birds [30,31]; fish [32]). By contrast, freestanding objects arranged in arrays would be used as local beacons within a frame of reference defined by global geometric information provided by extended surfaces. Effective use of freestanding objects as landmarks would require extensive training experience and the activation of a separated neuronal circuitry. Functional magnetic resonance imaging experiments have shown that humans trained in a virtual reality maze to locate a hidden object relative either to an intramaze landmark or distal cues in the scene showed an increased activation, respectively, of the dorsal striatum and the hippocampus [33]. Comparative research in rodents has shown that freestanding objects in arrays fail to control the place cells location field in the rats’ hippocampus [34].
Interestingly, however, two recent studies have shown that both domestic chicks [35] and homing pigeons [36] can reorient according to geometric cues in a rectangular array of identical cylinders located centrally within a circular arena, provided that birds are tested within a stable panoramic view of the surrounding at reward sites. This was obtained by training the birds to have access to the reward from an opening on the cylinders. Although the cylinders maintained stable geometric relationships in the array, the position of the opening could be experimentally manipulated by rotating the cylinders on-site, so as to force the birds to perceive the configuration from a particular perspective at the time of the choice. The results showed that birds trained with a stable position of opening on cylinders, and therefore exposed to a stable view of the surrounding at reward, successfully learned the task. By contrast, birds trained by changing the position of openings between the training sessions failed to reorient. The results have been interpreted within a view-based framework for navigation [37–41]. According to this theoretical approach, movements in space are deduced by comparing specific contents of panoramic views (visual snapshot) at the target and at the current location, following a minimal mismatch criterion. Importantly, the hypothesis of a view-based strategy for spatial reorientation does not make any assumption of separate computation processes between information provided by extended surfaces and those provided by freestanding objects in an array. A question then arises as to whether a stable panoramic view of the surrounding is crucial for spatial reorientation also in the case of a three-dimensional layout of extended surfaces. If this would be the case, then the apparent physical extension of the visual cues should be no longer considered a discriminant property on the basis of which geometric representation of an environment are constructed. The hypothesis was investigated in the present series of experiments by training chicks to access a food-reward from an opening on cylinders arranged in arrays at the centre of an arena, a procedure that keeps the panoramic view perceived by the subjects at reward sites under control. The macroscopic surface layout of the arena of unambiguous geometric cues was provided (i.e. rectangular rather than circular arenas; figure 1). The subjects’ performances were tested against predictions based on the hypothesis of a primacy of these cues for visuo-spatial reorientation. We showed that exposure to a stable panoramic view of the surrounding at reward site is a crucial factor for successful geometric-based learning also in this spatial context, suggesting that geometric cues provided both by extended surfaces and freestanding objects in an array are encoded similarly in an egocentred frame of reference.
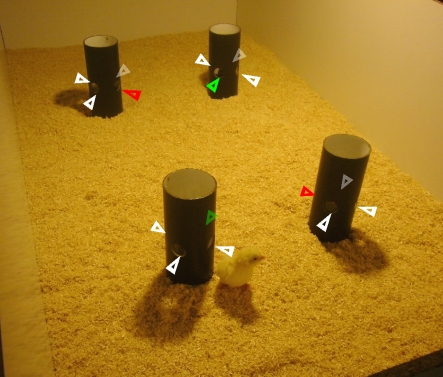
Figure 1.
Experimental set-up. Inner view of the arena used for the training. The arrangement of the accessible openings (correct cylinders, green arrows; incorrect cylinders, red arrows) and the blocked openings (white arrows) on cylinders used in experiment 3 for a given training series is shown schematically.
2. Material and methods
(a). Subjects and housing
Seventy-three domestic chicks of the Hybro strain (a local variety derived from the White Leghorn breed) were used for the experiments. Chicks were obtained from a commercial hatchery (Agricola Berica, Montegalda, Vicenza, Italy) on their first day of life and were reared individually in metal cages (22.5 cm wide × 30 cm high × 40 cm deep). The cages were located in a temperature-controlled room (30°C) and were lit from above by fluorescent lights (Philips Aquarelle 36 W, 12 L: 12 D schedule). Water was delivered ad libitum during the entire training period. Ten hours before starting every training session, the chicks were food-deprived to obtain the necessary motivational status.
(b). Experimental set-up
In all experiments, chicks were trained to locate a food-reward in an array of four cylindrical objects located centrally within a uniformly white-painted rectangular arena (160 cm long, 80 cm wide, 50 cm high). The arena was mounted on a desk 75 cm above the floor and it was illuminated by a light spot (75 W) hung above its centre. A food-cup was located inside each of the cylinders and it could be accessed by a circular opening on each pipe. The correct landmark(s) in the array contained a food-reward, which consists of larvae (Tenebrio molitor) that were killed before trials preventing them from escaping the food-cups and becoming visible from the outside by simple inspection of the cylinders.
The first experiment aimed at testing whether chicks trained to locate a reward in a distinctively coloured cylinder could learn the geometric cues specified by the shape of the arena in a comparable way as in the traditional version of the rectangular room task, where the rewarded spot typically occupies one of the corners of the apparatus (figure 2a). Sixteen chicks were used for the experiment. The cylinders (diameter (Ø) = 7.5 × 26 cm) were arranged at the centre of the arena to form a square-shaped array (42 × 42 cm) with sides aligned with the walls of the enclosure. The cylinders were coloured and textured masking tapes as follows: (i) a homogeneous blue masking tape with one spiral yellow stripe (2 cm wide); (ii) alternating white and red horizontal stripes; (iii) dark green discs (Ø = 1 cm) randomly disposed over an orange background; and (iv) a homogeneous black masking tape. Type (i) feature was selected to serve as the rewarded landmark during training. The position of each differently coloured cylinder was maintained constant for the same chick, but it was changed across chicks. All of the cylinders were presented with one circular opening (Ø = 2 cm) 13 cm from the floor. The openings pointed at the centre of the arena and were maintained stable throughout the training and the test trials. After training, chicks were observed in the absence of the reward in a series of 12 consecutive trials carried out in a square-shaped array (42 × 42 cm) of cylinders of the type previously rewarded.
