Abstract
Calprotectin has been recently described as a novel marker of obesity. The aim of this study was to determine the circulating concentrations and expression levels of calprotectin subunits (S100A8 and S100A9) in visceral adipose tissue (VAT), exploring its impact on insulin resistance and inflammation and the effect of weight loss. We included 53 subjects in the study. Gene expression levels of the S100A8/A9 complex were analyzed in VAT as well as in both adipocytes and stromovascular fraction cells (SVFCs). In addition, circulating calprotectin and soluble receptor for the advanced glycation end product (sRAGE) concentrations were measured before and after weight loss achieved by Roux-en-Y gastric bypass (RYGB) (n = 26). Circulating concentrations and VAT expression of S100A8/A9 complex were increased in normoglycemic and type 2 diabetic obese patients (P < 0.01) and associated with markers of inflammation (P < 0.01). Oppositely, concentrations of sRAGE were significantly lower (P < 0.001) in both obese groups compared to lean volunteers. Elevated calprotectin levels in obese patients decreased (P < 0.00001) after RYGB, whereas sRAGE concentrations tended to increase. Calprotectin was mainly expressed by SVFCs, and its expression was significantly correlated (P < 0.01) with mRNA levels of the monocyte-macrophage–related molecules macrophage-specific antigen CD68 (CD68), monocyte chemotactic protein 1 (MCP1), integrin α-M (CD11B), and NADPH oxidase 2 (NOX2). Tumor necrosis factor-α treatment significantly enhanced (P < 0.05) the mRNA levels of S100 calcium-binding protein A8 (S100A8) of human visceral adipocytes. The increased levels of calprotectin in obesity and obesity-associated type 2 diabetes, its positive association with inflammation as well as the higher expression levels in the SVFCs in VAT suggests a potential role of this protein as a chemotactic factor in the recruitment of macrophages to VAT, increasing inflammation and the development of obesity-associated comorbidities.
INTRODUCTION
Epidemiologic evidence has shown that chronic activation of intracellular proinflammatory pathways related to obesity can lead to the development of insulin resistance and cardiovascular diseases (1). In this sense, the inflammatory protein calprotectin was recently described as a novel marker of obesity, with its circulating levels being increased not only in obese subjects but also in type 1 diabetic patients (2,3). Calprotectin is a heterodimer comprising the S100A8 (calgranulin A, MRP8) and S100A9 (calgranulin B, MRP14) subunits, which are low-molecular-weight members of a subfamily of S100 calcium-binding proteins called calgranulins (4). The S100A8/A9 complex is expressed in a tissue/cell-specific manner mainly in cells of the myeloid lineage, including monocytes, neutrophils and early-differentiation states of macrophages (5). However, the S100A8/A9 expression is also inducible in mature macrophages, osteoclasts, keratinocytes, fibroblasts and microvascular endothelial cells (6). Besides extracellular functions related to antiinfectious host defense mechanisms, calprotectin is also involved in the regulation of kinase activities, cytoskeletal rearrangement, differentiation and cell migration, with recent attention being focused on the involvement of S100A8/A9 in cancer (6,7). In line with these functions, phagocytes reportedly express S100A8 and S100A9 under multiple inflammatory conditions, i.e., inflammatory bowel disease, rheumatoid arthritis or allograft rejection (8–10), whereas numerous pathological conditions associated with inflammation in humans are associated with elevated S100A8 and S100A9 levels (11). Furthermore, the inflammatory factors interleukin (IL)-1, interferon (IFN)-γ and tumor necrosis factor (TNF)-α induce the expression and secretion of the S100A8/A9 heterodimer (12–14). It has been shown that the release of S100A8/A9 from monocytes during the inflammatory stimulation takes place via an energy-dependent pathway involving protein kinase C activation (12). Circulating calprotectin may be involved in inflammation by enhancing CD11b expression in human monocytes and by participating in the transendothelial migration mechanism, thereby contributing to an accumulation of monocytes at the site of inflammation (15). During the progression of inflammation, due to the generation of reactive oxygen species and the synthesis of nitric oxide, irreversible oxidative modifications of S100A8/A9 may affect its functions (16). S100A8/A9 also serves as a regulator for NADPH oxidase (NOX) and may facilitate its activation to increase reactive oxygen species generation. The hetero-complex S100A8/A9 also interacts with heparin and heparan sulfate glycosaminoglycans, the receptor for the advanced glycation end product (RAGE), the scavenger receptor CD36 and the toll-like receptor 4 (17). Moreover, by sequestration of zinc, calprotectin is able to regulate many important processes in the body. It has been described that calprotectin inhibits matrix metalloproteinases, zinc-dependent enzymes that are important in angiogenesis and inflammation (7).
Visceral adipose tissue (VAT) is an important initiator of the inflammatory response in obesity because of its ability to produce and secrete a variety of proteins involved in the development of obesity-related derangements (18). Because calprotectin has emerged as an important mediator of chronic inflammation (19), the aim of the present study was to determine gene expression levels of the S100A8/A9 complex in VAT together with circulating concentrations in healthy control subjects, obese normoglycemic (NG) individuals and obese patients with type 2 diabetes (T2D). To gain insight the molecular links, the relation of calprotectin with key genes and circulating proteins involved in inflammation as well as the effect of TNF-α in human adipocyte culture was further explored. To corroborate the functional relation between obesity and inflammation, the present study also analyzed the effect on plasma calprotectin concentrations of Roux-en-Y gastric bypass (RYGB), an intervention with proven weight loss and antiinflammatory effects.
MATERIALS AND METHODS
Patient Selection
To analyze the effect of obesity and T2D on the concentrations of calprotectin, 53 females were recruited from healthy volunteers and patients attending the Departments of Endocrinology and Surgery at the Clínica Universidad de Navarra. Patients underwent a clinical assessment including medical history, physical examination, body composition analysis and comorbidity evaluation by a multidisciplinary consultation team. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters, and body fat (BF) was estimated by air-displacement plethysmography (Bod-Pod®; Life Measurements, Concord, CA, USA) (20). Waist-to-hip ratio (WHR) was measured as the quotient between the circumference of the waist (at the midway level between the margin of the lowest rib and the iliac crest) and the hip (at the widest trochanters). Obese patients were further subclassified according to the established diagnostic thresholds for diabetes (NG: fasting plasma glucose concentration <100 mg/dL and plasma glucose <140 mg/dL 2 h after an oral glucose tolerance test [OGTT]; T2D: fasting plasma glucose >126 mg/dL or plasma glucose ≥200 mg/dL 2 h after OGTT) (21). T2D subjects were not on insulin therapy or on medications likely to influence endogenous insulin levels. Furthermore, gene expression levels were assessed in VAT in a subgroup of subjects (n = 29). The samples were collected from patients undergoing either Nissen fundoplication (for hiatus hernia repair in lean [LN] volunteers) or RYGB (for morbid obesity treatment in obese subjects) at the Clínica Universidad de Navarra. Both interventions were carried out via a laparoscopic approach. Tissue samples were immediately frozen in liquid nitrogen and stored at −80ºC for subsequent analyses. In addition, a group of 26 obese female patients was selected to investigate the effect of weight loss achieved by RYGB (after a mean of 13 months) on circulating concentrations of calprotectin. The study was approved, from an ethical and scientific standpoint, by the hospital’s ethical committee responsible for research, and written informed consent of participants was obtained.
