Abstract
The effects of a humanized monoclonal antibody (mAb) having high affinity and specificity for cocaine in animal models are reviewed. The mAb reduced the concentration of cocaine in the brain of mice after intravenous injection of cocaine. In addition, the mAb increased the concentration of cocaine required to reinstate cocaine self-administration. These effects may predict clinical efficacy of a passive immunotherapy for reducing the probability of cocaine-induced relapse. However, in the presence of the mAb, once cocaine self-administration was reinstated, the consumption rate of cocaine was increased. This effect is hypothesized to result from a pharmacokinetic/pharmacodynamic interaction. A humanized mAb should minimize adverse events related to the immunogenicity of the mAb protein, and the specificity for cocaine should avoid adverse events related to interactions with physiologically relevant endogenous proteins.
Keywords: addiction, cocaine, drug abuse, immunotherapy, monoclonal antibody, relapse prevention, vaccine
Pharmacotherapeutic approaches to the treatment of cocaine addiction target the sites in the brain through which cocaine exerts its effects. Despite decades of research, during which dopaminergic neurotransmission has been identified as a primary mediator of the actions of cocaine, there is no effective pharmacological treatment available for cocaine abuse. An alternative approach is to target the cocaine itself using an agent that prevents the rapid entry of cocaine into the brain [1] and thereby acts as a pharmacokinetic antagonist. The sequestration of cocaine in the peripheral circulation by its binding by an anticocaine monoclonal antibody (mAb) prevents its access to all sites of action in the brain, which confers therapeutic efficacy. Anticocaine antibodies can be elicited by active immunization with a vaccine, which produces a polyclonal antibody response, or, alternatively, monoclonal anticocaine antibodies that have been manufactured exogenously can be used to provide passive immunity.
Confirmation of the potential efficacy of immunotherapy for cocaine abuse comes from recent clinical studies demonstrating that a cocaine vaccine generating anticocaine antibodies decreases cocaine use in the cocaine abusers that generated the highest levels of anticocaine antibodies [2]. This promising indicator of efficacy provides proof-of-principle for immunotherapy. The active immunization approach has a number of advantages over the proposed passive immunotherapy. In particular, the vaccine is simple to administer and inexpensive, and with booster vaccinations its effects can be relatively long lasting. However, the response to the vaccine is slow to develop, and the levels of antibodies generated, their affinities and specificities have been shown to differ widely between individuals [2–4]. In contrast to this unpredictability, passive immunization confers immediate immunity by a well-characterized mAb with a uniform high affinity and specificity for cocaine [1,5]. Furthermore, the dosage and pharmacokinetics of that mAb will be well established and the treatment responses should be more predictable. Clearly, in patients with low responses to the anticocaine vaccine, that mAb can also be complementary, supplementing the endogenous anticocaine antibodies to therapeutic levels.
Treating the acute toxicity caused by a cocaine overdose would typically require a single treatment with an anticocaine mAb. By contrast, relapse prevention would require the mAb to be present prior to the intake of cocaine, necessitating the long-term presence of the medication. Although the anticocaine mAb, which has a preclinical designation of 2E2, should be effective for both clinical indications, we have focused here on relapse prevention due to the mAb’s long-lasting effects, which should confer a competitive advantage over traditional small-molecule therapeutics that are typically rapidly cleared from the body.
In order to generate an antibody suitable for this drug-targeted approach, a cocaine-based hapten–carrier conjugate was designed and transgenic mice that produce human sequence antibodies [6] were immunized. The lead candidate mAb, 2E2, was selected on the basis of its high affinity and selectivity for cocaine over the major inactive cocaine metabolites [5]. This selectivity is important because high affinity for the inactive metabolites would decrease the mAb’s efficiency. Interestingly, the mAb 2E2 also has high affinity for the active cocaine metabolite cocaethylene, which is formed in vivo in the presence of cocaine and ethanol [7]. This is a potentially useful clinical feature of 2E2 since addicts often co-abuse ethanol and cocaine.
In this article the mechanisms underlying the effects of anticocaine mAbs in animal models of cocaine abuse will be reviewed with an emphasis on extrapolating this to the anticipated effects in human cocaine abusers. It may be expected that antibodies will have some adverse events associated with their use, which may be related to the mechanisms underlying their clinically relevant effects and also to their potential immunogenicity in humans. This article will also review the structural characteristics and targets of mAbs that may produce problems during clinical use and how targeting an exogenous drug, such as cocaine, rather than an endogenous protein, and having a humanized structure may minimize any adverse events. It should also be noted that an immunotherapeutic intervention for cocaine abuse would be suitable only for those individuals who are actively committed to abstaining from cocaine intake. This is because of the potential for individuals to attempt to circumvent the therapy through increased cocaine consumption in the presence of anticocaine antibodies, or for them to switch to abusing other stimulants, rendering the highly specific anticocaine antibodies ineffective.
