Abstract
Patients with COPD are frequently prescribed inhaled corticosteroids (ICS); however, it is unclear whether the treatment with ICS might modify responses to inhaled bronchodilators. Two 6-month, randomized, placebo-controlled, double-blind, double-dummy, parallel-group studies of tiotropium 18 μg once daily, compared with salmeterol, 50 μg bid, had been conducted in patients with moderate-to-severe COPD. Efficacy was assessed by spirometry, transition dyspnea index (TDI), St. George’s Respiratory Questionnaire (SGRQ), and exacerbations. Data from both studies were combined to form subgroups with regard to concurrent use of ICS. 796 patients receiving ICS were separately analyzed from 390 patients not receiving ICS. Mean age was 64 years, and pre-bronchodilator FEV1 was 1.06 L (ICS group) and 1.13 L (non-ICS group). Both bronchodilators increased morning mean ± SE pre-dose FEV1 compared with placebo (ICS groups: tiotropium 110 ± 20 mL, salmeterol 80 ± 20 mL; non-ICS groups: tiotropium 150 ± 30 mL, salmeterol 110 ± 30 mL; p > 0.05 for tiotropium vs salmeterol). Improvements in TDI and SGRQ and frequency of exacerbations also tended to be more profound for tiotropium. Treatment with tiotropium in patients with moderate-to-severe COPD was superior to salmeterol in lung function, irrespective of concurrent use of ICS.
Keywords: chronic obstructive pulmonary disease, inhaled corticosteroids, salmeterol, tiotropium, fluticasone
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by a chronic progressive deterioration in lung function and commonly results in dyspnea on activity, and impaired health status. In addition, periodic worsenings of the disease, referred to as exacerbations, occur with increasing frequency as the disease advances and are felt to adversely affect health status and to hasten the already accelerated decline in lung function seen in COPD patients (Seemungal et al 1998; Kanner et al 2001; Donaldson et al 2002). COPD guidelines currently advocate aggressive smoking cessation, certain non-pharmacologic management strategies such as exercise and pulmonary rehabilitation, and a stepped approach to pharmacotherapy. The latter includes initial treatment with short-acting bronchodilators, progressing to the addition of long-acting anticholinergic bronchodilators or long-acting beta2-adrenergic bronchodilators, or both, for patients with more advanced airway obstruction and persisting symptoms.
In contrast to current asthma guidelines, where inhaled corticosteroids (ICS) are central to therapy of all patients with regular symptoms (GINA 1995), current COPD-guidelines are more cautious regarding the role of ICS, recommending their use only in symptomatic COPD patients with a documented spirometric or clinical response to ICS, or in those patients with an FEV1 <50% predicted and who suffer repeated exacerbations requiring antibiotics and/or therapy with oral corticosteroids (Pauwels 2001; O’Donnell et al 2003; NICE 2004). Despite what guidelines recommend, ICS are commonly prescribed for patients with COPD, while the merits of this practice continue to be debated (Barnes 2000; Calverley 2000; Suissa 2006). This empiric practice may have some justification, as it has been demonstrated that withdrawal of ICS from patients with COPD is associated with an increase in exacerbation rates and worsening health status, as well as a deterioration in lung function and increased exertional breathlessness (O’Brien et al 2001; van der Valk et al 2002; Wouters et al 2005). A few studies (Paggiaro et al 1998; Weir et al 1999; Burge et al 2000; Lung Health Study Research Group 2000; Pauwels 2002) have suggested a beneficial effect from ICS at reducing the number of COPD exacerbations for patients with advanced disease (FEV1 <50% predicted), and a meta-analysis of such trials reported a 30% reduction in COPD exacerbations with ICS (Alsaeedi et al 2002). However, other randomized clinical trials have not shown benefit from ICS in COPD (Bourbeau et al 1998; Calverley et al 2003a; Szafranski et al 2003). Recently, the methodology of statistical analysis used in many of these trials has been questioned (Suissa 2006). According to this line of reasoning, conclusions concerning the effect of ICS on the frequency of COPD exacerbations should be considered flawed whenever the overall rate of COPD exacerbations has been calculated by treating all patients equally and simply averaging individual patient exacerbation rates. This so-called unweighted approach is overly sensitive to individual follow-up times which usually vary significantly within a given trial, and can therefore lead to a biased estimate of exacerbation rate ratios (Suissa 2006).
While long-acting beta2-adrenergics appear to be effective when added to inhaled steroids for many outcomes in patients with advanced COPD (Mahler et al 2002; Calverley et al 2003a, b, 2007), there are no data yet available assessing the efficacy of adding tiotropium, a long-acting anticholinergic inhaled bronchodilator for once-daily inhalation (Maesen et al 1995; Disse et al 1999; Littner et al 2000; Gross 2004) to ICS alone in COPD patients. At least in theory, if ICS could reduce airway inflammation, peripheral mucus plugging and airway wall compliance might be expected to improve, leading to a greater response to bronchodilator therapy. Whether differential effects might also be observed between different classes of bronchodilators is also unknown.
