Abstract
PARACEST redox sensors containing the NAD+/NADH mimic N-methylated quinolinium moiety as redox active functional group have been designed and synthesized. The Eu3+-complex with two quinolinium moieties was nearly completely silent in CEST in the oxidized form, but “turns on” upon reduction with β-NADH. The CEST effect of the Eu3+- complex containing only one quinolinium group was much less redox responsive but showed an unexpected sensitivity to pH in the physiologically relevant pH range.
Keywords: PARACEST, Redox, Europium, MRI
Proper regulation of the cellular redox state is critical to many fundamental biochemical processes. Localized changes in the redox potential have been shown to play an important role in signal transduction influencing a wide variety of cellular functions.1 Despite its biological significance, currently there is no convenient non-invasive method to map the ambient redox potential in living tissues. Magnetic resonance imaging (MRI) is a powerful noninvasive diagnostic tool and promising results have been achieved in the development of various redox sensitive Gd3+ and Mn2+/3+ based (shortening) contrast agents.2–4 Over the past decade, chemical T1 exchange saturation transfer (CEST) agents have evolved as a viable alternative to traditional (T1) agents, especially in the adaptation of MRI to molecular imaging.5,6 DOTA-tetraamide complexes of paramagnetic hyperfine shifting Ln3+-ions, in particular Eu3+, induce large paramagnetic shifts of the metal bound water protons and have sufficiently slow water exchange rates to satisfy the slow to intermediate exchange condition (kex≤Δω) for CEST.6,7
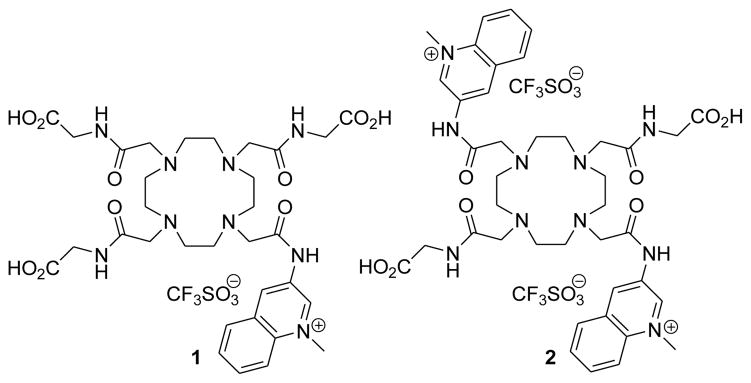
It has been demonstrated that comparatively small changes in the structure of the coordinating amide side arms of Eu3+-DOTA tetraamide complexes can generate large changes in the frequency and/or magnitude of the CEST effect originating from the metal bound water molecule.8–10 Our approach to develop redox sensitive MRI agents relies on this sensitivity of the CEST signal on the water exchange rate.11 By attaching one or more redox active group to the coordinating pendant arms of a Eu3+-chelate, the redox status of the reporter group should have a detectable influence on the CEST signal. We have shown previously that the oxidized and reduced form of a Eu3+-DOTA-tetraamide containing a p-nitrophenylamide functionality could be discriminated by CEST spectroscopy and MR imaging.8 However, this complex cannot be used as an in vivo redox sensor because the redox potential of this system is outside the biologically relevant range. Our design for a potentially biocompatible redox active CEST agent is based on the reversible redox reactivity of the nicotinamide moiety in the nicotinamide adenine dinucleotide (NAD+/NADH) coenzyme system.12,13 Several nicotinamide, quinoline and acridine derivatives have been reported that are capable of mimicking the function of NAD+/NADH. While the pyridinium/1,4-dihydropyridine redox systems are the closest analogs to the NAD+/NADH, these compounds are extremely susceptible to degradation in aqueous solutions. Quinoline derivatives are significantly more stable and still display reasonable reactivity in biomimetic reductions.14 Our hypothesis was that reduction of the quinolinium moiety to the dihydroquinoline derivative would induce a sufficient change in the coordination environment around the lanthanide ion to modulate the water exchange lifetime of the Eu3+ bound water molecule. Using these principles, we have designed and synthesized two Eu3+-DOTA-tetramide complexes (Eu3+-(1) and Eu3+-(2), Figure 1) containing one or two quinolinium groups attached to the pendant arm of the DOTA-tetraamide moiety, respectively. The ligands were synthesized by alkylating the tert-butyl esters of DO3A-(gly)3 or DO2A-(gly)2 with 3-bromoacetamidoquinoline followed by quaternarization of the quinoline N atom with methyl triflate (synthetic details are given in the Supporting Information, Schemes S1–S3).