Effect of anticocaine mAbs on cocaine pharmacokinetics
The pharmacokinetics of 2E2 in mice and rats was measured after an intravenous injection of 2E2, and demonstrated an elimination half-life of approximately 8 and 11 days in mice [8] and rats [9], respectively. Thus, the in vivo effects of 2E2 are likely to be protracted. The mAb 2E2’s low volume of distribution at steady state, 0.28 l/kg for mice and 0.5 l/kg for rats, indicated that it was not widely distributed and was largely restricted to the blood volume.
In mice, 2E2 produced dose-dependent increases in plasma cocaine concentrations and decreases in brain cocaine concentrations when measured 5 min after the cocaine injection. The mAb 2E2 was very effective and at the highest dose of 2E2 tested (360 mg/kg), cocaine was not detectable in the brain and all of the injected cocaine could be accounted for in the plasma [8]. These dramatic results provided an initial demonstration of the in vivo efficacy of 2E2. Additional studies in mice showed that 2E2 (120mg/kg) produced a 4.5-fold (78%) decrease in the area under the time–concentration curve of cocaine in the brain [8]. Not only did 2E2 reduce the exposure of the brain to cocaine, it restricted the distribution of cocaine to, essentially, the blood volume. Interestingly, despite being bound and sequestered in the peripheral blood, cocaine was still eliminated rapidly from this compartment. This lack of inhibition of cocaine clearance, if it also occurs in humans, would be predicted to be a clinically useful phenomenon, as antibodies may also be used effectively along with a new generation of enzyme therapeutics that rapidly hydrolyze cocaine in vivo [10,11].
The dramatic 2E2-induced reduction in brain cocaine concentrations suggests in vivo efficacy for decreasing the probability of relapse in cocaine abusers. Therefore, the effect of 2E2 in a rat model of relapse was investigated.
Increased priming threshold as a model of relapse prevention
In the rat self-administration paradigm animals press a lever and receive a rapid intravenous injection of cocaine. Typically, the dose of cocaine that is administered after each lever press is controlled by the investigator while the animal controls when each dose is administered. Other parameters can be controlled by the investigator, such as the number of times the lever must be pressed before each dose of cocaine is delivered or a minimum time after the last injection before another dose can be administered. Once the behavior is acquired, the animals will self-administer cocaine for many hours, with the intervals between self-injections being regular and proportional to the dose administered. This model of maintained drug self-administration represents a useful model of cocaine abuse. Once access to cocaine is terminated the behavior extinguishes after several minutes. Importantly, cocaine reliably reinstates self-administration behavior in these animals and this cocaine-induced priming of behavior may represent a partial model of relapse. In the authors’ novel quantitative rat model of cocaine-induced relapse, the concentration of cocaine required to reinstate cocaine self-administration (priming threshold) was measured [9]. Antagonism of the cocaine-induced reinstatement of self-administration behavior manifests as an increase in the priming threshold and would be interpreted as decreasing the probability of cocaine-induced relapse.
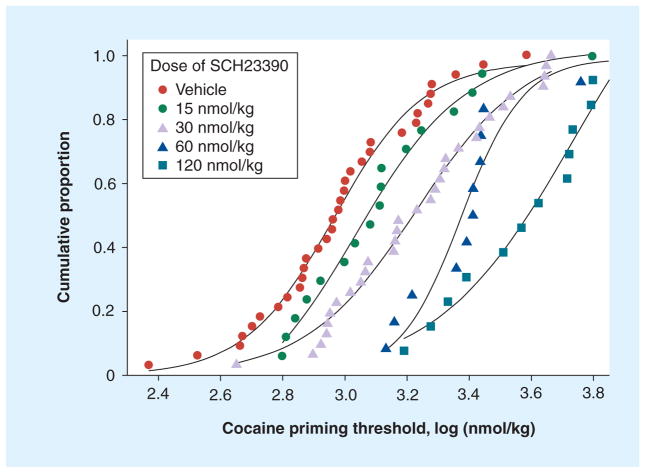
It has been shown that the variation in the priming threshold values in a group of rats that have acquired self-administration behavior is log-normally distributed [12]. Furthermore, the variability of the values measured for individual animals is almost as large as the variability between animals. This has the important consequence that measuring the priming threshold in a single rat many times has almost equal validity as measuring this value in many rats a single time. Therefore, the geometric mean or median value from a few measurements of the cocaine priming threshold from a relatively small number of rats can provide a good estimation of the population distribution. Using this method it has been shown that the D1 dopamine receptor antagonist SCH23390 produced a dose-dependent increase in the median and geometric mean cocaine priming threshold (Figure 1). This increase in the priming threshold would be expected to reduce the probability of relapse produced by a dose of cocaine. However, given the involvement of dopaminergic neurotransmission in a wide variety of physiological systems, this class of antagonist unfortunately produces a wide side-effect profile. By contrast, antibodies, with their exquisite specificity for cocaine, rather than acting on dopamine receptors or the dopamine transporter, will almost certainly have fewer pharmacodynamic side effects and, consequently, should be better tolerated.