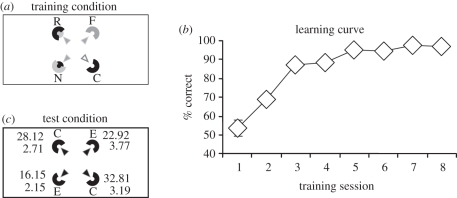
Figure 2.
Experiment 1. (a) Schematic of the rectangular arena and the square-shaped array of distinctively coloured cylinders used during the training. The inner arrows represent the arrangement of the accessible openings on cylinders (empty arrows represent correct cylinder; grey arrows represent incorrect cylinders; black arrows represent unrewarded cylinders at test). For convenience, the rewarded position is represented at one position only. (b) Mean percentage of the correct choices (±s.e.m.) made by the chicks during the training. (c) Mean percentage of the choices (±s.e.m.) made by the chicks to the geometrically correct (C) and the incorrect cylinders (E) at the experimental test.
The second experiment aimed at dissociating the relative contribution of the geometric cues provided by extended surfaces and discrete objects for visuo-spatial reorientation. Forty-seven chicks took part in the experiment. Four indistinguishable cylinders of type (i) already used in the first experiment were arranged at the centre of the arena to form a rectangular-shaped array (30 × 60 cm). The openings pointed at the centre of the arena and were maintained stable throughout the training and the test trials. The rewarded cylinders occupied geometrically equivalent locations in the array (same diagonal). As a consequence, multiple geometric cues specified both by the shape of the arena and the shape of the array defined the rewarded locations. The rewarded diagonal was maintained the same for the same chick, but it was changed across chicks (figure 3a). At the end of the training, the chicks’ performances were assessed according to the following criterion. If the chicks chose the correct landmarks, at least 60 per cent of the trial during the training session 7 and at least 70 per cent of the trial during the training session 8, then they were considered as subjects for subsequent transformational tests. Twenty-three chicks failed to reach the criterion and were discarded. The remaining chicks were assigned independently to three transformational test conditions. (i) Global-only (GO) geometry test (n = 8): the cylinders were moved to a novel position in order to form a square-shaped array (42 × 42 cm) at the centre of the arena; (ii) local-only (LO) geometry tests (n = 8): the rectangular array was located centrally within a circular arena (Ø 130 cm; 50 cm height); and (iii) global plus local (GL) geometry tests (n = 8): the chicks were tested in the same array and in the same arena as during training. In all of the transformational test conditions, 12 consecutive unrewarded trials were administered to the chicks.
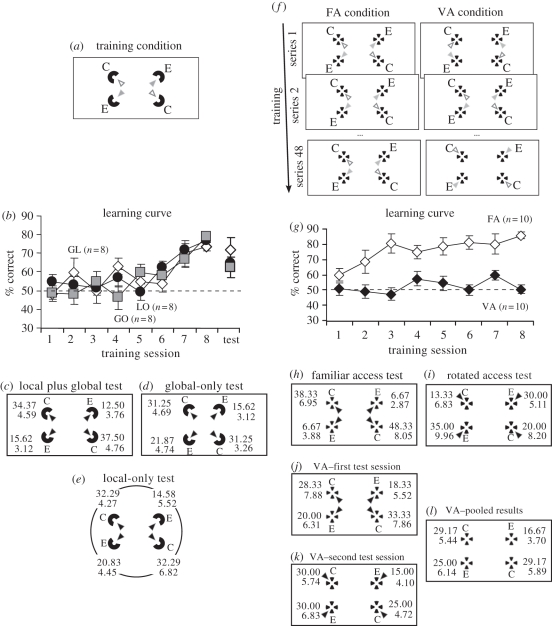
Figure 3.
Experiments 2 and 3. Schematic of the arena and the accessible openings on cylinders (empty arrows represent correct cylinder; grey arrows represent incorrect cylinders) used during training in experiment 2 (a) and in experiment 3 (f). (b) Mean percentage of the geometrically correct choices (± s.e.m.) made by the chicks during the training sessions in experiment 2. Data are reported separately for each group (unfilled diamonds, global plus local; filled squares, global-only; filled circles, local-only). After training, the chicks were observed after selective removal of informative geometric cues provided either by the perimeter walls of the arena (e) or the shape of the array (d) or with both informative geometric shape available (c). Mean percentage of the choices (± s.e.m.) made by the chicks to the geometrically correct (C) and the incorrect cylinders (E) in the array is reported in the corresponding panels (black arrows denote position of the accessible openings on the unrewarded cylinders at test). (g) Mean percentage of the geometrically correct choices (± s.e.m.) made by the chicks during the training sessions in experiment 3 (unfilled diamonds, fixed access; filled diamonds, variable access). Mean percentage of choices (± s.e.m.) made by the FA birds to the four cylinders in the array with a familiar position of the accessible openings on cylinders (h) and with a rotated position of the accessible openings (i) is also reported. Mean percentage of choices (± s.e.m.) made by the VA birds to the four cylinders in the array in both the first (j) and the second test session (k) is reported (pooled results in (l)).