Blood Assays
Plasma samples were obtained by venipuncture after an overnight fast. Glucose was analyzed on the basis of enzymatic spectrophotometric reactions by an automated analyzer (Hitachi Modular P800; Roche, Basel, Switzerland). Insulin was measured by means of an enzyme-amplified chemiluminescence assay (IMMULITE®; Diagnostic Products, Los Angeles, CA, USA) with intra- and interassay coefficients of variation of 4.2% and 5.7%, respectively. Insulin resistance and sensitivity were calculated using the homeostatic model assessment (HOMA) and quantitative insulin sensitivity check index (QUICKI) indices, respectively (22,23). Total cholesterol, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol levels were calculated as previously described (23). Uric acid, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyltransferase and creatinine were measured by enzymatic tests (Roche) in an automated analyzer (Roche/Hitachi Modular P800). High-sensitivity C-reactive protein (CRP), fibrinogen and von Willebrand factor (vWF) antigen concentrations were determined as previously reported (23). Leptin was measured by a double-antibody radioimmunoassay method (Linco Research, St. Charles, MO, USA); intra- and interassay coefficients of variation were 5.0% and 4.5%, respectively. Adiponectin was determined using a commercially available enzyme-linked immunosorbent assay kit (Biovendor, Heidelberg, Germany), with the intra- and interassay coefficients of variation being 6.7% and 7.8%, respectively. Calprotectin and the soluble RAGE (sRAGE) levels were assessed using commercially available enzyme-linked immunosorbent assay kits (R&D Systems Europe, Abingdon, U.K.) according to the manufacturer’s instructions. The intra- and interassay coefficients of variation were 3.7% and 6.5%, respectively, for the former and 5.7% and 7.7% for the latter.
Multiplex Immunoassays
The circulating concentrations of different inflammatory markers were quantified with a Luminex™ 200 platform (Luminex, Austin, TX, USA) using microsphere-based multiplexing technology. The human immunoassay kit (Millipore Iberica, Madrid, Spain), encompassing analyte-specific components for the measurement of E-selectin, intercellular adhesion molecule 1 (ICAM-1), IL-6, plasminogen activator inhibitor-1 (PAI-1), vascular cell adhesion protein-1 and TNF-α, was used in the study. The standard curve was calculated using five- parameter-curve fitting, and results were analyzed using the Luminex IS™ 2.3 software (Luminex). Intraassay precision ranged from 4.5% to 12.3%, whereas interassay precision was <16.3%. Calibrators, controls and samples were run in duplicate throughout the study.
RNA Extraction and Real-Time Polymerase Chain Reaction
Adipose tissue RNA isolation was performed by homogenization with an ULTRA-TURRAX® T 25 basic (IKA Werke, Staufen, Germany) using QIAzol® Reagent (Qiagen, Valencia, CA, USA). Samples were purified with the RNeasy Lipid Tissue Mini-Kit (Qiagen) according to the manufacturer’s directions and treated with DNase I (RNase-free DNase Set; Qiagen) to remove any trace of genomic DNA. For first-strand cDNA synthesis, constant amounts of 1 μg total RNA were reverse-transcribed in a 40-μL final volume using random hexamers (Roche) as primers and 200 units Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen, Carlsbad, CA, USA) (24).
The transcript levels for S100 calcium-binding protein A8 (S100A8), S100 calcium-binding protein A9 (S100A9), integrin α-M (CD11B), macrophage-specific antigen CD68 (CD68), monocyte chemotactic protein 1 (MCP1), NADPH oxidase 2 (NOX2) and tumor necrosis factor-α (TNFA) were quantified by real-time polymerase chain reaction (PCR) (7300 Real Time PCR System; Applied Biosystems, Foster City, CA, USA). Primers and probes (Supplementary Table 1) were designed using the software Primer Express 2.0 (Applied Biosystems) and purchased from Genosys (Sigma-Aldrich, Madrid, Spain). Primers or TaqMan® probes encompassing fragments of the areas from the extremes of two exons were designed to ensure the detection of the corresponding transcript avoiding genomic DNA amplification. The cDNA was amplified at the following conditions: 95°C for 10 min, followed by 45 cycles of 15 s at 95°C and 1 min at 59°C, using the TaqMan® Universal PCR Master Mix (Applied Biosystems). The primer and probe concentrations for gene amplification were 300 and 200 nmol/L, respectively. All results were normalized to the levels of the ribosomal 18S rRNA (Applied Biosystems), and relative quantification was calculated using the ΔΔCt formula (Ct, cycle threshold) (25). Relative mRNA expression was expressed as fold expression over the calibrator sample (average of gene expression corresponding to the LN group), as previously described. All samples were run in triplicate, and the average values were calculated (25).
Table 1.
Anthropometric and biochemical characteristics of subjects included in the study.
| Lean | Obese NG | Obese T2D | |
|---|---|---|---|
| n | 16 | 20 | 17 |
| Age (years) | 32 ± 5 | 34 ± 2 | 49 ± 3a |
| BMI (kg/m2) | 20.4 ± 0.8 | 44.0 ± 1.3b | 47.0 ± 2.4b |
| Body fat (%) | 26.2 ± 2.0 | 53.8 ± 0.9b | 54.1 ± 1.2b |
| Waist circumference (cm) | 66.8 ± 1.3 | 112.8 ± 3.6b | 130.6 ± 4.7b,c |
| WHR | 0.76 ± 0.03 | 0.86 ± 0.02a | 0.90 ± 0.02b |
| Fasting glucose (mg/dL) | 88.8 ± 5.8 | 90.7 ± 3.2 | 131.2 ± 12.9b,d |
| 2-h OGTT glucose (mg/dL) | 101.0 ± 14.1 | 120.0 ± 8.4 | 238.1 ± 37.5b,d |
| Fasting insulin (μU/mL) | 7.2 ± 1.4 | 12.8 ± 1.7 | 28.5 ± 4.1b,d |
| 2-h OGTT insulin (μU/mL) | 8.5 ± 1.9 | 87.5 ± 13.9 | 132.6 ± 33.5b,d |
| HOMA | 1.7 ± 1.0 | 2.9 ± 0.4 | 9.8 ± 2.4b,d |
| QUICKI | 0.367 ± 0.043 | 0.333 ± 0.006b | 0.288 ± 0.008b,d |
| Triglycerides (mg/dL) | 76 ± 13 | 98 ± 8 | 130 ± 14a |
| Cholesterol (mg/dL) | 182 ± 11 | 194 ± 10 | 202 ± 11 |
| LDL cholesterol (mg/dL) | 102 ± 11 | 130 ± 9 | 129 ± 12 |
| HDL cholesterol (mg/dL) | 61 ± 4 | 44 ± 2b | 48 ± 5a |
| Leptin (ng/mL) | 8.3 ± 1.6 | 64.7 ± 7.0b | 67.3 ± 7.0b |
| Adiponectin (μg/mL) | 21.4 ± 5.4 | 8.1 ± 1.2a | 10.0 ± 0.6a |
| Uric acid (mg/dL) | 4.0 ± 0.2 | 5.1 ± 0.3 | 5.9 ± 0.4b |
| CRP (mg/L) | 1.1 ± 0.3 | 8.4 ± 1.3b | 13.3 ± 3.9b |
| Fibrinogen (mg/dL) | 208 ± 24 | 363 ± 27b | 385 ± 25b |
| vWF (%) | 65 ± 10 | 97 ± 12 | 125 ± 17a |
| Homocysteine (μmol/L) | 6.8 ± 0.9 | 6.8 ± 0.5 | 8.3 ± 0.6 |
| IL-6 (pg/mL) | 1.6 ± 0.1 | 2.5 ± 0.2b | 2.8 ± 0.3b |
| TNF-α (pg/mL) | 2.05 ± 0.06 | 2.40 ± 0.16 | 2.67 ± 0.13b |
| PAI-1 (ng/mL) | 0.10 ± 0.01 | 0.22 ± 0.02b | 0.50 ± 0.08b,d |
| E-selectin (ng/mL) | 0.11 ± 0.01 | 0.17 ± 0.02 | 0.28 ± 0.04b,d |
| ICAM-1 (ng/mL) | 0.75 ± 0.05 | 0.81 ± 0.06 | 0.81 ± 0.6 |
| Vascular cell adhesion protein-1 (ng/mL) | 5.73 ± 0.61 | 5.22 ± 0.26 | 7.12 ± 0.52c |
| Alanine aminotransferase (UI/L) | 7 ± 1 | 20 ± 3 | 33 ± 6b,c |
| Aspartate aminotransferase (UI/L) | 11 ± 1 | 13 ± 1 | 21 ± 5 |
| Alkaline phosphatase (UI/L) | 88 ± 9 | 109 ± 8 | 122 ± 10 |
| γ-Glutamyltransferase (UI/L) | 9 ± 1 | 18 ± 2 | 33 ± 6b,c |
Data are mean ± SEM. Differences between groups were analyzed by one-way ANOVA followed by Tukey post hoc tests.
