Abstract
The failure of chemotherapeutic regimens to eradicate cancers often results from the outgrowth of minor subclones with more dangerous genomic abnormalities or with self-renewing capacity. To explore such intratumor complexities in B-cell chronic lymphocytic leukemia (CLL), we measured B-cell kinetics in vivo by quantifying deuterium (2H)-labeled cells as an indicator of a cell that had divided. Separating CLL clones on the basis of reciprocal densities of chemokine (C-X-C motif) receptor 4 (CXCR4) and cluster designation 5 (CD5) revealed that the CXCR4dimCD5bright (proliferative) fraction contained more 2H-labeled DNA and hence divided cells than the CXCR4brightCD5dim (resting) fraction. This enrichment was confirmed by the relative expression of two cell cycle–associated molecules in the same fractions, Ki-67 and minichromosome maintenance protein 6 (MCM6). Comparisons of global gene expression between the CXCR4dimCD5bright and CXCR4brightCD5dim fractions indicated higher levels of pro-proliferation and antiapoptotic genes and genes involved in oxidative injury in the proliferative fraction. An extended immunophenotype was also defined, providing a wider range of surface molecules characteristic of each fraction. These intraclonal analyses suggest a model of CLL cell biology in which the leukemic clone contains a spectrum of cells from the proliferative fraction, enriched in recently divided robust cells that are lymphoid tissue emigrants, to the resting fraction enriched in older, less vital cells that need to immigrate to lymphoid tissue or die. The model also suggests several targets preferentially expressed in the two populations amenable for therapeutic attack. Finally, the study lays the groundwork for future analyses that might provide a more robust understanding of the development and clonal evolution of this currently incurable disease.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is a relatively frequent, incurable B-cell malignancy (1,2). Even though some patients live for long periods with the disease, many undergo progressive decline, leading to demise. Progression to a more aggressive disease is often associated with genomic changes (3), suggesting that clonal evolution is a key factor in the disease.
We previously found that CLL clones are composed of subpopulations of cells that proliferate at different rates (4), as measured by in vivo deuterium (2H)- incorporation into newly synthesized DNA of dividing cells (5,6). The most proliferative fraction of a cancer clone is of major interest for several reasons. First, the “proliferative compartment” may contain cells that developed new structural DNA abnormalities leading to more lethal disease. Furthermore, the most recently born fraction may be progeny of putative leukemic stem cells. Finally, such cells would be potential targets for therapies to abort clonal evolution.
We therefore studied the kinetic complexity of individual CLL clones to decipher fundamental insights about the pathophysiology of the disease. In particular, we focused on further characterizing the proliferative and resting compartments using differences in the densities of a surface membrane molecule upregulated after normal B-cell activation (cluster designation 5 [CD5]) and another involved in maintaining B-cell contact with stromal elements of solid lymphoid tissues (chemokine [C-X-C motif] receptor 4 [CXCR4]). Using samples from patients for which CLL cells had been labeled in vivo with 2H, we divided clones into sub-fractions enriched in the most proliferative and most quiescent compartments. These fractions were then further characterized by comparing expression of genes encoding molecules usually upregulated in dividing or resting populations. Finally, to provide a robust membrane map of these compartments that might be used for further characterization and therapeutic targeting in patients, an extended surface phenotype was defined with a larger patient cohort.
MATERIALS AND METHODS
Patients
The Institutional Review Board of the North Shore–LIJ Health System approved these studies. After obtaining informed consent in accordance with the Declaration of Helsinki, venous blood was collected from randomly chosen CLL patients diagnosed by established criteria. A total of 15 subjects participating in the 2H2O protocols were studied. Patients drank 2H2O for 6–12 weeks, depending on the protocol, and cells were studied at two time points during this period.
2H Measurements by Gas Chromatography/Mass Spectrometry and Calculation of the Fraction of Labeled Cells
Peripheral blood mononuclear cells were separated from heparinized venous blood and leukocyte-enriched fractions by density gradient centrifugation using Ficoll-Paque (Pharmacia LKB Biotechnology, Piscataway, NJ, USA) and cryo-preserved until use. Calculation of the fraction of newly divided cells was performed after determination of 2H enrichment in plasma or saliva and of 2H enrichment in deoxyadenosine of genomic DNA as described (4).
Isolation of Cell Fractions on the Basis of Expression of CXCR4 and CD5
Peripheral blood mononuclear cells were incubated with murine anti–human monoclonal antibodies (mAbs): CD5-FITC (fluorescein isothiocyanate), CXCR4-PE (phycoethrin), CD3–peridininchlorophyll-protein (CD3-PerCP) and CD19-allophycocyanin (CD19-APC) (all from BD Biosciences, San Jose, CA, USA). After gating on CD19+CD3−CD5+ events, cells were sorted with a BD FACSAria™ (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) on the basis of intensity of CXCR4 and CD5 (Figures 1A, B), and cell pellets were stored at −80°C until performing gas chromato graphy/mass spectrometry or RNA extraction.
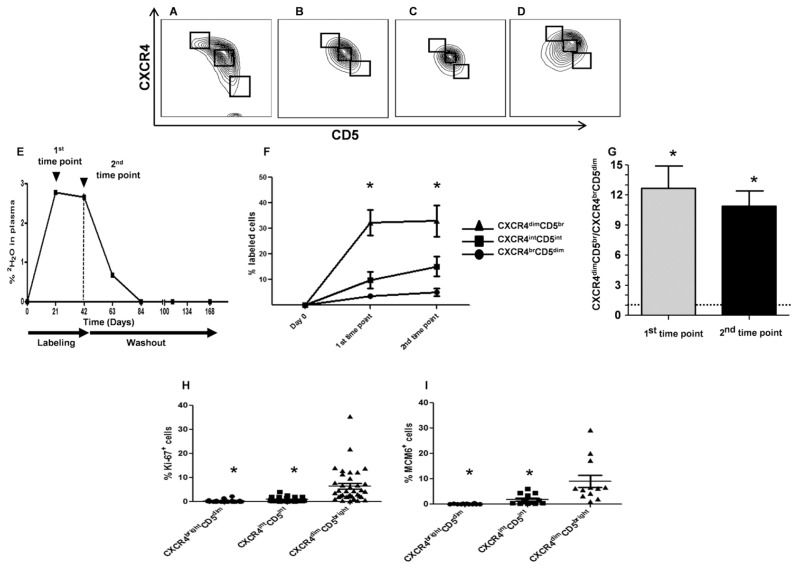
Figure 1.