If the chicks reorient exclusively on the basis of a mental representation of the geometric cues provided by the macroscopic three-dimensional surface layout [10–12,26], a generalization decrement should be expected specifically in the LO geometry condition, in which the geometric contribution provided by the walls of the arena were no longer available. An alternative hypothesis has been tested that chicks rely on a mental representation of the environmental axis to reorient and therefore on the basis of both the spatial distribution of extended surfaces and discrete objects in the scene [42]. If the chicks reorient on the basis of that geometric feature to reorient, comparable accuracy between the training and the test sessions should be expected in all test conditions, in that the environmental axis were the same between the training and the test sessions in all of the experiments.
The third experiment aimed at investigating whether chicks rely on purely egocentred strategy to reorient. Twenty chicks took part in the experiment. The chicks were trained with multiple geometric cues as in experiment 2. Four indistinguishable cylinders (8.3 cm Ø; 26 cm high) covered by homogeneous black masking tape were arranged in a rectangular-shaped array (30 × 60 cm) at the centre of the rectangular arena already used in the previous experiments. The rewarded landmarks occupied geometrically equivalent locations in the array (same diagonal). The rewarded diagonal was maintained the same for the same chick, but it was changed across chicks. The cylinders were provided each of four circular openings (Ø = 2 cm) aligned 13 cm from the floor and spaced each other by 90°. One of the openings on each cylinder was aligned with the bisecting line of each corner of the array. The position of the openings on pipes relative to the array was maintained stable throughout the training and the test trials. However, three of the four pipe's openings were blocked by a transparent screen. The chicks could access the pipe’s contents from the remaining opening that was left uncovered by a hole in the transparent screen (figure 1). The chicks were then assigned independently to one out of two training conditions. In the fixed access (FA) position group (n = 10), chicks were trained with a fixed position of the accessible opening on pipes throughout the training. The position of the accessible openings was maintained the same for a given chick but it was changed across chicks. In the variable access (VA) condition (n = 10), the position of the accessible opening on pipes was changed between the training series (figure 3f). In doing so, the chicks were exposed either to a stable or a variable view of the surrounding at reward. After the training, both FA- and VA-trained chicks received two consecutive test sessions of six trials each. In the FA group of chicks, the position of the accessible opening was the same as during the training in one test session (familiar access position) and it was rotated by 180° with respect to the training in the other session (rotated access position). The test order was changed across chicks. In the VA group, one position of the accessible opening was chosen for one test session and it was rotated by 180° in the other session, changing the choice across chicks. The first position of the accessible openings was the one not presented to the chicks in the last training session.
(c). Procedure
The birds were familiarized to the environment by means of two pre-training sessions, respectively, at day 2 and day 3 post hatch. During the first pre-training session, the chicks were allowed to move freely within the enclosure in the presence of the array. All of the landmarks contained the reward at this stage of the procedure. A wooden stick, manually moved by the experimenter, was used to encourage the chicks to peck at the reward. The same procedure was repeated at day 3 post hatch, during the second pre-training session. The pre-training was considered ended as soon as the chicks spontaneously insert their head and successfully gain a reward from all of the cylinders in the array in two consecutive trials.
Training started at day 4 and continued until day 12 after hatching, with one interruption at day 7. Every daily training session consisted of 30 trials distributed into three series of 10 trials each. Overall, 240 trials were administered during the training to each individual. Within a series, each chick was released twice from the centre of the arena and twice from each side of the array, near the wall of the arena, facing different directions and following a pseudo-random order. A choice was scored as soon as the chick inserted its beak through an opening on the cylinders. In the case of a correct choice, the chicks were allowed to consume the reward. Only the first choices at every trial, during both the training and the test trials, were scored for the analysis. Between two consecutive trials, the chicks were moved into a cardboard box (32 cm wide × 13 cm high × 21 cm deep) located outside the arena, where they were slowly rotated in order to obtain a complete spatial disorientation.
The cylinders were cleaned and interchanged whenever possible (the cylinders were not interchanged in experiment 1 because of the different colours characterizing them) before starting each training series. Trials at test were administered following the same procedure as described for the training. The percentage of correct choices at every training session was considered as the individual performance. In all of the experiments, a non-parametric statistics (Wilcoxon signed-rank test for two related samples or repeated measurements on a single sample) was chosen for its robustness to examine the distribution of the chicks’ choices at test. Null hypotheses were always rejected with α < 0.05.
3. Results
(a). Experiment 1
(i). Training
Chicks learnt to locate the reward in the array on the basis of the featural cues (figure 2b). ANOVA, with session as the within-subject factor and position of the rewarded cylinders relative to the shape of the arena as the between-subject factor, revealed a significant effect of session, indicating that the mean percentage of the correct choices increased significantly during training (F7,98 = 63.588, p < 0.001). The main effect of position was neither significant (F1,14 = 0.112, p = 0.743), nor significant interaction emerged between session and position (F7,98 = 1.166, p = 0.329; figure 2b).
(ii). Test
In the transformational test, the cylinders used for the training were replaced by four indistinguishable cylinders of the type previously rewarded. The results showed that chicks retrieved the geometric cues specified by the shape of the arena to reorient (figure 2c). The chicks chose significantly more often the cylinders at the geometrically correct positions than the cylinders at the geometrically incorrect positions in the array (Wilcoxon signed-rank test: n = 16, z = −2.346, p = 0.019). No significant differences emerged when comparing the mean percentage of choices directed to the cylinders that occupied the geometrically equivalent locations in the array (Wilcoxon signed-rank test—correct versus rotational: n = 16, z = 1.295, p = 0.195; far versus near: n = 16, z = −1.428, p = 0.153).
Overall, the results demonstrated incidental learning of the geometric cues specified by the shape of the arena and confirmed previous findings in this avian species [14,18–20,43–46]. Our method proved to be sufficiently reliable to allow further examination on the relative contribution of extended surfaces and freestanding objects for visuo-spatial reorientation.
