Abstract
New drug-eluting stent (DES) methods have recently been demonstrated to improve outcomes of intravascular interventions. A novel technique is the design of gene-silencing stents that elute specific small-interfering RNAs (siRNAs) for better vascular wall regeneration. Although siRNAs used to alter gene expression have surpassed expectations in in vitro experiments, the functional and local delivery of siRNAs is still the major obstacle for the in vivo application of RNA interference. In this preliminary in vitro study we investigated a surface-immobilized siRNA delivery technique that would be readily adaptable for local intravascular applications in vivo. The transfection potency of gelatin coatings consisting of a specific siRNA complexed with polyethylenimine (PEI) was examined in primary human endothelial cells by flow cytometry and quantitative real-time polymerase chain reaction. Several media conditions, such as the presence or absence of serum during cultivation, were investigated. Furthermore, different siRNA and PEI amounts, as well as nitrogen/phosphate ratios, were tested for their transfection efficiency. Gelatin coatings consisting of PEI and siRNA against an exemplary endothelial adhesion molecule receptor achieved a significant knockdown of around 70%. The transfection efficiency of the coatings was not influenced by the presence of serum. The results of this preliminary study support the expectation that this novel coating may be favorable for local in vivo gene silencing (for example, when immobilized on stents or balloons for percutanous transluminal coronary angioplasty). However, further animal experiments are needed to confirm the translation into clinical practice. This intriguing technology leads the way to more sophisticated and individualized coatings for the post-DES era, toward silencing of genes involved in the pathway of intimal hyperplasia.
INTRODUCTION
During recent decades considerable advances have been achieved in intravascular interventions for the treatment of arterial disease. In particular, drug-eluting stents (DES)—–although not as perfect as initially expected—–partly replaced bare metal stents (1). Meanwhile, several new stent coatings, like the CD34 antibody-coated endothelial progenitor cell capture stent, are under verification in patient studies (2). Results of several clinical studies indicate that the ideal stent is not yet available, and therefore industries as well as the scientific community continue to work hard on the development of more sophisticated solutions for the post-DES era. A novel technique may arise through the design of “gene silencing stents” that elute specific small-interfering RNAs (siRNAs) to improve vascular wall regeneration by silencing of genes involved in the pathway of intimal hyperplasia development.
Gene silencing by RNA interference (RNAi) has become established as a powerful technique for the down-regulation of any target gene of interest in basic research, drug discovery, and therapeutic applications (3–6). With this technique, optimized small interfering RNAs (siRNAs) are incorporated into the RNA-induced silencing complex, which mediates the degradation of complementary mRNAs. This technique is not considered as gene therapy because no alterations of the genome occur, and the silencing effect is transient (7,8). When siRNA duplexes are introduced by transfection, RNAi has been shown to efficiently silence endogenous genes in a variety of mammalian cells (9,10). Therapeutic knockdown of relevant target genes, which are up-regulated under pathological conditions, is a promising option for numerous therapeutic applications (11–14). The local delivery of siRNAs to the respective target cells remains a major issue, particularly in vivo, and is the greatest barrier for the success of RNA-based therapeutics (15–18).
In this study, we used a well-established siRNA, siRNA for E-selectin (siSELE), that silences the expression of E-selectin on human primary endothelial cells (ECs) (19). Selectins are calcium-dependent transmembrane glycoproteins that play a pivotal role in leukocyte–endothelial interactions during the early phases of atherogenesis (20). To achieve delivery of siRNAs into the cells, we examined the synthetic cationic polymer polyethylenimine (PEI) at different nitrogen/phosphate (N/P) ratios. The N/P ratio describes the ratio of moles of the amine groups of cationic polymer (PEI) to moles of the phosphate groups of DNA or RNA and is a measure of the ionic balance. The N/P ratio influences the transfection efficiency and toxicity (21). Because of the high cationic charge density of PEI under physiological pH, which is based on the partial protonation of the amino groups in every third position and results in a positive charge, PEI forms noncovalent complexes with negatively charged nucleic acids and thereby confers protection of siRNA against enzymatic degradation (22,23). In addition, PEI is postulated to exert a “proton sponge effect” upon endocytosis (PEI acts as proton acceptor and thus buffers low pH in the endosomal/lysosomal system, which leads to osmotic swelling and rupture of endosomes and release of the oligonucleotides into the cytoplasm) (24). Although PEI condenses oligonucleotides like DNA and siRNA, these complexes aggregate immediately under physiological conditions, such as salt or bovine serum albumin (25).
Thin films and coatings that sustain the release of siRNA from surfaces could play an important role in engineering surfaces for local gene silencing. Multilayered architectures have been shown to deliver drugs (26–29) and DNA (30–34), but little is known about whether this technique is feasible for the sustained delivery of siRNAs. Dimitrova et al. (2008) used multilayered polyelectrolyte films consisting of polylysine and polyglutamic acid as a precursor film and a hyaluronan-chitosan layer for the adsorption of PEI siRNA complexes that delivered siRNA to Huh7.5 cells (35). Interestingly, Neumann et al. (2006) used a gelatin layer with Lipofectamine™ 2000 and siRNAs for high-throughput RNAi screening on microarray for mainly HeLa cells (36).
Endothelial cells (ECs) play a key role in tumor growth and atherosclerosis. Therefore, they are a major target for numerous therapeutic applications. However, ECs are neither easy to culture nor easy to transfect and require special culture media supplemented with growth factors and serum (37). The in vitro growth of ECs is dependent on the presence of suitably coated surfaces (38). Relou et al. (1998) showed that coating culture dishes with fibronectin, collagen, gelatin or hyaluronan potentiated basic fibroblast growth factor activity (39). In addition, ECs cover a huge surface area of the whole vasculature and therefore are useful for local application only at the diseased sections of the vessel wall.
In this preliminary study, we examined the potential of novel strategies for the delivery of surface immobilized PEI/siRNA complexes to human primary ECs, a key issue for overcoming obstacles to the clinical application of siRNA-based local gene-silencing strategies (for example, when immobilized on stents or balloons for percutaneous transluminal coronary angioplasty for treatment of vascular diseases).
MATERIALS AND METHODS
Cells
ECs were isolated from saphenous vein specimens obtained from patients undergoing elective coronary artery bypass grafting. The study protocol conformed to the ethics guidelines of the 1975 Declaration of Helsinki. All patients gave their written consent, and the study was approved to by the clinical ethics committee of the University of Tuebingen.
Isolation and Cultivation of Human Venous ECs
ECs were harvested by collagenase treatment of saphenous veins as previously described (40), then cultured in VascuLifeTM EnGS cell culture medium (Lifeline Cell Technology, Walkersville, MD, USA) containing supplements and antibiotics.