Donohue et al reported results from a placebo-controlled study over 6 months comparing tiotropium and the long-acting beta2-adrenergic, salmeterol, in patients with moderately advanced COPD (Donohue et al 2002, 2003). Tiotropium provided sustained and superior improvement in 12-hour lung function compared to salmeterol. When the data from this trial were combined with a similar 6-month trial in COPD patients for the analysis of patient-centred outcomes, additional benefits for tiotropium over salmeterol were indicated in symptoms, health status and the frequency of COPD exacerbations (Brusasco et al 2003). In these latter two studies, 66% of the patients enrolled had been prescribed ICS on a regular basis. The completion of these two placebo-controlled 6-month clinical studies has presented the opportunity to retrospectively analyze the relative efficacy of tiotropium and salmeterol as a function of the concomitant use of ICS in patients with moderately advanced COPD.
Patients and methods
Study design
This is a retrospective analysis of the pooled results of two similar 6-months, randomized, double-blind, double-dummy, placebo-controlled, parallel group studies of inhaled tiotropium bromide (18 μg once daily via the HandiHaler®) and salmeterol (50 μg twice daily via a CFC metered dose inhaler) in the treatment of patients with COPD (study codes 205.130 and 205.137). The only difference in the two studies was the duration of serial spirometry in the clinic (12 hours in one study, 3 hours in the second) (Brusasco et al 2003; Donohue et al 2002, 2003). The studies were conducted in 18 countries. All patients provided written consent and the studies were approved by the respective ethics committees and institutional review boards.
The study design has previously been described (Brusasco et al 2003; Donohue et al 2002, 2003). In brief, following an initial screening to assess eligibility, patients had entered a 2-week baseline period. Patients who successfully completed this phase were randomized into the double-blind portion of the study during which they received tiotropium, salmeterol or placebo for a period of 24 weeks. The drugs were administered in a double-dummy design with tiotropium or placebo inhalation capsules taken once daily in the morning and salmeterol or placebo inhalation aerosol taken twice daily (every 12 hours). Clinic visits were scheduled after 2, 8, 16, and 24 weeks following randomization.
Study patients
The main inclusion criteria were: age ≥40 years, a diagnosis of COPD with pre-bronchodilator FEV1 at screening visit of ≤60% predicted (calculated according to European Commission for Steel and Coal criteria and using predicted values from Morris) (Morris et al 1988; Quanier et al 1993) and screening FEV1/FVC <0.70. Patients were required to have a smoking history of ≥10 pack-years, and be able to perform all study related tests including acceptable pulmonary function tests and maintenance of daily diary card records. The main exclusion criteria were clinically significant diseases other than COPD which, in the opinion of the investigator, had the potential to impact on the patient’s ability to participate in the study, as well as known symptomatic prostatic hypertrophy, bladder neck obstruction, or narrow-angle glaucoma. Additionally, patients with a history of asthma, allergic rhinitis, or atopy or who had a total blood eosinophil count of ≥600/μL, patients requiring use of supplemental oxygen therapy for more than 1 hour per day, a history of life-threatening pulmonary obstruction, a history of cystic fibrosis or bronchiectasis, thoracotomy with pulmonary resection, upper respiratory infection in the 6 weeks prior to the screening visit or during the run-in period were also excluded.
Treatment with long-acting beta2-adrenergics and anticholinergics, other than study treatment, was discontinued at randomization. Patients were allowed to continue treatment with stable doses of ICS, theophyllines, and short-acting beta2-adrenergic medication as scheduled prior to the study. Patients using oral corticosteroids within or 6 weeks prior to the study were not included in the analysis.
Efficacy evaluations
Pulmonary function testing was conducted prior to the start of therapy at 60 and 10 minutes pre-dose at the randomization visit and at 30 minutes, 60 minutes, 2, and 3 hours post-dosing. As only one study collected spirometry after 3 hours during clinic visits, only spirometry up to 3 hours post-dosing has been considered for the present analysis. Pulmonary function tests were repeated at the same time intervals after 2, 8, 16, and 24 weeks of therapy. The bronchodilator efficacy of tiotropium and salmeterol was primarily determined by trough FEV1 and FVC response, which was defined as the change from baseline at the end of the dosing interval, approximately 24 hours post drug administration for tiotropium and approximately 12 hours post drug administration for salmeterol. Baseline values were the mean of the two pre-treatment values measured in the morning of the randomization visit prior to administration of the first dose of study medication. Other spirometric parameters analyzed included the peak response and AUC0–3 response.
Patient and investigator administered questionnaires, including the St. George’s Respiratory Questionnaire (SGRQ) (Jones et al 1991) and Mahler’s Dyspnea Index (Mahler et al 1984), were administered at baseline and after 8, 16, and 24 weeks of therapy. Requirement for as-needed salbutamol (albuterol) was monitored throughout the study period. Exacerbations were reported as adverse events and were defined as a complex of respiratory symptoms (ie, at least one of cough, sputum, dyspnea, wheeze, chest discomfort) lasting at least 3 days and usually associated with a therapeutic intervention.