Figure 1. The SCH23390-induced increase in the cocaine priming threshold.
Data points represent individual priming thresholds, after the injection of vehicle or various doses of SCH23390, presented as cumulative proportion distributions. Each of the seven rats in the group contributed between one and six measurements to each of the curves. Priming threshold values are typically log-normally distributed. Data taken from [12].
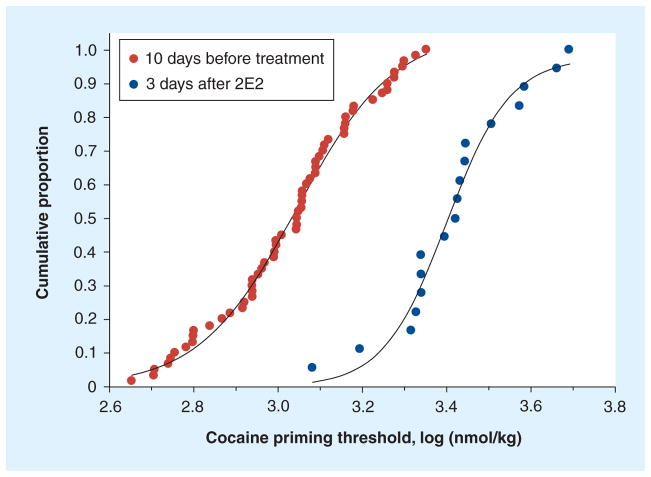
Using the same technique used to measure the effects of dopamine receptor antagonists the authors have demonstrated that the anticocaine mAb 2E2 increases the cocaine priming threshold in rats [9]. This would also be predicted to result in a decreased probability of maintaining a relapse event after a dose of cocaine. As shown in Figure 2, 2E2 shifted the distribution of the priming threshold values measured in a group of six rats in ten daily sessions prior to the infusion of 2E2 and over the 3 days after the infusion of 2E2 in these same rats. The median value for the concentration of cocaine required to reinstate self-administration was increased from 1.1 to 2.7 μmol/kg of cocaine. As can be seen from the distribution of priming threshold values (Figure 2), a concentration of cocaine that reinstated self-administration behavior in approximately 90% of sessions prior to the treatment with 2E2 reinstated self-administration behavior in only approximately 12% of cases during the 3 days after 2E2 treatment. By comparing the magnitude of the effects of SCH23390 (Figure 1) and 2E2 (Figure 2) on the cocaine priming threshold, it can be seen that the dose of 2E2 used in these studies was approximately equivalent to the effect produced by a 60 nmol/kg intravenous dose of SCH23390. Thus, 2E2 acting as a chemical antagonist of the effects of cocaine (by binding/chelation) has an effect that is comparable to that of a competitive dopamine receptor antagonist. These data in a rat model of relapse provide an indication of the potential for immunotherapy to decrease the probability of a dose of cocaine inducing relapse in human cocaine abusers.
Figure 2. The anticocaine monoclonal antibody-induced increase in the cocaine priming threshold.
Each data point represents a priming threshold value measured during one session. Each of a group of six rats contributed ten values, one from each daily session during the ten sessions prior to the infusion of the anticocaine monoclonal antibody 2E2. The cumulative proportion represents the distribution of these priming threshold values (n = 60 measurements, red circles) during the approximately 2-week baseline period. The distribution of the priming threshold values from the same six rats over the 3 days after the infusion of 2E2 are shown (n = 18 measurements, blue circles). The median priming threshold values were 1.1 and 2.7 μmol/kg of cocaine prior to and following the infusion of 2E2, respectively. After 3 days, the magnitude of the priming threshold was lower as the priming threshold values gradually declined towards baseline.
Data presented here are re-analyzed from those presented in [9].
