Abstract
Animals should continuously assess the threat of predation. Alarm calls inform on predation risk and are often used as cues to shape behavioural responses in birds and mammals. Hitherto, however, the ecological consequences of alarm calls in terms of organization of animal communities have been neglected. Here, we show experimentally that calls of a resident nocturnal raptor, the little owl Athene noctua, triggered a response in terms of breeding habitat selection and investment in current reproduction in conspecifics and heterospecifics. Little owls preferred to settle in territories where calls of conspecifics, irrespective of their type (i.e. alarm versus contact calls), were broadcasted, indicating that either conspecific attraction exists or calls are interpreted as foreign calls, eliciting settlement as a mode of defence against competitors. Also, we found that little owls seemed to invest more in current reproduction in safe territories as revealed by conspecific calls. Innovatively, we reported that a second owl species, the migratory scops owl Otus scops, preferred to breed in safe territories as indicated by little owls' calls. These results evidence that the emission of alarm calls may have, apart from well-known behavioural effects, ecological consequences in natural communities by inducing species-specific biases in breeding habitat selection. This study demonstrates a previously unsuspected informative role of avian alarm calls which may modulate the spatial structure of species within communities.
Keywords: alarm calls, breeding habitat selection, conspecifics and heterospecifics, ecological consequences, eavesdropping, predation risk
1. Introduction
Every animal is exposed to predation at some time through its life [1]. Predation is a major evolutionary force that shapes life histories and behaviours of prey and affects community organization [2]. Thus, animals should identify, evaluate and cope with predation risk to maximize survival [2,3]. Prey may trust on their own capabilities to detect potential predators or may rely on indirect cues revealing a threat [4]. Indeed, when encountering a predator many animals produce anti-predator signals (sensu [5]) that may function by: (i) alerting conspecifics (usually relatives or mates) of potential danger [6] inducing freezing or fleeing to cover [7]; (ii) recruiting nearby individuals for mobbing defence [8]; (iii) warning the predator that it has been detected [9], which may either confuse it [10], show it that the prey is prepared to escape [11] or deter pursuing [12]; and finally, (iv) attracting the predator of the predator [8]. Also, because non-relative conspecifics and heterospecifics may share predators with the signal emitter, anti-predator signals could provide information to non-targeted receivers (i.e. non-related conspecifics or heterospecifics).
Alarm calls, as specific vocalizations produced by animals when facing impending danger, are alarm signals frequently emitted by many species of birds and mammals [3]. Alarm calls can encode very rich information about the type and intensity of threat allowing receivers to give the more appropriate behavioural response [13]. For instance, alarm calls emitted by vervet monkeys Cercopithecus aethiops and social mongooses Suricata suricata provide information on both type of predator [14,15] and level of urgency it imposes [16], eliciting different behavioural responses in conspecifics [14,15]. Similarly, among birds, alarm calls of black-capped chickadees Poecile atricapilla, white-browed scrub wren Sericornis frontalis and Siberian jays Perisoreus infaustus inform conspecifics on size [17], distance [7] and behaviour (e.g. perching versus aerial searching; [18]) of aerial predators. Alarm calls can also alert of predation risk to non-related conspecifics and heterospecifics (i.e. eavesdroppers). Indeed, heterospecific eavesdroppers may gather information on predation risk and use it to modulate their behavioural response against predators in the short term. Examples of heterospecific eavesdropping on predation risk cues can be found between species within the same taxa, as between bird [19] and mammal [20] species, or even between non-closely related taxa, such as reptiles and birds [21] or mammals and birds [22].
Although evidence suggests that eavesdroppers may tune their anti-predator behaviours in relation to others' alarm calls, it remains unknown as to whether perception of alarm calls may influence the organization of communities. Eavesdropping has been shown to have important ecological consequences in various contexts [23,24] such as in breeding territory choice [25–27]. On the other hand, local predation risk is an essential component of habitat suitability [28,29]. However, it is unknown whether coexisting preys could rely on alarm calls from both con- and hetero-specifics to evaluate predation risk when choosing a breeding territory. Here, we demonstrate for the first time, to our knowledge, one ecological consequence of reactions to alarm calls by testing whether two medium-sized coexisting raptors, one resident and one migratory species, cue on alarm calls of the resident species to assess breeding site quality. Specifically, we studied the little owl (Athene noctua)—scops owl (Otus scops) dyad, two nocturnal birds of prey that breed sympatrically in the south of Spain [30] and that base intraspecific communication on vocal cues [31,32]. Before bird settlement, we experimentally increased the perception of predation risk at a patch scale by broadcasting little owl alarm calls. Resident little owls are supposed to be more familiar with and more accurately reveal habitat quality than migrant scops owls because they have winter time to update the information gathered during the previous year [26]. We expected that settling individuals of the two species avoided areas with playbacks of alarm calls because these calls are likely to indicate dangerous areas. In addition, individuals finally settled in dangerous areas are expected to lay smaller clutches in accordance with life-history theory [33]. Whether these expectations should affect more conspecifics or heterospecifics is not clear for us a priori. On the one hand, heterospecifics may provide secondary information compared with conspecifics because conspecifics share more needs than heterospecifics [34]. On the other hand, information produced by heterospecifics has been suggested to be more likely used when individuals of one species are worse informed than the other species [35], as scops owls must be compared with little owls owing to their migratory status. Therefore, our experimental set-up allowed us to determine whether little owl alarm calls are used as a proxy of patch predation risk influencing breeding territory choice and subsequent reproductive strategies in conspecific and heterospecific owls.