Statistical analysis
Data from both studies were pooled for analysis. Patients receiving ICS at the time of randomization were separately analyzed from those patients without concurrent ICS treatment. For the spirometry measurements, SGRQ total score and TDI focal score, analysis of covariance was performed with the baseline as a covariate. In order to include the same patients at each time point in the 3 hour spirometry summaries, missing values were estimated using other values recorded for the patient on that test day. Linear interpolation between the two adjacent measurements was used to estimate random, middle, and missing spirometry measurements. For values at the end of the profiles that were missing because rescue medication was taken, the minimum observed FEV1 value on that test day was used as the estimate. The last available value was used as the estimate for data that were missing for other reasons.
For the SGRQ, missing individual questions were imputed according to the guidelines established by the questionnaire developer. Data from missing SGRQ questionnaires and TDI questions were imputed using last observation carried forward, unless the patient discontinued due to worsening of COPD, in which case the least favorable observation was carried forward. The numbers of exacerbations over the 6 months period were compared across treatment groups using Poisson regression with logarithm of exposure as offset and correction for overdispersion as recommended by Suissa (2006).
Unless otherwise specified, data are presented as means ± standard error. Statistical significance was considered at p < 0.05.
Results
A total 796 of 1207 patients (66%) in the two trials were concurrently treated with ICS. Demographic data and baseline characteristics for the treatment groups were comparable (Tables 1a, b). For patients concurrently treated with ICS, mean pre-bronchodilator FEV1 values were 39%, 38%, and 40% of predicted in the tiotropium, salmeterol, and placebo groups respectively. In general, at baseline, the group of patients taking ICS had slightly more airway obstruction and poorer health status scores than patients not on ICS (p < 0.05). The median daily doses and dose ranges of ICS used by the majority of these patients for at least 3 months prior to the study are shown in Table 2. Fluticasone was the most commonly used ICS (approximately 35%–40% in each group) with a median daily dose of 1000 μg. The apparent large dose ranges observed reflect extreme ICS dosing by one or two patients in each treatment group. Twenty-one patients from the original data sets, not taking ICS, but receiving regular treatment with oral corticosteroids, were excluded from the analysis. There were 168 patients concurrently taking ICS who were prematurely withdrawn from the studies for various reasons. Significantly fewer patients taking ICS withdrew in the tiotropium (17%) and salmeterol (19%) groups compared with placebo (27%) (p < 0.05). Similarly, 68 patients not taking ICS treatment did not complete the studies. Of those not taking ICS, significantly fewer patients in the tiotropium group (11%) withdrew compared with those in the placebo group (23%) (p < 0.05). The percentages of patients who withdrew in the salmeterol group (18%) were not significantly different from placebo. Overall, adverse events leading to discontinuation from the studies occurred in 9%, 16%, and 18% of ICS patients, and in 4%, 13%, and 15% of patients not using ICS, but these differences were not statistically significant. The main adverse event causing early withdrawal was worsening of COPD (ICS: 6% tiotropium, 12% salmeterol, 12% placebo; non-ICS groups: 3%, 6%, and 10%, respectively).
Table 1.
Patient characteristics at baseline
| (a) Patient characteristics (ICS users) at baseline (mean [SD]) | |||
| Tiotropium | Salmeterol | Placebo | |
| N | 259 | 278 | 259 |
| Mean age (yrs) | 64 (8) | 64 (8) | 65 (8) |
| Male (%) | 79 | 76 | 78 |
| Current smokers (%) | 41 | 29 | 36 |
| Smoking history (pack years) | 43 (23) | 44 (24) | 41 (23) |
| FEV1 (L) | 1.08 (0.37) | 1.03 (0.39) | 1.09 (0.40) |
| FEV1 (%predicted)a | 39 (12) | 38 (13) | 40 (13) |
| FVC (L) | 2.53 (0.71) | 2.51 (0.78) | 2.60 (0.80) |
| BDI (0–12) | 6.3 (2.2) | 6.2 (2.3) | 6.3 (2.2) |
| SGRQ (Total) (0–100) | 47 (17) | 47 (17) | 46 (16) |
| (b) Patient characteristics (ICS nonusers) at baseline (mean [SD]) | |||
| Tiotropium | Salmeterol | Placebo | |
| N | 135 | 120 | 135 |
| Mean age (yrs) | 64 (8) | 65 (8) | 64 (9) |
| Male (%) | 74 | 73 | 73 |
| Current smokers (%) | 46 | 52 | 53 |
| Smoking history (pack years) | 46 (22) | 47 (24) | 46 (23) |
| FEV1(L) | 1.18 (0.41) | 1.14 (0.39) | 1.07 (0.38) |
| FEV1 (%predicted)a | 43 (13) | 42 (12) | 40 (13) |
| FVC (L) | 2.63 (0.78) | 2.61 (0.73) | 2.48 (0.73) |
| BDI (0–12) | 6.7 (2.4) | 7.1 (2.2) | 6.9 (2.4) |
| SGRQ (Total) (0–100) | 42 (16) | 40 (17) | 43 (18) |
(Morris 1998).
(Morris 1998).
Table 2.
