Abstract
In Sweden, human cases of tularemia caused by Francisella tularensis holarctica are assumed to be transmitted by mosquitoes, but how mosquito vectors acquire and transmit the bacterium is not clear. To determine how transmission of this bacterium occurs, mosquito larvae were collected in an area where tularemia is endemic, brought to the laboratory, and reared to adults in their original pond water. Screening of adult mosquitoes by real-time PCR demonstrated F. tularensis lpnA sequences in 14 of the 48 mosquito pools tested; lpnA sequences were demonstrated in 6 of 9 identified mosquito species. Further analysis confirmed the presence of F. tularensis holarctica–specific 30-bp deletion region sequences (FtM19inDel) in water from breeding containers and in 3 mosquito species (Aedes sticticus, Ae. vexans, and Ae. punctor) known to take blood from humans. Our results suggest that the mosquitoes that transmit F. tularensis holarctica during tularemia outbreaks acquire the bacterium already as larvae.
Keywords: Mosquitoes, vector-borne infections, culicidae, Francisella tularensis holarctica, bacteria, Aedes sticticus, transstadial transmission, tularemia, Sweden, research
Outbreaks of tularemia are caused by the bacterium Francisella tularensis holarctica throughout the Northern Hemisphere and by F. tularensis tularensis in North America only. Routes of infection include transmission from blood-sucking arthropods and through contact with infected dead or live animals, as well as from aerosols, dust, and water (1). Two primary disease manifestations, ulceroglandular and glandular tularemia, are associated with vector-borne transmission of the bacterium (1). Traditionally, mosquitoes are considered the primary vectors of F. tularensis holarctica to humans in Russia and Scandinavia (2–4,5). Moreover, mosquito-borne transmission of tularemia may be becoming more common in central Europe; evidence shows that this infection has reemerged during the past decade (6,7).
The ulceroglandular form of tularemia is by far the most common in Sweden; most human cases occur in late summer and early fall and are assumed to be transmitted by mosquitoes (4,8). A total of 5,754 human cases of tularemia were reported during 1931–1993, and the incidence of infection varies greatly among these years, ranging from a few cases in some years to >2,700 cases during 1967 (8). In the Örebro area of central Sweden, widespread mosquito-associated tularemia outbreaks first occurred during 2000 and 2003 (4,5), after which human cases have continued to occur in this new area where tularemia is endemic (www.smi.se/statistik/harpest). However, how vector mosquitoes acquire the bacterium is still not clear.
The demonstrated ability of F. tularensis holarctica strains to survive in association with protozoa indicates that ubiquitous aquatic protozoa might be an important environmental reservoir for the bacterium (9–11). Moreover, mosquito larvae, mainly the species A. sticticus and other floodwater mosquitoes, exert a predatory effect on aquatic protozoan populations (12). These factors indicate that mosquito larvae may be exposed to F. tularensis in their natural aquatic environment. We investigated the natural occurrence of F. tularensis in mosquitoes hatched from larvae collected in an area where tularemia was endemic. Because of unknown mechanisms, the bacterium F. tularensis is extremely difficult to isolate directly from environmental samples. Thus, our study focuses entirely on molecular techniques.
Materials and Methods
Sample Collection
Mosquito larvae were sampled on August 28, 2008, in Örebro, an area where tularemia is endemic. Nine human tularemia cases (3.24/100,000 persons) were reported from Örebro County in June–September 2008 (www.smi.se/statistik/harpest). Two sampling locations were selected, Ormesta (WGS84; 59°16′12′′N, 15°16′48′′E) and Vattenparken (WGS84; 59°16′55′′N, 15°15′00′′E), on the basis of a geographic distribution study of human tularemia cases in the area (13). Both locations are situated at Lake Hjälmaren near the city of Örebro and are characterized by lush vegetation of reed belts and deciduous trees and bushes. Using a standard dipper, we collected mosquito larvae from shallow temporary water bodies in the transition zone between reed and willow bush habitats (Ormesta 1), in the deciduous forest (Ormesta 2), and in a ditch covered by bush and grass (Vattenparken).