The rate of cocaine consumption is increased if relapse is maintained
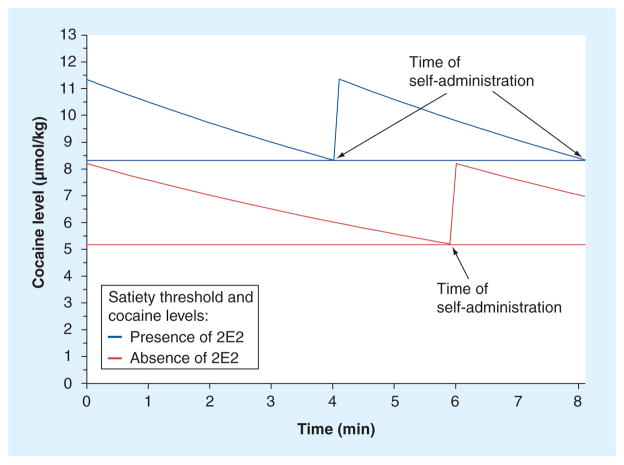
One of the potential problems with antagonist-based pharmacotherapies is that antagonists are known to increase the rate of consumption of cocaine in animal self-administration studies. It seemed possible that anticocaine antibodies would have the same effect. This issue was investigated in the same experiments in which the effect of 2E2 on the cocaine priming threshold was measured. In these studies it was observed that, once cocaine self-administration was reinstated, the intervals between successive cocaine injections remained constant at a particular cocaine dose. This indicates that the normal regulation of cocaine intake was not negated by 2E2. However, the dosing intervals, while being regular, were shorter than they were before 2E2 was administered and, consequently, the rate of cocaine consumption was increased [9]. A potential mechanism for this phenomenon is likely to be a pharmacokinetic/pharmacodynamic interaction resulting from an increased plasma cocaine concentration and the first-order kinetics of cocaine elimination. Each successive self-administration occurs when the cocaine concentration falls to a minimum that has been termed the satiety threshold [13,14]. This is a pharmacodynamic parameter that should not be affected by anticocaine antibodies as they do not enter the brain. However, the cocaine concentration in the brain produced by a given intravenous dose is decreased in the presence of the anticocaine mAb. Therefore, in order for a rat to maintain the same brain concentration of cocaine, the concentration in the plasma would need to be increased. Importantly, because cocaine elimination is first-order, the rate of elimination of cocaine is proportionally increased at the higher plasma cocaine concentrations. This results in the cocaine concentration in the brain decreasing to the satiety threshold faster. This, in turn, results in a shorter inter-injection interval and a concomitant increase in the rate of cocaine consumption. This model of the increase in the rate of cocaine self-administration is adapted from the model published previously [15] and is shown in Figure 3. This model also nicely explains why the inter-injection intervals remain regular in the presence of the authors’ anticocaine mAb [9]. If relapse does occur in the presence of an immunotherapeutic agent and the patient overrides the pharmacokinetic antagonism produced by immunotherapy, then there is a likelihood of an increase in cocaine consumption, which could be considered an adverse event. However, the important issue is whether this increase in cocaine consumption produces additional deleterious effects on health. Indeed, in one human study, a modest increase in cocaine-induced heart rate was reported in human cocaine abusers who were immunized with a cocaine vaccine. This effect was observed only in the subjects with the highest titers of anticocaine polyclonal antibodies [4]. However, this increased cardiovascular effect of cocaine is in contrast to the demonstrated ability of antidigoxin and anticolchicine antibody fragments to protect the heart from drug overdoses (a more detailed discussion is presented below and see [16–19]). Based on these latter results it would be expected that anticocaine antibodies would ameliorate the cardiovascular effects of cocaine. Therefore, the effects reported in vaccinated humans [4] are an important reminder that the immunotherapeutic strategy for mitigating the effects of drugs of abuse is still at an early stage of investigation.
Figure 3. A pharmacokinetic/pharmacodynamic model of the antibody-induced increase in the rate of cocaine self-administration.
The descending lines represent the total cocaine level in the body after a self-administration of 3.0 μmol/kg of cocaine during the maintenance phase of a self-administration session. The horizontal lines represent the minimum cocaine levels (satiety threshold) in the absence and presence of anticocaine antibodies. The antibody-induced increase in the satiety threshold results in an increase in the cocaine concentration in the body. First-order elimination kinetics dictate a faster rate of cocaine elimination at the higher concentrations of cocaine, even if the cocaine elimination half-life does not change in the presence of the antibody. Consequently, the cocaine levels decline to the satiety threshold more rapidly, resulting in a shorter inter-injection interval. In this model it is assumed that the cocaine elimination half-life is 9 min, the injection and subsequent distribution of cocaine is instantaneous and a single-compartment pharmacokinetic model applies to the distribution and elimination of cocaine.
Adapted with permission from Wiley [15].
Immunotherapeutic safety & potential adverse effects
Clearly, in addition to the requirement for high efficacy of an anticocaine mAb to prevent the entry of cocaine into the brain, there is the issue of the safety of repeated intravenous administrations of a high-molecular-weight biologic and its potential for adverse effects. The therapeutic use of pooled preparations of human IgG designated as intravenous IgG [20] in patients with various hematological, inflammatory and primary immune deficiency diseases has been very successful. Furthermore, in some regimens the antibody doses infused (70–210 g per 70 kg patient, given every 3–4 weeks) far exceed those likely to be required to successfully treat cocaine relapse patients (approximately 1–10 g per patient, given every 3–4 weeks) and they are generally well tolerated. However, patients must be closely monitored and serious renal dysfunction can occur owing to excessive rates of antibody infusion, the presence of multimeric antibody aggregates and the use of sucrose as a product stabilizer. Therefore, based on clinical experience with intravenous IgG, it is expected that patients with pre-existing renal dysfunctions, taking nephrotoxic drugs or with diabetes would need to be excluded from treatment [20,21]. For HIV-infected cocaine abuse patients, especially those having progressed to advanced immunodeficiencies and who would likely not respond to anticocaine vaccination, the passive immunotherapy might be the only, or the preferred, treatment approach. However, it is not clear whether their overall poor health would make them high-risk patients for renal failure or aseptic meningitis syndrome.