Median daily dose and ranges of ICS in treatment groups for patients concurrently taking ICS
| ICS type and median dose (μg/day) |
Tiotropium |
Salmeterol |
Placebo |
|||
|---|---|---|---|---|---|---|
| % patients | ICS dose range (μg/day) | % patients | ICS dose range(μg/day) | % patients | ICS dose range (μg/day) | |
| fluticasone (1000) | 41% | 100–1500 | 36% | 220–2000 | 44% | 100–2000 |
| budesonide (800) | 33% | 160–2400 | 35% | 50–2400 | 29% | 400–1800 |
| beclomethasone (1000) | 23% | 200–1600 | 25% | 100–2000 | 22% | 100–2250 |
Note: Percentages do not add up to 100% because of the use of other ICS (flunisolide, triamcinolone) by 2%–3% of patients in each treatment group.
Lung function responses
Patients treated with ICS
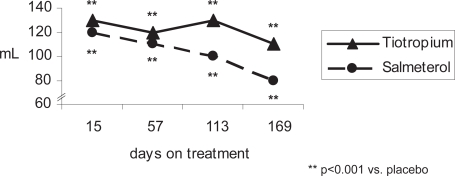
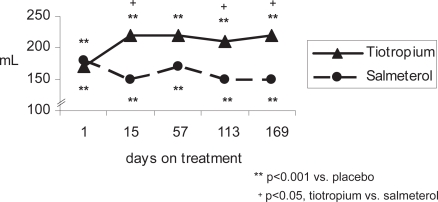
Compared with placebo, patients in both the tiotropium and salmeterol groups concurrently treated with ICS achieved statistically significant improvements in FEV1 and FVC (trough, peak and AUC0–3 hours) throughout the study (p ≤ 0.001, all comparisons at day 15, 57, 113, and 169) (Figure 1 and Table 3a). After 169 days of treatment, the mean ± SE improvement above placebo in trough FEV1 was 110 ± 20 mL for tiotropium (p < 0.001) and 80 ± 20 mL for salmeterol (p < 0.001) (Table 3a). The numerical difference (10–30 mL) in trough FEV1 between tiotropium and salmeterol was not significant at 6 months (p = 0.11 tiotropium vs salmeterol). Tiotropium was superior in improving trough FVC of patients using ICS from day 57 to 169 (p < 0.05, tiotropium vs salmeterol), and differences between active treatments were also statistically significant in favor of tiotropium for peak FEV1 and FVC at the first dose and at all subsequent visits (p < 0.05, tiotropium vs salmeterol). In addition, FEV1 AUC0–3, as well as FVC AUC0–3 were consistently statistically significant in favor of tiotropium (p < 0.01, tiotropium vs salmeterol) from day 15 to day 169.
Figure 1a.
Mean trough FEV1 response of active treatment groups above placebo (patients concurrently using ICS).
Table 3.
Outcomes in patients using ICS (tiotropium: n = 259; salmeterol: n = 278; placebo: n = 259)
| (a) Treatment response (spirometry, TDI, and SGRQ score) after 169 days | |||
| Tiotropium vs placebo | Salmeterol vs placebo | ||
| FEV1 | Trough | 110 ± 20** | 80 ± 20** |
| Peak | 220 ± 30**† | 150 ± 30** | |
| AUC 0–3 | 190 ± 20**† | 130 ± 20** | |
| FVC | Trough | 220 ± 40**† | 130 ± 40** |
| Peak | 400 ± 40**† | 220 ± 40** | |
| AUC0–3 | 370 ± 40**† | 230 ± 40** | |
| TDI focal score | 1.03 ± 0.4* | 0.57 ± 0.4 | |
| SGRQ total score | −3.27 ± 1.2* | −1.12 ± 1.2 | |
| (b) Treatment response (exacerbations) after 169 days | |||
| Tiotropium | Salmeterol | Placebo | |
| % patients with ≥1 exacerbation | 40% | 40% | 45% |
| Exacerbations/patient-year (% reduction vs placebo) | 1.36 (−23%)* | 1.46 (−17%)** | 1.76 |
| Exacerbation days/patient-year | 21.9 | 26.9 | 29.3 |
p < 0.05,
p < 0.001 vs placebo;
p < 0.05, tiotropium vs salmeterol.
p = 0.047,
p = 0.135 vs placebo.
Patients not treated with ICS
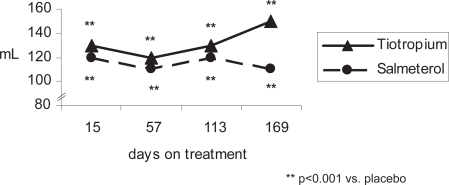
In those patients not taking ICS, the patterns of spirometric response according to treatment group were similar to those observed with the ICS group (Figure 2 and Table 4a). However, because of the smaller sample size (n = 360) relative to the ICS treated group (n = 796), there was insufficient power to detect statistically significant differences between bronchodilators in this group of patients.
Figure 2a.
Mean trough FEV1 response of active treatment groups above placebo (patients without ICS treatment).
Table 4.