Mosquito larvae from each water body were reared to adults in their original pond water (Ormesta 1, containers A and B; Ormesta 2, containers C, D, E, F, and G; and Vattenparken, container H). At the start of this study, time-zero samples of the original water from each container were collected and stored at –20°C. Emerging adult mosquitoes were collected by a mechanical aspirator, killed by freezing, and stored at –80ºC until species was identified. During identification, adult mosquitoes were kept cold on a chill table, illuminated by a cold light lamp, and identified to species based on morphologic features. Identified mosquitoes were sorted by area, species, and sex in pools of 1–50 specimens and returned to the –80ºC freezer.
DNA Extraction from Mosquitoes and Water Samples
For DNA extraction, 10 μL of 2.8 M NH4OH solution and 450 mg each of 1.0-mm and 0.1-mm silica beads were added to each pooled mosquito sample. Samples were homogenized for 60 s (BeadBeater FastPrep; BioSpec Products, Inc., Bartlesville, OK, USA). The homogenized samples were incubated at room temperature for 15 min, and 60 μL of sterile water was added. DNA extraction was performed by using SoilMaster DNA Extraction Kit (Epicentre Biotechnologies, Madison, WI, USA) according to the manufacturer’s instructions. The resulting DNA pellet was resuspended in 60 μL Tris EDTA buffer.
DNA extraction from water samples was performed as previously described (14). Two milliliters of each water sample was centrifuged at 16,000 × g for 1 h, 1.9 mL of the resulting supernatant was discarded, and DNA was extracted from the remaining volume by using a SoilMaster DNA Extraction Kit (Epicentre Biotechnologies).
PCR Analysis of Mosquitoes and Water
Mosquitoes and water samples were screened for F. tularensis by using a modified real-time PCR SYBR-based assay (Quanta BioSciences, Gaithersburg, MD, USA) for detection of the F. tularensis-specific lpnA gene. The PCR assay was modified from the methods of Thelaus et al. (11). Each reaction consisted of 1 µL template, 1× Quanta PerfeCTa SYBR Green FastMix (Quanta BioSciences), 400 nmol/L for each of the lpnA2F/R-primers (5′-CGCAGGTTTAGCGAGCTGTT-3′ and 5′-GAGCAGCAGCAGTATCTTTAGC-3′), and Milli-Q (Millipore, Billerica, MA, USA) up to 20 µL. An initial denaturation at 95°C for 5 min was followed by 40 cycles at 95°C for 10 s, 60°C for 30 s, and a melt curve 60°C–95°C on Mastercycler (Eppendorf, Hamburg, Germany).
Mosquitoes and water samples then underwent a F. tularensis holarctica–specific PCR, based on the 30-bp–deletion region FtM19 and using the FtM19InDelF/R primer pair, and modified from PCR (14). Each reaction consisted of 1–3 µL templates, 1x SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA) 400 nmol/L for each of the primers FtM19Indel F/R (5′-GAATTACATAAAGTTCATGGTCCAGTAC-3′ and 5′-GTTTCAGAATTCATTTTTGTCCGTAA-3′) and Milli-Q (Millipore) water to give a final volume of 20 µL. An initial denaturation at 98°C for 2 min was followed by 50 cycles at 98°C for 5 s, 60°C for 5 s, and a melt curve 65°C–95°C on a Bio-Rad CFX96. Positive control mixtures, using DNA from F. tularensis holarctica and negative control mixtures without a template, were included in each PCR run.
Sequencing
The lpnA gene and FtM19InDel amplicons were cloned with TOPO TA cloning kit PCR4 (Invitrogen, Carlsbad, CA, USA) and sequenced. The acquired sequences were deposited in GenBank under accession nos. GY97987–GU97997 and HQ289871–HQ289876 (lpnA and FtM19InDel, respectively).