The first mAbs generated as potential therapeutics were derived using the now classic murine hybridoma technology of Kohler and Milstein that enabled the first isolation of high-affinity, target-specific homogeneous species of mAbs [22]. Unfortunately, as immunotherapeutics, murine mAbs proved highly immunogenic, generating neutralizing human antibodies along with the potential for serious allergic reactions including anaphylactic shock. For example, the first clinically approved mAb, OKT3, directed against the T-cell receptor CD3 complex, can generate strong human antimouse antibody responses even while being used as a component of immunosuppressive treatments in organ transplant patients [23]. Such results prompted the use of current molecular biology technologies to re-engineer murine-derived mAbs and achieve the transition from murine mAbs, to chimeric mAbs with human heavy- and light-chain constant region sequences replacing murine constant region sequences, to humanized mAbs with all but critical target-binding site loci residues of the murine hypervariable regions replaced with human sequences, and eventually to fully human sequence mAbs. Fully human mAbs can be generated through the use of transgenic mice with humanized immune systems [24,25] and human sequence recombinant single-chain Fv phage display libraries [26].
The success of these mAb humanization approaches in reducing mAb immunogenicity in patients is illustrated by the fact that the current success rate for US FDA approval of clinical candidate mAbs is approximately 20%, with nearly 150 mAbs now undergoing clinical trials, versus approximately 5% for traditional low-molecular-weight chemical entities [27].
Achieving a nearly or fully human sequence, however, does not guarantee clinical success, but the risks of serious adverse effects due to immunogenicity do appear significantly reduced. It is important to note that recent reviews of US and European safety-related regulatory actions for biologics seem to indicate that safety issues for re-engineered mAbs are most often related to the mAb’s mechanism(s) of action and/or the targeting of cell membrane-bound protein targets that result in untoward immunomodulation or suppression of critical anti-infective immune responses [27–29].
Alternatively, chimeric and humanized mAbs may still prove to be immunogenic owing to the presence of strong human T-cell epitope sequences that may be naturally present or have been constructed into the antibody sequence [25,28]. Currently, there are several different computational algorithms available to aid investigators in the identification and modification of potentially immunogenic sequence motifs [30].
Thus far, the majority of the approximately 20 FDA-approved mAbs currently in clinical use have demonstrated a potential for serious side effects that complicates their usage, but these effects largely result from the mAbs’ mechanisms of action [27–29]. An illustrative example is infliximab, which binds and ‘inactivates’ the proinflammatory cytokine TNF-α and is used to moderate rheumatoid and psoriatic arthritis. Unfortunately, excessive decreases in TNF-α levels can allow the reactivation of dormant TB or histoplasmosis infections with fatal consequences. Furthermore, receptor-directed mAbs such as trastuzumab, which acts as a therapeutic antagonist when binding the HER2 receptor in ERBB2-positive breast cancer cells, can prove cardiotoxic when the mAb binding cardiac cells prompts alterations in mitochondrial membrane permeability and proapoptotic responses [27]. Alternatively, mAb-mediated over-stimulation of immune responses can provoke near-fatal cytokine storms, as occurred in healthy volunteers infused with the clinical candidate anti-CD28 receptor mAb TGN1412 [31].
The potential for serious side effects is also present for mAbs acting through either complement-dependent cytotoxicity or ADCC, the two principle mechanisms for anticancer or antilymphoproliferative disease mAb treatments. The T- and B-cell CD52-directed alemtuzumab and the anti-IL-2 receptor α-subunit-directed basilizimab are examples of mAbs acting through these mechanisms. They have considerable potential for excessive cell lysis, immunosuppression, hypersensitivity reactions and cytokine release syndrome.
In contrast to the immunotherapeutics discussed above, mAbs, or their Fab fragments, directed against exogenous low-molecular-weight plasma-accessible toxins/targets such as digoxin, colchicine, nicotine or cocaine represent a distinct and simpler class of ‘antidotal’ immunotherapeutic [16]. These mAbs/Fabs, by binding the free unbound drug in circulation, block both the drug’s action and distribution to its site(s) of action and possibly (especially for Fab fragments) alter the drug’s elimination rate, potentially without affecting normal physiological processes. A key finding relative to the safe use of this immunotherapeutic approach towards drug overdose treatment is the fact that, while Fab infusion causes an immediate 10–30-fold increase in plasma levels of digoxin [17] or colchicine [18], the cardiac and/or cardiovascular toxic effect(s) of these drugs is not increased but rather rapidly and dramatically decreased. This is because the plasma levels of free drug are significantly lowered and substantial amounts of the drug are also removed from vascular and peripheral sites of action and redistributed back to the vascular/extracellular space as an inactive drug–antibody complex.