Outcomes in patients not using ICS (tiotropium: n = 135; salmeterol: n = 120; placebo: n = 135)
| (a) Treatment response (spirometry, TDI, and SGRQ score) after 169 days | |||
| Tiotropium vs placebo | Salmeterol vs placebo | ||
| FEV1 | Trough | 150 ± 30* | 110 ± 30* |
| Peak | 220 ± 30*† | 150 ± 30* | |
| AUC 0–3 | 220 ± 30*† | 150 ± 30* | |
| FVC | Trough | 190 ± 50* | 130 ± 50* |
| Peak | 300 ± 60* | 200 ± 60* | |
| AUC 0–3 | 290 ± 50* | 200 ± 50* | |
| TDI focal score | 1.35 ± 0.5* | 1.02 ± 0.55 | |
| SGRQ total score | −2.05 ± 1.7 | −1.66 ± 1.8 | |
| (b) Treatment response (exacerbations) after 169 days | |||
| Tiotropium | Salmeterol | Placebo | |
| % patients with ≥1 exacerbation | 17% | 26% | 27% |
| Number of exacerbations/patient-year (% reduction vs. placebo) | 0.52* (−48%) | 0.74 (−36%) | 1.00 |
| Exacerbation days/patient-year | 8.0* | 18.4 | 16.9 |
p < 0.05 vs placebo;
p < 0.05, tiotropium vs salmeterol.
p < 0.05 vs placebo.
Dyspnea responses
Patients treated with ICS
The mean baseline dyspnea index (BDI) of the patients using ICS was 6.2–6.3 in the various treatment groups, reflecting a moderate level of dyspnea for all patients at the start of the study (Table 1a). Compared with placebo, ICS patients treated with tiotropium demonstrated a clinically and statistically significant improvement in TDI focal score throughout the study (p < 0.01 at all time points). Patients treated with salmeterol also achieved a statistically significant improvement in TDI focal score compared with placebo, only after 8 weeks of therapy (p < 0.01), but failed to sustain this improvement compared with placebo at 16 and 24 weeks. The difference in TDI focal score between the tiotropium and salmeterol groups was not statistically significant. However, only in patients treated with tiotropium did the improvement in TDI reach the minimal clinically important difference of 1 unit after 169 days of treatment (Table 3a).
Patients not treated with ICS
In patients not being treated with ICS, the mean improvement in dyspnea as measured by the TDI focal score after 169 days of treatment was above the minimal clinically important difference of 1.0 for both tiotropium and salmeterol, but only in patients receiving tiotropium was this difference statistically significantly different from placebo (Table 4a).
Health status responses
Patients treated with ICS
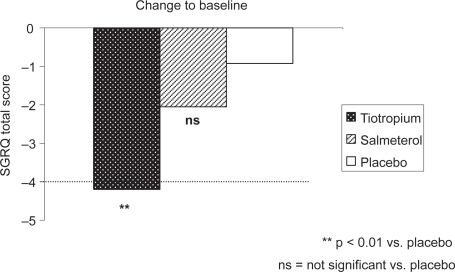
Health status, as quantified by the change in SGRQ total score after 169 days on treatment, improved significantly in patients on ICS treated with tiotropium (−3.27 ± 1.22 vs placebo; p < 0.01), but not in patients receiving salmeterol (−1.12 ± 1.21; p > 0.05 vs placebo) (Table 4a). Although only patients receiving tiotropium experienced a statistically significant improvement in SGRQ vs placebo, there was no significant difference in SGRQ total score after 6 months between the tiotropium and salmeterol groups. When the change in SGRQ total score from baseline is considered, only the tiotropium group showed an improvement equal to or greater than the accepted minimally important clinical difference of 4 units (Figure 3).
Figure 3.
Improvement in health-related quality of life in active treatment groups compared to baseline SGRQ Total score (patients concurrently using ICS).
Patients not treated with ICS
In patients without concurrent ICS treatment, the improvement in health status achieved by active study drugs after 169 days of treatment did not reach the level of significance (Table 4a).
Exacerbations
Patients treated with ICS
In patients concurrently treated with ICS there was a trend towards reduced exacerbation frequency and number of exacerbation days experienced by the tiotropium and salmeterol groups compared with the placebo group (Table 3b). Fewer patients in both the tiotropium and salmeterol groups experienced 1 or more exacerbations during the 6 months treatment period compared with placebo (40% receiving tiotropium or salmeterol, 45% receiving placebo), although the differences were not statistically significant (Table 3b).
Patients not treated with ICS
In patients not receiving concurrent treatment with ICS (Table 4b), only tiotropium use was associated with a significant reduction in the number of COPD exacerbations (p < 0.05) and in the number of exacerbation days (p < 0.05). The reduction in the proportion of patients experiencing an exacerbation of COPD approached statistical significance (p = 0.08). Salmeterol use was associated with a significant numerical reduction in exacerbations in this subgroup of patients (p = 0.81).
Safety
A safety summary of the two studies was published previously (Brusasco et al 2003). Both active treatments were well tolerated, with dry mouth the predominant adverse event reported in 8% of patients receiving tiotropium (salmeterol and placebo 2%, each).
Discussion
A pooled analysis from two studies comparing the long-acting anticholinergic tiotropium to the long-acting beta2-adrenergic salmeterol in patients with COPD had previously demonstrated a sustained and superior improvement in lung function with tiotropium 18 μg once-daily compared to salmeterol 50 μg twice-daily regardless of concurrent use of ICS (Brusasco et al 2003; Donohue et al 2002, 2003). In these two studies, 66% of the patients enrolled had been prescribed ICS on a regular basis, providing the opportunity to retrospectively analyze the relative efficacy of tiotropium and salmeterol as a function of the concomitant use of ICS in patients with moderately advanced COPD.