Intraclonal kinetic differences in CLL fractions defined by CXCR4 and CD5. (A–D) Contour plots of CLL cells on the basis of CXCR4 and CD5 levels. An inverse relationship giving a crescent shape is clear in ~35% of analyses (A), evident in ~50% (B), suggested in ~10% (C) and absent in a few patients (D). (E) Representative example of 2H2O enrichment in plasma (CLL875) of patients drinking 2H2O. CLL cells were flow-sorted on the basis of CXCR4 and CD5 densities at time points indicated by arrows. The vertical dashed line indicates end of 2H2O consumption. (F) Average fraction of 2H-labeled cells in the three fractions defined by CXCR4/CD5 densities. *CXCR4dimCD5bright fraction contains significantly higher levels of 2H-labeled DNA compared with CXCR4intCD5int and CXCR4brightCD5dim fractions (P < 0.01 for both). (G) Plot indicates the ratio of 2H-labeled cells in CXCR4dimCD5bright over CXCR4brightCD5dim fractions, indicating that CXCR4dim CD5bright cells proliferated ~11 times more than CXCR4brightCD5dim cells (*P < 0.0001). (H, I) CXCR4dimCD5bright fractions are significantly enriched in cells expressing Ki-67 (H) and MCM6 (I) compared with CXCR4intCD5int and CXCR4brightCD5dim fractions (*P < 0.01 for both).
Gene Expression Profiling and Gene Expression Data Analyses
The quality of RNA extracted from sorted cells using the RNeasy minikit (Qiagen, Valencia, CA, USA) was assessed using an Agilent RNA 6000 Pico Kit (Agilent Technologies, Colorado Springs, CO, USA). A total of 50 ng total RNA was subjected to first- and second-strand reverse transcription followed by a single in vitro transcription amplification that incorporated biotin-labeled nucleotide to yield biotinylated antisense RNA (a-RNA) using a TargetAmp Nano-g Biotin-aRNA labeling kit (Epicentre Biotechnologies, Madison, WI, USA). Purified a-RNA was quantified, and the fragment size was ascertained on a Bioanalyzer (Agilent Technologies) using an Agilent RNA 6000 Nano Kit. Labeled a-RNA was hybridized to a Human WG-6 v3.0 Expression BeadChip containing 48,804 probes and stained using streptavidin-Cy3 as per the manufacturer’s instructions. The signal generated was detected by high-performance laser optics of the BeadArray Reader (Illumina, San Diego, CA, USA). Data were normalized using quantile normalization by BeadStudio software (Illumina). To determine differentially expressed genes, the CXCR4dimCD5bright versus CXCR4bright CD5dim expression value ratio was computed for each patient and log-transformed, and a Student t test was performed using R (www.Rproject.org). Significant gene differences had 1.3 or greater fold change and P < 0.01; for fold change, we used the nth root of the product of all individual ratios, or the geometric mean of the ratio, for n samples.
Genes were assigned to specific functional categories using Ingenuity Pathway Analysis (IPA, www.ingenuity.com), Panther Classification System (Panther, www.pantherdb.org), DAVID Bioinformatics Resources 6.7, National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) (DAVID, http://david.abcc.ncifcrf.gov/home.jsp) and GeneCards Batch Queries (GeneALaCart, www.genecards.org/BatchQueries/index.php). Significantly different gene expression values were clustered hierarchically on the basis of average linkage on a correlation-based distance (defined at http://arxiv.org/abs/cs/0402061). Heatmaps of gene expression values for identified relevant functional categories were generated using heatmap.2 in the gplots package of R.
Measurements of Surface and Intracellular Antigens by Flow Cytometry
Peripheral blood mononuclear cells were incubated with different combinations of murine anti–human mAbs directly conjugated with the indicated fluorochrome: anti–CD5-FITC or -APC; CXCR4-PE, -APC, or -PECy7; CD3-PerCP; CD19-PerCP, -APC, -Pacific blue or -AmCyan; CD23, CD27, CD52, CD38, CCR7, CXCR3, BAFF-R, CTLA4, CD11a, CD11c, DR5, CD49d, CD62L and FcγRIIb, all in PE; and CD20-FITC (all from BD Biosciences). Unconjugated rabbit IgG antibody to receptor tyrosine kinase-like orphan receptor 1 (ROR-1) was provided by Christoph Rader (National Cancer Institute/NIH, Bethesda, MD, USA). Secondary staining was performed with PE-conjugated goat anti–rabbit IgG antibodies (Southern Biotech, Birmingham, AL, USA).
For surface membrane immunofluorescence, cells (2 × 105) in FACS buffer (PBS + 10% fetal bovine serum + 1% sodium azide) were incubated with primary antibody for 30 min at 4°C and then exposed to secondary antibody for 25 min at 4°C, followed by fixation with 0.1% formaldehyde in PBS. For intracellular detection of Ki-67 and minichromosome maintenance protein 6 (MCM6), after membrane staining with anti–CXCR4-PE, –CD3-PerCP, –CD5-APC and –CD19- Pacific Blue (BD Biosciences), cells were fixed and permeabilized (Cytofix/Cytoperm, BD Biosciences) and incubated with murine anti–Ki-67-FITC or anti–MCM6-FITC mAbs (BD Biosciences). Data were acquired with a BD LSRII flow cytometer or a FACS calibur (both Becton Dickinson Immunocytometry systems) and analyzed by FlowJo v7.2.4 version. Within each CLL clone, relative expression in terms of percent positive cells and mean fluorescent intensity of each surface or intracellular marker was determined and compared.