Results
F. tularensis in Mosquitoes Hatched from Field-collected Larvae
The 334 adult mosquitoes of 9 species hatched from mosquito larvae collected in the tularemia-endemic Örebro area were analyzed in 48 pools; 14 pools (29%) were positive for the F. tularensis lpnA gene (Table 1). Eleven of the 14 lpnA-positive samples were possible to sequence (Figure 1). All obtained sequences showed high sequence similarity (>97%) with F. tularensis in alignment with published sequences from representatives of subspecies of F. tularensis and their closest known relatives (i.e., Francisella-like endosymbionts).
Table 1. Presence of Francisella tularensis lpnA and F. tularensis holarctica–specific 30-bp–deletion region (FtM19InDel) in female and male mosquitoes, Örebro area, Sweden*.
| Group | Species | Female mosquitoes |
Male mosquitoes |
|||||
|---|---|---|---|---|---|---|---|---|
| Pools | F. tularensis | F. tularensis holarctica | Pools | F. tularensis | F. tularensis holarctica | |||
| 2a | Aedes communis | 5 (10) | 1 | ND | – | – | – | |
| 2a | Ae. intrudens | 2 (2) | ND | ND | – | – | – | |
| 2a | Ae. punctor | 5 (5) | 1 | 1 | – | – | – | |
| 2a | Aedes spp.† | – | – | – | 10 (31) | 2 | ND | |
| 2b | Ae. cinereus | 7 (135) | 3 | ND | 7 (109) | 2 | ND | |
| 2b | Ae. sticticus | 4 (17) | 2 | ND | 2 (12) | 1 | 1 | |
| 2b |
Ae. vexans
|
2 (2) |
1 |
1 |
|
– |
– |
– |
| 1c | Culiseta alaskaensis | 1 (1) | ND | ND | – | – | – | |
| 1c | Cs. annulata | 1 (1) | ND | ND | – | – | – | |
| 1d |
Culex pipiens/torrentium
|
1 (5) |
ND |
ND |
|
1 (4) |
1 |
1 |
| Total | 28 (178) | 8 | 2 | 20 (156) | 6 | 2 | ||
*Mosquitoes were collected as larvae in the Örebro area, central Sweden, reared to adults, and analyzed by real-time PCR in pools of up to 50 specimens. Numbers in parenthesis refer to total specimens within pools. ND, not detected; –, not analyzed. †Male mosquitoes identified as Aedes spp. could belong to any species within functional group 2a and were not identified further. Mosquito functional groups are as defined by Schäfer et al. (15).
Figure 1.
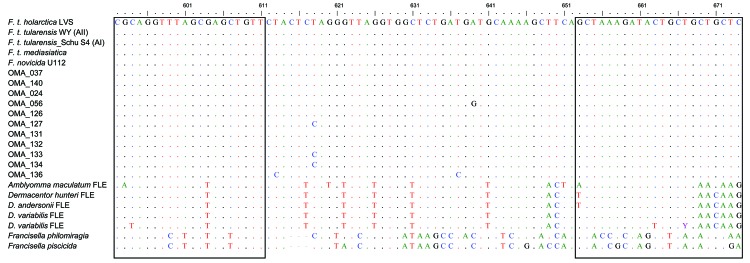
Multiple alignment of the 11 Francisella lpnA sequences obtained from mosquitoes in Sweden (hatched from field-collected larvae) with previously published sequences of Francisella species and subspecies, and Francisella-like endosymbionts (FLE). Boxed nucleotides represent target sequences of lpnA primers. The nucleotide positions 592–674 refer to F. tularensis holarctica live vaccine strain (LVS). Colors indicate individual nucleotides to clearly delineate those diverging from the F. tularensis holarctica LVS sequence. Reference sequences from GenBank; F. tularensis holarctica LVS (M32059), F. tularensis tularensis strain WY96-3418 (CP000608), F. tularensis tularensis strain Schu S4 (NC_006570), F. tularensis mediasiatica strain FSC147 (NC_010677), F. tularensis novicida strain U112 (CP000439), Amblyomma maculatum FLE (AY375422), Dermacentor hunteri FLE (AY375417), D. andersonii FLE (AY375413), D. variabilis FLE (AY375420), D. variabilis FLE (AY375421), F. philomiragia (AY243030), and F. piscicida (DQ825765).