(b). Experiment 2
(i). Training
The chicks successfully learnt to locate the reward during the training (figure 3b). ANOVA with session as the within-subject factor, and both the rewarded diagonal and group as the between-subjects factors, revealed a significant main effect of session (F7,126 = 14.962, p < 0.001). The main effects of group and rewarded diagonal were not statistically significant (group: F2,18 = 0.154, p = 0.859; rewarded diagonal: F2,18 = 1.374, p = 0.256). No significant interaction emerged from the analysis.
(ii). Test
In order to assess the impact of selective removal of the geometric cues specified either by the shape of the arena or the shape of the array, the mean percentage of the geometrically correct choices made by the three groups of chicks during the last 12 training trials and the test sessions was compared, separately for each group, using non-parametric statistics. This analysis failed to reveal a significant difference between performances and test in the GL group (Wilcoxon signed-rank test: n = 6, T+ = 13, ties = 2; p = 0.349). Conversely, GO chicks were significantly less accurate at test than during training (Wilcoxon signed-rank test: n = 8, T+ = 31, p = 0.039). A generalization decrement, though not statistically significant, also emerged in the LO group of chicks (Wilcoxon signed-rank test: n = 8, T+ = 29, p = 0.074). Therefore, the results suggest that the geometric cues provided by the array of cylinders were at least as effective as those provided by the perimeter walls of the arena in controlling the chicks’ choices.
(iii). Global plus local (rectangular enclosure–rectangular array)
GL chicks reoriented successfully at test, carried out in the absence of the reward (figure 3c). Chicks focused their searches significantly more often at the geometrically correct cylinders in the array (geometrically correct landmarks versus geometrically incorrect landmarks: Wilcoxon signed-rank test: n = 7, T+ = 28, ties = 1; p = 0.008). The mean percentage of choices was equally distributed across the geometrically equivalent locations, both within the geometrically correct and the incorrect diagonal (Wilcoxon signed-rank test—correct versus rotational: n = 6, T+ = 13 ties = 2, p = 0.344; far versus near: n = 5, T+ = 12, ties = 3, p = 0.156).
(iv). Global-only (rectangular enclosure–square-shaped array)
GO chicks focused their searches significantly more often at the geometrically correct cylinders than at the geometrically incorrect cylinders in the array, providing evidence that this group was still oriented with residual geometric cues specified by the shape of the arena (figure 3d). Statistical comparison revealed a significant difference between the mean percentage of the geometrically correct choices and the mean percentage of the geometrically incorrect choices in the array (geometrically correct cylinders versus geometrically incorrect cylinders: Wilcoxon signed-rank test: n = 6, T+ = 20, ties = 2; p = 0.031). Any significant differences between the mean percentage of choices directed towards the cylinders that occupied geometrically equivalent positions in the arena were found neither within the geometric correct (correct versus rotational—Wilcoxon signed-rank test: n = 6, T+ = 10, ties = 2, p > 0.5) nor within the geometrically incorrect diagonal (Wilcoxon signed-rank test—far versus near: n = 6, T+ = 15, ties = 2, p = 0.219).
(v). Local-only (circular enclosure–rectangular array)
LO chicks reoriented successfully with residual geometric cues specified by the shape of the array. The chicks directed their searches significantly more often at the geometrically correct cylinders than at the geometrically incorrect cylinders in the array (geometrically correct cylinders versus geometrically incorrect cylinders—Wilcoxon signed-rank test: n = 6, T+ = 19, ties = 2, p = 0.047; figure 3e). No significant differences emerged when comparing the mean percentage of choices directed to the geometrically equivalent cylinders in the array (correct versus rotational—Wilcoxon signed-rank test: n = 6, T+ = 9, ties = 2, p > 0.5; far versus near: n = 6, T+ = 13, ties = 2, p = 0.344).
The generalization decrement observed after training in both the transformational test conditions with a single informative geometric shape stands in contrast to the prediction based on the hypothesis of a primacy of the three-dimensional surface layout geometry for spatial reorientation [10–12,26]. Since the environmental axis was not altered by the transformations in any respect, it appears unlikely that chicks relied on those features to reorient (see also [47–50]). We hypothesized that chicks might have relied on egocentred panoramic views of the surrounding to reorient. The mismatch of the panoramic views perceived at the opening on cylinders between the training and the test sessions may have been responsible for the generalization decrement observed at test, when a single informative geometric shape was provided. The third experiment examined this possibility in further detail.
(c). Experiment 3
(i). Training
The chicks’ performances during the training are reported in figure 3g. ANOVA, with session as the within-subject factor and both access condition and the rewarded diagonal as the between-subject factors, revealed a significant main effect of session (F7,112 = 2.609, p = 0.016). The main effect of access condition turned out to be statistically significant (F1,16 = 17.618, p = 0.001). A significant interaction between session and access condition emerged from the analysis (F7,112 = 2.149, p = 0.044). Further analysis revealed that birds trained in the FA condition learnt to locate the reward in the array (session F7,56 = 3.246, p = 0.006). Conversely, chicks trained in the VA condition failed to learn the task (session F7,56 = 1.209, p = 0.303).