In patients with moderate-to-severe COPD, our present post-hoc analysis demonstrated superior spirometric responses with tiotropium plus ICS compared to salmeterol plus ICS. Both long-acting bronchodilators demonstrated an improvement in lung function, similar to that observed in the original study populations which included patients with and without ICS, and it is noteworthy that in the present sub-group analysis ICS did not appear to protect against the loss of bronchodilator activity over time that was observed to occur for salmeterol in the original data set (Donohue et al 2002, 2003). The findings of these two studies comparing tiotropium, salmeterol, and placebo are consistent with many published studies addressing efficacy of bronchodilators in COPD and add weight to the modern concept that COPD is not necessarily a disease with fixed airflow obstruction, but rather that COPD patients often demonstrate considerable bronchodilator response (Nisar et al 1992; Cazzola et al 1999; O’Donnell et al 2001, 2004a, b; Tashkin and Cooper 2004). Regarding dyspnea and health status in patients concurrently using ICS, in the current analysis, only those receiving tiotropium showed statistically significant improvement after 24 weeks on treatment, and only in the tiotropium group did these improvements reach accepted levels of clinical importance.
In the original study population there was a relatively low number of exacerbations, and in the current subgroup analysis only 27% of placebo patients without ICS and 45% of placebo patients concurrently treated with ICS experienced any exacerbation during the 6-month observation period. Although in the original study patients using ICS had experienced more exacerbations (1.76 exacerbations per patient per year in the placebo group) than patients without ICS (1.0 exacerbations/patient/year, placebo), because patients were not randomized to ICS or non-ICS groups, it should be stressed that a comparison of these sub-groups is of limited value. This apparent difference in exacerbation rates according to concurrent ICS use may simply indicate that the patients’ physicians had prescribed ICS for those patients they felt to have the most unstable COPD. The trend towards a reduced number of exacerbations experienced by the patients of this subgroup analysis receiving tiotropium (−23%) and salmeterol (−17%) did not reach statistical significance, but the magnitude of effect resembled that observed in the original study population (Brusasco et al 2003). A recently reported prospective trial comparing the addition of combined salmeterol/fluticasone, salmeterol alone, or placebo with COPD patients receiving tiotropium was also unable to demonstrate a statistically significant difference in COPD exacerbation frequency amongst the treatment groups, but compared with placebo, the group treated with tiotropium plus salmeterol/fluticasone had significantly fewer hospitalizations (Aaron et al 2007). Recent prospective studies have observed a positive impact of adding an ICS to a long-acting beta2-adrenergic on spirometric response, health status, and COPD exacerbations (Calverley et al 2003a, 2007; Mahler et al 2002; Szafranski et al 2003). The effect of this treatment strategy on mortality in COPD has also been recently studied, but although an absolute reduction in mortality of 2.6% was observed, with the combination therapy, this did not achieve statistical significance (Calverley et al 2007). Prospective trials designed to assess the effect of adding an ICS to an anticholinergic bronchodilator alone have not been published.
The role of ICS without long-acting bronchodilators in the treatment of COPD continues to be a matter of debate. Many patients with COPD appear to be steroid-resistant based on in vitro studies (Barnes et al 2004, 2005; Culpitt et al 2003). However, because clinical trials usually report only mean treatment responses, it could be postulated that ICS are beneficial in COPD, but only for certain sub-sets of patients who are not otherwise identified in clinical trials. For example, ICS may have beneficial effects by modulating inflammation driven by eosinophils when present (Pizzichini et al 1998; Leigh et al 2006), or mast cells (Hattotuwa et al 2002). ICS have also been shown to reduce some of the systemic markers of inflammation in COPD patients (Sin et al 2004) and to modulate oxidative damage in COPD (Sadowska et al 2005).
The parasympathetic nervous system is felt to be the main determinant of airway smooth muscle tone (Douglas et al 1979; Cazzola et al 1997; Barnes 2004; Gross 2004) and there is evidence that cholinergic tone is increased in COPD (Gross and Skorodin 1984; Gross et al 1989). It has therefore been suggested that COPD could be more effectively treated with anticholinergic drugs than by sympathomimetics (Barnes 2004; Gross 2004). Clinical data about the co-administration of tiotropium with inhaled long-acting beta2-adrenergics is also beginning to emerge and it would seem that such additivity does indeed occur (Cazzola et al 2004a, b; Tashkin and Cooper 2004; van Noord et al 2000, 2005, 2006).
In summary, the present post-hoc analysis of two large-scale trials, comparing the M3 receptor antagonist tiotropium and the long-acting beta2-adrenergic salmeterol to placebo in COPD subgroups with or without concurrent treatment with ICS, confirms that both, tiotropium and salmeterol are effective bronchodilators. Clinically important endpoints such as dyspnea, health status and frequency of exacerbations were also observed and were superior with tiotropium compared to salmeterol, regardless of concomitant ICS use. While the direct effect of ICS on COPD outcomes was not studied in this post-hoc analysis, we conclude that concurrent use of ICS neither augmented nor impeded the relative efficacy of the two bronchodilators tested.