Confirmation that this immunotherapeutic anticocaine approach is safe thus far appears to be provided by recent clinical trials of an anticocaine vaccine [2–4], designated as TA-CD (succinylnorcocaine linked to recombinant cholera toxin B), in which patients were immunized from three- to five-times over a 12-week period and monitored up to 52 weeks. In these studies, while some minor site of injection and immune response stimulation side effects were reported, no issues emerged relative to the circulating anti-cocaine antibodies raised and their interaction with cocaine.
As for passive immunotherapy, antidigoxin Fab fragments such as Digibind® (GlaxoSmithKline, Brentford, UK), even while being of foreign species origin (ovine), proved quite safe with allergic responses reported in fewer than 1% of patients [19]. Long-term treatment with an intact mAb is clearly more problematic but the mAb 2E2, as previously discussed, is comprised of a fully human sequence γ1 heavy chain and a murine λ-light chain and the authors have now re-engineered 2E2 to have a human λ-light chain constant region (designated as h2E2) with no loss of affinity or specificity [Ball WJ, Norman AB, Unpublished Data]. As the heavy-chain sequence is generally the predominant contributor to antibody immunogenicity and the 2E2 heavy chain is a product of the HuMAb-Mouse® transgenic mouse developed by Medarex, Inc. [24], formerly GenPharm, now part of Bristol-Myers Squibb (Milpitas, CA, USA) the authors are optimistic about the likelihood of success of 2E2/h2E2. At least 22 human sequence mAbs derived from HuMAb transgenic mice have undergone initial clinical trials with, thus far, a notable lack of immunogenicity [32].
Therefore, the primary indications of safety as well as efficacy for an IgG1 anticocaine mAb will be the demonstration that it has low in vivo immunogenicity, even upon repeat administrations, and exhibits normal FcRn binding with a clearance half-life of approximately 3 weeks and no demonstrable cross-reactivity binding to human blood or tissue cells. Indeed, initial in vitro testing of the mAb 2E2 has shown no indication of cross-reactivity with human cells/tissues, nor has analysis of its heavy- and light-chain sequences revealed any potentially strong human T-cell epitopes [Ball WJ, Norman AB, Unpublished Data].
Another cause for concern sometimes expressed by clinicians is that of drug-induced nephrotoxicity, resulting during mAb elimination as the mAb–drug complex is transported into the urinary space of the glomerulus, reabsorbed and concentrated in the proximal tubules of the kidneys, and the drug released as the mAb either re-enters circulation or is catabolized [33]. This is conceptually possible given the very high affinity (dissociation constant in the sub-nM range) mAb– or Fab–drug binding of toxic drugs, such as digoxin or colchicine, which undergo little in vivo metabolic inactivation, but this issue does not arise for cocaine. Cocaine is rapidly metabolized to inactive derivatives that do not significantly compete with cocaine for mAb h2E2 binding. Furthermore, as the affinity of mAb 2E2 and h2E2 for cocaine is approximately 4 nM, cocaine has an exchange- or off-rate of minutes and in vivo pharmacokinetic studies performed by the authors in mice have demonstrated that, while mAb 2E2 dramatically increases plasma cocaine concentrations and limits its access to the brain, it does not inhibit cocaine’s rate of elimination or metabolism. Thus, current evidence indicates that the re-engineered mAb h2E2 should be both efficacious and safe.
Future perspective
Within the next 2 years the ongoing multi-center Phase III clinical trials of the cocaine vaccine should be completed. It is likely that they will continue to show substantial inter-individual variability in the levels of anticocaine immune responses and that the clinical efficacy is dependent on high antibody titers. This will confirm the need for the development of a more effective cocaine vaccine, which is likely to be ready for clinical testing within 5 years. The results of these clinical trials will provide a better understanding of the antibody concentrations and affinity requirements for a reliably effective immunotherapy. In parallel with this new vaccine development, the current work to generate a mammalian cell-based production platform for the large-scale manufacture of h2E2, a further humanized variant of 2E2, as needed to support an investigational new drug application and subsequent clinical trials, should have been completed. We predict that both active and passive immunotherapies will be approved for clinical use and that these two complementary approaches will be combined to fine tune the optimal anticocaine antibody binding activity over protracted treatment periods. Once this combination therapy is optimized, these approaches will be, in addition, combined with a new generation of high-efficiency recombinant cocaine hydrolases. Ideally, within the next decade, this triple-combination therapy can be further complemented with a pharmacotherapeutic agent that will act in the brain to ameliorate environmental cue- or stressor-induced cravings for cocaine. This additional treatment component may be important because the immunotherapies and cocaine hydrolases have no effect on the systems in the brain involved in centrally-mediated responses. As no single therapy will be effective under all circumstances, it can reasonably be expected that the effectiveness of different therapies will be additive and, hopefully, synergistic. In addition to their obvious clinical potential, these therapeutic agents will continue to provide useful tools for defining the pharmacokinetic/pharmacodynamic mechanisms underlying cocaine’s in vivo effects.