Chemicals and siRNAs
Branched PEI (800 Da) was purchased from Sigma-Aldrich (Seelze, Germany). Type B gelatin with gel strength of 160 was provided by Fluka (Seelze, Germany) and dissolved in double-distilled water to make a 0.1% solution.
siSELE was obtained from CureVac GmbH (Tuebingen, Germany) and had the following sequence: sense 5′-GGU UGA AUG CAC CAC UCA AdTdT-3′, antisense 5′-UUG AGU GGU GCA UUC AAC CdTdT-3′. Control nonsense siRNA (scrRNA) and control nonsense siRNA labeled with Alexa Fluor 488 were provided by Qiagen (Hilden, Germany). Qiagen does not provide the sequence of their nonsilencing siRNAs but ensures that they have no homology to any known mammalian gene. These nonsilencing siRNAs are validated by using Affymetrix GeneChip arrays and a variety of cell-based assays and shown to ensure minimal nonspecific effects on gene expression and phenotype.
PEI-siRNA Complex Preparation for Coatings or Conventional Transfection
Complexes of PEI and siRNA were obtained by diluting siRNAs and PEI separately for 10 min in 150 mmol/L NaCl and 20 mmol/L Hepes, pH 7.4. N/P ratios of 3, 6 and 8 were obtained, and it was assumed that 1 μg of siRNA contains 3 nmol of phosphate, and that 1 μL of 10 mmol/L PEI solution contains 10 nmol of amine nitrogen. After 10 min, the siRNA and PEI solutions were mixed together, vortexed, and incubated for another 10 min. Afterward, the mixture was added to 500 μL 0.1% gelatin solution for one well of a 12-well plate and dried in 12-well plates for employment as a coating.
Conventional Transfection of ECs
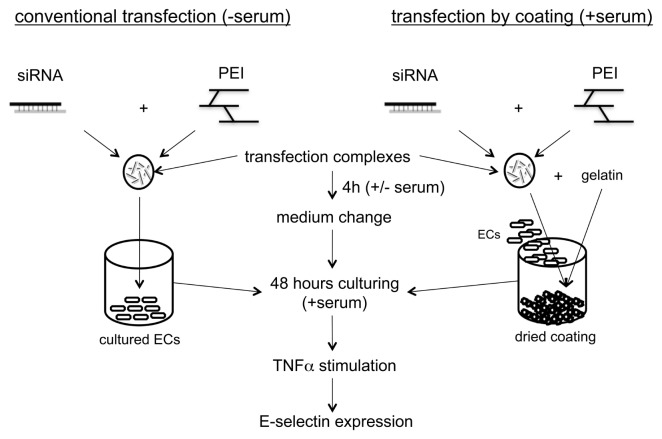
Herein, conventional transfection describes the general procedure to transfect cells with an siRNA that is introduced on seeded cells. Twenty-four hours before transfection, the ECs were seeded in 12-well plates with VascuLife™ EnGS cell culture medium. After reaching 70% confluency, the cells were transfected with 1 μg siRNA complexed with PEI at an N/P ratio of 8 in 50 μL 150 mmol/L NaCl and 20 mmol/L Hepes, pH 7.4. Then complexes of 50 μL were pipetted into 350 μL Vasculife EnGS basal medium (not containing supplements or serum), and given to cells of one well of a 12-well plate. After 2 h, medium was changed to VascuLife™ EnGS cell culture medium again, and 48 h after transfection, cells were stimulated with 2.5 ng/mL tumor necrosis factor (TNF)-α (Immunotools, Friesoy, Germany). E-selectin protein expression was measured by flow cytometry. Figure 1 shows an illustration of the conventional transfection compared with transfection of ECs on coatings with immobilized siRNA.
Figure 1.
Illustration of the conventional transfection methodology compared with immobilized siRNA transfection by coating.
Transfection of ECs on Coatings with Immobilized siRNA
When coatings were dried, ECs were seeded onto the plates with a minimum of 100,000 cells in each well of a 12-well plate in VascuLife™ EnGS cell culture medium containing supplements, serum and antibiotics. After 4 h, the medium was replaced with fresh medium. Forty-eight hours after transfection, cells were stimulated with 2.5 ng/mL TNF-α, and E-selectin protein expression was measured by flow cytometry.
Influence of Serum on siRNA Delivery
ECs were cultured for 12 h with VascuLife™ EnGS complete cell culture medium (with supplements and serum) on coatings containing PEI and siRNA at an N/P ratio of 8 with 2, 4 or 6 μg siSELE or scrRNA per well of a 12-well plate to adhere to the surface of the coating. Afterward, half of the cells were changed to VascuLife™ EnGS cell culture medium without serum and supplements for 4 h, and changed back to VascuLife™ EnGS cell culture medium containing supplements and serum until examination by flow cytometry for E-selectin receptor expression.
Effects of Coating Components in Solution
PEI/siRNA complexes at an N/P ratio of 8 with 2, 4 and 6 μg siSELE per well of a 12-well plate were prepared and added to gelatin. This solution was mixed with 1 mL VascuLife™ EnGS cell culture medium and detached ECs in one well of a 12-well plate. After 4 h of cultivation, medium was replaced by VascuLife™ EnGS cell culture medium containing supplements and serum.
Washing of the Coatings Prior to Cultivation of ECs
Coatings were prepared with PEI and siRNA at an N/P ratio of 8 with 4 or 6 μg siRNA per well of a 12-well plate. Prior to cultivation of ECs, half of the coatings were rinsed with 500 μL phosphate-buffered saline (PBS), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to assess toxicity.
Optimization of Coating-Mediated Transfection through Different siRNA Amounts and N/P Ratios
N/P ratios of 3, 6 and 8 were prepared with 10, 12 and 14 μg siSELE; 4, 6 and 8 μg siSELE; and 2, 4 and 6 μg siSELE for each well, respectively. These solutions were added to 500 μL 0.1% gelatin, for each well of a 12-well plate. Cells were cultivated 48 h on coatings, and E-selectin expression was examined by flow cytometry.
Comparison of E-Selectin Knockdown Duration between Coating or Conventional Mediated Transfection
One layer with PEI and siSELE at an N/P ratio of 3 with 14 μg siRNA per well of a 12-well plate was prepared and pipetted to 500 μL 0.1% gelatin and dried. In addition, some of the prepared wells received a second layer with the same PEI, siSELE and 0.1% gelatin amounts. The second layer was also dried; afterward, SECs were seeded on the coated wells. In parallel, ECs were conventionally transfected with PEI and siSELE at an N/P ratio of 8 with 1 μg siRNA per well. E-selectin knockdown was examined by flow cytometry from d 2 to 7 after cultivation.