Figure 1.
Quinolinium DOTA tetraamide derivatives examined in this work.
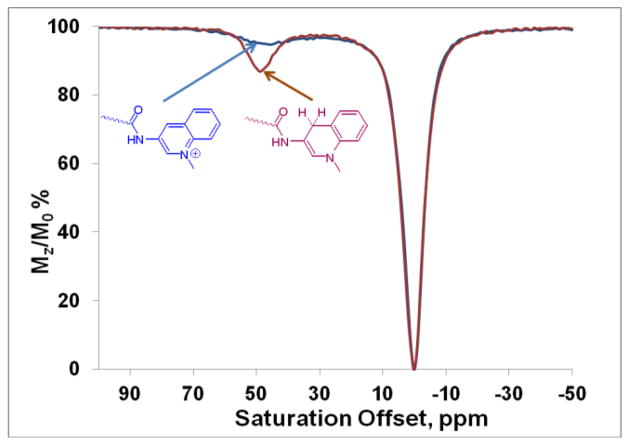
The 1H NMR spectra of the Eu3+-(1) and Eu3+-(2) in D2O showed a resonance at 25 ppm characteristic of four H4 macrocyclic protons in Eu3+ complexes of this type that exist in solution in a square antiprism (SAP) coordination geometry as the major isomer (Supporting information, Figure S1).7,11 Non-enzymatic reduction of the quinolinium moiety of Eu3+-(1) occurred rapidly after addition of one equivalent of β-NADH (pH 7) resulting in the formation of the reduced form of the complex (Supporting information Figure S2).15 Other reducing agents such as Na2S2O4 and NaBH4 also reduced the quinolinium moiety in the Eu3+-(1) resulting in a similar change in CEST.16,17 In analogy to NADH model compounds, we suggest that the quinolinium ring on the pendant arm of the DOTA scaffold is reduced predominantly to the 1,4-dihydroquinoline derivative, even though we could not establish the structure of the reduced products unequivocally.15 Interestingly, the Eu3+complex, in which the quinoline N-atom is not methylated does not undergo reduction by β-NADH and shows no change in the CEST (Supporting Information Figure S3,). This observation indicates that the quaternary nitrogen of the quinolinium ring is essential for reduction to occur. The CEST signal arising from exchange of the Eu3+-bound water molecule in Eu3+-(1) increased in intensity somewhat, became sharper (indicative of slower exchange) and shifted from 48 ppm to 53 ppm upon reduction. The bound water life-times (τM) of the oxidized and reduced form estimated by fitting the experimental CEST spectra to the Bloch equations modified for exchange were 78 μs and 130 μs, respectively. In comparison, the τM of the parent Eu[DOTA-(gly)4]− agent is about 160 μs. One can conclude then that substitution of aminoquinoline for a single glycine on the sidearm of DOTA-(gly)4 results in about a two fold increase in the water exchange rate in the resulting Eu3+ complexes. It is worth noting that the slower water exchange rate of the reduced form is somewhat counterintuitive as one might have anticipated the oxidized species to have slower water exchange due to the presence of the more electron withdrawing positively charged methylquinolinium moiety.11
The reaction of Eu3+-(2) with two equivalents of β-NADH in water at pH 7 also gave a reduced complex. Interestingly, the CEST signal increased in intensity much more dramatically in Eu3+-(2) (from 2% to 15 %) upon reduction than that observed previously for Eu3+(1). The CEST peak of Eu3+(2) also displayed a larger 7 ppm shift (from 43 to 50 ppm, see Figure 2). As expected, the water exchange lifetime of the reduced form of Eu3+-(2) (90 μs) was found to be significantly shorter than that in reduced Eu3+-(1). The τM for the oxidized form could not be determined due to the small magnitude of the CEST effect even at high B1 power but its broad width indicates that water exchange was faster in the oxidized form of Eu3+-(2) than in Eu3+-(1).