Executive summary.
Effect of anticocaine monoclonal antibodies on cocaine pharmacokinetics
A humanized anticocaine monoclonal antibody (mAb) having a high affinity (dissociation constant: 4 nM) and specificity for cocaine over its inactive metabolites decreases brain concentrations of cocaine produced by an intravenous injection. By binding cocaine and sequestering it in the circulating blood, the mAb acts as both a chemical and a pharmacokinetic antagonist of the central effects of cocaine.
This mAb, 2E2, has a long elimination half-life in rodents, potentially predicting a long duration of effects during clinical use.
Increased priming threshold as a model of relapse prevention
The cocaine priming threshold determination represents a quantitative measure of the ability of the humanized anticocaine mAb to antagonize the cocaine-induced reinstatement of cocaine self-administration.
The magnitude of the mAb-induced increases in the cocaine priming threshold in rats is comparable with the effects of a competitive dopamine receptor antagonist.
The mAb-induced increase in the cocaine priming threshold predicts clinical efficacy through a reduction in the probability of a dose of cocaine inducing relapse.
The rate of cocaine consumption is increased if relapse is maintained
When sufficient cocaine is administered to overcome the presence of the anticocaine mAb, cocaine self-administration is reinstated and the rate of cocaine consumption is increased in rats.
The mechanism for this modest acceleration of cocaine self-administration is hypothesized to result from a pharmacokinetic/pharmacodynamic interaction where the brain concentration at which cocaine induces lever pressing (satiety threshold) is unaltered, but the whole-body cocaine concentration required to overcome the anticocaine mAb and maintain the same brain concentration is increased. As cocaine elimination from the body is first-order, the rate of cocaine clearance is increased at the higher concentrations. Consequently, the cocaine concentrations decline more rapidly in a shorter time between cocaine self-injections.
This may predict that cocaine addicts will take more cocaine if relapse is maintained during immunotherapy with a humanized anticocaine mAb. This therapy is most suitable for use in addicts committed to remaining abstinent.
Immunotherapeutic safety & potential adverse effects
Murine-derived mAbs were the first developed, but they provoke a strong immune response in patients that may neutralize the mAb, compromise efficacy and pose safety issues that contraindicate repeated treatments.
Humanized mAbs typically minimize immune responses and are better tolerated during repeated treatments.
Humanized mAbs that target endogenous human proteins can disrupt the physiology of important systems compromising safety.
Humanized anticocaine mAbs with high specificity minimize potential immune responses, have a low probability of disrupting endogenous physiological systems and should be well tolerated during long-term treatment for relapse prevention in cocaine addicts.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors acknowledge funding from the NIH (grant number DP1DA031386). The authors are named as co-inventors on a pending patent application concerning 2E2 and h2E2. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Kosten TR, Owens SM. Immunotherapy for the treatment of drug abuse. Pharmacol Ther. 2005;108:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2▪.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. roundbreaking report showing that anticocaine antibodies, which are produced in response to a cocaine vaccine, can decrease cocaine intake in human addicts. It also shows that responses to the vaccine can vary widely between individuals. [DOI] [PubMed] [Google Scholar]
- 3▪▪.Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone maintained patients. Arch Gen Psychiatry. 2009;66(10):1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. Pivotal study reporting that there is a threshold anticocaine antibody titer that is necessary to confer clinical efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paula S, Tabet MR, Farr CD, Norman AB, Ball WJ. Modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47:133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- 6▪.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23:1117–1125. doi: 10.1038/nbt1135. Reviews the technology that was used to generate a humanized anticocaine monoclonal antibody (mAb), the effects of which are reviewed herein. [DOI] [PubMed] [Google Scholar]
- 7.Harris DS, Everhart ET, Mendelson J, Jones RT. The pharmacology of cocaethylene in humans following cocaine and ethanol administration. Drug Alcohol Depend. 2003;72:169–182. doi: 10.1016/s0376-8716(03)00200-x. [DOI] [PubMed] [Google Scholar]
- 8▪.Norman AB, Tabet MR, Norman MK, Buesing WR, Pesce AJ, Ball WJ. A chimeric human/murine anticocaine monoclonal antibody inhibits the distribution of cocaine to the brain in mice. J Pharmacol Exp Ther. 2007;320:145–153. doi: 10.1124/jpet.106.111781. Reports the in vivo effects of a novel humanized anticocaine mAb and shows that the mAb acts as a pharmacokinetic antagonist of the distribution of cocaine. [DOI] [PubMed] [Google Scholar]