Quantitative Real-Time Polymerase Chain Reaction
To perform quantitative real-time poly-merase chain reaction (qRT-PCR), we purified total RNA from cultured ECs by use of an Aurum™ total RNA minikit from Bio-Rad (Hercules, CA, USA). Consecutively, 200 ng of each RNA sample was reverse transcribed with an iScript™ cDNA Synthesis Kit from Bio-Rad according to the manufacturer’s instructions. Primer design was done with the software Primer3 (41) and Primer Premier 5 (PREMIER Biosoft International, Palo Alto, CA, USA). The following sequences were used: E-selectin forward 5′-GCCCAGAGCCTTCAGTGTACC-3′, reverse 5′-GGAATGGCTGCACCT CACAG-3′; TNF-α forward 5′-CCGTC TCCTACCAGACCAAGG-3′, reverse 5′-CTGGAAGACCCCTCCCAGATAG-3′; nuclear factor (NF)κB1 forward 5′-CCTCC ACAAGGCAGCAAATAG-3′, reverse 5′-TGAGTTTGCGGAAGGATGTCTC-3′; NFκB2 forward 5′-GGACCTCGGC GTTGTCAAC-3′, reverse 5′-ACTCG TCTGCCCCGTGATC-3′; interleukin (IL)-1β forward 5′-CCCACAGACC TTCCAGGAGA-3′, reverse 5′-CGGAG CGTGCAGTTCAGTG-3′; IL-12 forward 5′-CAACATGCTCCAGAAGGCCA GACA-3′, reverse 5′-TGGTAAACAG GCCTCCACTGTGC-3′; IL-6 forward 5′-CACACAGACAGCCACTCACCTC-3′, reverse 5′-CTGCCAGTGCCTCTT TGCTG-3′; IL-8 forward 5′-GACTT CCAAGCTGGCCGTG-3′, reverse 5′-CTCCTTGGCAAAACTGCACC-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward 5′-TCAACAGCGA CACCCACTCC-3′, reverse 5′-TGAGG TCCACCACCCTGTTG-3′; intercellular adhesion molecule (ICAM)-1 forward 5′-CTTGAGGGCACCTACCTCTGTC-3′, reverse 5′-CGGCTGCTACCACAG TGATG-3′. All primers were synthesized by Operon (Köln, Germany). All PCR reactions contained IQTMSYBR®Green Supermix from Bio-Rad, 400 nmol/L forward and reverse primer and 2 ng of cDNA in a total volume of 15 μL. All PCR reactions were performed in triplicates. Normalized gene expression was calculated by the ΔCt method using GAPDH as a reference.
Flow Cytometry
Forty-eight hours after transfection through coatings or conventional transfection, ECs were stimulated with 2.5 ng/mL TNF-α for another 4 h to induce E-selectin expression. The cells were stained for 1 h at 4°C with a PE-Cy5- labeled E-selectin antibody (Becton Dickinson GmbH, Heidelberg, Germany) diluted in 0.5 % fetal calf serum. After washing and detaching of cells, they were fixed with 2.5% paraformaldehyde in PBS. Flowcytometric analyses (5000 cells/measurement) were performed in a FACScan™ (Becton Dickinson GmbH) and evaluated with the CellQuestPro software (Becton Dickinson GmbH). The geometric mean values were used for further analyses.
Cell Viability Assay
Cell viability was determined by MTT reduction assay performed with a Vybrant® MTT Cell Proliferation Assay Kit (Invitrogen, Karlsruhe, Germany). Thus, MTT was reduced by the cells to insoluble formazan. The growth medium was replaced by 300 μL RPMI without phenol red, containing 0.5 mg/mL MTT. After 4 h incubation at 37°C, the medium was removed from the cultures and the blue formazan products were solubilized by adding 150 μL of dimethyl-sulphoxide to each well for 10 min at 37°C. Absorbance at 540 nm was measured by a microplate (enzyme-linked immunosorbent assay) reader.
Release of Fluorescent Labeled siRNA
One microgram of Alexa Fluor 488 fluorescent labeled siRNA was complexed with PEI at N/P 8 and dried with 200 μL 0.1% gelatin in one well of a 48-well plate. After the coating was dried, 200 μL VascuLifeTM EnGS cell culture medium was added to the 48-well plates and incubated at 37°C. At time 0, 30 min, 60 min, 4 h, 12 h and 24 h, the supernatant was used to measure fluorescence intensity at excitation 485 nm and emission 535 nm, with a fluorescence reader TriStar LB 941, from Berthold Technologies (Bad Herrenalb, Germany). The amount of released siRNA in the supernatant was calculated by comparison to a concentration series of N/P 8 build complexes.
Statistics
Experiments were carried out three times independently with three different EC populations originating from three individual patients. Each single experimental approach was executed in triplicate. Geometric means of fluorescence measured by flow cytometry were used to calculate the knockdown of the E-selectin receptor, at which untreated cells stimulated with TNF-α were set to 100%. The Shapiro-Wilk test was used to proof for gaussian distribution. Groups were compared by unpaired two-sample t tests, and for the overall significance level we adopted the value of 5%.
RESULTS
Influence of Serum on siRNA Delivery
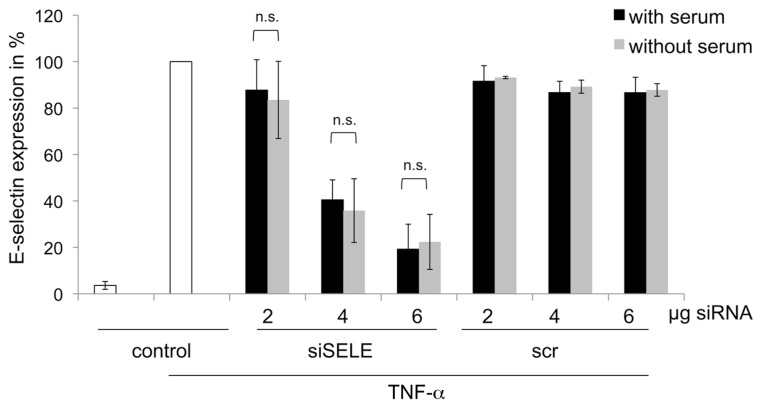
As known from previous experiments, the presence of serum may have a negative influence on siRNA delivery (37). Therefore, we compared the knockdown of the E-selectin receptor of ECs cultivated with and without serum on gelatineimmobilized siRNA/PEI coatings. Transfection efficiency of the coating revealed best knockdown of the E-selectin receptor at 6 μg siSELE with an N/P ratio of 8 (Figure 2). There was no significant difference in the knockdown of the E-selectin receptor in cells that were cultivated for 4 h without serum after their adhesion to the well plates, compared with cells growing continually in the presence of serum. These results show that coatings of gelatin with PEI and siRNA perform well in the presence of serum. Coatings prepared with scrRNA, PEI and gelatin did not result in a significant knockdown of the E-selectin receptor.
Figure 2.