We observed no difference in the F. tularensis detection rate between male (6/20 pools, 30%) and female (8/28 pools, 29%) mosquitoes (Table 1). Using the definitions in Schäfer et al. (15), we determined that the mosquito species belonged to 4 of 10 defined mosquito functional groups. Functional group 2a (snow-pool mosquitoes) and functional group 2b (floodwater mosquitoes) constitute most of the mosquitoes tested. Notably, the F. tularensis detection rate in female floodwater mosquitoes (6/13 pools, 46%) was higher than in female snow-pool mosquitoes (2/12 pools, 17%).
F. tularensis in Water
Water samples from 5 of the 8 water containers used for rearing were positive for the F. tularensis lpnA gene (Table 2). Four of these containers yielded mosquitoes that were positive for the F. tularensis lpnA gene. However, 3 water containers used for rearing that were negative for the F. tularensis lpnA gene, all yielded adult mosquitoes positive for the F. tularensis lpnA gene. Thus, there was no correlation between detection of F. tularensis in water from a specific container used for rearing and detection of F. tularensis in adult mosquitoes hatched from the container (Table 2).
Table 2. Presence of Francisella tularensis lpnA and F. tularensis holarctica–specific 30-bp–deletion region in water samples and in pools of mosquitoes hatched from larvae collected in the Orebro area, Sweden*.
| Pond |
Water |
|
Mosquitoes | ||||
|---|---|---|---|---|---|---|---|
| Container |
F. tularensis
|
F. tularensis holarctica
|
Pools |
F. tularensis
|
F. tularensis holarctica
|
||
| Ormesta 1 | A | Yes | Yes | 2 (2) | 1 | ND | |
|
|
B |
Yes |
ND |
|
6 (28) |
ND |
ND |
| Ormesta 2 | C | ND | ND | 6 (74) | 2 | ND | |
| D | Yes | ND | 9 (79) | 4 | 3 | ||
| E | ND | ND | 6 (45) | 3 | ND | ||
| F | Yes | ND | 8 (61) | 2 | ND | ||
|
|
G |
ND |
ND |
|
5 (27) |
1 |
ND |
| Vattenpark |
H |
Yes |
Yes |
|
6 (18) |
1 |
1 |
| Total | 8 | 5 | 2 | 48 (334) | 14 | 4 | |
*Water samples were collected from each mosquito-breeding container on arrival at the laboratory. Numbers in parentheses refer to number of mosquito specimens within pools. Sampling locations are Ormesta and Vattenpark. ND, not detected.
Detection of Francisella tularenis spp. holarctica
Using the FtM19InDel primers, we detected sequences specific for F. tularensis holarctica in 2 of the 8 water samples analyzed and 4 of the 48 mosquito pools (Figure 2; Table 2). Mosquito species positive for F. tularensis holarctica sequences were A. punctor (snow-pool mosquito), Ae. vexans, Ae. sticticus (both floodwater mosquitoes), and Culex pipens/torrentium (Table 1).
Figure 2.
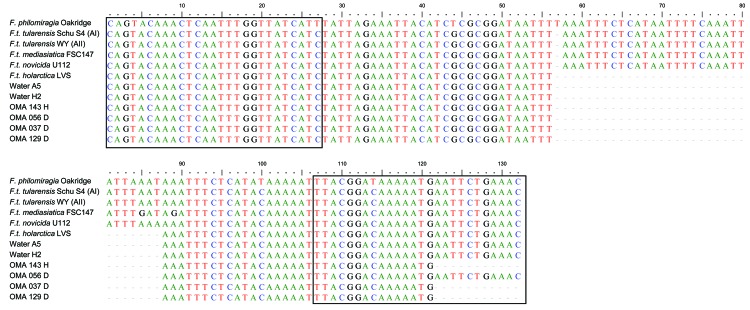
Multiple alignments of the 11 Francisella lpnA sequences (designated OMA_xxx) obtained from mosquitoes and water samples with previously published sequences of Francisella species and subspecies. Boxed nucleotides represent target sequences of FtM19InDel primers. In this alignment the F. tularensis subsp. holarctica specific deletion is located from position 57 to 87. Colors indicate individual nucleotides. Reference sequences from GenBank: F. tularensis holarctica LVS(M32059), F. tularensis subsp. tularensis strain WY96-3418 (CP000608), F. tularensis subsp. tularensis strain Schu S4 (NC_006570), F. tularensis mediasiatica strain FSC147 (NC_010677), F. tularensis novicida strain U112 (CP000439), and F. philomiragia (AY243030).