All supplementary materials are available online at www.molmed.org.
RESULTS
CLL clones can be divided into distinct fractions on the basis of inverse surface expression of CXCR4 and CD5. By screening a panel of chemokine receptors expressed on clones from a series of CLL patients (4), we found an inverse relationship between CXCR4 and CD5 densities, with differing shapes among patients (Figures 1A–D). The abundance of cells representing the extremes of these two populations (CXCR4dimCD5bright versus CXCR4brightCD5dim) was relatively small and varied between patients (1–8%, not shown), with the majority of cells (>90%) falling into an intermediate category (CXCR4intCD5int; Figures 1A–D).
We reasoned that high CD5 density would reflect cellular activation as in normal human B cells (7), and low CXCR4 levels would identify cells that internalized the receptor because of an activation event and thereby passaged from a lymphoid tissue to the periphery (8). As detailed elsewhere (5), incorporation of 2H into cellular DNA in patients given 2H2O is a direct measure of newly synthesized DNA and hence cell division, thereby allowing study of birth rates of CLL cells in vivo (6,9,10). Therefore, we sorted CLL cells from nine patients consuming 2H2O (Figure 1E) into three fractions and quantified 2H-labeled deoxyadenosine in each fraction by gas chromatography/mass spectrometry. This step indicated that at 21 d, the CXCR4dimCD5bright fraction was markedly enriched in divided cells compared with the remainder of the clone, and this result lasted through 42 d (Figure 1F); this disparity was reflected by significantly different CXCR4dimCD5bright to CXCR4brightCD5dim enrichment ratios at the two time points (12.6 at 21 d and 10.9 at 42 d, Figure 1G; P < 0.0001). See Supplementary Figure 1 for data describing the kinetics of each fraction for each patient.
These findings were verified by analyzing ex vivo, in the CXCR4/CD5 fractions, two cell cycle–related molecules, Ki-67 and MCM6, both expressed from the G1 to M phase. Comparisons of CXCR4dimCD5bright versus CXCR4intCD5int versus CXCR4brightCD5dim fractions in an expanded cohort of CLL patients were significantly different (P < 0.01) for both Ki-67 and MCM6 (Figures 1H–I).
Collectively, these in vivo and ex vivo findings indicate that the CXCR4dim CD5bright and CXCR4brightCD5dim fractions of CLL clones are highly enriched compared with the total circulating leukemic load, in recently divided/young and more resting/older members, respectively. For simplicity, we refer to these as the “proliferative” and “resting” compartments going forward, even though the dominant intermediate population contributes cells with similar phenotypes to a significant extent.
Proliferative and Resting Fractions Exhibit Gene Expression Differences Consistent with Their Putative Contrasting Proliferative Histories
Global gene expression profiling (GEP) was performed on isolated fractions from the same patients. Using Illumina HumanWG-6 v3.0 expression arrays to measure the relative expression of 25,440 genes and selecting genes for ≥1.3-fold difference in expression and P ≤ 0.01, we defined 1,299 genes differentially expressed between the two compartments; 715 genes were more highly expressed in the proliferative and 584 in the resting fraction (Supplementary Table 1). These genes were then segregated into categories that would support their difference in time from cellular activation and division and in migratory capacities using a number of bioinformatic programs and databases (see Materials and Methods).
Genes involved in cell proliferation
We determined the extent that the two fractions differed in expression of genes involved in normal or abnormal cell proliferation. Consistent with the CXCR4dimCD5bright fraction being enriched in divided cells, 20 pro-proliferation genes were found in this compartment (Figure 2A, top) and 10 in the CXCR4brightCD5dim fraction. In contrast, a larger number of genes with an antiproliferation function were found in the CXCR4brightCD5dim fraction than the CXCR4dimCD5bright fraction (12 genes versus 7; Figure 2A, bottom).
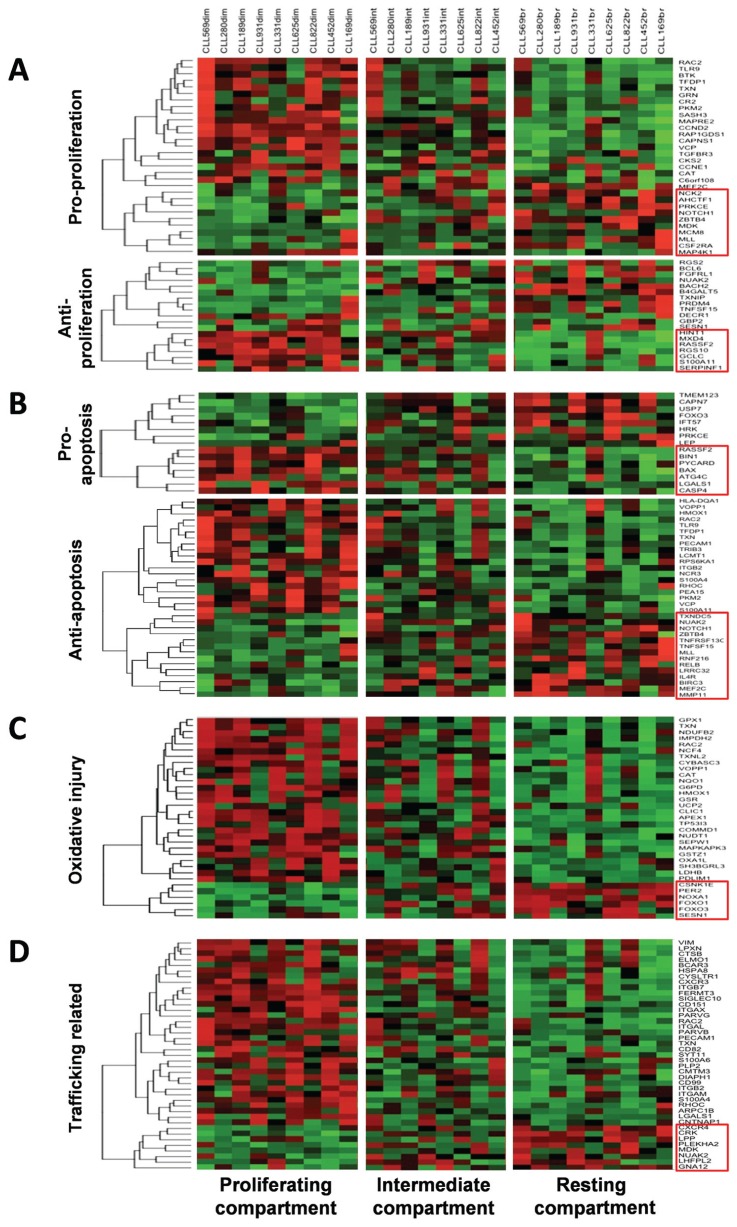
Figure 2.