Influence of serum on transfection efficiency. Cells were cultivated on different coatings, prepared with 2, 4 or 6 μg siSELE or scrRNA at N/P 8 ratio. After 12 h of cultivation, half of the cells were supplied to medium without serum for 4 hours. 48 h after seeding, cells were analyzed by FACS. The y axis reflects E-selectin receptor expression in % untreated cells stimulated with TNF-α set to 100%. Each bar represents the mean ± standard deviation (SD) of n = 3. n.s. indicates not significant.
In Culture Medium, Dissolved Coating Components Do Not Achieve Transfection
When mixtures of PEI/siSELE and gelatin were given to detached ECs in -solution, only 23% knockdown of the E-selectin receptor could be achieved with 6 μg siSELE (Figure 3). The maximum knockdown (49%) of the E-selectin receptor was gained with coatings of PEI/siSELE in gelatin with 6 μg siSELE. These results show that coatings of 4 and 6 μg siSELE gave a significant knockdown of the E-selectin receptor compared with mixtures of the same PEI and siSELE amounts given to detached cells.
Figure 3.
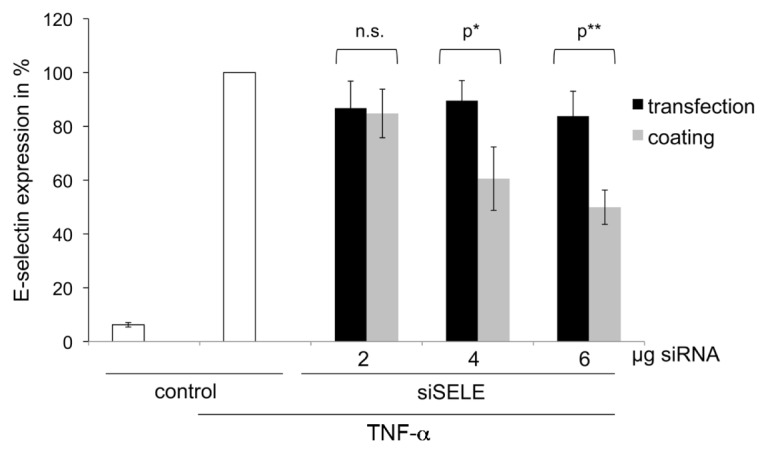
Comparison of the transfection efficiency between PEI-siRNA complexes with gelatin dried (coating) or as mixture given to detached cells (transfection). The y axis reflects the E-selectin receptor expression in %; untreated cells stimulated with TNF-α were set to 100%. Each bar represents the mean ± SD of n = 3. n.s. indicates not significant, p* indicates statistical significance at a level of P < 0.05; p** indicates statistical significance at a level of P < 0.01.
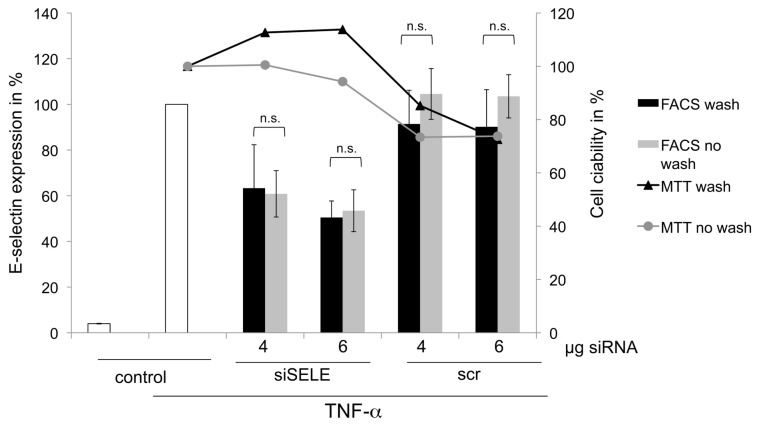
Washing of the Coatings Prior to Cultivation of ECs Achieves Similar siRNA Delivery Efficiency with Less Toxicity
Coatings washed with PBS before cultivation of the ECs showed no significant aberration of the E-selectin knockdown compared with coatings that were not washed (Figure 4). The results of the viability assay with MTT showed less toxicity when coatings were washed with PBS prior to seeding, but the statistical analysis showed no significant reduction of the toxicity. Furthermore, coatings containing scrRNA did not show a significant knockdown of the E-selectin receptor.
Figure 4.
Comparison between coatings of 4 and 6 μg siSELE/scrRNA washed with PBS prior to cultivation of cells, to unwashed coatings. The primary y axis reflects E-selectin receptor expression in %, whereby untreated cells stimulated with TNF-α were set to 100%. The secondary y axis reflects the viability of ECs compared with untreated cells. Each bar represents the mean ± SD of n = 3. n.s. indicates not significant.
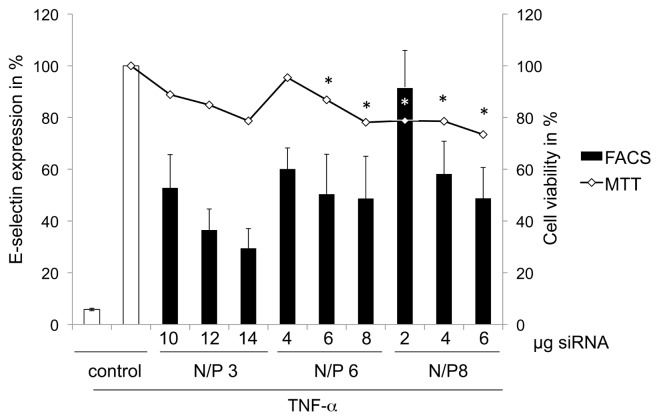
Optimization with Different N/P Ratios and Examination of the Toxicity
For the optimization of siRNA delivery, we tested several N/P ratios and siRNA amounts. The highest knockdown of the E-selectin receptor of around 70% was achieved by coatings that contained 14 μg siRNA per well of a 12-well plate at an N/P ratio of 3 (Figure 5). The viability decreased significantly at N/P ratios of 6 with 6 and 8 μg siRNA, and at N/P ratios of 8 with 2, 4 and 6 μg siRNA compared with untreated cells.
Figure 5.
SiRNA delivery optimization by different coatings shows the transfection efficiency with different amounts of siRNAs and different N/P ratios. The first y axis reflects the E-selectin receptor expression in %, whereby untreated cells stimulated with TNF-α were set to 100%. The second y axis shows the viability of ECs in % seeded on coating composed of different siRNA amounts and N/P ratios. The viability of untreated cells was set to 100%. Each bar represents the mean ± SD of n = 3. p* indicates statistical significance at a level of P < 0.05 compared with untreated cells.