Discussion
We detected F. tularensis holarctica DNA in adult mosquitoes hatched from field-collected larvae sampled in an area in Sweden endemic for tularemia. This finding suggests that mosquitoes came in contact with the causative agent of the disease, F. tularensis holarctica, in the aquatic habitat of the mosquito larvae. We have previously shown that F. tularensis holarctica persists in natural aquatic environments between outbreaks (14) and in association with protozoa (10,11). Mosquito larvae of floodwater mosquitoes (i.e., A. sticticus) are predators on protozoa in temporary wetland environments (12). Our results suggest natural transstadial transmission of F. tularensis holarctica from its water reservoir via female mosquitoes to their vertebrate blood-meal hosts, including humans. The observation of water containers negative for F. tularensis that yielded mosquitoes positive for the bacterium and vice versa suggests that mosquitoes were truly positive for F. tularensis and not cross-contaminated with water that tested positive; however, we cannot exclude varying sensitivity of the real-time PCR analysis for water and mosquito samples. Further studies of the tissue tropism of the bacterium within the mosquito body are needed to confirm how F. tularensis holarctica is transmitted by mosquito vector.
Transstadial transmission by mosquitoes after ingestion of pathogenic microorganisms as larvae has previously been shown for Rift Valley fever virus (16). The virus was transstadially transmitted to emerging adult mosquitoes (laboratory strain of Cx. pipiens and natural strains of Ae. circumluteolus and Abydosaurus mcintoshi from Kenya) that were capable of transmitting Rift Valley fever virus to hamsters (16). Transstadial transmission of F. tularensis subspecies has also been reported in several species of ticks (3,17).
In a recent study, transmission of F. tularensis novicida was tested in laboratory strains of the tropical mosquitoes Anopheles gambiae and Ae. aegypti (18). However, the bacterium was not transmitted transstadially to adult mosquitoes, and female mosquitoes exposed to F. tularensis novicida in a blood meal were not able to transmit the bacterium to mice. Results of this study, along with our results, contribute to the growing body of data that indicate differences in the ecology, including vectors and reservoirs, of Francisella species, subspecies, and even populations (3,14,19,20).
We detected F. tularensis DNA in 29% of the pooled samples of adult mosquitoes hatched from field-collected larvae, indicating that transmission of the bacterium from water can generate a relatively high proportion of infected adult mosquitoes in an area endemic for tularemia. In line with our results, the F. tularensis fopA gene was detected in 30% of mosquito pools sampled in Alaska (18). However, further studies of host-seeking female mosquitoes in areas where tularemia is endemic are required to identify the range of mosquito species naturally infected with F. tularensis holarctica and the temporal distribution of the bacterium in these potential vector species in relation to the onset of outbreaks.
We detected F. tularensis holarctica in the floodwater mosquito species Ae. sticticus and Ae. vexans, the snow-pool mosquito species Ae. punctor, and a mixture of Cx. pipiens and Cx. torrentium mosquitoes. The 3 Aedes spp. mosquitoes feed primarily on mammals and commonly take blood meals from humans; the 2 Culex spp. mosquitoes feed on birds (15). With respect to blood-feeding habits, and the detection of F. tularensis holarctica DNA, all 3 Aedes spp. mosquitoes are potential vectors for transmission of F. tularensis holarctica to humans. Human cases of tularemia in Sweden (ulceroglandular and glandular) occur mainly in late summer and fall (13), a period when floodwater mosquito species are dominating the Swedish mosquito fauna (21). Notably, the detection rate of F. tularensis was higher in floodwater pools of female mosquitoes (46%) than in snow-pool pools of female mosquitoes (17%). The observation that F. tularensis holarctica occur in the floodwater mosquito Ae. sticticus is especially noteworthy because this nuisance species is now increasing its geographic range within Sweden (22).