Heatmaps illustrating genes typically expressed differentially between the proliferative versus resting fractions of CLL clones. (A) Pro- and antiproliferation genes up- or downregulated in the proliferative versus resting fractions of the nine CLL clones analyzed. (B) Antiapoptotic genes. (C) Genes related to oxidative injury. (D) Trafficking-related genes. (A–D) Genes presented are significantly differentially expressed (Supplementary Table 1) and were assigned to categories using Ingenuity Pathway Analysis, Panther Classification System, DAVID Bioinformatics Resources 6.7 and GeneCards Batch Queries. Significantly differentially expressed gene expression values for each set were clustered using hierarchical clustering on the basis of the average linkage on a correlation-based distance.
Among the pro-proliferation genes up-regulated in the CXCR4dimCD5bright fraction were two canonical molecules involved in cell cycle progression: Cyclin E1 (CCNE1) and cyclin D2 (CCND2). CCND2 plus Ki-67 and MCM6, which were enriched in this fraction on the basis of flow cytometry (Figure 3), have been found higher in CLL lymph nodes than peripheral blood (11). Notably, of the pro-proliferation genes that were higher in resting cells (Figure 2A, top), myocyte-specific enhancer factor 2C (MEF2C) and midkine (MDK; neurite growth-promoting factor 2) promote growth of cell types that are often in a resting state: hematopoietic progenitors (12) and embryonic stem cells (13), respectively.
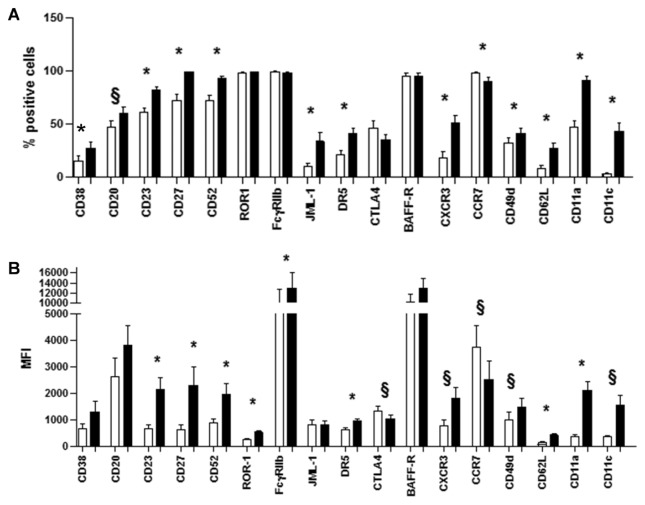
Figure 3.
Extended immunophenotype of proliferative and resting CLL fractions. Surface markers were analyzed by flow cytometry in CXCR4dimCD5bright (X) and CXCR4brightCD5dim (G) fractions. All surface molecules are depicted but CCR7 and CTLA4 were greater in CXCR4dimCD5bright fractions, in terms of percent positive cells (A) or mean fluorescence intensity (B) or both. CCR7 and CTLA4 are higher in the CXCR4brightCD5dim fraction. *P < 0.01 and §P < 0.05 (paired Student t test).
Of the antiproliferative genes higher in the CXCR4brightCD5dim fraction (Figure 2A, bottom), BCL6, BACH2 and RGS2 are of interest because they control checkpoints in B-cell activation and maturation (14–16).
Genes involved in cell survival
Recently born/divided cells would also likely receive prosurvival/antiapoptotic signals, whereas cells of the resting compartment would be less likely to receive these, being temporally further from an activation signal and trophic support of tissue microenvironments. A total of 25 antiapoptotic genes are higher in the proliferative versus 8 in the resting fraction (Figure 2B, bottom). Of note, MEF2C appears necessary for B-cell proliferation and survival after in vitro B-cell receptor (BCR) stimulation (17). Several genes up-regulated in the resting fraction are of interest. TNF receptor superfamily 13C (TNFRSF13C) is the receptor for the B-cell activating factor that mediates survival in normal and non-Hodgkin lymphoma B lymphocytes (18,19). TNFSF15 codes a ligand for TNFRSF25 and TNFRSF6B that is not known to be expressed in B cells and that inhibits growth in other cells (20). Finally, interleukin (IL)-4R, which promotes normal B-cell and CLL cell survival (21) and proliferation (22) and is expressed by CLL cells (23), is upregulated in this fraction.
Prodeath genes are slightly more abundant in the resting fraction, suggesting that this compartment contains older, less robust cells (Figure 2B, top). For example, harakiri (HRK), forkhead box O3 (FOXO3) and leptin (LEP) (24–26) stimulate apoptosis in various cell types, and transmembrane 123 (TMEM123) encodes a mucinlike gene that leads to oncotic cell death upon cross-linking (27).