2. Methods
(a). Study system
The study was conducted in the breeding season (April–July) of 2010 in southeastern Spain (37°18′ N, 3°11′ W). The area covers approximately 756 ha of holm oak Quercus ilex wooded landscape where cork oak nest-boxes (roof surfaces of 24 × 24 cm, 40 cm height and an opening of 6 cm diameter) were held on trees. The site harbours a cavity-nesting bird community that includes the two study owl species (little and scops owls) and the diurnal European roller Coracias garrulus. Breeding densities of little and scops owls in the study are 0.024 and 0.02 pairs per hectare, respectively (see [30] for details).
Little and scops owls are medium-sized (157 g and 91 g, respectively) territorial species differing in migratory status. Little owls are year-round residents, whereas scops owls are transaharian migrants arriving throughout April into the study area [36]. Thus, scops owls should have less accurate information on breeding habitat suitability than little owls owing to time constraints imposed by migration [26]. Both species are secondary hole nesting birds that readily use nest-boxes for breeding [36]. Little and scops owls also share predators. Indeed, these two owl species are common prey of tawny Strix aluco [37,38] and eagle owls Bubo bubo [39]. Moreover, serpents and rodents are common predators of their nest contents in our study area (D. Parejo, J. M. Avilés & J. Rodríguez 2010, unpublished data).
Up to 22 types of vocalizations, which vary according to sex, age and context, have been described in the little owl [40]. Thus, little owl vocalizations seem to encode valuable information and their use appears to be comparable to that reported in oscine birds. As alarm calls, little owls emit a loud, chattering ‘kek kek’ or a short, explosive, high-pitched ‘chi chi chi-chi’ when disturbed at the nests. Calls include ‘queb’ and/or ‘keck’ sounds said to express unease or fear [36].
(b). Experimental design
Nest-boxes were assigned by proximity to plots (the mean number of nest-boxes per plot was 5.4 and ranged from three to nine nest-boxes, n = 161 nest-boxes in 30 plots). Plots were separated by at least 300 m and nest-boxes within each plot were separated by 50–100 m of each other. Plots were spatially grouped in triads to avoid possible spatial influence. That is, by spatially matching the treatments, we wanted to remove the possible effect of the area on the effect of the treatment if plots assigned to the same treatment were spatially grouped. Within each triad, plots were randomly assigned to one of the following treatments: (i) ‘Risky’, in which we artificially increased perceived predation risk by broadcasting little owl alarm calls; (ii) ‘Non-risky’, in which we did not modify perception of predation risk but played back little owl contact calls as a control of playback experiments; and, (iii) ‘Control’, in which we did not broadcast any playback but visited as frequently as Risky and Non-risky plots.
The experiment started on 20 April, when the two owl species are evaluating breeding territories but have not started reproduction yet [30]. Treatments were applied during 15 days, i.e. from 20 April to 5 May, in alternative days in Risky and Non-risky plots and half of the Control plots. We confirmed that the manipulation was perceived by the two owl species because little owls began breeding in most of the plots during the two manipulation weeks (mean laying dates ± se: 29 April ± 1.6 days) and because we recorded vocal displays of scops owls in most occupied plots between 20 April and 5 May.