Comparison of E-Selectin Knockdown Duration between Coating or Conventional Mediated Transfection
To evaluate the duration of the siRNA delivery, we compared conventional transfection of PEI and siRNA at N/P 8 with 1 μg siSELE against 1- or 2-layered coatings of PEI and siRNA at N/P 3 (14 μg siRNA per well of a 12-well plate) for one layer. We decided to use N/P 8 with 1 μg siRNA for conventional transfection, because we observed maximum knockdown at this concentration in other experiments (data not shown) but without the use of serum during transfection. For the coating we decided to use an N/P ratio of 3 with 14 μg of siRNA, because with this ratio we revealed maximum knockdown (see Figure 5). Conventional transfection achieved the highest knockdown (of 80%) of the E-selectin receptor 2 d after transfection. At the same time, one-layered and two-layered coatings showed a decrease in the E-selectin expression of around 50% and 60%, respectively (Figure 6). Seven days after transfection by coatings or conventional transfection of ECs, the knockdown of the E-selectin receptor was similar in 1- or 2-layered coatings compared with general transfected cells.
Figure 6.
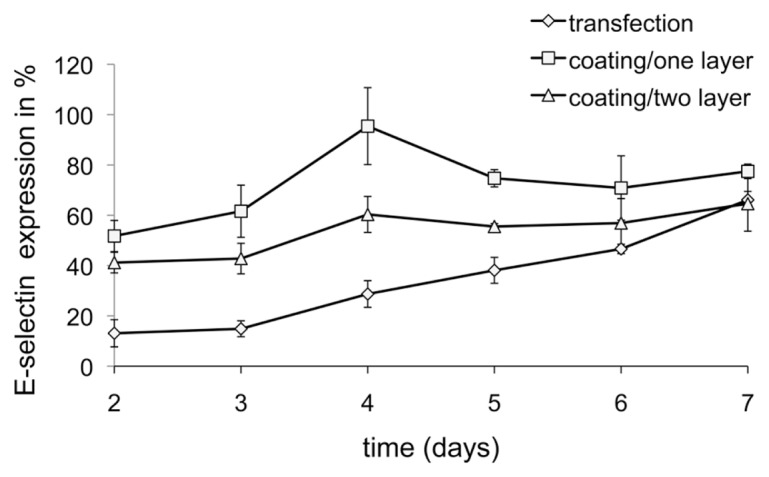
Duration of siRNA delivery. The x axis reflects the time of analysis of E-selectin expression after cultivation on coatings of one or two layers, with 14 μg siRNA at N/P 3. The y axis reflects E-selectin receptor expression in %, whereby untreated cells stimulated with TNF-α were set to 100%. Mean ± SDs of three independent experiments are shown.
Release of siRNA from Coatings
The release of siRNA from gelatin coatings passes rapidly at 37C° in VascuLife™ EnGS cell culture medium; after 30 min most of the siRNA is in the supernatant (Figure 7). These results provide evidence that the delivery of siRNA to the ECs has occurred within the first 30 min after cultivation on coatings.
Figure 7.
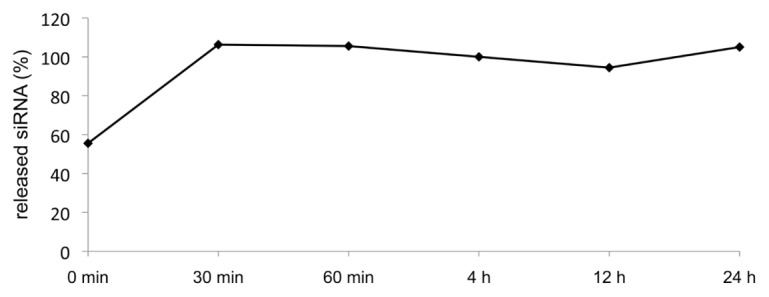
Release of fluorescent-labeled siRNA. The x axis shows the time of analysis of the fluorescence in the supernatant. The y axis reflects the release of siRNA from coatings, whereby the entire volume in one well was set to 100%.
mRNA Expression of siSELE- or scrRNA-Transfected ECs
The conventional transfection of siSELE showed a decrease of the E-selectin mRNA level measured by qRT-PCR of approximately 90%. The scrRNA showed no reduction of the E-selectin mRNA level (Figure 8). Furthermore, neither siSELE-nor scrRNA-transfected ECs show a reduction of the EC adhesion molecule ICAM-1.
Figure 8.
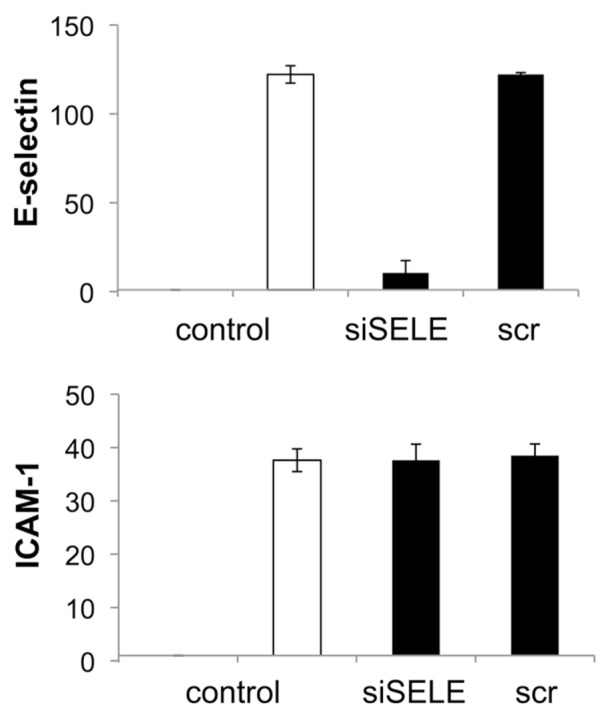
qRT-PCR measurement of siSELE mediated knockdown of E-selectin and ICAM. Conventional transfection was conducted by PEI and siSELE or scrRNA. The expression of nontreated cells was set to one. Each bar represents the mean ± SD of n = 1.