P < 0.05 and
P < 0.01 versus LN.
P < 0.05 and
P < 0.01 versus obese NG.
CRP was logarithmically transformed for statistical analysis because of its nonnormal distribution.
Adipocyte Culture
Human stromovascular fraction cells (SVFCs) were isolated from omental adipose tissue from obese normoglycemic subjects as previously described (26). SVFCs were seeded at 2 × 105 cell/cm2 and grown in adipocyte medium (DMEM/F-12 [1:1] [Invitrogen], 17.5 mmol/L glucose, 16 μmol/L biotin, 18 μmol/L panthotenate, 100 μmol/L ascorbate and antibiotic-antimycotic) supplemented with 10% newborn calf serum. After 4 d, the medium was changed to adipocyte medium supplemented with 3% newborn calf serum, 0.5 mmol/L 3-isobutyl-1-methylxanthine (IBMX), 0.1 μmol/L dexamethasone, 1 μmol/L BRL49653 and 10 μg/mL insulin. After a 3-d induction period, cells were fed every 2 d with the same medium but without IBMX and BRL49653 supplementation for the remaining 7 d of adipocyte differentiation.
Differentiated human omental adipocytes were serum-starved for 24 h and then treated with increasing concentrations of TNF-α (1, 10 and 100 ng/mL) (Sigma) for 24 h.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Because of their nonnormal distribution, CRP concentrations were logarithmically transformed. The normal distribution of the other variables was adequate for the use of parametric tests. Differences between groups were assessed by one-way ANOVA followed by Tukey post hoc tests and two-tailed paired t tests as appropriate. Differences between groups adjusted for age were analyzed by analysis of covariance (ANCOVA). Pearson correlation coefficients (r) were used to analyze the association between variables. The calculations were performed using the SPSS/Windows version 15.0 statistical package (SPSS, Chicago, IL, USA). A P value <0.05 was considered statistically significant.
All supplementary materials are available online at www.molmed.org.
RESULTS
Increased Circulating Calprotectin Levels in Human Obesity and Obesity-Associated T2D Decrease After Weight Loss
The biochemical and hormonal characteristics of the subjects included in the study are shown in Table 1. Patients from both obese groups were anthropometrically similar between them exhibiting significantly higher (P < 0.01) BMI, BF, waist circumference and WHR compared to the LN volunteers. As expected, the high BF values were accompanied by hypoad-iponectinemia (P < 0.05) and hyper-leptinemia (P < 0.01) together with reduced circulating concentrations of HDL cholesterol (P < 0.05). Obese patients with T2D exhibited a lower insulin sensitivity than both LN and obese NG individuals, as evidenced by the increased glucose concentrations in the fasting state (P < 0.01) and 2 h after an OGTT (P < 0.01) as well as the higher HOMA (P < 0.01) and lower QUICKI (P < 0.01) indices.
Significant differences in circulating calprotectin concentrations between the three experimental groups were observed (P < 0.001), being significantly increased in both obese groups compared to LN subjects (Figure 1A). Because the obese T2D group was significantly older, an ANCOVA with age as a covariable was performed to investigate the effect of age on plasma calprotectin levels. We observed similar results, with calprotectin levels being significantly increased (P < 0.001) in both obese NG and T2D patients compared to LN subjects. In this regard, plasma calprotectin levels were positively associated (P < 0.05) with BMI, BF, waist circumference and WHR as well as with leptin and fasting insulin concentrations, while being negatively correlated with the QUICKI index (Table 2). Because calprotectin constitutes a ligand of sRAGE, the circulating concentrations of sRAGE were also measured. We found that concentrations of sRAGE were significantly lower (P < 0.001) in both obese groups compared to LN volunteers (Figure 1B). These differences were maintained (P < 0.001) after age adjustment. Noteworthy, a statistically significant inverse correlation between circulating levels of sRAGE and calprotectin (r = −0.35; P = 0.013) was found. Moreover, sRAGE concentrations were negatively correlated (P < 0.01) with BMI, BF, waist circumference and WHR as well as with leptin and fasting glucose and insulin concentrations. A positive association (P < 0.0001) was found between sRAGE and the QUICKI index (Table 2).
Figure 1.
Circulating levels of calprotectin and sRAGE of LN, obese NG and obese T2D volunteers as well as the effect of weight loss in obese patients achieved by RYGB. Bars represent the mean ± SEM of calprotectin and sRAGE plasma concentrations under basal conditions (LN: n = 16; NG: n = 20; T2D: n = 17) (A, B) and 13 months after RYGB (n = 26) (C, D). Differences between groups were analyzed by one-way ANOVA followed by Tukey tests or by two-tailed paired Student t tests. **P < 0.01 versus LN and versus presurgery values.
Table 2.