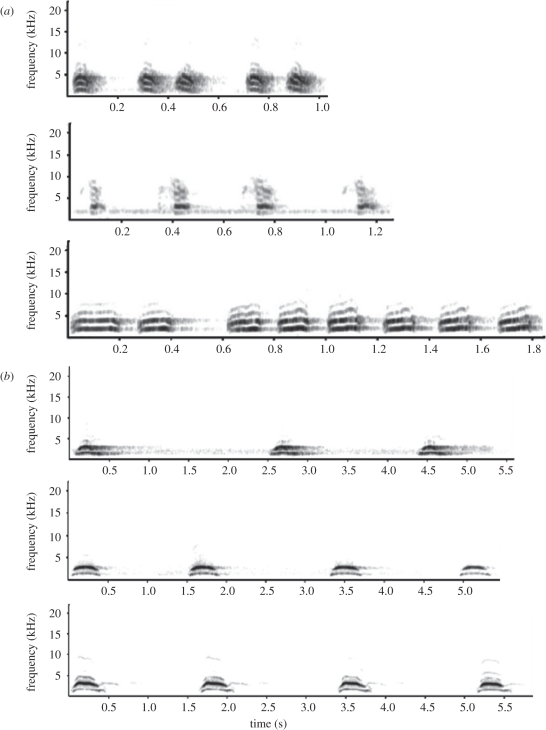
Little owl vocalizations (alarm calls in Risky plots and contact calls in Non-risky plots) were broadcast during 3 h at dusk on alternative days at the core of plots. For that purpose, we used portable amplified speakers connected to digital audio players equipped with track repeat and random track selection functions. We used recordings of little owl alarm calls (figure 1a) and of contact calls (figure 1b) extracted from Llimosa et al. [41]. Spectrograms of alarm calls, although showing some degree of variation, are clearly different from contact calls. Alarm calls all have syllables with wider bands that are repeated at a higher rate than contact calls (figure 1). Among the available recordings of little owl calls we selected the highest quality ones to be broadcast, which led us to use in playbacks three recordings of each type. We disregarded recording calls of local little owls as this could have induced data biases as a consequence of discrimination of neighbour versus stranger calls in our experiment [32]. To completely avoid pseudoreplication we would have needed 80 different alarm and 80 contact little owl calls (i.e. playbacks were broadcasted eight times in each plot and there were 10 plots assigned to each experimental treatment) which is not practical given that recordings of owl calls are so rare. Therefore, as the best way to reduce the chance of pseudoreplication given the small number of available recordings, we decided to use the three alarm calls or the three contact calls in all Risky and Non-risky playback tracks. The use of the three recordings in each treatment produces an unique assortment of calls by their randomized presentation and combination with silence tracks for each day of treatment and plot. Similar approaches have been used in previous studies [42–44]. Approximately, 1.5–3 min of uncompressed audio files with the recordings of the various calls were interspersed with periods of silence that varied from 3 to 8 min. Little owl calls and silent periods were recorded in separate tracks so that the exact sequence of calls and silences was randomly determined by selecting the random playback mode. We measured playbacks amplitude by using a sound-level metre (Velleman Inc., AVM2050, weighting level A, fast response). Average playback volume was 89.1 (±1.1) dB measured 1 m away from the speaker. Such playback volume was judged by ear to be similar to the natural production of real calls.
Figure 1.
Spectrograms of the three alarm (a) and the three contact (b) calls of little owls used in this study. Calls were prepared in Avisoft, with settings as described in the text.
Plots were visited weekly from 20 April to the end of July to record occupation (a nest-box was defined as occupied when at least one egg was laid in it), owl species, laying dates and reproductive parameters.
Response to the broadcast experiment was evaluated using first three estimators of breeding habitat preference: (i) the earliest laying date of each owl species per plot in the knowledge that laying date correlates with habitat preference as high-quality habitats are occupied first [45,46]; (ii) the occupation probability of a plot by each owl species, i.e. whether at least one breeding pair settled in a plot, because it reflects actual territory choice; and (iii) the species specific rate of occupation of all nest-boxes in a plot (i.e. the proportion of nest-boxes occupied by each species per plot) in the knowledge that high-quality habitats should be more occupied [46]. Additionally, as birds may respond to predation risk by modifying their breeding strategy [30,42], we also measured clutch size for each reproductive event of the two owl species.
(c). Statistical Analyses
Analyses were performed using SAS v. 9.1 statistical software (SAS 2001 Institute, Cary, NC, USA).
General linear models (GLM SAS procedure) were used to investigate whether the plot treatment affected (i) the earliest laying date of each owl species within each plot, (ii) the plot occupation rate (arcsin transformed) by each species, and (iii) the mean clutch size of the two owl species. In these last analyses, laying date was introduced as a covariate because it is usually correlated to clutch size. We checked for differences between pairs of treatments by performing Scheffé tests.
We performed generalized linear models (Genmod SAS procedure) to contrast plot occupation probability between different treatments by the two owl species. We compared least-squared means of each treatment to check for pairwise differences.
3. Results
Twenty-eight out of the 30 plots were occupied by at least one of the two owl species. Mean number of occupied territories per plot was 1.93, rendering an average occupation of 38%.
(a). Response to conspecific alarm cues
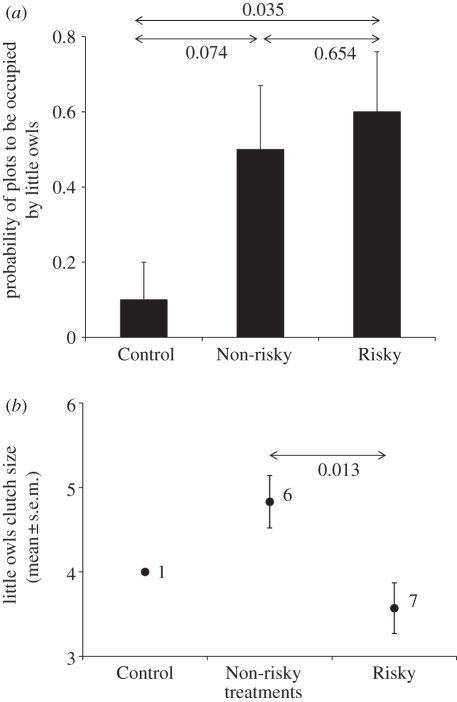
The earliest laying date of little owls in each plot was not affected by the treatment (general linear model: F2,9 = 0.36, p = 0.71). However, the probability of a plot to be occupied by little owls differed among experimental treatments (figure 2a, generalized linear model: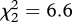 , p = 0.038). Probability of occupation by little owls was higher in Risky than in Control plots and tended to be also higher in Non-risky than in Control plots (figure 2a). Plot occupation rate by little owls was not affected by the treatment (general linear model: F2,27 = 0.29, p = 0.75).