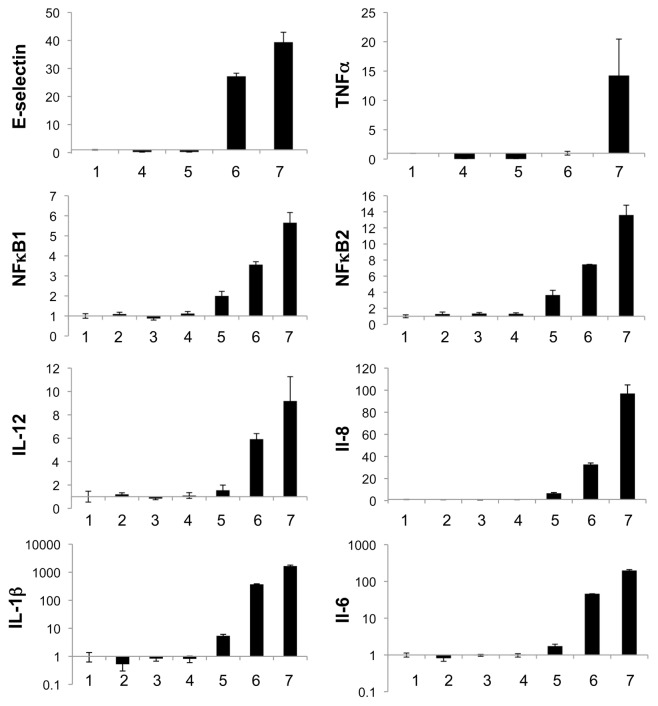
Additionally, the mRNA level of inflammatory markers was measured after transfection of ECs with scrRNA, siSELE or poly (IC) double-stranded RNA (dsRNA). The transfection of the RNAs was carried out with and without PEI and was performed to study the response of ECs to double-stranded siRNAs (see Figure 9). Therein, the relative expression of the mRNA of E-selectin, TNF-α, NFκB1, NFκB2, IL-12, IL-8, IL-1β and IL-6 is shown. The poly (IC) dsRNA alone or complexed with PEI showed a strong increase of the inflammatory markers E-selectin, TNF-α, IL-12, IL-8, IL-1β and IL-6 in ECs; in contrast the transfection of siSELE or scrRNA showed only a slight increase of the mRNA level.
Figure 9.
qRT-PCR measurement of relative expression of E-selectin, TNF-α, NFκB1, NFκB2, IL-12, IL-8, IL-1β and IL-8 compared with untreated cells (1) after conventional transfection with scrRNA, siSELE or poly (IC) dsRNA; (2) scrRNA without PEI; (3) scrRNA with PEI; (4) siSELE without PEI; (5) siSELE with PEI; (6) poly (IC) dsRNA without PEI and (7) poly (IC) dsRNA with PEI. The expression of untreated cells was set to one. Each bar represents the mean ± SD of n = 1.
DISCUSSION
Gene silencing by RNA interference has a huge potential for the development of numerous nucleic acid–based therapeutics. However, the in vivo application of siRNAs for specific targeting of disease-relevant genes has been limited by several technical obstacles, such as instability of the siRNAs in body fluids, nonfunctional intracellular uptake, and poor bioavailability. Secondly, the main drawback of in vivo RNAi applications is the efficiency of transfection to primary cells and the local administration.
The focus of this study was the development of a novel technique useful for designing gene-silencing stents or balloons that elute specific siRNAs to improve vascular wall regeneration by silencing of genes involved in the pathway of intimal hyperplasia, such as E-selectin. For us it seems to be quite attractive to focus on the early processes involved in the vascular inflammation, as Ley et al. have postulated: “The activation of ECs at atherosclerotic lesion-prone sites in the arterial tree results in the up-regulation of cell adhesion molecules and chemokines, which mediate the recruitment of circulating monocytes. Accumulation of monocytes and monocyte-derived phagocytes in the wall of large arteries leads to chronic inflammation and the development and progression of atherosclerosis” (42,43). We succeeded in creating a coating that locally delivers PEI-encapsulated siRNA molecules to primary human ECs. Our gelatin coatings released siRNA and delivered it to ECs, even in the presence of serum (Figure 2), whereas it is known for conventional transfection that complexes of PEI and siRNA aggregate immediately in the presence of bovine serum albumin (25). A recently reported work of Neumann et al. 2006 showed the use of Lipofectamin 2000 instead of PEI to generate a gelatin coating for siRNA delivery to mainly HeLa cells (36). From our experience with primary human ECs, the use of Lipofectamin 2000 is not advisable, because we achieved E-selectin knockdown of 40% with Lipofectamin 2000 alone, without the use of siRNA in case of conventional transfection (37). From these results, we can conclude that the efficiency and side effects of transfection reagents is dependent on the cell line that is used. We confirmed a 90% knockdown of the protein level by flow cytometry (Figure 6) and also a 90% knockdown of the mRNA level by qRT-PCR (Figure 8) after conventional transfection with siSELE and PEI, whereas scrRNA-mediated transfection showed no significant reduction of the E-selectin expression. These results lead to the conclusion that PEI is a suitable transfection reagent and siSELE shows no side effects. Additionally, as shown in Figure 9, we demonstrated that the conventional transfection of siSELE or scrRNA compared with poly (IC) dsRNA mediates no significant increase of inflammatory markers. Therefore, we propose that siSELE and scrRNA do not signal through toll-like receptors and do not mediate the release of inflammatory markers after transfection.
Solved components of PEI-siRNA with gelatin gave no significant knockdown of the E-selectin receptor compared with coatings with the same amounts, which prompts the conclusion that the solved components of the coating were not able to transfect ECs (Figure 3).
These results prove that the delivery of the siRNA directly came from the coating and not by dissolved components that mediate transfection. Furthermore, our coating showed a local delivery of siRNA.
We revealed that washing of the coatings with PBS did not result in lower transfection efficiency (Figure 4), a finding that may be explained by the fact that gelatin does not dissolve until temperatures reach 37°C. We have to point out that the toxicity of the coatings could be reduced by washing them prior to transfection (Figure 4). Moreover, a coating consisting of scrRNA shows a decrease of EC viability, but no decrease of E-selectin receptor expression. This result could be attributable to a toxic side effect of the scrRNA.
Our results revealed that the transfection efficiency of gelatin coatings is dependent on the siRNA amount and the N/P ratio. Increasing amounts of siRNA with the same N/P ratio tended toward higher transfection efficiencies (Figure 5). When we examined the lowest N/P ratio of 3, we found out that much higher siRNA amounts were needed to achieve a similar knockdown compared with N/P ratios of 6 or 8. This result can be explained by the fact that these complexes are less positively charged, which results in reduced cell-surface binding. The use of low N/P ratios (like 3) with a high siRNA amount resulted in no significant toxicity compared with ECs that were cultivated on coatings consisting of PEI/siRNA complexes with a ratio of N/P 8 and 2–6 μg siRNA (Figure 5). This toxicity might be a consequence of endosomal/lysosomal enzyme release into the cytoplasm consecutive to feeding a cell with many membrane-disrupting particles (24).
When we examined the duration of the transfection from one- or two-layered coatings, we found out that a knockdown of 30%–50% could still be achieved after 6 d of cultivation (Figure 6). Whereas conventional transfection achieves a higher knockdown in the first day after transfection, the E-selectin expression starts to increase faster, compared with the one- or two-layered coatings. Although conventional transfection achieves a similar knockdown with lower siRNA amounts compared with our coating, the conventional transfection had to be performed without the use of serum during transfection. From this finding, we conclude that local delivery by PEI/siRNA complexes under in vivo conditions will not work efficiently. In addition, we propose encapsulating PEI/siRNA complexes into gelatin and drying as a coating.