Genes involved in oxidative injury
Cells of the proliferative fraction upregulated more genes involved in the generation or repair of oxidative injury than in resting cells (27 genes versus 6; Figure 2C), likely because of increased metabolic activity in the dividing/recently divided cells. In addition, oxidative injury is a known feature of CLL cells (28). Products of catalase (CAT), glucose-6-phosphate dehydrogenase (G6PD), glutathione peroxidase 1 (GPX1), heme oxygenase (decycling) 1 (HMOX1) and thioredoxin (TXN) have a role in scavenging free radicals.
Genes involved in cell trafficking
Lastly, 33 trafficking-related molecules were expressed at higher levels in the proliferative and 8 in the resting fractions (Figure 2D). Consistent with cells of the proliferative compartment having recently exited lymphoid tissues is the heightened expression of integrin, beta 7 (ITGB7) and CXCR3. ITGB7 remains on the surface of cells after leaving solid tissues and is involved in passage of cells into mucosa-associated lymphoid tissues (29,30); CXCR3 is involved in CLL cell migration along interferon (IFN)- inducible protein 10 and IFN-γ–induced monokine gradients (31). However, a number of other genes do not readily fit the profile of recent emigrants of lymphoid tissues. In particular, overexpression of adhesion molecules platelet endothelial cell adhesion molecule 1 (PECAM1), cluster designation 11A (lymphocyte function-associated antigen 1)/integrin, alpha L (CD11a/ITGAL) and cluster designation 11c/integrin, alpha X (complement component 3 receptor 4 subunit) (CD11c/ITGAX), the latter two of which were confirmed by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) (Supplementary Figure 2) and flow cytometry (Figure 3), seems paradoxical, since these are usually expressed on cells docking on the endothelium and exiting the circulation.
Cells of the resting compartment expressed more pleckstrin homology domain containing, family A member 2 (PLEKHA2) mRNA (Figure 2D) and CCR7 and CTLA4 protein (Figure 3). CCR7 promotes homing of normal B lymphocytes and CLL cells to lymph nodes and mediates neoplastic B-cell homing in patients with widespread nodal dissemination (32). Also, CCR7 and CXCR4 promote metastasis of solid tumors (33). PLEKHA2 has been found to be highly expressed in poor outcome CLL cells (unmutated IGHV or high ZAP-70 levels) and to have a role in adhesion to fibronectin and laminin (34).
qRT-PCR Analyses of Selected Genes Confirm Their Overexpression in Proliferative or Resting Fractions
We performed qRT-PCR for genes coding molecules expressed on or at B-cell surfaces to provide a guide for developing an extended surface membrane phenotype and to corroborate the GEP data. A total of 67.8% (38/56) of genes were confirmed as significantly different between the two fractions (Supplementary Figure 2). Expression levels of CD5 and CXCR4 served as internal controls.
Surface Molecules Further Distinguish the Proliferative and Resting Compartments
Finally, to permit an even more precise definition of the two fractions in CLL and to define molecules that might be valuable therapeutic targets for these fractions, the CXCR4/CD5 subsets were subjected to a detailed multiparameter phenotypic analysis (Figures 3A, B). Molecules were studied because they are markers of B-cell activation, survival and migration or are targets of mAbs in use in humans. The proliferative compartment contained significantly more cells that expressed, at higher densities, CD38, CD20, CD23, CD27, CD52, JML-1, DR5, CXCR3, CD49d, CD62L, CD11a and CD11c (Figures 3A, B). Only ROR-1, FcγRIIb and BAFF-R exhibited differences solely on the basis of density. Two molecules were higher in the resting compartment: CCR7 (both in the percent of positive cells and intensity) and CTLA4 (intensity). It is noteworthy that several of these molecules are targets of mAbs or compounds already in clinical use (35–41) or are being tested in human subjects (23,42–46).
DISCUSSION
The aim of this study was to further delineate and isolate cells with different kinetics in CLL clones, focusing on cells that had recently divided or are resting. Using 2H-incorporation into newly synthesized DNA of dividing cells as a measure of proliferation, we previously demonstrated intraclonal kinetic complexity in CLL, defining a small population (~0.1–1% of the leukemic clone) that divided daily (6). Because the 2H- incorporation approach does not permit visualization of individual cells that have divided, we subsequently enriched for cells of the proliferative fraction using surface expression of a candidate molecule, CD38, that is expressed on activated human B cells (47) and is associated with poor clinical outcome in CLL (48). These studies indicated that within each CLL clone, the CD38+ fraction contained ~2.5 times more recently divided cells than the CD38− fraction (4). In the present study, using differential surface membrane densities of CXCR4 and CD5, we isolated fractions differing by ~11-fold in the percentage of recently divided cells (Figure 1G). In line with our previous study, CD38 expression was significantly higher in the CXCR4dim CD5bright proliferative subset (Figure 3A). Finding increased numbers of cells expressing Ki-67 and MCM6 in the same fraction confirmed the accuracy of this approach (Figures 1H, I). Finally, global GEP on the same CXCR4/CD5 subsets further validated that our approach identified subsets of CLL clones differing in proliferative histories, since the CXCR4dimCD5bright fraction overexpressed more genes that support cell division (Figure 2A), block apoptosis (Figure 2B) and induce/repair oxidative damage (Figure 2C) than the resting fraction, which contained more genes that inhibit cell division (Figure 2A) and survival (Figure 2B). Nevertheless, these fractions remain heterogeneous, likely containing cells from the intermediate fraction with characteristics distinct from those we are describing. Thus, the situation is complex, and a more expansive collection and analysis of such data will better elucidate unique features of the two compartments. Still, the detection of gene (Figure 2) and protein (Figures 1 and 3) expression differences consistent with recently divided and resting cells, combined with the direct in vivo demonstration of significant enrichment in cycled cells in the proliferative fraction and a markedly reduced abundance in the resting fraction (Figure 1), confirm that using CXCR4/CD5 densities on CD19+ cells is a major improvement in the delineation of these two compartments.