, p = 0.038). Probability of occupation by little owls was higher in Risky than in Control plots and tended to be also higher in Non-risky than in Control plots (figure 2a). Plot occupation rate by little owls was not affected by the treatment (general linear model: F2,27 = 0.29, p = 0.75).
Figure 2.
Evidence of conspecific eavesdropping on alarm cues for breeding habitat choice. (a) Probability of occupation of plots by at least one little owl breeding pair in each treatment (n = 30 plots, 10 plots per treatment). (b) Mean clutch size of little owls in relation to treatments. Numbers near mean values indicate n = number of nests. Pairwise differences are shown, with arrows designating pairs, with the exception of the Control treatment in (b) (small sample size).
Owing to positive selection of little owls by Risky and Non-risky plots when compared with Control plots, sample size for clutch size in Control plots was insufficient to perform statistical analyses (figure 2b). Therefore, we only could compare mean clutch size of little owls between Risky and Non-risky plots. We found that clutches were larger in Non-risky than in Risky plots (general linear model: treatment effect, F1,11 = 8.64, p = 0.01; laying date effect: F1,10 = 0.52, p = 0.49; figure 2b).
(b). Response to heterospecific alarm cues
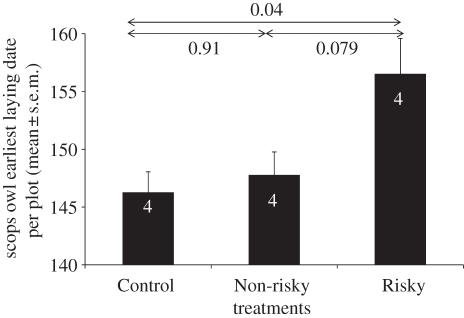
The treatment affected the date of first occupation by scops owls in each plot (general linear model: F2,9 = 5.45, p = 0.028). Scops owls installed first in Control and Non-risky plots than in Risky ones (figure 3). On the other hand, neither the probability of a plot to be occupied by scops owls (generalized linear model: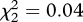 , p = 0.98), plot occupation rate by this species (general linear model: F2,27 = 0.01, p = 0.99), nor clutch size varied in relation to treatments (general linear model: treatment effect, F2,7 = 1.31, p = 0.33; laying date effect: F1,9 = 5.66, p = 0.04).
, p = 0.98), plot occupation rate by this species (general linear model: F2,27 = 0.01, p = 0.99), nor clutch size varied in relation to treatments (general linear model: treatment effect, F2,7 = 1.31, p = 0.33; laying date effect: F1,9 = 5.66, p = 0.04).
Figure 3.
Evidence of heterospecific eavesdropping on alarm cues for breeding habitat choice. Mean scops owl earliest laying dates per plot are shown for each experimental treatment applied. Numbers inside bars are n = number of plots. Pairwise differences are shown, with arrows designating pairs.
4. Discussion
Numerous studies have investigated eavesdropping on mammal and bird alarm calls and showed that such a mechanism may elicit short-term behavioural responses against predation. Previous studies, however, have disregarded the ecological consequences of eavesdropping on alarm calls in terms of community organization. Our objective here was to examine whether alarm calls of a resident species in an owl community, the little owl, are used to assess habitat quality, and subsequently choose breeding sites by conspecifics, and by one migratory bird species, the scops owl. We aimed to manipulate owl perception of predation risk at the patch scale by broadcasting either little owl alarm or contact calls during the pre-breeding period in breeding areas.
(a). Breeding habitat selection and conspecific eavesdropping on alarm cues
Contrary to our expectations, little owls preferred to settle down in plots in which conspecifics' vocalizations were broadcast (Risky and Non-risky plots) because these plots showed higher probabilities to be occupied by little owls compared with plots where information on conspecifics was unavailable (Control plots). Several non-exclusive explanations can be given to this result. (i) Little owls are unable to interpret information conveyed by the different calls of conspecifics and their preference for plots with little owl's vocalizations is only based on conspecific attraction. The presence of conspecifics can be easily detected and individuals could use this information to make rapid settlement decisions [47]. At moderate population densities, relying on local conspecific abundance to assess habitat quality may be a good strategy [48] which may provide advantages such as enhanced mating success and other positive density dependence effects (i.e. Allée effect [47]). However, clutch size of little owls differed between Risky and Non-risky plots, suggesting that little owls are able to decode the information conveyed in the two calls. (ii) Alternatively, little owls could be able to interpret information encoded in conspecific calls but they could choose to settle down in Risky and Non-risky plots because conspecific vocalizations indicate high conspecific densities in the two types of plots. This would mean that the advantages of breeding near conspecifics surpass the disadvantages of high local predation risk. This would be also the case if little owls perceived vocalizations of foreigner little owls as a measure of intrusion risk and hence responded to them by settling in those plots with a high perceived competition risk, i.e. in Risky and Non-risky plots, to defend their ownership. This possibility is plausible since little owls distinguish between vocalizations of neighbours and strangers [32]. (iii) Finally, the different little owl calls used in the experiment could not reliably convey information on differences in predation risk. However, little owls laid larger clutches in Non-risky than in Risky plots. As different plots only differed in the experimental treatment applied, it seems clear that different little owl calls used in this study are informative to conspecifics bystanders.