Figure 2.
CEST spectra of the oxidized (blue) and reduced forms (red) of Eu3+-(2) (20 mM) collected at 9.4 T, pH 7, 298 K, B1=10 μT, with a presaturation time of 5 s.
The redox properties of these complexes were examined by cyclic voltammetry. Two well separated redox peaks corresponding to the two electron reduction of the quinolinium ring to the dihydroquinoline were detected for Eu3+-(1). The redox couple however, appeared to be quasi-reversible since the reduced form is not completely oxidized when an anodic current is applied. It is likely that the fully reduced dihydroquinoline derivative or its precursor species are susceptible to degradation in aqueous solutions and the incomplete oxidation during the anodic scan is due to the partial decomposition of the reduced derivative. Eu3+-(2) displayed a similar cyclic voltammogram under identical experimental conditions (Supporting Information Figure S5). In addition, this behavior was also seen in the cyclic voltammogram of the free ligand (2) indicating that the redox reaction occurs at the quinolinium moiety and the Eu3+ ion is not involved in the redox reaction.18
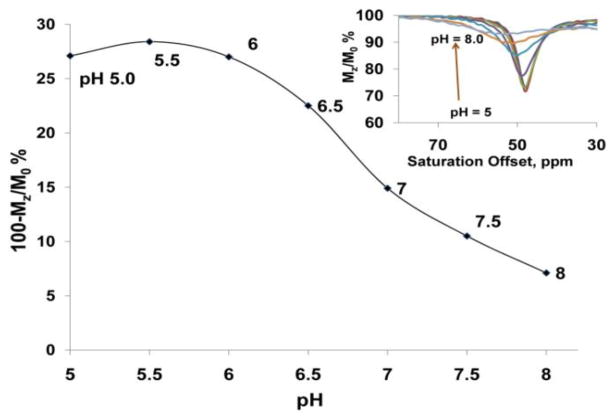
While CEST from the exchanging water molecule is generally independent of pH,6 the CEST signal of Eu3+-(1) showed a surprisingly strong pH-sensitivity (Figure 3). The bound water CEST peak at 48 ppm gradually broadened and decreased in intensity when the pH was increased from 5 to pH 8 (Figure 3 inset). Although the exact mechanism of this pH-responsiveness is yet unknown, the broadening of the CEST peak at higher pH suggests that this effect may be due to the base catalyzed deprotonation of the amide NH attached to the strongly electron withdrawing quaternarized quinoline ring, which in turn accelerates water exchange rate. This assumption is supported by the fact that the CEST signal of non-methylated form of Eu3+-(1) showed only negligible pH-dependence over the same pH range. The pH-dependent CEST is an interesting feature of Eu3+-(1) and may provide ideas for the design of future pH responsive agents. In comparison, Eu3+-(1) after reduction with β-NADH did not show a significant pH responsive behavior.
Figure 3.
The pH dependence of the metal bound water CEST signal of Eu3+-(1) (20 mM) at 9.4 T, 298 K, B1=10 μT, and irradiation time of 5 s). The inset shows CEST spectra collected at each condition.
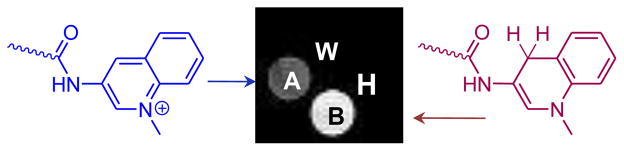
To demonstrate that the oxidized and reduced forms can be distinguished by MRI, CEST imaging of a phantom containing the oxidized and reduced species (generated by adding two equivalents of NADH) of Eu3+-(2) was performed. The images were collected at 9.4 T using a modified fast spin echo sequence at 298 K. A 10 μT saturation pulse was applied for 3 s prior to collection of the image. The CEST images were generated by subtracting the saturation-on image from the saturation-off image. In agreement with the CEST spectra, the tube containing the reduced form showed the highest image intensity in the difference images compared to that of the oxidized form (Figure 4).
Figure 4.
PARACEST images acquired for a phantom containing four tubes A: Eu3+-(2) (20 mM) in HEPES buffer (pH 7, 0.25M); B: Eu3+-(2) (20 mM) plus two equivalents of β-NADH in HEPES buffer (pH 7, 0.25M); W: water; H: HEPES buffer at pH 7.