However, the release of the fluorescent-labeled siRNA occurs within 30 min after medium addition, and therefore proves that the transfection of the ECs happens within this time (Figure 7). Gelatin is soluble in aqueous solution, and a few minutes of storage in physiological solution is sufficient to induce considerable swelling of the coatings (about 700% after 1 h) (44). The inconsistency of having more released siRNA in the solution (see Figure 7) seems to be caused by small amounts of free siRNA that are not complexed and no longer have their fluorescence quenched by PEI; free siRNA causes a much higher fluorescence. This burst release of functional PEI-siRNA complexes with the ability to be delivered to primary cells in the presence of serum could be suitable for RNAi therapeutics in local intravascular applications. Furthermore, the encapsulation of siRNA with PEI shields the siRNA from degradation by RNases (22,23). To stop the leukocyte–endothelial interaction, it is necessary to reduce the expression of E-selectin, but also of ICAM-1 and vascular cell adhesion molecule-1 (45). Gelatin with PEI and siRNA cocktails consisting of siRNAs against different adhesion molecules responsible for leukocyte migration could be coated easily on balloons used for percutaneous transluminal coronary angioplasty. The inflammatory process that will take place after the balloon application may be inhibited by the cytosol-delivered siRNAs. Adhesion molecule production will be reduced and stop the leukocyte migration and muscle cell proliferation. We designed a coating consisting of different siRNAs for the specific knockdown of several targets simultaneously. It is possible to target not only EC receptors but also smooth muscle cell targets. Thus, we expect to reduce the high risk of restenosis. It is known that siRNAs are diluted with every cell division, and therefore the silencing of the desired protein decreases (46). But the division of cells in tissue culture is much faster compared with in vivo ECs. Our results may have a high clinical impact, because we expect a prolonged silencing of the adhesion molecules in vivo compared with our in vitro tissue culture results.
The short vessel-wall contact of a balloon-coated drug in contrast to a stent-coated drug may not be a disadvantage, because Tepe et al. have shown that paclitaxel-coated balloons significantly reduced restenosis in patients undergoing angioplasty of femoropopliteal arteries (47,48).
In addition, more sophisticated coatings are under development to achieve even better transfection of the stabilized siRNA. Alternatively, by use of short hairpin RNA, an extremely long-lasting silencing can be attained; however, this approach is classified as gene therapy and thus involves many hurdles for regulatory affairs and certification.
With this coating it was sufficient to use a standardized inflation time of 1 min. We suggest that this short inflation time could also be effectual for our coating.
We found that different gelatin species used for the coating showed different E-selectin receptor knockdowns (data not shown). The use of gelatin that contains only short collagen fragments and has low gel strength showed higher transfection efficiencies than high gel strength gelatin. The gelatin that was used in this study had a gel strength of 160, whereas gelatin with a gel strength of around 300 showed 20% less E-selectin receptor knockdown. Therefore, we hypothesize that the burst release of siRNA out of low-strength gelatin is suitable to transfect ECs. We propose that the transfection time of around 30 min can be shortened with lower gel strength gelatin to have a shorter angioplasty procedure. Furthermore, there was no difference observed in E-selectin knockdown between gelatins with different isoelectric points and basic-or acid-digested gelatin. If the gelatin coating is used on a balloon for percutaneous coronary angioplasty, we suggest using a mechanical protective cap around the balloon, which could be disconnected immediately before inflation of the balloon. This procedure could circumvent the detachment of the coating before it is inflated at the side of the plaque.
Very recently, San et al. (2009) used cationized pullulan coated stents and delivered siRNA against matrix metalloproteinase 2 (MMP-2) to the vascular wall. However, their results showed poor transfection efficiency; thus only the pro–MMP-2 was significantly reduced but not MMP-2 (49). In comparison, our coating significantly improved transfection efficiency to the vascular wall of arteries because we were able to achieve a knockdown of the E-selectin receptor between 50% and 70%. Additionally, E-selectin expression increased more slowly in the following days of cultivation on the coatings compared with conventional transfected cells.
CONCLUSIONS
This study provided proof of the principle of a local gene-silencing strategy that uses surface coatings composed of gelatin, PEI and specific siRNA by which a highly significant knockdown of the E-selectin receptor in human primary ECs was achieved. The transfection efficiency of our coating was around 70%, without a significant reduction of the cell viability. The transfection efficiency was not inhibited by the presence of serum, which is an important prerequisite for intravascular applications. These results demonstrate that this coating may be suitable for local RNAi in vivo applications. Further animal experiments with the use of siRNA-coated stents or balloons for percutaneous transluminal coronary angioplasty must be performed to confirm the positive effects on reduction of inflammatory reactions and consecutive atherogenesis.
The results of this preliminary study support the vision of designing gene-silencing intravascular devices for more sophisticated and individualized solutions for the post-DES era.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Greenhalgh J, et al. Drug-eluting stents versus bare metal stents for angina or acute coronary syndromes. Cochrane Database Syst Rev. 2010;5:CD004587. doi: 10.1002/14651858.CD004587.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Wendel HP, Avci-Adali M, Ziemer G. Endothelial progenitor cell capture stents: hype or hope? Int. J. Cardiol. 2010;5;145:115–7. doi: 10.1016/j.ijcard.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004;3:318–29. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- 4.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 5.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 6.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–9. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 7.Chi JT, et al. Genomewide view of gene silencing by small interfering RNAs. Proc Natl Acad Sci U S A. 2003;100:6343–6. doi: 10.1073/pnas.1037853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan A, et al. Small interfering RNA-mediated functional silencing of vasopressin V2 receptors in the mouse kidney. Physiol Genomics. 2005;21:382–8. doi: 10.1152/physiolgenomics.00147.2004. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu H, Fujita T. New short interfering RNA-based therapies for glomerulonephritis. Nat Rev Nephrol. 2011;7:407–15. doi: 10.1038/nrneph.2011.61. [DOI] [PubMed] [Google Scholar]