- 9▪.Norman AB, Norman MK, Buesing WR, Tabet MR, Tsibulsky VL, Ball WJ. The effect of a chimeric human/murine anti-cocaine monoclonal antibody on cocaine self-administration in rats. J Pharmacol Exp Ther. 2009;328:873–881. doi: 10.1124/jpet.108.146407. Provides quantitative measures of the magnitude of effect of a novel humanized anticocaine mAb in a standard model of addictive behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brimijoin S, Gao Y. Lasting reduction of cocaine action in neostriatum – a hydrolase gene therapy approach. J Pharmacol Exp Ther. 2009;330:449–457. doi: 10.1124/jpet.109.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll ME, Gao Y, Brimijoin S, Anker JJ. Effect of cocaine hydrolase on cocaine self-administration under a PR schedule and during extended access (escalation) in rats. Psychopharmacology. 2011;213:817–829. doi: 10.1007/s00213-010-2040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norman AB, Welge JA, Tsibulsky VL. Characterization of the distribution of the cocaine priming threshold and the effect of SCH23390. Brain Res. 2002;946:253–261. doi: 10.1016/s0006-8993(02)02893-7. [DOI] [PubMed] [Google Scholar]
- 13.Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- 14.Norman AB, Tsibulsky VL. The compulsion zone: a pharmacological theory of acquired cocaine self-administration. Brain Res. 2006;1116:143–152. doi: 10.1016/j.brainres.2006.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman AB, Norman MK, Tabet MR, Tsibulsky VL, Pesce AJ. Competitive dopamine receptor antagonists increase the equiactive cocaine concentration during self-administration. Synapse. 2011;65:404–411. doi: 10.1002/syn.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baud FJ, Borron SW, Scherrmann JM, Bismuth C. A critical review of antidotal immunotherapy for low molecular weight toxins. Arch Toxicol Suppl. 1997;19:271–287. doi: 10.1007/978-3-642-60682-3_25. [DOI] [PubMed] [Google Scholar]
- 17.Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab therapy in the management of digitalis toxicity. Clin Pharmacokinet. 1995;28:483–493. doi: 10.2165/00003088-199528060-00006. [DOI] [PubMed] [Google Scholar]
- 18.Baud F, Sabouraud A, Vicaut E, et al. Treatment of severe colchicine overdose with colchicine specific Fab fragments. N Engl J Med. 1995;332:642–645. doi: 10.1056/NEJM199503093321004. [DOI] [PubMed] [Google Scholar]
- 19.Bowden CA, Krenzelok EP. Clinical applications of commonly used contemporary antidotes. Drug Safety. 1997;16(1):9–47. doi: 10.2165/00002018-199716010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Gammagard Liquid®. Physicians’ Desk Reference. 61. Thomson PDR; Montvale, NJ, USA: 2007. pp. 721–724. [Google Scholar]
- 21.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 22.Kohler G, Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- 23.Cosimi AB. Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Tranplantation. 1981;32(6):535–539. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Lonberg N, Taylor LD, Harding FA, et al. Antigen-specific human monoclonal antibodies from mice comprising four distinct genetic modifications. Nature. 1994;368:856–859. doi: 10.1038/368856a0. [DOI] [PubMed] [Google Scholar]
- 25.Green LL, Hardy MC, Maynard-Currie CE, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nature Genet. 1994;7:13–21. doi: 10.1038/ng0594-13. [DOI] [PubMed] [Google Scholar]
- 26.Marks JD, Hoogenboom HR, Bonnert TP, et al. By-passing immunization: human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 27▪.Hansel TT, Kropshofer S, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338. doi: 10.1038/nrd3003. Provides an extensive overview of the issues related to the safety of mAbs currently approved for clinical use. [DOI] [PubMed] [Google Scholar]
- 28.Hai SH, McMurray JA, Knopf PM, Martin W, De Groot AS. Immunogenicity screening using in silico methods. In: An Z, editor. Therapeutic Monoclonal Antibodies: From Bench to Clinic. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2009. pp. 417–437. [Google Scholar]
- 29.Gibson CR, Sandhu P, Hanley WD. Monoclonal antibody pharmacokinetics and pharmacodynamics. In: An Z, editor. Therapeutic Monoclonal Antibodies: From Bench to Clinic. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2009. pp. 439–460. [Google Scholar]
- 30.Van Walle I, Gansemans Y, Parren PW, et al. Immunogenicity screening in protein drug development. Expert Opin Biol Ther. 2007;7:405–418. doi: 10.1517/14712598.7.3.405. [DOI] [PubMed] [Google Scholar]
- 31.Eastwood D, Findlay L, Poole S, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol. 2010;161:512–526. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fishwild DM, O’Donnell SL, Bengoechea T, et al. High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat Biotechnol. 1996;14:845–851. doi: 10.1038/nbt0796-845. [DOI] [PubMed] [Google Scholar]
- 33.Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]