(ii). Test
At the experimental test, VA chicks failed to reorient in the array at the experimental test in both the first (figure 3j) and the second test session (figure 3k). The pooled results showed that the mean percentage of the geometrically correct choices did not differ significantly from the mean percentage of the geometrically incorrect choices (Wilcoxon signed-rank test—geometrically correct cylinders versus geometrically incorrect cylinders: n = 10, T+ = 31, ties = 1, p = 0.385; correct versus rotational: T+ = 27, ties = 1; p > 0.5; far versus near: T+ = 29, ties = 2, p = 0.461) (figure 3l). On the contrary, FA chicks reoriented successfully with a familiar position of the accessible openings on pipes (Wilcoxon signed-rank test—geometrically correct cylinders versus geometrically incorrect cylinders: n = 10, T+ = 45, ties = 1; p = 0.042; correct versus rotational: T+ = 7, ties = 5, p > 0.5; far versus near: T+ = 1, ties = 8, p > 0.5) (figure 3h). However, FA birds failed to reorient in the array when the position of openings was rotated with respect to training by 180° (Wilcoxon signed-rank test—geometrically incorrect cylinders versus geometrically correct cylinders: n = 8, T+ = 41, ties = 1; p = 0.097; correct versus rotational: T+ = 13, ties = 4, p > 0.5; far versus near: T+ = 21, ties = 2, p > 0.5) (figure 3i). A tendency towards reversal of performance was apparent, suggesting that chicks tend to discriminate the cylinders on the basis of their reciprocal arrangement relative to the agent rather than in relation to the shape of the arena.
Overall, the results indicate that chicks were able to locate the correct pipes provided that the position of openings giving the access to the reward was maintained stable throughout the experiment. Therefore, it appears that a stable panoramic view of the surrounding was critical for successful visuo-spatial reorientation.
4. Discussion
Domestic chicks were trained in a reference memory task to locate a food-reward according to the geometric cues specified by a rectangular enclosure under experimental conditions that kept the panoramic view perceived by the birds at the reward sites under control. The chicks exposed to a stable panoramic view of the surrounding reoriented successfully according to the environmental geometry in all of the experiments. In contrast, chicks exposed to a variable view of the surrounding at reward sites failed to reorient. The results do not agree with predictions based on the hypothesis of an obligatory process for spatial reorientation based on a geocentric representation of the three-dimensional surface layout geometry [26]. By contrast, the results parallel earlier findings in this species obtained in a task with informative geometric cues provided only by discrete objects in an array [35,36] (see §1). Collectively, the results suggest that the stability of the panorama perceived at the target is a critical cue for successful spatial reorientation, regardless of whether geometric information are specified by extended surfaces or freestanding objects.
The lack of integration across multiple panoramic views of the surrounding observed in the VA group of chicks during training as well as failure of the FA group of chicks to retrieve the geometrically correct positions in the arena in the rotation test supports the use of local strategies for spatial reorientation in this avian species [47–50] (see also [51]). Local strategies for spatial reorientation may reflect a view-matching process operating on partial views of the surrounding. It has been recently hypothesized that a global-matching strategy, based on a pixel-by-pixel matching between oriented visual snapshot, could provide a satisfactory account of spatial reorientation in ants [38] (see also [41]). Contrary to the prediction based on global-matching, however, it has been shown that children fail to reorient by sharp contrast borders produced by a two-dimensional rectangle flashed against the floor of a circular-shaped room [12]. Children also fail to reorient by geometry in an array of freestanding objects, including cylinders [9–11] and conspicuous rectangular boxes [8], even in the case of objects connected together by a thin cord [12]. Similar results have been obtained in chicks in a working memory task [52].
An alternative hypothesis to global matching could be that a view-based strategy in vertebrates operates on a subset of information contents from egocentred (non-oriented) representation of the visual scene, with featural and geometrical cues each providing separate contribution to the process. The hypothesis that vertebrates reorient on the basis of an egocentred spatial representation of the scene would be consistent with findings indicating that the relative reliance of featural and geometrical cues is modulated by the size of the experimental space in a rectangular-shaped arena [43–45,53–57]. It has been suggested that local views of the surrounding taken at a distance to a corner of a rectangular arena conveys different amounts of details concerning local features and local geometric cues, respectively, depending on the size of the arena, with local geometric information being increasingly available within the enclosure of small dimensions [43].
We suggest that the different outcomes of studies investigating spatial reorientation in environments characterized by extended surfaces and spatially isolated objects could be reconciled within an egocentred framework for spatial reorientation (see also [58]). Neurons specifically activated by proximal boundaries have been recently identified in both the entorhinal cortex and the subiculum in rats [59,60]. These cells may be instrumental in anchoring the grid cells’ grids and the place cells’ fields representations to the external world [29]. We found that chicks reoriented on the basis of egocentred spatial representation of the surroundings built upon direct exploration of local physical constrains. The local physical constrains imposed by an object, rather than its apparent vertical extension, is a discriminating property of obstacles [61] and appear to provide critical cues to reorient. Previous studies investigating the relative contribution of the geometry of extended surfaces and freestanding objects for spatial reorientation typically did not keep under control the impact of the panoramic view perceived at the goal location. Note that this is crucial in the case of freestanding objects that could be approached from several directions, but less so in the case of the classical rectangular room task. In the latter case, the reward site occupies one of the corners of the arena, a condition that forces the approach direction to a limited set of alternatives, thus facilitating the encoding of a stable, though local, view of the correct site [35,36] (see also [62]).
The same point may hold correct for locating the centre of an enclosure, in which no obvious physical constrains are provided locally. The panoramic views of the surrounding taken from the centre of a symmetric arena are in fact comparable across gaze directions. Thus, the edge conjoining two walls could provide useful (distal) guides to align visual memories within the arena for shapes other than cylindrical ones [23].
Acknowledgements
The authors wish to thank Nataša Sabadoš for the help provided with animal testing. This work has been realized thanks to the support from the Provincia Autonoma di Trento and the Fondazione Cassa di Risparmio di Trento e Rovereto.