Univariate analysis of the correlations between circulating concentrations of calprotectin and sRAGE with variables of glucose metabolism and inflammatory markers.
| Calprotectin | sRAGE | |||
|---|---|---|---|---|
|
|
|
|||
| r | P | r | P | |
| Calprotectin | — | — | −0.35 | 0.013 |
| sRAGE | −0.35 | 0.013 | — | — |
| BMI | 0.52 | <0.001 | −0.57 | <0.001 |
| BF | 0.52 | <0.001 | −0.64 | <0.001 |
| Waist circumference | 0.62 | 0.002 | −0.75 | <0.001 |
| WHR | 0.63 | 0.002 | −0.72 | <0.001 |
| Glucose | 0.30 | 0.090 | −0.36 | 0.031 |
| Insulin | 0.38 | 0.030 | −0.44 | 0.008 |
| QUICKI | −0.46 | 0.009 | 0.60 | <0.001 |
| Leptin | 0.52 | 0.002 | −0.40 | 0.019 |
| Fibrinogen | 0.40 | 0.029 | −0.51 | 0.003 |
| CRP | 0.42 | 0.025 | −0.73 | <0.001 |
| IL-6 | 0.29 | 0.063 | −0.54 | <0.001 |
| TNF-α | 0.22 | 0.173 | −0.31 | 0.039 |
| PAI-1 | 0.57 | <0.001 | −0.43 | 0.002 |
| Adiponectin | −0.27 | 0.252 | 0.49 | 0.022 |
Bold values are statistically significant P values.
Once the association between calprotectin and its receptor with variables indicative of obesity and inflammation was established, we further analyzed the functional relation between them in an intervention with proven weight loss and insulin-sensitizing effects as well as known for ameliorating the low-grade chronic inflammation such as the RYGB. After an average postsurgical period of 13 months from the RYGB, patients experienced a significant decrease (P < 0.0001) in body weight, BMI, BF and waist circumference as well as a significant improvement in the presurgery glycemia (P = 0.006), insulinemia (P = 0.047), HOMA (P = 0.007) and QUICKI (P < 0.0001) indices (Supplementary Table 2). Of note, our data show that weight loss after RYGB was accompanied by a statistically significant reduction (P < 0.01) in the circulating concentrations of calprotectin in both NG and T2D obese patients (Figure 1C). The improvement in weight control achieved by bariatric surgery also resulted in a modest increase of sRAGE levels in both obese groups, although the differences fell out of statistical significance (Figure 1D). Interestingly, the differences in calprotectin concentrations were positively correlated with the differences in the WHR (r = 0.88; P = 0.004) as well as with the differences in the concentrations of the inflammatory markers CRP (r = 0.93; P = 0.044) and von Willebrand factor (r = 0.98; P = 0.038).
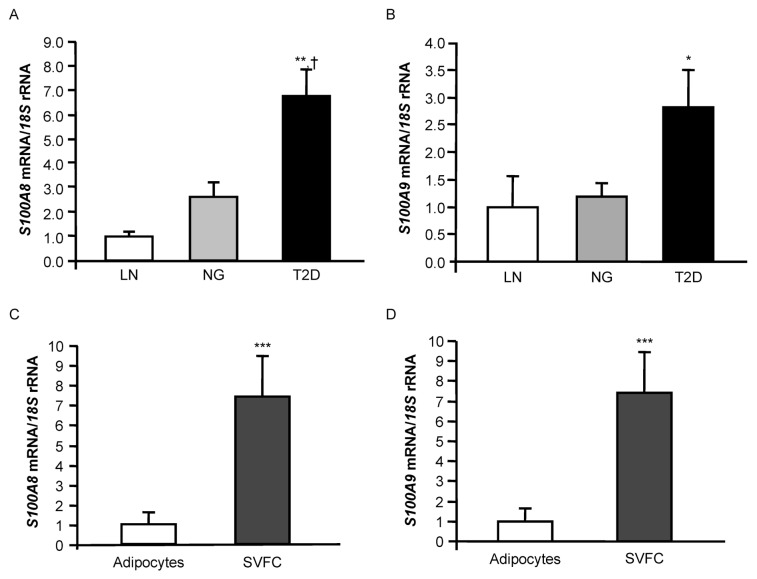
Calprotectin Gene Expression Levels in VAT Are Increased in Obesity and Obesity-Associated T2D
To gain more insight into the molecular mechanism involved in the increased circulating calprotectin concentrations observed in obese individuals, we further explored in VAT the gene expression levels of calprotectin in these pathophysiological conditions. Real-time PCR analysis indicated that S100A8 mRNA expression levels in VAT were significantly higher in obese patients with T2D than in both LN (P < 0.001) and obese NG volunteers (P = 0.003) (Figure 2A). S100A9 mRNA expression in VAT followed a similar trend, being significantly increased in obese T2D patients (P = 0.037) compared to the LN group, but no statistically significant differences were detected compared to the obese NG subjects (Figure 2B). A positive correlation between S100A8 and S100A9 gene expression in VAT was found (r = 0.60; P < 0.001). However, no significant correlation of circulating calprotectin with either S100A8 mRNA (P=0.220) or S100A9 mRNA (P = 0.166) expression levels in VAT was observed in the common samples of both studies (n = 39).
Figure 2.
Analysis of the calprotectin subunits S100A8 and S100A9 in VAT. (A, B) Gene expression levels of S100A8 and S100A9 in VAT of LN, obese NG and obese T2D volunteers. Bars represent the mean ± SEM of the ratio between the gene expression to 18S rRNA. The expression level in LN subjects was assumed to be 1 (LN: n = 9; NG: n = 10; T2D: n = 10). Differences between groups were analyzed by one-way ANOVA followed by Tukey tests. *P < 0.05, **P < 0.01, versus LN, and †P < 0.05 versus NG. (C, D) Comparison of S100A8 and S100A9 gene expression in adipocytes and SVFCs isolated from VAT of obese patients. Bars represent the mean ± SEM of the ratio between the gene expression to 18S rRNA. The expression level in adipocytes was assumed to be 1 (adipocytes: n = 9; SVFCs: n = 11). Statistical differences were assessed by two-tailed unpaired Student t tests. ***P < 0.001 versus adipocytes.
An increased macrophage infiltration in adipose tissue of obese patients (especially those with a high BMI) such as the patients included in this study is a well-established feature. To identify which cell type preferentially contributed to the observed calprotectin levels, adipocytes and SVFCs were isolated from VAT samples obtained from 15 morbidly obese patients and the expression of S100A8 and S100A9 was determined. Although S100A8 and S100A9 expression was evident in the mature adipocytes, gene expression levels were mainly detected in SVFCs (P < 0.001) (Figures 2C, D).
Calprotectin Gene Expression Levels in VAT in Relation to Macrophages
Because macrophages represent a relevant cell type source in proinflammatory cytokine production and release and both S100A8 and S100A9 were mainly expressed in SVFCs, the macrophage-specific marker CD68 was used to evaluate the impact of resident macrophages to the overall expression of S100A8 and S100A9 in the SVFCs of adipose tissue. As expected, the mRNA of CD68 was up-regulated in VAT of obese NG individuals compared to LN volunteers (P = 0.010), being further increased in obese T2D patients (P = 0.003) (Figure 3A). A positive association between both S100A8 (r = 0.62; P < 0.001) and S100A9 (r = 0.62; P < 0.001) with CD68 mRNA levels was also detected (Table 3).
Figure 3.