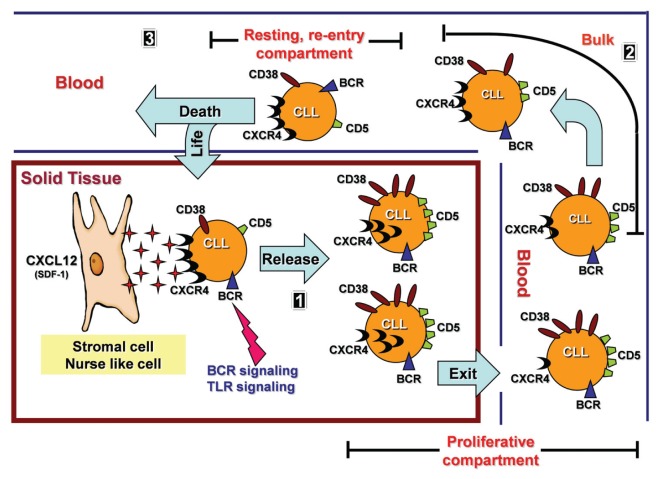
The data conjure a lifecycle for individual CLL cells representing a continuum between the CXCR4dimCD5bright, CXCR4intCD5int and CXCR4 brightCD5dim fractions (Figure 4). At one extreme is the proliferative fraction, highly enriched in young, vital cells that recently left a solid lymphoid tissue where activation and proliferation occurred. At the other end is the resting compartment, containing older, less robust cells that may have been circulating in the periphery longer and are attempting through high CXCR4 levels to migrate into a solid tissue niche to avoid death. In this model, the intermediate fraction, which is the bulk of the clone, links the extremes and is the fraction from which most of our current knowledge on circulating CLL cells is derived. This relationship is supported by the fact that BCR stimulation leads to downregulation of CXCR4 expression (49), as depicted in Figure 4, part 1. Although our data are consistent with this model, the process remains hypothetical because we have only studied cells in the peripheral circulation. Notably, it was recently shown by comparative GEP of bone marrow and lymph node cells that lymph nodes may host most of the CLL proliferative events (11). In our study, cells with low CXCR4 and high CD5 levels could include emigrants from any site, and only one of the differentially expressed molecules involved in trafficking that we defined is site specific: ITGB7 suggests specific passage to mucosa-associated lymphoid tissues. Considering that at least some CLL cells may be derived from a human B-1 cell equivalent (50,51), the potential of migration to such tissues, which is favored for murine B-1 cells (52), is provocative.
Figure 4.
Hypothetical model of the lifecycle of a CLL B cell. Part 1: CLL cells rest on the stroma because of at least CXCR4-CXCL12 interactions. When stimulated, cells are activated and divide, upregulating CD5, internalizing CXCR4 and detaching from stroma. The process could be ligand-induced (for example, BCR or toll-like [TLR] or other pathways) or spontaneous. Low CXCR4 levels increase the chances of recently divided CLL cells (CXCR4dimCD5bright phenotype) exiting solid tissue and reaching peripheral blood. Part 2: Recently born/divided CLL cells reach peripheral blood as members of the CXCR4dimCD5bright fraction. Over time, possibly because of a lack of trophic input from the solid tissue microenvironment, cells begin to reexpress CXCR4 to trek back to nutrient-rich niches. This leads to expression of a CXCR4intCD5int and then CXCR4brightCD5dim membrane phenotype. The model considers the three fractions to be linked as a continuum. Part 3: CXCR4brightCD5dim CLL cells have the greatest chance of detecting and following a CXCL12/SDF1 gradient, thereby reentering lymphoid solid tissue and receiving prosurvival stimuli. Those that do not reenter die by exhaustion.
This hypothetical lifecycle also suggests “stem-like” or “initiating” capabilities of CLL subclones (53). For instance, the CXCR4brightCD5dim cells could be nestled on stromal elements (Figure 4, part 1) before being released and giving rise to the dividing CXCR4dimCD5bright cells of the proliferative and bulk fractions (Figure 4, part 2); therefore, these CXCR4brightCD5dim resting cell fractions could be a distinct self-renewing subset from which all clonal members emanate. In a related scenario, these same cells could be members of the CXCR4bright CD5dim fraction of the resting compartment of the blood on their way back to a sustaining niche in a lymphoid tissue (Figure 4, part 3), where they might divide again. The former possibility would be consistent with a B-1–like cell, because in mice these cells have self-renewal capacity (54), implying that the ability to migrate effectively would be the key factor determining survival of the clone. If the latter possibility is the case, a careful analysis of the resting compartment might yield a subset of cells with stem-like/initiating capacities.
Delineation of intratumor kinetic heterogeneity may also have therapeutic implications. Although most anticancer therapies are predicated on eliminating the entire neoplastic clone, preferentially targeting the proliferative and resting compartments might be efficacious. Thus, preferentially eliminating the most recently born/divided cells would limit the fount of clonal evolution; preventing cells from reentering solid tissue would thwart the less robust, apoptosis-prone resting cells from receiving survival signals. Collectively, these approaches would lock a clone into a steady state of genetic abnormalities and eventually lead to clonal shrinkage based on spontaneous cell death (6,55) and death due to survival signal deprivation (56).