Life-history theory predicts a reduced investment in the current reproduction in response to increased predation risk [33]. Accordingly, we found that little owls laid larger clutches in Non-risky than in Risky plots. Reduced investment might aim to shorten the time the nest is exposed to predation [49], to diminish the number of parental feeding trips to nests that could attract predators [50], and to save energy for re-nesting in the event of failure [51]. Alternatively, differences in clutch size may be explained by the non-random distribution of individuals among plots differing in predation risk [52] whenever plots with elevated predation risk were mainly occupied by suboptimal breeders that would lay smaller clutches. In any case, this result evidences that little owls can interpret information conveyed by conspecifics calls and use it in their reproductive decisions.
(b). Breeding habitat selection and heterospecific eavesdropping on alarm cues
Scops owls preferred safe (Non-risky and Control plots) to risky (Risky plots) territories as revealed by little owls' calls. Heterospecific attraction has been shown between several bird species [26,53,54], mainly between resident and migratory birds [26]. However, although scops owls are migrants that could potentially rely on cues provided by resident little owls, our results cannot be owing to scops owls copying little owl preferences because little owls preferred Risky and Non-risky plots (i.e. plots in which vocalizations were broadcasted) to Control plots, whereas scops owls preferred Non-risky plots. Alternatively, scops owls could be avoiding competition with little owls by choosing the less preferred plots by this last species (i.e. Control plots). However, this is not a possible explanation to our results because scops owls should have chosen Control plots instead of Non-risky plots. Therefore, our result includes that scops owls are able to extract valuable information from little owls' calls during the assessment of breeding sites. Previous studies have shown that both conspecifics and heterospecifics may easily recognize others' alarm calls [17,19,55], and make finely tuned adjustments of their behavioural responses [6,19,20,54]. Furthermore, scops owls are migrant birds that could be attentive to the anti-predator cues of residents to gain information about local predation risk [56]. As the use of this kind of information by migrants is less costly than inspecting predation risk directly, it is likely to be common in nature [56]. Therefore, here we illustrate for the first time, to our knowledge, one possible ecological consequence of reactions to heterospecific alarm calls by showing how little owls' alarm calls are used by scops owls as a source of social information on local predation risk during the breeding habitat selection process.
The fact that we used recordings of foreign little owls in our experimental design gives an additional dimension to the informative content of calls. Thereby, little owls' calls might provide information on conspecific intrusion risk as well as on predation risk, which could lead to an unexpected response of conespecifics to the experimental treatment as is the case here. For scops owls, however, little owls' calls are likely to inform on only predation risk and thus heterospecific scops owls are likely to respond only to that information.
Our findings may have important implications for community organization. The use of information provided by heterospecifics in breeding habitat selection might either increase competition by the coincident choice of breeding sites by different species, or it might facilitate species coexistence, thereby affecting the entire community dynamics [35]. Additionally, these findings also have a conservationist side because, whenever competitors provide one another useful information, declines in the abundance of one species may prejudice the other. This could help to understand scenarios in which different species within a community show parallel population declines. Moreover, conservation strategies manipulating social information to promote settlement of declining species in suitable habitats [57,58] may have non-target effects on other members of the community [48].
Finally, this study raises an interesting additional question. Heterospecific eavesdropping on alarm calls may have consequences on the evolution of alarm calls as signals. Eavesdropping might lead to increased levels of intra- and interspecific competition, and consequently to reduced fitness, owing to the choice of the same sites by individuals of the same and different species [58]. Therefore, heterospecifics and predators eavesdropping, could synergistically act as selective forces diminishing alarm calls' conspicuousness.
Acknowledgements
The study was conducted under license of the Junta de Andalucia, Spain.
We are grateful to Andrew Radford, who acted as Associate Editor, and two anonymous referees for constructive and useful comments on the manuscript. Financial support was received from the Spanish Ministry of Science and Innovation/FEDER (Projects CGL2008-00718 and CGL2011-27561) to J.M.A. and D.P. and PIE No. 200930I029 to J.M.A. and J.R.