Gene expression levels of CD68 (A), CD11B (B), MCP1 (C) and NOX2 (D) in VAT of LN, obese NG and obese T2D volunteers. Bars represent the mean ± SEM of the ratio between the gene expression to 18S rRNA. The expression level in LN subjects was assumed to be 1 (LN: n = 9; NG: n = 10; T2D: n = 10). Differences between groups were analyzed by one-way ANOVA followed by Tukey tests. *P < 0.05, **P < 0.01, versus LN, and †P < 0.05 versus NG.
Table 3.
Univariate analysis of the correlations between mRNA expression levels of S100A8 and S100A9 in VAT with inflammatory markers.
| S100A8 mRNA | S100A9 mRNA | |||
|---|---|---|---|---|
|
|
|
|||
| r | P | r | P | |
| S100A8 mRNA | — | — | 0.60 | <0.001 |
| S100A9 mRNA | 0.60 | <0.001 | — | — |
| CD68 mRNA | 0.62 | <0.001 | 0.62 | <0.001 |
| CD11B mRNA | 0.56 | 0.003 | 0.51 | 0.006 |
| MCP1 mRNA | 0.64 | <0.001 | 0.63 | <0.001 |
| NOX2 mRNA | 0.74 | <0.001 | 0.88 | <0.001 |
| TNFA mRNA | 0.61 | <0.001 | 0.65 | <0.001 |
| Waist circumference | 0.82 | 0.014 | 0.31 | 0.454 |
| WHR | 0.80 | 0.018 | 0.60 | 0.118 |
| IL-6 | 0.51 | 0.017 | 0.63 | 0.002 |
| E-selectin | 0.53 | 0.024 | 0.56 | 0.013 |
| ICAM-1 | 0.72 | <0.001 | 0.74 | <0.001 |
Bold values are statistically significant P values.
We also evaluated the gene expression levels of CD11B, since a rapid increase of this antigen in the surface of monocytes after its stimulation with S100A8 or S100A9 was observed. We showed that mRNA expression levels of CD11B in VAT were significantly upregulated (P = 0.030) in both obese NG and T2D patients compared to LN volunteers (Figure 3B). Furthermore, a significant positive association between gene expression levels of S100A8 (r = 0.56; P = 0.003) and S100A9 (r = 0.51; P = 0.006) with CD11B was observed (Table 3). We analyzed the mRNA levels of MCP1, a monocyte chemotactic protein that plays an important role in the recruitment of monocytes to adipose tissue, finding a significant upregulation (P = 0.004) in T2D obese patients compared to LN volunteers, together with a positive correlation with S100A8 and S100A9 mRNA levels (Figure 3C, Table 3).
Given that the calprotectin subunits are particularly susceptible to oxidative modification, we next investigated the role of NOX2, a catalytic subunit of the macrophage NADPH oxidase (27), in obesity and obesity-associated T2D as well as the association with both S100A subunits. The expression levels of NOX2 in VAT were significantly increased (P = 0.007) in T2D patients compared with LN and obese NG volunteers (Figure 3D). Moreover, a highly significant association (P < 0.001) between gene expression levels of NOX2 and both calprotectin subunits (Table 3) was observed.
Circulating and mRNA Adipose Calprotectin Levels Correlate with Chronic Low-Grade Obesity-Associated Inflammation: Role of TNF-α in the Gene Expression Levels of S100A in Human Adipocytes
Circulating concentrations of calprotectin were positively correlated (P < 0.01) with the inflammatory and prothrombotic markers leptin, CRP, PAI-1 and fibrinogen (Table 2). We also found a significant negative correlation between sRAGE levels and leptin, CRP, IL-6, PAI-1, fibrinogen and TNF-α concentrations as well as a positive association with adiponectin levels (Table 2). Analogously, S100A8 and S100A9 gene expression levels in VAT were positively associated (P < 0.01) with the inflammatory markers IL-6, E-selectin and ICAM-1 (Table 3).
TNF-α is linked to the inflammatory response in obesity, and it is known to regulate the production of certain adipokines. We found that gene expression levels of the inflammatory marker TNFA in VAT were threefold higher in obese individuals than in LN volunteers, being further upregulated (P < 0.01) in T2D obese patients (Figure 4A). In addition, a positive correlation of the TNFA gene expression with the mRNA levels of S100A8 and S100A9 was also found (P < 0.01) (Table 3). On the basis of the positive correlation of the S100A8 and S100A9 transcripts with TNFA, to establish a causative link between them, the effect of TNF-α on S100A8 and S100A9 expression in human adipocytes was examined. Cells were stimulated with increasing concentrations of TNF-α for 24 h. As shown in Figures 4B and C, TNF-α treatment significantly enhanced the mRNA levels of S100A8 (P < 0.05). We also detected an increased gene expression of S100A9, although the differences were not statistically significant.
Figure 4.
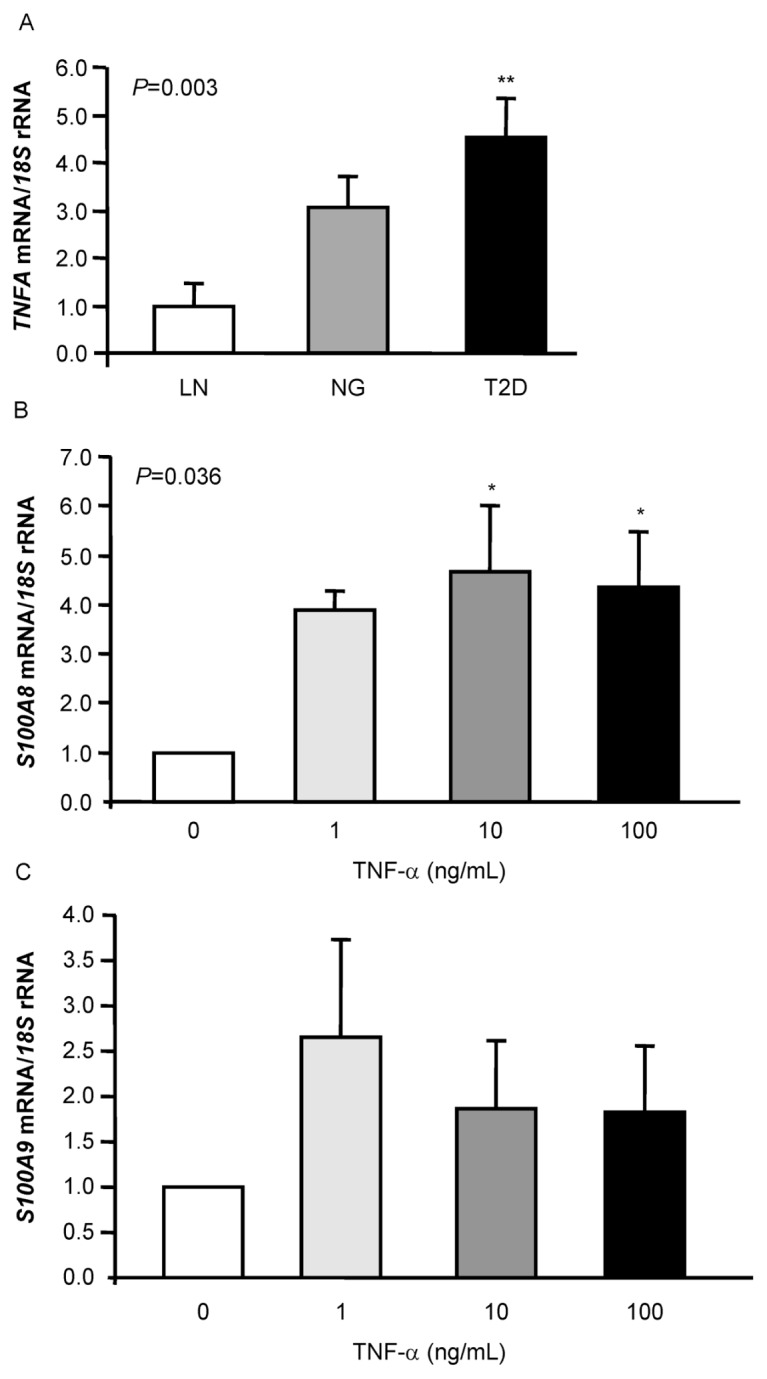
Effect of TNF-α on the expression of both calprotectin subunits S100A8 and S100A9 in human omental adipocytes. Gene expression levels of TNFA (A) in VAT of LN, obese NG and obese T2D volunteers. Bars represent the mean ± SEM of the ratio between the gene expression to 18S rRNA. The expression level in LN subjects was assumed to be 1 (LN: n = 9; NG: n = 10; T2D: n = 10). Differences between groups were analyzed by one-way ANOVA followed by Tukey tests. **P < 0.01 versus LN. Bar graphs show the effect of coincubation for 24 h of TNF-α with omental adipocytes on the transcript levels of S100A8 (B) and S100A9 (C). The gene expression levels in unstimulated cells was assumed to be 1. Values are the mean ± SEM (n = 6 per group). Differences between groups were analyzed by one-way ANOVA followed by Tukey tests. *P < 0.05 versus unstimulated cells.