References
- 1.Cheng K. 1986. A purely geometric module in the rat's spatial representation. Cognition 23, 149–178 10.1016/0010-0277(86)90041-7 (doi:10.1016/0010-0277(86)90041-7) [DOI] [PubMed] [Google Scholar]
- 2.Gallistel C. R. 1990. The organization of learning. Cambridge, MA: MIT Press [Google Scholar]
- 3.Cheng K., Newcombe N. S. 2005. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon. Bull. Rev. 12, 1–23 10.3758/BF03196346 (doi:10.3758/BF03196346) [DOI] [PubMed] [Google Scholar]
- 4.Spelke E. S., Kinzler K. D. 2007. Core knowledge. Dev. Sci. 10, 89–96 10.1111/j.1467-7687.2007.00569.x (doi:10.1111/j.1467-7687.2007.00569.x) [DOI] [PubMed] [Google Scholar]
- 5.Vallortigara G. 2009. Animals as natural geometers. In Cognitive biology: evolutionary and developmental perspectives on mind, brain and behaviour (eds Tommasi L., Nadel M., Peterson), pp. 83–104 Cambridge, UK: MIT Press [Google Scholar]
- 6.Haun D. B. M., Jordan F. M., Vallortigara G., Clayton N. S. 2010. Origins of spatial, temporal and numerical cognition: insights from comparative psychology. Trends Cogn. Sci. 14, 552–560 10.1016/j.tics.2010.09.006 (doi:10.1016/j.tics.2010.09.006) [DOI] [PubMed] [Google Scholar]
- 7.Hermer L., Spelke E. 1994. A geometric process for spatial reorientation in young children. Nature 370, 57–59 10.1038/370057a0 (doi:10.1038/370057a0) [DOI] [PubMed] [Google Scholar]
- 8.Gouteux S., Spelke E. S. 2001. Children's use of geometry and landmarks to reorient in an open space. Cognition 81, 119–148 10.1016/S0010-0277(01)00128-7 (doi:10.1016/S0010-0277(01)00128-7) [DOI] [PubMed] [Google Scholar]
- 9.Lee S. A., Shusterman A., Spelke E. S. 2006. Reorientation and landmark-guided search by young children: evidence for two systems. Psychol. Sci. 17, 577–582 10.1111/j.1467-9280.2006.01747.x (doi:10.1111/j.1467-9280.2006.01747.x) [DOI] [PubMed] [Google Scholar]
- 10.Lee S. A., Spelke E. S. 2008. Children's use of geometry for reorientation. Dev. Sci. 11, 743–749 10.1111/j.1467-7687.2008.00724.x (doi:10.1111/j.1467-7687.2008.00724.x) [DOI] [PubMed] [Google Scholar]
- 11.Lee S. A., Spelke E. S. 2010. A modular geometric mechanism for reorientation in children. Cogn. Psychol. 61, 152–176 10.1016/j.cogpsych.2010.04.002 (doi:10.1016/j.cogpsych.2010.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S. A., Spelke E. S. 2011. Young children reorient by computing layout geometry, not by matching images of the environment. Psychon. Bull. Rev. 18, 192–198 10.3758/s13423-010-0035-z (doi:10.3758/s13423-010-0035-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrad-Cole F., Lew A. R., Bremner J. G., Whitaker C. J. 2001. Use of cue configuration geometry for spatial reorientation in human infants (Homo sapiens). J. Comp. Psychol. 115, 317–320 10.1037/0735-7036.115.3.317 (doi:10.1037/0735-7036.115.3.317) [DOI] [PubMed] [Google Scholar]
- 14.Vallortigara G., Zanforlin M., Pasti G. 1990. Geometric modules in animal's spatial representation: a test with chicks. J. Comp. Psychol. 104, 248–254 10.1037/0735-7036.104.3.248 (doi:10.1037/0735-7036.104.3.248) [DOI] [PubMed] [Google Scholar]
- 15.Pecchia T., Vallortigara G. 2010. Reorienting strategies in a rectangular array of landmarks by domestic chicks (Gallus gallus). J. Comp. Psychol. 124, 147–158 10.1037/a0019145 (doi:10.1037/a0019145) [DOI] [PubMed] [Google Scholar]
- 16.Kelly D. M. 2010. Features enhance the encoding of geometry. Anim. Cogn. 13, 453–462 10.1007/s10071-009-0296-y (doi:10.1007/s10071-009-0296-y) [DOI] [PubMed] [Google Scholar]
- 17.Doeller C. F., Burgess N. 2008. Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proc. Natl Acad. Sci. USA 105, 5909–5914 10.1073/pnas.0711433105 (doi:10.1073/pnas.0711433105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiandetti C., Vallortigara G. 2008. Is there an innate geometric module? Effects of experience with angular geometric cues in spatial re-orientation based on the shape of the environment. Anim. Cogn. 11, 139–146 10.1007/s10071-007-0099-y (doi:10.1007/s10071-007-0099-y) [DOI] [PubMed] [Google Scholar]
- 19.Chiandetti C., Vallortigara G. 2010. Experience and geometry: controlled-rearing studies with chicks. Anim. Cogn. 13, 463–470 10.1007/s10071-009-0297-x (doi:10.1007/s10071-009-0297-x) [DOI] [PubMed] [Google Scholar]
- 20.Vallortigara G., Sovrano V. A., Chiandetti C. 2009. Doing Socrates experiment right: controlled-rearing studies of geometrical knowledge in animals. Curr. Opin. Neurobiol. 19, 20–26 10.1016/j.conb.2009.02.002 (doi:10.1016/j.conb.2009.02.002) [DOI] [PubMed] [Google Scholar]
- 21.Langston R. F., Ainge J. A., Couey J. J., Canto C. B., Bjerknes T. L., Witter M. P., Moser E. I., Moser M. B. 2010. Development of the spatial representation system in the rat. Science 328, 1576–1580 10.1126/science.1188210 (doi:10.1126/science.1188210) [DOI] [PubMed] [Google Scholar]