References
- 1.Elton C. 1927. Animal ecology. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Lima S. L., Dill L. M. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 (doi:10.1139/z90-092) [DOI] [Google Scholar]
- 3.Caro T. 2005. Antipredator defenses in birds and mammals. Chicago, IL: Chicago University Press [Google Scholar]
- 4.Dall S. R. X., Giraldeau L. A., Olsson O., McNamara J. M., Stephens D. W. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 10.1016/j.tree.2005.01.010 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 5.Searcy W. A., Nowicki S. 2005. The evolution of animal communication. New York, NY: Princeton University Press [Google Scholar]
- 6.Weary D. M., Kramer D. L. 1995. Response of eastern chipmunks to conspecific alarm calls. Anim. Behav. 49, 81–93 10.1016/0003-3472(95)80156-1 (doi:10.1016/0003-3472(95)80156-1) [DOI] [Google Scholar]
- 7.Leavesley A. J., Magrath R. D. 2005. Communicating about danger: urgency alarm calling in a bird. Anim. Behav. 70, 365–373 10.1016/j.anbehav.2004.10.017 (doi:10.1016/j.anbehav.2004.10.017) [DOI] [Google Scholar]
- 8.Curio E., Ernst U., Vieth W. 1978. Cultural transmission of enemy recognition: one function of mobbing. Science 202, 899–901 10.1126/science.202.4370.899 (doi:10.1126/science.202.4370.899) [DOI] [PubMed] [Google Scholar]
- 9.Hasson O. 1991. Pursuit-deterrent signals: communication between prey and predator. Trends Ecol. Evol. 6, 325–329 10.1016/0169-5347(91)90040-5 (doi:10.1016/0169-5347(91)90040-5) [DOI] [PubMed] [Google Scholar]
- 10.Walther F. R. 1969. Flight behaviour and avoidance of predators in Thomsons gazelle (Gazella thomsoni Guenther 1884). Behaviour 34, 184–186 10.1163/156853969X00053 (doi:10.1163/156853969X00053) [DOI] [Google Scholar]
- 11.Dawkins R. 1976. The selfish gene. Oxford, UK: Oxford University Press [Google Scholar]
- 12.Zuberbuhler K., Jenny D., Bshary R. 1999. The predator deterrence function of primate alarm calls. Ethology 105, 477–490 10.1046/j.1439-0310.1999.00396.x (doi:10.1046/j.1439-0310.1999.00396.x) [DOI] [Google Scholar]
- 13.Fallow P. M., Magrath R. D. 2010. Eavesdropping on other species: mutual interspecific understanding of urgency information in avian alarm calls. Anim. Behav. 79, 411–417 10.1016/j.anbehav.2009.11.018 (doi:10.1016/j.anbehav.2009.11.018) [DOI] [Google Scholar]
- 14.Manser M. B., Bell M. B., Fletcher L. B. 2001. The information that receivers extract from alarm calls in suricates. Proc. R. Soc. Lond. B 268, 2485–2491 10.1098/rspb.2001.1772 (doi:10.1098/rspb.2001.1772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyfarth R. M., Cheney D. L., Marler P. 1980. Monkey responses to 3 different alarm calls: evidence of predator classification and semantic communication. Science 210, 801–803 10.1126/science.7433999 (doi:10.1126/science.7433999) [DOI] [PubMed] [Google Scholar]
- 16.Manser M. B. 2001. The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. Lond. B 268, 2315–2324 10.1098/rspb.2001.1773 (doi:10.1098/rspb.2001.1773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Templeton C. N., Greene E., Davis K. 2005. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937 10.1126/science.1108841 (doi:10.1126/science.1108841) [DOI] [PubMed] [Google Scholar]
- 18.Griesser M. 2008. Referential calls signal predator behavior in a group-living bird species. Curr. Biol. 18, 69–73 10.1016/j.cub.2007.11.069 (doi:10.1016/j.cub.2007.11.069) [DOI] [PubMed] [Google Scholar]
- 19.Templeton C. N., Greene E. 2007. Nuthatches eavesdrop on variations in heterospecific chickadee mobbing alarm calls. Proc. Natl Acad. Sci. USA 104, 5479–5482 10.1073/pnas.0605183104 (doi:10.1073/pnas.0605183104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shriner W. M. 1998. Yellow-bellied marmot and golden-mantled ground squirrel responses to heterospecific alarm calls. Anim. Behav. 55, 529–536 10.1006/anbe.1997.0623 (doi:10.1006/anbe.1997.0623) [DOI] [PubMed] [Google Scholar]
- 21.Vitousek M. N., Adelman J. S., Gregory N. C., St Clair J. J. H. 2007. Heterospecific alarm call recognition in a non-vocal reptile. Biol. Lett. 3, 632–634 10.1098/rsbl.2007.0443 (doi:10.1098/rsbl.2007.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyfarth R., Cheney D. 1990. The assessment by vervet monkeys of their own and another species alarm calls. Anim. Behav. 40, 754–764 10.1016/S0003-3472(05)80704-3 (doi:10.1016/S0003-3472(05)80704-3) [DOI] [Google Scholar]