We suggest that the transmission of the bacterium F. tularenis holarctica via blood-feeding mosquitoes to humans in areas of Sweden where tularemia is endemic originates from the aquatic habitat of mosquito larvae. However, further studies are needed to confirm transmission of the bacterium from its aquatic reservoir by blood-feeding female mosquitoes to their vertebrate hosts. The finding of F. tularensis holarctica DNA in adult mosquitoes, hatched from larvae collected in an area where tularemia is endemic, indicates that disease transmission in outbreaks originates in the pond habitats of the mosquito larvae.
Acknowledgments
We thank Thomas Vinnersten for help with rearing mosquitoes and Martin Pfeffer for valuable comments on the manuscript.
This study was supported by the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (Formas No.209-2006-1311), the Swedish Armed Forces, and the Swedish Civil Contingencies Agency.
Biography
Dr Lundström is a researcher at the Department of Ecology and Genetics, in the Evolutionary Biology Centre of Uppsala University, Sweden. His research interests include mosquitoes as vectors of viral and bacterial infections and the involvement of birds in the local and intercontinental dissemination of human pathogens.
Footnotes
Suggested citation for this article: Lundström JO, Andersson A-C, Bäckman S, Schäfer ML, Forsman M, Thelaus J. Transstadial transmission of Francisella tularensis holarctica in mosquitoes, Sweden. Emerg Infect Dis [serial on the Internet]. 2011 May [date cited]. http://dx.doi.org/10.3201/eid1705.100426
References
- 1.Eliasson H, Broman T, Forsman M, Bäck MD. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20:289–311. 10.1016/j.idc.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 2.Olin G. Occurrence and mode of transmission of tularemia in Sweden. Acta Pathol Microbiol Scand. 1942;19:220–47. 10.1111/j.1699-0463.1942.tb03345.x [DOI] [Google Scholar]
- 3.Petersen JM, Mead PS, Schriefer ME. Francisella tularensis: an arthropod-borne pathogen. Vet Res. 2009;40:7. 10.1051/vetres:2008045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eliasson H, Lindbäck J, Nuorti P, Arneborn M, Giesecke J, Tegnell A. The 2000 tularemia outbreak: a case-control study of risk factors in disease-endemic and emergent areas, Sweden. Emerg Infect Dis. 2002;8:956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliasson H, Bäck E. Tularemia in an emergent area in Sweden: an analysis of 234 cases in five years. Scand J Infect Dis. 2007;39:880–9. 10.1080/00365540701402970 [DOI] [PubMed] [Google Scholar]
- 6.Hanke CA, Otten J-E, Berner R, Serr A, Splettstoesser W, von Schnakenburg C. Ulceroglandular tularemia in a toddler in Germany after a mosquito bite. Eur J Pediatr. 2009;168:937–40. 10.1007/s00431-008-0862-3 [DOI] [PubMed] [Google Scholar]
- 7.Splettstoesser WD, Piechotowski I, Buckendahl A, Frangoulidis D, Kaysser P, Kratzer W, et al. Tularemia in Germany: the tip of the iceberg? Epidemiol Infect. 2009;137:736–43. 10.1017/S0950268808001192 [DOI] [PubMed] [Google Scholar]
- 8.Tärnvik A, Sandström G, Sjöstedt A. Epidemiological analysis of tularemia in Sweden 1931–1993. FEMS Immunol Med Microbiol. 1996;13:201–4. 10.1016/0928-8244(95)00104-2 [DOI] [PubMed] [Google Scholar]
- 9.Konstantinova ND, Kormilitsyna MI, Didenko LV, Meshcheriakova IA. The ultrastructural characteristics of Francisella tularensis interaction with Tetrahymena pyriformis. Zh Mikrobiol Epidemiol Immunobiol. 2000;1:10–5. [PubMed] [Google Scholar]