In conclusion, we report here the first activatable PARACEST redox sensor. We have incorporated an NAD+/NADH analog, N-methylated quinolinium moiety as redox active functional group onto the pendant arm of the DOTA-tetraamide scaffold. The CEST signal of Eu3+-(2) with two quinolinium moieties was nearly completely silent while in the oxidized form, but “turns on” upon reduction with β-NADH. This makes Eu3+-(2) a good candidate for further development as an responsive redox sensor. The CEST signal of the Eu3+-complex containing only one quinolinium group was much less redox responsive but showed an unexpected sensitivity to pH over the physiologically relevant pH range.
Supplementary Material
Acknowledgments
The authors acknowledge partial financial support for this work from the National Institutes of Health (CA-115531, CA-126608, RR-02584, and EB-00482) and the Robert A. Welch Foundation (AT-584).
Footnotes
Supporting Information Available: The synthetic procedure, experimental details, 1H NMR spectroscopy, CEST spectra as a function of applied B1, CEST fitting data, and electrochemical data. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Stryer L. Biochemistry. 3. Freeman and co; New York: 1988. [Google Scholar]
- 2.Tu C, Nagao R, Louie AY. Angewandte Chemie, International Edition. 2009;48:6547–6551. S6547/1–S6547/13. doi: 10.1002/anie.200900984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghunand N, Jagadish B, Trouard TP, Galons JP, Gillies RJ, Mash EA. Magnetic Resonance in Medicine. 2006;55:1272–1280. doi: 10.1002/mrm.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aime S, Botta M, Gianolio E, Terreno E. Angewandte Chemie, International Edition. 2000;39:747–750. doi: 10.1002/(sici)1521-3773(20000218)39:4<747::aid-anie747>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Ward KM, Aletras AH, Balaban RS. Journal of Magnetic Resonance. 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Chemical Reviews (Washington, DC, United States) 2010;110:2960–3018. doi: 10.1021/cr900284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Winter P, Wu K, Sherry AD. Journal of the American Chemical Society. 2001;123:1517–1518. doi: 10.1021/ja005820q. [DOI] [PubMed] [Google Scholar]
- 8.Ratnakar SJ, Woods M, Lubag AJM, Kovacs Z, Sherry AD. Journal of the American Chemical Society. 2008;130:6–7. doi: 10.1021/ja076325y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanathan S, Ratnakar SJ, Green KN, Kovacs Z, De Leon-Rodriguez LM, Sherry AD. Angewandte Chemie, International Edition. 2009;48:9330–9333. S9330/1–S9330/21. doi: 10.1002/anie.200904649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mani T, Tircso G, Togao O, Zhao P, Soesbe TC, Takahashi M, Sherry AD. Contrast Media & Molecular Imaging. 2009;4:183–191. doi: 10.1002/cmmi.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woessner DE, Zhang S, Merritt ME, Sherry AD. Magnetic Resonance in Medicine. 2005;53:790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 12.Gomez E, Miguel M, Jimenez O, De la Rosa G, Lavilla R. Tetrahedron Letters. 2005;46:3513–3516. [Google Scholar]
- 13.Ostovic D, Lee ISH, Roberts RMG, Kreevoy MM. Journal of Organic Chemistry. 1985;50:4206–11. [Google Scholar]
- 14.Mikata Y, Mizukami K, Hayashi K, Matsumoto S, Yano S, Yamazaki N, Ohno A. Journal of Organic Chemistry. 2001;66:1590–1599. doi: 10.1021/jo000829w. [DOI] [PubMed] [Google Scholar]
- 15.Creighton DJ, Hajdu J, Mooser G, Sigman DS. J Amer Chem Soc. 1973;95:6855–7. doi: 10.1021/ja00801a067. [DOI] [PubMed] [Google Scholar]
- 16.Wong YS, Marazano C, Gnecco D, Das BC. Tetrahedron Letters. 1994;35:707–10. [Google Scholar]
- 17.Bunting JW, Meathrel WG. Tetrahedron Letters. 1971:133–6. [Google Scholar]
- 18.Blaedel WJ, Haas RG. Analytical Chemistry. 1970;42:918–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.