- 22.Wills T. J., Cacucci F., Burgess N., O'Keefe J. 2010. Development of the hippocampal cognitive map in preweaning rats. Science 328, 1573–1576 10.1126/science.1188224 (doi:10.1126/science.1188224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tommasi L., Vallortigara G., Zanforlin M. 1997. Young chickens learn to localize the centre of a spatial environment. J. Comp. Psychol. A180, 567–572 10.1007/s003590050073 (doi:10.1007/s003590050073) [DOI] [PubMed] [Google Scholar]
- 24.Hartley T., Trinkler I., Burgess N. 2004. Geometric determinants of human spatial memory. Cognition 94, 39–75 10.1016/j.cognition.2003.12.001 (doi:10.1016/j.cognition.2003.12.001) [DOI] [PubMed] [Google Scholar]
- 25.Pearce J. M. 2009. An associative analysis of spatial learning. Q. J. Exp. Psychol. 62, 1665–1684 10.1080/17470210902805589 (doi:10.1080/17470210902805589) [DOI] [PubMed] [Google Scholar]
- 26.Wang R. F., Spelke E. S. 2002. Human spatial representations: insights from animals. Trend Cogn. Sci. 6, 376–382 10.1016/S1364-6613(02)01961-7 (doi:10.1016/S1364-6613(02)01961-7) [DOI] [PubMed] [Google Scholar]
- 27.O'Keefe J., Nadel L. 1978. The hippocampus as a cognitive map. Oxford, UK: Claredon Press [Google Scholar]
- 28.O'Keefe J., Burgess N. 1996. Geometric determinants of the place fields of the hippocampal neurons. Nature 381, 425–428 10.1038/381425a0 (doi:10.1038/381425a0) [DOI] [PubMed] [Google Scholar]
- 29.Moser E. I., Moser M. B. 2008. A metric for space. Hippocampus 18, 1142–1156 10.1002/hipo.20483 (doi:10.1002/hipo.20483) [DOI] [PubMed] [Google Scholar]
- 30.Tommasi L., Gagliardo A., Andrew R. J., Vallortigara G. 2003. Separate processing mechanisms for encoding geometric and landmark information in the avian brain. Eur. J. Neurosci. 17, 1695–1702 10.1046/j.1460-9568.2003.02593.x (doi:10.1046/j.1460-9568.2003.02593.x) [DOI] [PubMed] [Google Scholar]
- 31.Vargas J. P., Petruso E. J., Bingman V. P. 2004. Hippocampal formation is required for geometric navigation in pigeons. Eur. J. Neurosci. 20, 1937–2004 10.1111/j.1460-9568.2004.03654.x (doi:10.1111/j.1460-9568.2004.03654.x) [DOI] [PubMed] [Google Scholar]
- 32.Vargas J. P., Bingman V. P., Portavella M., Lopez J. C. 2006. Telencephalon and geometric space in goldfish. Eur. J. Neurosci. 24, 2870–2878 10.1111/j.1460-9568.2006.05174.x (doi:10.1111/j.1460-9568.2006.05174.x) [DOI] [PubMed] [Google Scholar]
- 33.Doeller C. F., King J. A., Burgess N. 2008. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memories. Proc. Natl Acad. Sci. USA 105, 5915–5920 10.1073/pnas.0801489105 (doi:10.1073/pnas.0801489105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cressant A., Muller R. U., Poucet B. 1997. Failure of centrally placed objects to control the firing fields of hippocampal place cells. J. Neurosci. 17, 2531–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pecchia T., Vallortigara G. 2010. View-based strategy for reorientation by geometry. J. Exp. Biol. 213, 2987–2996 10.1242/jeb.043315 (doi:10.1242/jeb.043315) [DOI] [PubMed] [Google Scholar]
- 36.Pecchia T., Gagliardo A., Vallortigara G. 2011. Stable panoramic views facilitate snap-shot like memories for spatial reorientation in homing pigeons. PLoS ONE 6, e22657. 10.1371/journal.pone.0022657 (doi:10.1371/journal.pone.0022657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cartwright B. A., Collett T. S. 1983. Landmark learning in bees: experiments and models. J. Comp. Psychol. 151, 521–543 10.1007/BF00605469 (doi:10.1007/BF00605469) [DOI] [Google Scholar]
- 38.Wystrach A. 2009. Ants in rectangular arenas: a support for the global matching theory. Commun. Integr. Biol. 2, 388–390 10.4161/cib.2.5.8717 (doi:10.4161/cib.2.5.8717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung A., Sturzl W., Zeil J., Cheng K. 2008. The information content of panoramic images II: view-based navigation arenas. Exp. Psychol. Anim. Behav. Process. 34, 15–30 10.1037/0097-7403.34.1.15 (doi:10.1037/0097-7403.34.1.15) [DOI] [PubMed] [Google Scholar]
- 40.Stürzl W., Cheung A., Zeil J., Cheng K. 2008. The information content of panoramic images I: the rotational errors and the similarity of views in a rectangular experimental arenas. J. Exp. Psychol. Anim. Behav. Process. 34, 1–14 10.1037/0097-7403.34.1.1 (doi:10.1037/0097-7403.34.1.1) [DOI] [PubMed] [Google Scholar]
- 41.Cheng K. 2008. Whither geometry? Troubles of the geometric module. Trends Cogn. Sci. 12, 355–361 10.1016/j.tics.2008.06.004 (doi:10.1016/j.tics.2008.06.004) [DOI] [PubMed] [Google Scholar]
- 42.Cheng K. 2005. Reflections on geometry and navigation. Connect. Sci. 17, 5–21 10.1080/09540090500138077 (doi:10.1080/09540090500138077) [DOI] [Google Scholar]