Although speculative, this approach could be tested, since relevant compounds are already in clinical use or evaluation. For example, the proliferative compartment expresses higher levels of CD20, CD23, CD38, CD49d, CD52, CD11a and DR5 (Figure 3), all of which can be targeted by specific, available mAbs (23,35–46). Other targets of potential interest in the proliferative fraction are CXCR3 (GEP, qRT-PCR, flow), CD27 (flow), ITGB7 (GEP and qRT-PCR), Ly96 (GEP), CD62L (flow) and CD11c/ITGAX (flow and GEP). For the resting compartment, the most relevant molecules appear to be CXCR4 and CCR7 (flow and GEP). Plerixaflor, a CXCL12 (CXCR4 ligand) inhibitor used for stem cell mobilization, is being tested in CLL treatment (39). Although no compound is presently available to target CCR7 in vivo, an anti-CCR7 mAb kills CLL cells in vitro without affecting T cells that also express CCR7 (57). Thus, an anti-CXCR4 and/or anti-CCR7 approach could limit the ability of CXCR4bright and CCR7bright cells to reenter solid tissues, fostering resting compartment apoptosis. Finally, targeting IL-4R and CTLA4 is feasible because a recombinant IL-4–Pseudomonas exotoxin fusion protein that binds IL-4R (23) and overcomes apoptosis resistance of CLL cells (58) is in clinical use, and mAbs reactive with CTLA4 (40,41) are currently being used to potentiate T-cell responses against tumors (59).
Supplemental Data
ACKNOWLEDGMENTS
This work was supported in part by the National Cancer Institute/NIH (CA81554) and by philanthropic contributions from The Karches Foundation, Prince Family Foundation, Marks Foundation, Jerome Levy Foundation, Leon Levy Foundation, Andrew and Mona Albert Fund Inc., and Joseph Eletto Leukemia Research Fund. We thank Aarti Damle and The Feinstein Institute’s microarray core facility for gene expression analyses.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Zenz T, Mertens D, Kuppers R, Dohner H, Stilgenbauer S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer. 2010;10:37–50. doi: 10.1038/nrc2764. [DOI] [PubMed] [Google Scholar]
- 3.Shanafelt TD, et al. Karyotype evolution on fluorescent in situ hybridization analysis is associated with short survival in patients with chronic lymphocytic leukemia and is related to CD49d expression. J. Clin. Oncol. 2008;26:e5–6. doi: 10.1200/JCO.2008.16.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calissano C, et al. In vivo intraclonal and interclonal kinetic heterogeneity in B-cell chronic lymphocytic leukemia. Blood. 2009;114:4832–42. doi: 10.1182/blood-2009-05-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neese RA, et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci U S A. 2002;99:15345–50. doi: 10.1073/pnas.232551499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messmer BT, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–64. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zupo S, et al. Expression of CD5 and CD38 by human CD5- B cells: requirement for special stimuli. Eur J Immunol. 1994;24:1426–33. doi: 10.1002/eji.1830240628. [DOI] [PubMed] [Google Scholar]
- 8.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116:1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Gent R, et al. In vivo dynamics of stable chronic lymphocytic leukemia inversely correlate with somatic hypermutation levels and suggest no major leukemic turnover in bone marrow. Cancer Res. 2008;68:10137–44. doi: 10.1158/0008-5472.CAN-08-2325. [DOI] [PubMed] [Google Scholar]
- 10.Defoiche J, et al. Reduction of B cell turnover in chronic lymphocytic leukaemia. Br J Haematol. 2008;143:240–7. doi: 10.1111/j.1365-2141.2008.07348.x. [DOI] [PubMed] [Google Scholar]
- 11.Herishanu Y, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-κB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2010;117:563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehling-Sun S, Dade J, Nutt SL, DeKoter RP, Camargo FD. Regulation of lymphoid versus myeloid fate ‘choice’ by the transcription factor Mef2c. Nat Immunol. 2009;10:289–96. doi: 10.1038/ni.1694. [DOI] [PubMed] [Google Scholar]
- 13.Yao X, et al. Promotion of self-renewal of embryonic stem cells by midkine. Acta Pharmacol Sin. 2010;31:629–37. doi: 10.1038/aps.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochiai K, Muto A, Tanaka H, Takahashi S, Igarashi K. Regulation of the plasma cell transcription factor Blimp-1 gene by Bach2 and Bcl6. Int Immunol. 2008;20:453–60. doi: 10.1093/intimm/dxn005. [DOI] [PubMed] [Google Scholar]
- 15.Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 105:193–210. doi: 10.1016/S0065-2776(10)05007-8. [DOI] [PubMed] [Google Scholar]
- 16.Reif K, Cyster JG. RGS molecule expression in murine B lymphocytes and ability to down-regulate chemotaxis to lymphoid chemokines. J Immunol. 2000;164:4720–9. doi: 10.4049/jimmunol.164.9.4720. [DOI] [PubMed] [Google Scholar]
- 17.Wilker PR, et al. Transcription factor Mef2c is required for B cell proliferation and survival after antigen receptor stimulation. Nat Immunol. 2008;9:603–12. doi: 10.1038/ni.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak AJ, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104:2247–53. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 19.Fu L, et al. BAFF-R promotes cell proliferation and survival through interaction with IKKβ and NF-κB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood. 2009;113:4627–36. doi: 10.1182/blood-2008-10-183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai Y, et al. VEGI, a novel cytokine of the tumor necrosis factor family, is an angiogenesis inhibitor that suppresses the growth of colon carcinomas in vivo. FASEB J. 1999;13:181–9. doi: 10.1096/fasebj.13.1.181. [DOI] [PubMed] [Google Scholar]
- 21.Coscia M, et al. IGHV unmutated CLL B cells are more prone to spontaneous apoptosis and subject to environmental prosurvival signals than mutated CLL B cells. Leukemia. 2011;25:828–37. doi: 10.1038/leu.2011.12. [DOI] [PubMed] [Google Scholar]
- 22.Porakishvili N, et al. CD180 functions in activation, survival and cycling of B chronic lymphocytic leukaemia cells. Br J Haematol. 2011;153:486–98. doi: 10.1111/j.1365-2141.2011.08605.x. [DOI] [PubMed] [Google Scholar]