DISCUSSION
It is now broadly accepted that obesity-associated low-grade chronic inflammation leads to the development of both insulin resistance and cardiovascular disease (28). In this sense, the S100 proteins are reportedly involved in a large number of cellular functions such as inflammatory response, cell growth and differentiation, cytoskeleton rearrangement, immune response or energy metabolism (17). The main findings in the present study are as follows: (i) the increased levels of calprotectin in obesity and obesity-associated T2D, which decrease after weight loss achieved by RYGB; (ii) the reduced levels of sRAGE in obesity and obesity-associated T2D as well as their inverse correlation with calprotectin concentrations; (iii) the elevated gene expression levels of the calprotectin subunits S100A8 and S100A9 in obesity, which are related to monocyte-macrophage content in VAT; and (iv) the correlation of both circulating and mRNA adipose calprotectin levels with chronic low-grade obesity-associated inflammation, establishing a causative link with TNF-α.
Calprotectin has been recently described as a novel marker of obesity (3). In agreement with previous results (3,29), we showed an increase of calprotectin concentrations in both obesity and obesity-associated T2D. These data together with the strong positive association with anthropometric variables such as BMI, BF, waist circumference and WHR highlight the link between calprotectin and obesity. It would be interesting to evaluate the influence of hormonal or sexual factors on the circulating levels of calprotectin. However, at our institution, as well as in all obesity specialized centers, the majority of morbidly obese subjects undergoing RYGB are females, and the relative percentage of males scheduled for RYGB is low. To avoid the potential additional confounding effect of gender differences, we designed the present study to assess the impact of calprotectin only in females undergoing RYGB. In this regard, it is also worthwhile to mention that no significant association was found between calprotectin levels and gender in a study including 139 subjects (30).
Our study further provides evidence that both obese NG individuals and obese T2D patients exhibit lower circulating concentrations of sRAGE, a soluble form of the putative receptor of calprotectin. In this sense, it has been described that obese subjects showed lower levels of sRAGE than nonobese subjects (31), but studies analyzing the relationship between obesity and sRAGE are scarce. Our data suggest that low levels of sRAGE may be another factor involved in the low-grade chronic inflammation associated with obesity. Recent studies have shown that plasma levels of sRAGE are downregulated in chronic hyperglycemia (32), with plasma sRAGE concentrations being decreased in patients with essential hypertension (33). RAGE is an important pathogenetic factor in the development of long-term renal changes in T2D (34), and the administration of sRAGE has been suggested to exert a protective effect against diabetic complications. In this sense, the progression of atherosclerosis in diabetic mice is inhibited by the competition of RAGE with its soluble form (35). Of note, a negative correlation of sRAGE with fasting glucose and insulin as well as a positive association with the QUICKI index was found in the present study. In this regard, the low sRAGE concentrations may represent a risk factor for the development or progression of diabetic complications. Indeed, sRAGE has been proposed as an early and sensitive biomarker for the occurrence of accelerated atherosclerosis mediated by oxidative stress in diabetes mellitus (36). These findings contrast with those of Nin et al. (37), reporting higher plasma sRAGE levels in incident cardiovascular disease as well as an association with all-cause mortality in individuals with T1D. In this regard, it has to be stressed that the patients included in our obese T2D group do not have a long diabetes history (<2–3 years, as evidenced from their anamnesis and biochemical determinations leading to the current diagnosis). Undoubtedly, the duration and progression of the disease, as well as the individual’s susceptibility together with the metabolic control achieved during the time course of the disease, are likely to exert a relevant impact on the circulating levels of sRAGE. In this context, a long diabetic history with poor diabetic control is likely to be associated with an increase in both circulating concentrations of the inflammatory cytokines and chemokines. The reduced sRAGE levels in our group of T2D patients with short duration of their disease suggests that sRAGE downregulation is an early event in the history of diabetes and is more related to metabolic control than to disease duration. In this sense, the median follow-up duration was 12.3 years in the study by Nin et al. (37) and 9.1 years in the study by Thomas et al. (38), establishing duration of diabetes as one of the main independent determinants of sRAGE concentrations. The inverse correlation between circulating levels of calprotectin and sRAGE found in our study may be pointing to a plausible implication of calprotectin in a negative-feedback mechanism in the RAGE signaling pathway, which is in line with previous results regarding S100A12, another member of the S100 protein family (32). Bariatric surgery is known to produce a marked weight loss and an improvement of the inflammatory state, specifically characterized by a decrease in several inflammatory mediators and an increase in the antiinflammatory marker adiponectin (39). Our results show a reduction in calprotectin concentrations after weight loss achieved by RYGB, indicating an association with adipose tissue and weight loss, which is in accordance with the study by Nijhuis et al. (29), although the specific bariatric surgery technique was not specified in that study. Of note, we observed a positive correlation between differences in the calprotectin levels and changes in the WHR and concentrations of the inflammatory markers CRP and vWF in the patients undergoing RYGB. This result highlights the role of calprotectin in relation to chronic low-grade obesity-associated inflammation.