- 10.Abd H, Johansson T, Golovliov I, Sandström G, Forsman M. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl Environ Microbiol. 2003;69:600–6. 10.1128/AEM.69.1.600-606.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thelaus J, Andersson A, Mathisen P, Forslund A-L, Noppa L, Forsman M. Influence of nutrient status and grazing pressure on the fate of Francisella tularensis in lake water. FEMS Microbiol Ecol. 2009;67:69–80. 10.1111/j.1574-6941.2008.00612.x [DOI] [PubMed] [Google Scholar]
- 12.Östman Ö, Lundström JO, Persson Vinnersten TZ. Effects of mosquito larvae removal with Bacillus thuringiensis israelensis (Bti) on natural protozoan communities. Hydrobiologia. 2008;607:231–5. 10.1007/s10750-008-9387-z [DOI] [Google Scholar]
- 13.Svensson K, Bäck E, Eliasson H, Berglund L, Granberg M, Karlsson L, et al. Landscape epidemiology of tularemia outbreaks in Sweden. Emerg Infect Dis. 2009;15:1937–47. 10.3201/eid1512.090487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broman T, Thelaus J, Andersson A-C, Bäckman S, Wikström P, Larsson E, et al. Molecular detection of persistent Francisella tularensis subspecies holarctica in natural waters. Int J Microbiol. 2011;2011:pii: 851946. PMID: 20885922 [DOI] [PMC free article] [PubMed]
- 15.Schäfer ML, Lundström JO, Pfeffer M, Lundkvist E, Landin J. Biological diversity versus risk for mosquito nuisance and disease transmission in constructed wetlands in southern Sweden. Med Vet Entomol. 2004;18:256–67. 10.1111/j.0269-283X.2004.00504.x [DOI] [PubMed] [Google Scholar]
- 16.Turell MJ, Linthicum KJ, Beaman JR. Transmission of Rift Valley fever virus by adult mosquitoes after ingestion of virus as larvae. Am J Trop Med Hyg. 1990;43:677–80. [DOI] [PubMed] [Google Scholar]
- 17.Reese SM, Dietrich G, Dolan MC, Sheldon SW, Piesman J, Petersen JM, et al. Transmission dynamics of Francisella tularensis subspecies and clades by nymphal Dermacentor variabilis (Acaari: Ixodae). Am J Trop Med Hyg. 2010;83:645–52. 10.4269/ajtmh.2010.10-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triebenbach AN, Vogl S, Lotspeich-Cole L, Sikes D, Happ G, Hueffer K. Detection of Francisella tularensis in Alaskan mosquitoes (Diptera: Culicidae) and assessment of a laboratory model for transmission. J Med Entomol. 2010;47:639–48. 10.1603/ME09192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen JM, Molins CR. Subpopulations of Francisella tularensis ssp. tularensis and holarctica: identification and associated epidemiology. Future Microbiol. 2010;5:649–61. 10.2217/fmb.10.17 [DOI] [PubMed] [Google Scholar]
- 20.Berrada ZL, Telford III Sr. Diversity of Francisella species in environmental samples from Martha’s Vineyard, Massachusetts. Microb Ecol. 2010;59:277–83. 10.1007/s00248-009-9568-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schäfer ML, Lundström JO, Petersson E. Comparizon of mosquito (Diptera: Culicidae) populations by wetland type and year in the lower River Dalälven region, central Sweden. J Vector Ecol. 2008;33:150–7. 10.3376/1081-1710(2008)33[150:COMDCP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 22.Schäfer ML, Lundström JO. The present distribution and the predicted geographic expansion of the floodwater mosquito Aedes sticticus in Sweden. J Vector Ecol. 2009;34:141–7. [DOI] [PubMed] [Google Scholar]