- 23.Kay NE, et al. A recombinant IL-4-Pseudomonas exotoxin inhibits protein synthesis and overcomes apoptosis resistance in human CLL B cells. Leuk Res. 2005;29:1009–18. doi: 10.1016/j.leukres.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura M, Shimada K, Konishi N. The role of HRK gene in human cancer. Oncogene. 2008;27(Suppl 1):S105–13. doi: 10.1038/onc.2009.48. [DOI] [PubMed] [Google Scholar]
- 25.Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol. 2009;665:227–41. doi: 10.1007/978-1-4419-1599-3_17. [DOI] [PubMed] [Google Scholar]
- 26.Samuel-Mendelsohn S, et al. Leptin signaling and apoptotic effects in human prostate cancer cell lines. Prostate. 2011;71:929–45. doi: 10.1002/pros.21309. [DOI] [PubMed] [Google Scholar]
- 27.Ma F, Zhang C, Prasad KV, Freeman GJ, Schlossman SF. Molecular cloning of Porimin, a novel cell surface receptor mediating oncotic cell death. Proc Natl Acad Sci U S A. 2001;98:9778–83. doi: 10.1073/pnas.171322898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
- 29.Parker CM, Cepek KL, Russell GJ, et al. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1924–8. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postigo AA, Sanchez-Mateos P, Lazarovits AI, Sanchez-Madrid F, de Landazuri MO. Alpha 4 beta 7 integrin mediates B cell binding to fibronectin and vascular cell adhesion molecule-1: expression and function of alpha 4 integrins on human B lymphocytes. J Immunol. 1993;151:2471–83. [PubMed] [Google Scholar]
- 31.Trentin L, et al. The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J Clin Invest. 1999;104:115–21. doi: 10.1172/JCI7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Giral S, et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol. 2004;76:462–71. doi: 10.1189/jlb.1203652. [DOI] [PubMed] [Google Scholar]
- 33.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 34.Costantini JL, et al. TAPP2 links phosphoinositide 3-kinase signaling to B-cell adhesion through interaction with the cytoskeletal protein utrophin: expression of a novel cell adhesion-promoting complex in B-cell leukemia. Blood. 2009;114:4703–12. doi: 10.1182/blood-2009-03-213058. [DOI] [PubMed] [Google Scholar]
- 35.Kappos L, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 2011;10:745–58. doi: 10.1016/S1474-4422(11)70149-1. [DOI] [PubMed] [Google Scholar]
- 36.Wiernik PH, Adiga GU. Single-agent rituximab in treatment-refractory or poor prognosis patients with chronic lymphocytic leukemia. Curr Med Res Opin. 2011;27:1987–93. doi: 10.1185/03007995.2011.615307. [DOI] [PubMed] [Google Scholar]
- 37.Nightingale G. Ofatumumab: a novel anti-CD20 monoclonal antibody for treatment of refractory chronic lymphocytic leukemia. Ann Pharmacother. 2011;45:1248–55. doi: 10.1345/aph.1P780. [DOI] [PubMed] [Google Scholar]
- 38.Rai KR, et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol. 2002;20:3891–7. doi: 10.1200/JCO.2002.06.119. [DOI] [PubMed] [Google Scholar]
- 39.Calandra G, Bridger G, Fricker S. CXCR4 in clinical hematology. Curr Top Microbiol Immunol. 2010;341:173–91. doi: 10.1007/82_2010_26. [DOI] [PubMed] [Google Scholar]
- 40.Cameron F, Whiteside G, Perry C. Ipilimumab: first global approval. Drugs. 2011;71:1093–104. doi: 10.2165/11594010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37:473–84. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajaj M, Heath EI. Conatumumab: a novel monoclonal antibody against death receptor 5 for the treatment of advanced malignancies in adults. Expert Opin Biol Ther. 2011;11:1519–24. doi: 10.1517/14712598.2011.610788. [DOI] [PubMed] [Google Scholar]
- 43.Byrd JC, et al. Phase 1 study of lumiliximab with detailed pharmacokinetic and pharmacodynamic measurements in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res. 2007;13:4448–55. doi: 10.1158/1078-0432.CCR-06-1463. [DOI] [PubMed] [Google Scholar]
- 44.Burger M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–30. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 45.Puri S, et al. A review of studies on targeting interleukin 4 receptor for central nervous system malignancy. Curr Mol Med. 2009;9:732–9. doi: 10.2174/156652409788970661. [DOI] [PubMed] [Google Scholar]
- 46.de Weers M, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 47.Pascual V, et al. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–39. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Damle RN, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–7. [PubMed] [Google Scholar]
- 49.Quiroga MP, Burger JA. BCR-mediated decrease of CXCR4 and CD62L in CLL. Cancer Res. 2010;70:5194. doi: 10.1158/0008-5472.CAN-09-3759. author reply 5195. [DOI] [PubMed] [Google Scholar]
- 50.Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 2011;117:1781–91. doi: 10.1182/blood-2010-07-155663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fagarasan S, et al. Mechanism of B1 cell differentiation and migration in GALT. Curr Top Microbiol Immunol. 2000;252:221–9. doi: 10.1007/978-3-642-57284-5_23. [DOI] [PubMed] [Google Scholar]
- 53.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 54.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–68. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiorazzi N. Cell proliferation and death: forgotten features of chronic lymphocytic leukemia B cells. Best Pract Res Clin Haematol. 2007;20:399–413. doi: 10.1016/j.beha.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–75. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alfonso-Perez M, et al. Anti-CCR7 mono-clonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol. 2006;79:1157–65. doi: 10.1189/jlb.1105623. [DOI] [PubMed] [Google Scholar]
- 58.Panayiotidis P, Ganeshaguru K, Jabbar SA, Hoff-brand AV. Interleukin-4 inhibits apoptotic cell death and loss of the bcl-2 protein in B-chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1993;85:439–45. doi: 10.1111/j.1365-2141.1993.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 59.Yang JC, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.