Because adipose tissue–derived adipokines play multiple roles in the body and can be considered as a link between obesity and the molecular events that lead to T2D development, we analyzed the gene expression levels of the calprotectin subunits S100A8 and S100A9 in VAT. To our knowledge, this is the first study describing elevated mRNA of both calprotectin subunits in VAT in human obesity-associated T2D. However, the lack of correlation between gene expression levels and the circulating concentrations of calprotectin suggests that in addition to VAT, a further source is contributing to the increased plasma calprotectin levels in obesity-associated T2D. In this sense, no evidence of dysregulation of mRNA levels of S100A8 in obese or T2D patients in skeletal muscle as well as no correlation with circulating calprotectin levels have been described (3). To gain more insight into the source of production, we further analyzed the gene expression levels of S100A8/A9 in isolated adipocytes and SVFCs, finding a higher expression in the SVFCs. Moreover, no differences in mRNA levels between obese NG volunteers and obese patients with T2D were found in isolated adipocytes, thereby suggesting a more pronounced contribution of SVFC to the overall expression of calprotectin subunits in VAT. Obesity-associated inflammation is characterized by an infiltration of macrophages in adipose tissue, which is likely a direct response to the abnormal fat metabolism caused by the increased adiposity. Because mRNA levels of S100A8/A9 and CD68 were positively correlated, S100A8/A9 expression levels may reflect the degree of VAT macrophage infiltration. It has been shown that calprotectin may participate in inflammation by enhancing CD11B expression in human monocytes and by participating in the transendothelial migration mechanism (40). Thus, calprotectin substantially contributes to the recruitment of monocytes to an inflammatory site. In this sense, it has been shown that circulating monocytes of T1D patients display increased CD11B and CD18 surface expression (2). Interestingly, we found an upregulation of CD11B in VAT of obese patients with T2D compared with LN volunteers. Moreover, a positive association between the gene expression levels of S100A8 and S100A9 with CD11B was also found. Taken together, these findings suggest that the presence of S100A8/A9 in inflamed VAT may be favoring the macrophage-monocyte migration and recruitment to inflammatory sites contributing to the obesity-associated low-grade chronic inflammation. It has been described that an increase in MCP1 expression in adipose tissue contributes to macrophage infiltration into this tissue and insulin resistance development (41). In this regard, an association of both calprotectin subunits with circulating MCP-1 was detected in our study. It has been proposed that the inactivation of the chemotactic activity of S100A8 by hypochlorite oxidation may provide a mechanism for limiting excess accumulation of leukocytes and terminating the progression of acute inflammation (42). In this sense, a putative role for calprotectin as a chemotactic factor might be put forward.
A hallmark of S100/calgranulin polypeptides is their association with chronic inflammation, such as human inflammatory bowel diseases (43). A positive association of calprotectin with CRP, IL-6, PAI-1 and TNF-α, well-established markers of chronic inflammation, was observed in the present study. Moreover, gene expression levels were positively correlated with IL-6, E-selectin and ICAM-1. Our findings in relation to inflammation are in line with previous reports where IL-6 infusion was found to induce mRNA expression of both S100A8 and S100A9 in skeletal muscle tissue (44). Calprotectin was also found to be associated with CRP (45,46) as well as with other acute-phase proteins such as fibrinogen and α1-antitrypsin (45,47). Increased oxidative stress in adipocytes causes dysregulated expression of adipokines, representing one of the causes of inflammatory changes in adipose tissue (48). Because S100A8 and S100A9 were proposed as regulators of the macrophage subunit NOX2 of the NADPH oxidase (49), we analyzed mRNA NOX2 levels in VAT, finding an upregulation in obese patients with T2D compared to LN and obese NG volunteers. In addition, a positive correlation of NOX2 with both calprotectin subunits was found. These findings further suggest a role for calprotectin in VAT inflammation through NOX2, increasing the generation of radical oxygen species.
A positive correlation between the mRNA levels of both S100A subunits with that of TNFA in VAT was also observed. In addition, our in vitro studies showed an induction of the S100A8 expression levels in human adipocyte culture upon stimulation with the cytokine TNF-α. In mice, TNF-α (14) and IL-1 (13) induced S100A8 and S100A9 expression in endothelial cells and macrophages. Taken together, these findings point to calprotectin’s implication in the development of insulin resistance via either direct or indirect effects on inflammation. In addition to its function as an inflammatory factor, murine S100A8 may also have antiinflammatory activity, depending on its extracellular concentration and circumstance of release (42). Most biological functions relevant to inflammation require the release of calprotectin into the extracellular space and the interaction with one or several signaling components. Although calprotectin interacts with RAGE, the role of this interaction is not clearly understood. In rheumatoid arthritis, calprotectin was shown to interact with RAGE, amplifying the proinflammatory cytokine production by macrophages via the activation of nuclear factor (NF)-κB and p38 mitogen-activated protein kinase (MAPK) (50). RAGE levels have been found to be decreased in chronic inflammatory diseases including atherosclerosis, diabetes, renal failure and aging. Regarding inflammation, a negative correlation between sRAGE and CRP, IL-6, PAI-1 and TNF-α together with a positive association with adiponectin was shown in the present study. Interestingly, serum sRAGE levels in rheumatoid arthritis patients were negatively associated with serum levels of CRP (51). It was also recently described that increased serum CRP together with reduced concentrations of sRAGE appear to be due to elevated TNF-α levels (52). In Il10 null mice, a murine model of colitis, no inflammation in rectosigmoid colon and a decrease in circulating concentrations of TNF-α were observed after treatment with sRAGE (53). Thus, the association of plasma sRAGE with components of inflammatory pathways highlights the role of the soluble form of the receptor in modulation of inflammation, probably acting as a decoy and competing with RAGE for its ligands. In this sense, anti-RAGE strategies including sRAGE-like molecules were proposed in relation to inflammation (54).
The increased levels of calprotectin in obesity and obesity-associated T2D together with its positive association with inflammatory markers as well as the higher expression levels in the stromovascular fraction in VAT suggests a role for this protein as a chemotactic factor in the recruitment of macrophages to VAT, increasing inflammation and the development of obesity-associated comorbidities.
ACKNOWLEDGMENTS
This work was supported by Fondo de Investigación Sanitaria (FIS) PI09/02330 from the Spanish Instituto de Salud Carlos III, by the Department of Health (20/2005 and 3/2006) of the Gobierno de Navarra of Spain and by Plan de Investigación de la Universidad de Navarra (PIUNA) (2009–2011).