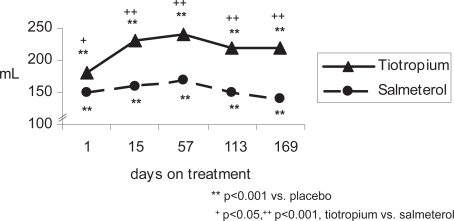
Figure 1b.
Mean peak FEV1 response of active treatment groups above placebo (patients concurrently using ICS).
Figure 2b.
Mean peak FEV1 response of active treatment groups above placebo (patients without ICS treatment).
Abbreviations
- AUC
area under the curve
- BDI
baseline dyspnea index
- FVC
forced vital capacity
- FEV1
forced expiratory volume in 1 second
- ICS
inhaled corticosteroids
- TDI
transition dyspnea index
- SGRQ
St. George Respiratory Questionnaire
Footnotes
Disclosures
Dr Steven Kesten (Corporate Department Medical Affairs. Ingelheim, Germany), Dr Shailendra Menjoge (Statistics and Data Management. Ridgefield, Connecticut, USA), and Klaus Viel (National Medicine. Ingelheim, Germany) are employees of Boehringer Ingelheim. Dr Rick Hodder is member of Scientific Advisory Boards established by Boehringer Ingelheim and Pfizer, has been speaker on scientific symposia sponsored by Boehringer Ingelheim and Pfizer, and has received grants from Boehringer Ingelheim to conduct clinical research trials.
References
- Aaron SD, Vandemheen KL, Fergusson D, et al. for the Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med. 2002;113(1):59–65. doi: 10.1016/s0002-9343(02)01143-9. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:342–344. doi: 10.1164/ajrccm.161.2.16125_2. [DOI] [PubMed] [Google Scholar]
- Barnes P. The role of anticholinergics in chronic obstructive pulmonary disease. Am J Med. 2004;117:24S–32S. doi: 10.1016/j.amjmed.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Barnes P, Adcock I, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25:552–63. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- Barnes P, Ito K, Adcock I. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet. 2004;363:731–3. doi: 10.1016/S0140-6736(04)15650-X. [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Rouleau M, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53:477–82. doi: 10.1136/thx.53.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusasco V, Hodder R, Miravitlles M, et al. Health outcomes in a 6-month placebo-controlled trial of once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58:399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge P, Calverley P, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM. Inhaled corticosteroids are beneficial in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:341–2. doi: 10.1164/ajrccm.161.2.16125_1. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003a;22:912–19. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003b;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- Calverley P, Anderson J, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Centanni S, Santus P, et al. The functional impact of adding salmeterol to tiotropium in patients with stable COPD. Respir Med. 2004a;98:1214–21. doi: 10.1016/j.rmed.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Donner C, Matera M. Long-acting β 2 agonists and theophyllines in stable chronic obstructive pulmonary disease. Thorax. 1999;54:730–6. doi: 10.1136/thx.54.8.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M, Marco F, Santus P, et al. The pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPD. Pul Pharmacol Ther. 2004b;17:35–9. doi: 10.1016/j.pupt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Spina D, Matera M. The use of bronchodilators in stable chronic obstructive pulmonary disease. Pul Pharmacol Ther. 1997;10:129–44. doi: 10.1006/pupt.1997.0087. [DOI] [PubMed] [Google Scholar]
- Culpitt SV, Rogers DF, Shah P, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:24–31. doi: 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
- Disse B, Speck GA, Rominger KL, et al. Tiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci. 1999;64:457–64. doi: 10.1016/s0024-3205(98)00588-8. [DOI] [PubMed] [Google Scholar]
- Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–52. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue JF, Menjoge S, Kesten S. Tolerance to bronchodilating effects of salmeterol in COPD. Respir Med. 2003;97:1014–20. doi: 10.1016/s0954-6111(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Donohue J, Van Noord J, Bateman E, et al. A 6-monthplacebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122:47–55. doi: 10.1378/chest.122.1.47. [DOI] [PubMed] [Google Scholar]
- Douglas N, Sudlow A, Julia-Serda G, et al. Effect of an inhaled atropine-like agent on normal airway function. J Appl Physiol. 1979;46:256–62. doi: 10.1152/jappl.1979.46.2.256. [DOI] [PubMed] [Google Scholar]
- GINA . Global Strategy for asthma management and prevention NHLBI/WHO Workshop Report. National Institutes of Health: National Heart Lung and Blood Institute. NIH Publication No.95; 1995. Global Initiative for Asthma (GINA) p. 3659. [Google Scholar]
- Gross NJ, Co E, Skorodin MS. Cholinergic bronchomotor tone in COPD. Estimates of its amount in comparison with that in normal subjects. Chest. 1989;96:984–7. doi: 10.1378/chest.96.5.984. [DOI] [PubMed] [Google Scholar]
- Gross N. Tiotropium bromide. Chest. 2004;126:1946–53. doi: 10.1378/chest.126.6.1946. [DOI] [PubMed] [Google Scholar]
- Gross N, Skorodin M. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311:421–5. doi: 10.1056/NEJM198408163110701. [DOI] [PubMed] [Google Scholar]
- Hattotuwa K, Gizycki M, Ansari T, et al. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1592–6. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164:358–64. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- Leigh R, Pizzichini M, Morris M, et al. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964–71. doi: 10.1183/09031936.06.00072105. [DOI] [PubMed] [Google Scholar]