- 43.Sovrano V. A., Vallortigara G. 2006. Dissecting the geometric module: different linkage for metric and landmark information in animals’ spatial reorientation. Psychol. Sci. 17, 616–621 10.1111/j.1467-9280.2006.01753.x (doi:10.1111/j.1467-9280.2006.01753.x) [DOI] [PubMed] [Google Scholar]
- 44.Chiandetti C., Regolin L., Sovrano V., Vallortigara G. 2007. Spatial reorientation, the effects of space size on the encoding of landmark and geometry information. Anim. Cogn. 10, 159–168 10.1007/s10071-006-0054-3 (doi:10.1007/s10071-006-0054-3) [DOI] [PubMed] [Google Scholar]
- 45.Chiandetti C., Vallortigara G. 2008. Spatial reorientation in large and small enclosures: comparative and developmental perspectives. Cogn. Process. 9, 229–238 10.1007/s10339-008-0202-6 (doi:10.1007/s10339-008-0202-6) [DOI] [PubMed] [Google Scholar]
- 46.Chiandetti C., Vallortigara G. 2010. Animals’ representation of enclosed spaces: evidence for use of a similar frame of reference following different disorientation procedures. J. Comp. Psychol. 124, 139–146 10.1037/a0017013 (doi:10.1037/a0017013) [DOI] [PubMed] [Google Scholar]
- 47.Tommasi L., Polli C. 2004. Representation of two geometric features of the environment in the domestic chick (Gallus gallus). Anim. Cogn. 7, 53–59 10.1007/s10071-003-0182-y (doi:10.1007/s10071-003-0182-y) [DOI] [PubMed] [Google Scholar]
- 48.Kelly D. M., Chiandetti C., Vallortigara G. 2011. Re-orienting in space: do animals use global or local geometry strategies? Biol. Lett. 7, 372–735 10.1098/rsbl.2010.1024 (doi:10.1098/rsbl.2010.1024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sturz B. R., Bodily K. D. 2011. Of global space or perceived place? Comment on Kelly et al. Biol. Lett. 7, 647–648 10.1098/rsbl.2011.0216 (doi:10.1098/rsbl.2011.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly D. M., Durocher S., Chiandetti C., Vallortigara G. 2011. A misunderstanding of principal and medial axes? Reply to Sturz and Bodily. Biol. Lett. 7, 649–650 10.1098/rsbl.2011.0482 (doi:10.1098/rsbl.2011.0482) [DOI] [Google Scholar]
- 51.Pearce J. M., Good M. A., Jones P. M., McGregor A. 2004. Transfer of spatial behavior between different environments. Implications for theories of spatial learning and for the role of the hippocampus in spatial learning. J. Exp. Psychol. Anim. Behav. Process. 30, 135–147 10.1037/0097-7403.30.2.135 (doi:10.1037/0097-7403.30.2.135) [DOI] [PubMed] [Google Scholar]
- 52.Lee S. A., et al. In preparation.
- 53.Learmonth A. E., Newcombe N. S., Huttenlocher J. 2001. Toddlers’ use of metric information and landmarks to reorient. J. Exp. Child. Psychol. 80, 225–244 10.1006/jecp.2001.2635 (doi:10.1006/jecp.2001.2635) [DOI] [PubMed] [Google Scholar]
- 54.Learmonth A. E., Nadel L., Newcombe N. S. 2002. Children's use of landmarks: implications for modularity theory. Psychol. Sci. 13, 337–341 10.1111/j.0956-7976.2002.00461.x (doi:10.1111/j.0956-7976.2002.00461.x) [DOI] [PubMed] [Google Scholar]
- 55.Vallortigara G., Feruglio M., Sovrano V. A. 2005. Reorientation by geometric and landmark information in environments of different size. Dev. Sci. 8, 393–401 10.1111/j.1467-7687.2005.00427.x (doi:10.1111/j.1467-7687.2005.00427.x) [DOI] [PubMed] [Google Scholar]
- 56.Sovrano V. A., Bisazza A., Vallortigara G. 2005. Animals’ use of landmarks and metric information to reorient: effects of the size of the experimental space. Cognition 97, 121–133 10.1016/j.cognition.2004.08.003 (doi:10.1016/j.cognition.2004.08.003) [DOI] [PubMed] [Google Scholar]
- 57.Maes J. H. R., Fontanari L., Regolin L. 2009. Spatial reorientation in rats (Rattus norvegicus): use of geometric and featural information as a function of arena size and feature location. Behav. Brain. Res. 201, 285–291 10.1016/j.bbr.2009.02.026 (doi:10.1016/j.bbr.2009.02.026) [DOI] [PubMed] [Google Scholar]
- 58.Lew A. R. 2011. Looking beyond the boundaries: time to put landmarks back on the cognitive map? Psychol. Bull. 137, 484–507 10.1037/a0022315 (doi:10.1037/a0022315) [DOI] [PubMed] [Google Scholar]
- 59.Solstad T., Boccara C. N., Kropff E., Moser M. B., Moser E. I. 2008. Representation of geometric borders in the entorhinal cortex. Science 322, 865–1868 10.1126/science.1166466 (doi:10.1126/science.1166466) [DOI] [PubMed] [Google Scholar]
- 60.Lever C., Burton S., Jeewajee A., O'Keefe J., Burgess N. 2009. Boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci. 29, 9771–9777 10.1523/JNEUROSCI.1319-09.2009 (doi:10.1523/JNEUROSCI.1319-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lorenz K. 1971. Studies in animal and human behaviour, vol. 2 London, UK: Butler & Tanner [Google Scholar]
- 62.Gibson B.M., Wilks T.J., Kelly D.M. 2007. Rats (Rattus norvegicus) encode the shape of an array of discrete objects. J. Comp. Psychol. 121, 130–144 10.1037/0735-7036.121.2.130 (doi:10.1037/0735-7036.121.2.130) [DOI] [PubMed] [Google Scholar]