- 23.Goodale E., Beauchamp G., Magrath R. D., Nieh J. C., Ruxton G. D. 2010. Interspecific information transfer influences animal community structure. Trends Ecol. Evol. 25, 354–361 10.1016/j.tree.2010.01.002 (doi:10.1016/j.tree.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 24.Schmidt K. A., Dall S. R. X., Van Gils J. A. 2010. The ecology of information: an overview on the ecological significance of making informed decisions. Oikos 119, 304–316 10.1111/j.1600-0706.2009.17573.x (doi:10.1111/j.1600-0706.2009.17573.x) [DOI] [Google Scholar]
- 25.Doligez B., Danchin E., Clobert J. 2002. Public information and breeding habitat selection in a wild bird population. Science 297, 1168–1170 10.1126/science.1072838 (doi:10.1126/science.1072838) [DOI] [PubMed] [Google Scholar]
- 26.Monkkönen M., Helle P., Soppela K. 1990. Numerical and behavioral-responses of migrant passerines to experimental manipulation of resident tits (Parus spp): heterospecific attraction in northern breeding bird communities. Oecologia 85, 218–225 10.1007/BF00319404 (doi:10.1007/BF00319404) [DOI] [PubMed] [Google Scholar]
- 27.Parejo D., White J., Clobert J., Dreiss A., Danchin E. 2007. Blue tits use fledgling quantity and quality as public information in breeding site choice: an experiment. Ecology 88, 2373–2382 10.1890/06-2000.1 (doi:10.1890/06-2000.1) [DOI] [PubMed] [Google Scholar]
- 28.Morosinotto C., Thomson R. L., Korpimaki E. 2010. Habitat selection as an antipredator behaviour in a multi-predator landscape: all enemies are not equal. J. Anim. Ecol. 79, 327–333 10.1111/j.1365-2656.2009.01638.x (doi:10.1111/j.1365-2656.2009.01638.x) [DOI] [PubMed] [Google Scholar]
- 29.Thomson R. L., Forsman J. T., Sarda-Palomera F., Monkkönen M. 2006. Fear factor: prey habitat selection and its consequences in a predation risk landscape. Ecography 29, 507–514 10.1111/j.0906-7590.2006.04568.x (doi:10.1111/j.0906-7590.2006.04568.x) [DOI] [Google Scholar]
- 30.Parejo D., Avilés J. M. 2011. Predation risk determines breeding territory choice in a Mediterranean cavity-nesting bird community. Oecologia 165, 185–191 10.1007/s00442-010-1723-0 (doi:10.1007/s00442-010-1723-0) [DOI] [PubMed] [Google Scholar]
- 31.Galeotti P., Sacchi R., Perani E. 1997. Cooperative defense and intrasexual aggression in scops owls (Otus scops): responses to playback of male and female calls. J. Raptor Res. 31, 353–357 [Google Scholar]
- 32.Hardouin L. A., Tabel P., Bretagnolle V. 2006. Neighbour–stranger discrimination in the little owl, Athene noctua. Anim. Behav. 72, 105–112 10.1016/j.anbehav.2005.09.020 (doi:10.1016/j.anbehav.2005.09.020) [DOI] [Google Scholar]
- 33.Roff D. A. 1992. The evolution of life histories . Theory and analyses London, UK: Chapman and Hall [Google Scholar]
- 34.Parejo D., Danchin E., Avilés J. M. 2005. The heterospecific habitat copying hypothesis: can competitors indicate habitat quality? Behav. Ecol. 16, 96–105 10.1093/beheco/arh136 (doi:10.1093/beheco/arh136) [DOI] [Google Scholar]
- 35.Seppänen J.-T., Forsman J. T., Mönkkönen M., Thomson R. L. 2007. Social information use is a process across space, time and ecology, reaching heterospecifics. Ecology 88, 1622–1633 10.1890/06-1757.1 (doi:10.1890/06-1757.1) [DOI] [PubMed] [Google Scholar]
- 36.Cramp S., Simmons K. E. L. 1988. The birds of the western Palearctic. Oxford, UK: Oxford University Press [Google Scholar]
- 37.Zuberogoitia I., Martínez J. A., Zabala J., Martínez J. E. 2005. Interspecific aggression and nest-site competition in an European owl community. J. Raptor Res. 39, 156–159 [Google Scholar]
- 38.Sergio F., Luigi M., Pedrini P. 2009. Conservation of scops owl Otus scops in the Alps: relationships with grassland management, predation risk and wider biodiversity. Ibis 151, 40–50 10.1111/j.1474-919X.2008.00865.x (doi:10.1111/j.1474-919X.2008.00865.x) [DOI] [Google Scholar]
- 39.Penteriani V., Delgado M. M. 2010. Búho real: Bubo bubo. In Enciclopedia virtual de los vertebrados españoles (eds Carrascal L. M., Salvador A.), pp. 1–39 Madrid, Spain: Museo Natural de Ciencia Naturales; (http://www.vertebradosibericos.org) [Google Scholar]