- 10.Tagalakis AD, He L, Saraiva L, Gustafsson KT, Hart SL. Receptor-targeted liposome-peptide nanocomplexes for siRNA delivery. Biomaterials. 2011;2:6302–15. doi: 10.1016/j.biomaterials.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Kato M, et al. The targeting of cyclophilin D by RNAi as a novel cardioprotective therapy: evidence from two-photon imaging. Cardiovasc Res. 2009;83:335–44. doi: 10.1093/cvr/cvp094. [DOI] [PubMed] [Google Scholar]
- 12.Valentim L, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–52. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 13.Yin C, Xi L, Wang X, Eapen M, Kukreja RC. Silencing heat shock factor 1 by small interfering RNA abrogates heat shock-induced cardioprotection against ischemia-reperfusion injury in mice. J Mol Cell Cardiol. 2005;39:681–9. doi: 10.1016/j.yjmcc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Zheng X, et al. Novel small interfering RNA-containing solution protecting donor organs in heart transplantation. Circulation. 2009;120:1099–107. doi: 10.1161/CIRCULATIONAHA.108.787390. 1091 p following 1107. [DOI] [PubMed] [Google Scholar]
- 15.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–32. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–9. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin X, et al. A robust in vivo positive-readout system for monitoring siRNA delivery to xenograft tumors. RNA. 2011;17:603–12. doi: 10.1261/rna.2546011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim MS, Kwon YJ. Efficient and targeted delivery of siRNA in vivo. FEBS J. 2010;277:4814–27. doi: 10.1111/j.1742-4658.2010.07904.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker T, et al. Inhibition of adhesion molecule expression on human venous endothelial cells by non-viral siRNA transfection. J Cell Mol Med. 2007;11:139–47. doi: 10.1111/j.1582-4934.2007.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins T, et al. Structure and chromosomal location of the gene for endothelial-leukocyte adhesion molecule 1. J Biol Chem. 1991;266:2466–73. [PubMed] [Google Scholar]
- 21.Zhao QQ, et al. N/P ratio significantly influences the transfection efficiency and cytotoxicity of a polyethylenimine/chitosan/DNA complex. Biol Pharm Bull. 2009;32:706–710. doi: 10.1248/bpb.32.706. [DOI] [PubMed] [Google Scholar]
- 22.Leng Q, Woodle MC, Lu PY, Mixson AJ. Advances in systemic siRNA delivery. Drugs Future. 2009;34:721. doi: 10.1358/dof.2009.034.09.1413267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werth S, et al. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Release. 2006;112:257–70. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Boussif O, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finsinger D, Remy JS, Erbacher P, Koch C, Plank C. Protective copolymers for nonviral gene vectors: synthesis, vector characterization and application in gene delivery. Gene Ther. 2000;7:1183–92. doi: 10.1038/sj.gt.3301227. [DOI] [PubMed] [Google Scholar]
- 26.Berg MC, Zhai L, Cohen RE, Rubner MF. Controlled drug release from porous polyelectrolyte multilayers. Biomacromolecules. 2006;7:357–64. doi: 10.1021/bm050174e. [DOI] [PubMed] [Google Scholar]
- 27.Thierry B, et al. Delivery platform for hydrophobic drugs: prodrug approach combined with self-assembled multilayers. J Am Chem Soc. 2005;127:1626–7. doi: 10.1021/ja045077s. [DOI] [PubMed] [Google Scholar]
- 28.Thierry B, Winnik FM, Merhi Y, Silver J, Tabrizian M. Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromolecules. 2003;4:1564–71. doi: 10.1021/bm0341834. [DOI] [PubMed] [Google Scholar]
- 29.Wood KC, Chuang HF, Batten RD, Lynn DM, Hammond PT. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc Natl Acad Sci U S A. 2006;103:10207–12. doi: 10.1073/pnas.0602884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blacklock J, You YZ, Zhou QH, Mao G, Oupicky D. Gene delivery in vitro and in vivo from bioreducible multilayered polyelectrolyte films of plasmid DNA. Biomaterials. 2009;30:939–50. doi: 10.1016/j.biomaterials.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jessel N, et al. Multiple and time-scheduled in situ DNA delivery mediated by beta-cyclodextrin embedded in a polyelectrolyte multilayer. Proc Natl Acad Sci U S A. 2006;103:8618–21. doi: 10.1073/pnas.0508246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jewell CM, Zhang J, Fredin NJ, Lynn DM. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J Control Release. 2005;106:214–23. doi: 10.1016/j.jconrel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Meyer F, Ball V, Schaaf P, Voegel JC, Ogier J. Polyplex-embedding in polyelectrolyte multilayers for gene delivery. Biochim Biophys Acta. 2006;1758:419–22. doi: 10.1016/j.bbamem.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Chua LS, Lynn DM. Multilayered thin films that sustain the release of functional DNA under physiological conditions. Langmuir. 2004;20:8015–21. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 35.Dimitrova M, et al. Sustained delivery of siRNAs targeting viral infection by cell-degradable multilayered polyelectrolyte films. Proc Natl Acad Sci U S A. 2008;105:16320–5. doi: 10.1073/pnas.0800156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann B, et al. High-throughput RNAi screening by time-lapse imaging of live human cells. Nat Methods. 2006;3:385–90. doi: 10.1038/nmeth876. [DOI] [PubMed] [Google Scholar]
- 37.Nolte A, et al. Optimized basic conditions are essential for successful siRNA transfection into primary endothelial cells. Oligonucleotides. 2009;19:141–50. doi: 10.1089/oli.2009.0182. [DOI] [PubMed] [Google Scholar]
- 38.Marin V, Kaplanski G, Gres S, Farnarier C, Bongrand P. Endothelial cell culture: protocol to obtain and cultivate human umbilical endothelial cells. J Immunol Methods. 2001;254:183–90. doi: 10.1016/s0022-1759(01)00408-2. [DOI] [PubMed] [Google Scholar]
- 39.Relou IA, Damen CA, van der Schaft DW, Groenewegen G, Griffioen AW. Effect of culture conditions on endothelial cell growth and responsiveness. Tissue Cell. 1998;30:525–30. doi: 10.1016/s0040-8166(98)80032-3. [DOI] [PubMed] [Google Scholar]
- 40.Walker T, Wendel HP, Tetzloff L, Heidenreich O, Ziemer G. Suppression of ICAM-1 in human venous endothelial cells by small interfering RNAs. Eur J Cardiothorac Surg. 2005;28:816–20. doi: 10.1016/j.ejcts.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 42.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 43.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–16. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bigi A, Cojazzi G, Panzavolta S, Roveri N, Rubini K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials. 2002;23:4827–32. doi: 10.1016/s0142-9612(02)00235-1. [DOI] [PubMed] [Google Scholar]
- 45.Walker T, et al. Graft protection in bypass surgery: siRNA-mediated silencing of adhesion molecules. Oligonucleotides. 2009;19:15–21. doi: 10.1089/oli.2008.0148. [DOI] [PubMed] [Google Scholar]
- 46.Dykxhoorn DM, Novina CD, Sharp PA. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2003;4:457–67. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 47.Tepe G, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–99. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 48.Werk M, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008;118:1358–65. doi: 10.1161/CIRCULATIONAHA.107.735985. [DOI] [PubMed] [Google Scholar]
- 49.San Juan A, et al. Development of a functionalized polymer for stent coating in the arterial delivery of small interfering RNA. Biomacromolecules. 2009;10:3074–80. doi: 10.1021/bm900740g. [DOI] [PubMed] [Google Scholar]