The authors gratefully acknowledge the valuable collaboration of all the members of the Multidisciplinary Obesity Team, Clínica Universidad de Navarra, Pamplona, Spain. CIBER de Fisiopatología de la Obesidad y Nutrición (CIBEROBN) is an initiative of the Instituto de Salud Carlos III, Spain.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 2.Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes. 2004;53:1979–86. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- 3.Mortensen OH, et al. Calprotectin: a novel marker of obesity. PLoS One. 2009;4:e7419. doi: 10.1371/journal.pone.0007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odink K, et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–2. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 5.Zwadlo G, Bruggen J, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988;72:510–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–66. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 7.Striz I, Trebichavsky I. Calprotectin: a pleiotropic molecule in acute and chronic inflammation. Physiol Res. 2004;53:245–53. [PubMed] [Google Scholar]
- 8.Frosch M, et al. Expression of MRP8 and MRP14 by macrophages is a marker for severe forms of glomerulonephritis. J Leukoc Biol. 2004;75:198–206. doi: 10.1189/jlb.0203076. [DOI] [PubMed] [Google Scholar]
- 9.Foell D, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216:183–92. doi: 10.1002/path.2394. [DOI] [PubMed] [Google Scholar]
- 10.Spagnuolo MI, et al. Relationship between severe obesity and gut inflammation in children: what’s next? Ital J Pediatr. 2010;36:66. doi: 10.1186/1824-7288-36-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera C, McNeil HP, Geczy CL. S100 Calgranulins in inflammatory arthritis. Immunol Cell Biol. 2009;88:41–9. doi: 10.1038/icb.2009.88. [DOI] [PubMed] [Google Scholar]
- 12.Rammes A, et al. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 13.Yen T, et al. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood. 1997;90:4812–21. [PubMed] [Google Scholar]
- 14.Xu K, Geczy CL. IFN-γ and TNF regulate macrophage expression of the chemotactic S100 protein S100A8. J Immunol. 2000;164:4916–23. doi: 10.4049/jimmunol.164.9.4916. [DOI] [PubMed] [Google Scholar]
- 15.Manitz MP, et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034–43. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SY, Raftery MJ, Goyette J, Hsu K, Geczy CL. Oxidative modifications of S100 proteins: functional regulation by redox. J Leukoc Biol. 2009;86:577–87. doi: 10.1189/jlb.1008608. [DOI] [PubMed] [Google Scholar]
- 17.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez A, Catalán V, Gómez-Ambrosi J, Frühbeck G. Visceral and subcutaneous adiposity: are both potential therapeutic targets for tackling the metabolic syndrome? Curr Pharm Des. 2007;13:2169–75. doi: 10.2174/138161207781039599. [DOI] [PubMed] [Google Scholar]
- 19.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–8. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 20.Ginde SR, et al. Air displacement plethysmography: validation in overweight and obese subjects. Obes Res. 2005;13:1232–7. doi: 10.1038/oby.2005.146. [DOI] [PubMed] [Google Scholar]
- 21.Genuth S, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Ambrosi J, Frühbeck G, Martínez JA. Leptin, but not a β3-adrenergic agonist, upregulates muscle uncoupling protein-3 messenger RNA expression: short-term thermogenic interactions. Cell Mol Life Sci. 1999;55:992–7. doi: 10.1007/PL00013203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalán V, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–74. doi: 10.1007/s11695-008-9424-z. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Ambrosi J, et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metab. 2007;92:3719–27. doi: 10.1210/jc.2007-0349. [DOI] [PubMed] [Google Scholar]
- 25.Catalán V, et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res. 2007;39:495–500. doi: 10.1055/s-2007-982502. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez A, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes. 2009;33:541–52. doi: 10.1038/ijo.2009.40. [DOI] [PubMed] [Google Scholar]
- 27.Griendling KK. NADPH oxidases: new regulators of old functions. Antioxid Redox Signal. 2006;8:1443–5. doi: 10.1089/ars.2006.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frühbeck G, Gómez-Ambrosi J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001;15:1996–2006. doi: 10.1096/fj.00-0829hyp. [DOI] [PubMed] [Google Scholar]
- 29.Nijhuis J, et al. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity. 2009;17:2014–8. doi: 10.1038/oby.2009.113. [DOI] [PubMed] [Google Scholar]
- 30.Sun X, et al. Analysis of plasma calprotectin and polymorphisms of S100A8 in patients with aggressive periodontitis. J Periodontal Res. 2011;46:354–60. doi: 10.1111/j.1600-0765.2011.01350.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim OY, et al. G allele at RAGE SNP82 is associated with proinflammatory markers in obese subjects. Nutr Res. 2009;29:106–13. doi: 10.1016/j.nutres.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Basta G, et al. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab. 2006;91:4628–34. doi: 10.1210/jc.2005-2559. [DOI] [PubMed] [Google Scholar]
- 33.Geroldi D, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23:1725–9. doi: 10.1097/01.hjh.0000177535.45785.64. [DOI] [PubMed] [Google Scholar]
- 34.Flyvbjerg A, et al. Long-term renal effects of a neutralizing RAGE antibody in obese type 2 diabetic mice. Diabetes. 2004;53:166–72. doi: 10.2337/diabetes.53.1.166. [DOI] [PubMed] [Google Scholar]
- 35.Park L, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–31. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 36.Devangelio E, et al. Soluble RAGE in type 2 diabetes: association with oxidative stress. Free Radic Biol Med. 2007;43:511–8. doi: 10.1016/j.freeradbiomed.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Nin JW, et al. Higher plasma soluble receptor for advanced glycation end products (sRAGE) levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes. 2010;59:2027–32. doi: 10.2337/db09-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas MC, et al. Soluble receptor for AGE (RAGE) is a novel independent predictor of all-cause and cardiovascular mortality in type 1 diabetes. Diabetologia. 2011;54:2669–77. doi: 10.1007/s00125-011-2186-5. [DOI] [PubMed] [Google Scholar]
- 39.Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21:117–33. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- 40.Eue I, Pietz B, Storck J, Klempt M, Sorg C. Transendothelial migration of 27E10+ human monocytes. Int Immunol. 2000;12:1593–604. doi: 10.1093/intimm/12.11.1593. [DOI] [PubMed] [Google Scholar]
- 41.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison CA, et al. Oxidation regulates the inflammatory properties of the murine S100 protein S100A8. J Biol Chem. 1999;274:8561–9. doi: 10.1074/jbc.274.13.8561. [DOI] [PubMed] [Google Scholar]
- 43.Lugering N, et al. The myeloic related protein MRP8/14 (27E10 antigen)-usefulness as a potential marker for disease activity in ulcerative colitis and putative biological function. Eur J Clin Invest. 1995;25:659–64. doi: 10.1111/j.1365-2362.1995.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 44.Mortensen OH, et al. Calprotectin is released from human skeletal muscle tissue during exercise. J Physiol. 2008;586:3551–62. doi: 10.1113/jphysiol.2008.153551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berntzen HB, Munthe E, Fagerhol MK. A longitudinal study of the leukocyte protein L1 as an indicator of disease activity in patients with rheumatoid arthritis. J Rheumatol. 1989;16:1416–20. [PubMed] [Google Scholar]
- 46.Hammer HB, et al. Calprotectin (a major S100 leucocyte protein) predicts 10-year radiographic progression in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:150–4. doi: 10.1136/ard.2008.103739. [DOI] [PubMed] [Google Scholar]
- 47.Brun JG, Madland TM, Gran JT, Myklebust G. A longitudinal study of calprotectin in patients with polymyalgia rheumatica or temporal arteritis: relation to disease activity. Scand J Rheumatol. 2005;34:125–8. doi: 10.1080/03009740410009931. [DOI] [PubMed] [Google Scholar]
- 48.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerkhoff C, et al. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005;19:467–9. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- 50.Sunahori K, et al. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor κB and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen YS, Yan W, Geczy CL, Brown MA, Thomas R. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoanti-body, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R39. doi: 10.1186/ar2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNair ED, et al. Modulation of high sensitivity C-reactive protein by soluble receptor for advanced glycation end products. Mol Cell Biochem. 2010;341:135–8. doi: 10.1007/s11010-010-0444-3. [DOI] [PubMed] [Google Scholar]
- 53.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 54.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–53. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]