- Littner MR, Ilowite JS, Tashkin DP, et al. Long-acting bronchodilation with once-daily dosing of tiotropium (Spiriva) in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1136–42. doi: 10.1164/ajrccm.161.4.9903044. [DOI] [PubMed] [Google Scholar]
- Lung Health Study Research Group Effects of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- Maesen FP, Smeets JJ, Sledsens TJ, et al. Tiotropium bromide, a new long-acting antimuscarinic bronchodilator: a pharmacodynamic study in patients with chronic obstructive pulmonary disease (COPD). Dutch Study Group. Eur Respir J. 1995;8:1506–13. [PubMed] [Google Scholar]
- Mahler DA, Weinberg DH, Wells CK, et al. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–58. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Wire P, Horstman D, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;1668:1084–91. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- Morris JF, Koski A, Temple WP, et al. Fifteen-year interval spirometric evaluation of the Oregon predictive equations. Chest. 1988;93:123–7. doi: 10.1378/chest.93.1.123. [DOI] [PubMed] [Google Scholar]
- [NICE] National Institute for Clinical Excellence (NICE) Chronic obstructive pulmonary disease: National clinical guidelines for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl 1):i1–i232. [PMC free article] [PubMed] [Google Scholar]
- Nisar M, Earis J, Pearson M, et al. Acute bronchodilator trials in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:555–9. doi: 10.1164/ajrccm/146.3.555. [DOI] [PubMed] [Google Scholar]
- O’Brien A, Russo-Magno P, Karki A, et al. Effects of withdrawal of inhaled corticosteroids in men with severe irreversible airflow obstruction. Am J Respir Crit Care Med. 2001;164:365–71. doi: 10.1164/ajrccm.164.3.2002052. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Forkert L, Webb K. Evaluation of bronchodilator responses in patients with „irreversible“ emphysema. Eur Respir J. 2001;18:914–20. doi: 10.1183/09031936.01.00216501. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004a;23:832–40. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, Voduc N, Fitzpatrick M, et al. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J. 2004b;24:86–94. doi: 10.1183/09031936.04.00072703. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease. Can Respir J. 2003;10(Suppl A):11A–65A. doi: 10.1155/2003/567598. [DOI] [PubMed] [Google Scholar]
- Paggiaro PL, Dahle R, Bakran I, et al. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet. 1998;351:773–80. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- Pauwels R. GOLD: Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Pauwels R. Inhaled glucocorticosteroids and chronic obstructive pulmonary disease: how full the glass? Am J Respir Crit Care Med. 2002;165:1579–80. doi: 10.1164/rccm.2204029. [DOI] [PubMed] [Google Scholar]
- Pizzichini E, Pizzichini M, Gibson P, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158:1511–17. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- Quanier P, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report working party; standardisation of lung function tests European Community for steel and coal. Eur Respir J. 1993;6(Suppl 6):5–40. [PubMed] [Google Scholar]
- Sadowska A, van Overveld F, Górecka D, et al. The interrelationship between markers of inflammation and oxidative stress in chronic obstructive pulmonary disease: modulation by inhaled steroids and antioxidant. Respir Med. 2005;99:241–9. doi: 10.1016/j.rmed.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–22. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:760–5. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:842–6. doi: 10.1164/rccm.200508-1338PP. [DOI] [PubMed] [Google Scholar]
- Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of COPD. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Cooper C. The role of long-acting bronchodilators in the management of stable COPD. Chest. 2004;125:249–59. doi: 10.1378/chest.125.1.249. [DOI] [PubMed] [Google Scholar]
- van der Valk P, Monninkhof E, van der Palen J, et al. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. The COPE study. Am J Respir Crit Care Med. 2002;166:1358–63. doi: 10.1164/rccm.200206-512OC. [DOI] [PubMed] [Google Scholar]
- van Noord JA, Aumann J-L, Jansenns E, et al. Comparison of tioropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26:214–22. doi: 10.1183/09031936.05.00140404. [DOI] [PubMed] [Google Scholar]
- Van Noord J, de Munck D, Bantje T, et al. Long-term treatment of COPD with salmeterol and the additive effect of ipratropium. Eur Respir J. 2000;15:878–85. doi: 10.1034/j.1399-3003.2000.15e11.x. [DOI] [PubMed] [Google Scholar]
- van Noord JA, Aumann JL, Janssens E, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyper-inflation in patients with COPD. Chest. 2006;129:509–17. doi: 10.1378/chest.129.3.509. [DOI] [PubMed] [Google Scholar]
- Weir D, Bale G, Bright P, et al. A double-blind, placebo-controlled study of the effect of inhaled beclomethasone dipropionate for 2 years in patients with nonasthmatic chronic obstructive pulmonary disease. Clin Exp Allergy. 1999;29:125–9. doi: 10.1046/j.1365-2222.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- Wouters EFM, Postma DS, Fokkens B, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005;60:480–7. doi: 10.1136/thx.2004.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]