- 40.Exo K. M., Scherzinger W. 1989. Voice and inventory of call-notes of the little owl (Athene noctua): description, context and habitat adaptation. Ecology of birds 11, 149–187 [Google Scholar]
- 41.Llimosa F., Matheu E., Roché J. 1999. Guía sonora de las Aves de España. Barcelona, Spain: Alosa [Google Scholar]
- 42.Eggers S., Griesser M., Nystrand M., Ekman J. 2006. Predation risk induces changes in nest-site selection and clutch size in the Siberian jay. Proc. R. Soc. B 273, 701–706 10.1098/rspb.2005.3373 (doi:10.1098/rspb.2005.3373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt K. A. 2006. Non-additivity among multiple cues of predation risk: a behaviorally-driven trophic cascade between owls and songbirds. Oikos 113, 82–90 10.1111/j.0030-1299.2006.14167.x (doi:10.1111/j.0030-1299.2006.14167.x) [DOI] [Google Scholar]
- 44.Bell M. B. V., Radford A. N., Rose R., Wade H. M., Ridley A. R. 2009. The value of constant surveillance in a risky environment. Proc. R. Soc. B 276, 2997–3005 10.1098/rspb.2009.0276 (doi:10.1098/rspb.2009.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alatalo R. V., Lundberg A., Glynn C. 1986. Female pied flycatchers choose territory quality and not male characteristics. Nature 323, 152–153 10.1038/323152a0 (doi:10.1038/323152a0) [DOI] [Google Scholar]
- 46.Fretwell S. D. 1972. Populations in a seasonal environment. New Jersey, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 47.Stamps J. A. 1988. Conspecific attraction and aggregation in territorial species. Am. Nat. 131, 329–347 10.1086/284793 (doi:10.1086/284793) [DOI] [Google Scholar]
- 48.Fletcher R. J., Jr 2007. Species interactions and population density mediate the use of social cues for habitat selection. J. Anim. Ecol. 76, 598–606 10.1111/j.1365-2656.2007.01230.x (doi:10.1111/j.1365-2656.2007.01230.x) [DOI] [PubMed] [Google Scholar]
- 49.Roff D. A., Remes V., Martin T. E. 2005. The evolution of fledging age in songbirds. J. Evol. Biol. 18, 1425–1433 10.1111/j.1420-9101.2005.00958.x (doi:10.1111/j.1420-9101.2005.00958.x) [DOI] [PubMed] [Google Scholar]
- 50.Martin T. E., Scott J., Menge C. 2000. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc. R. Soc. Lond. B 267, 2287–2293 10.1098/rspb.2000.1281 (doi:10.1098/rspb.2000.1281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin T. E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127 10.2307/2937160 (doi:10.2307/2937160) [DOI] [Google Scholar]
- 52.Carrete M., Tella J. L. 2010. Individual consistency in flight initiation distances in burrowing owls: a new hypothesis on disturbance-induced habitat selection. Biol. Lett. 6, 167–170 10.1098/rsbl.2009.0739 (doi:10.1098/rsbl.2009.0739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elmberg J., Poysa H., Sjoberg K., Nummi P. 1997. Interspecific interactions and co-existence in dabbling ducks: observations and an experiment. Oecologia 111, 129–136 10.1007/s004420050216 (doi:10.1007/s004420050216) [DOI] [PubMed] [Google Scholar]
- 54.Goodale E., Kotagama S. W. 2005. Testing the roles of species in mixed-species bird flocks of a Sri Lankan rain forest. J. Trop. Ecol. 21, 669–676 10.1017/S0266467405002609 (doi:10.1017/S0266467405002609) [DOI] [Google Scholar]
- 55.Hurd C. R. 1996. Interspecific attraction to the mobbing calls of black-capped chickadees (Parus atricapillus). Behav. Ecol. Sociobiol. 38, 287–292 10.1007/s002650050244 (doi:10.1007/s002650050244) [DOI] [Google Scholar]
- 56.Nocera J. J., Taylor P. D., Ratcliffe L. M. 2008. Inspection of mob-calls as sources of predator information: response of migrant and resident birds in the Neotropics. Behav. Ecol. Sociobiol. 62, 1769–1777 10.1007/s00265-008-0605-5 (doi:10.1007/s00265-008-0605-5) [DOI] [Google Scholar]
- 57.Betts M. G., Hadley A. S., Rodenhouse N., Nocera J. J. 2008. Social information trumps vegetation structure in breeding-site selection by a migrant songbird. Proc. R. Soc. B 275, 2257–2263 10.1098/rspb.2008.0217 (doi:10.1098/rspb.2008.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward M. P., Schlossberg S. 2004. Conspecific attraction and the conservation of territorial songbirds. Conserv. Biol. 18, 519–525 10.1111/j.1523-1739.2004.00494.x (doi:10.1111/j.1523-1739.2004.00494.x) [DOI] [Google Scholar]