Abstract
Introduction
Giant pituitary adenomas of excessive size, fibrous consistency or unfavorable geometric configuration may be unresectable through conventional operative approaches. We present our select case series for operative resection and long-term follow-up for these unusual tumors, employing both a staged procedure and a combined transsphenoidal-transcranial above and below approach.
Method
A retrospective chart review was performed on patients operated via the staged, and combined approaches by the senior author (J.N·B.). Pre-operative characteristics and postoperative outcomes were reviewed. A detailed description of the operative technique and perioperative management is provided.
Results
Between 1993 and 1996, two patients harboring giant pituitary adenomas underwent an intentionally staged resection, and between 1997 and 2006, nine patients harboring giant pituitary adenomas underwent surgery via a single-stage above and below approach. Nine patients (82%) presented with non-secreting adenomas and two patients (18%) presented with prolactinomas refractory to medical management. Gross total resection was achieved in six patients (55%), near total resection in 1 (9%), and subtotal removal in 4 (36%). Seven patients (64%) experienced visual improvement postoperatively and no major complications occurred. Long-term follow-up averaged 51.6 months. Panhypopituitarism was observed in four patients, partial hypopituitarism in four, persistent DI in two, and persistent SIADH in one.
Conclusions
The addition of a transcranial component to the transsphenoidal approach offers additional visualization of critical neurovascular structures during giant pituitary adenoma resection. Complications rates are similar to other series in which complex pituitary adenomas are resected by other means. The above and below approach is both safe and effective and the immediate and long-term advantages of a single-stage approach justify its utility in this select group of patients.
Keywords: Adenoma, Approach, Pituitary, Transsphenoidal, Treatment, Tumor
Introduction
A relatively rare subset of pituitary adenomas grows to a giant size before reaching clinical diagnosis. Aggressive surgical resection is indicated since they are usually histologically benign tumors. Complete resection obviates the need for radiation therapy, but even when gross total resection is not possible, aggressive resection and decompression of critical neurological structures improves the response to radiation therapy while minimizing its risks. More importantly, large tumors often compress the optic chiasm, and when incompletely resected, are at risk for perioperative bleeding into residual tumor. Although this bleeding is of a low-pressure venous etiology, it may be sufficient to cause significant deterioration in an already compromised visual apparatus.
The transsphenoidal approach to pituitary tumors has proved to be very versatile since its development in the 1970s [1–3] and experienced transsphenoidal surgeons can successfully remove most tumors even when large. However, giant pituitary adenomas present a surgical challenge that is not easily managed through standard transsphenoidal approaches. Among the most challenging are giant tumors with excessive extension superiorly or laterally and so-called “dumbbell-shaped” or “hourglass-shaped” tumors with a constriction at the level of the opening in the diaphragm sellae [4–6]. Additionally, removal of tumors that are very firm or fibrous may be limited by conventional surgical approaches although this may not be recognizable preoperatively except with recurrent tumors [7–9]. For this unusual subset of giant pituitary adenomas, a craniotomy is often necessary to achieve surgical goals [3–6, 8, 10–13]. In rare instances, a craniotomy may be performed prior to a staged transsphenoidal procedure [3, 8, 10, 11, 13]. Others have preferred using the transsphenoidal approach first [14].
Giant adenomas are a challenge for the neurosurgeon because their proximity to optic pathway, intracavernous carotid artery, the circle of Willis, and oculomotor nerve makes radical surgical removal more difficult and associates it with a higher complication rate than in nongiant adenomas [14]. Both planned two-stage operations and combined, single-stage, transsphenoidal-craniotomy approaches have been previously described for unique tumors that are contraindicated, by size and geometric configuration, to conventional surgery [4–6, 14]. We present a series of patients with giant pituitary adenomas that required this unusual combined, “above and below”, surgical approach. We also present our earlier series of patients who have undergone an intentionally staged operation, employing a transsphenoidal approach followed by a craniotomy for similar, giant pituitary tumors. The operative approach is described with operative nuances emphasizing the benefits of simultaneous procedures with two surgeons to achieve aggressive resection in a single stage. This strategy facilitates simultaneous decompression of the intracranial portion without violating the tumor capsule with removal of the tumor through the transsphenoidal approach. Bleeding is minimized and the arachnoid plane along the tumor interface is preserved which allows for aggressive resection and avoidance of postoperative hemorrhage.
Methods
Preoperative evaluation
A Columbia University Medical Center Institutional Review Board approved retrospective chart review was performed on all patients undergoing a simultaneous above and below approach as well as intentionally staged surgeries to complex, giant pituitary adenomas by the senior author (J.N·B.). Patient demographics, presenting signs and symptoms, neurological status, visual field abnormalities, and endocrine abnormalities were recorded. Preoperative magnetic resonance imaging (MRI) studies were reviewed and extrasellar tumor extension was described based on anatomical location. For direct comparison to historical series, lesions were also classified based on the system first described by Hardy and Vezina [2] with modifications as later suggested by Wilson [15]. Under this system, tumors were classified into five groups (A–E) based on supra- or extrasellar extension. Hardy configuration A represents small, superior tumor extension protruding into the suprasellar cistern; configuration B represents medium-size extension indenting the floor of the third ventricle; configuration C represents large symmetrical, superior extension filling the anterior portion of the third ventricle; configuration D represents asymmetrical superior extension sprouting anteriorly, laterally, or posteriorly (intracranial, intradural); and configuration E represents lateral extension into the adjacent cavernous sinus (extracranial, extradural).
Patient selection
Simultaneous above and below resection was indicated for patients with: (1) a giant, non-functioning pituitary adenoma or a giant adenoma refractory to medical management; or (2) giant adenoma with significant supra-or parasellar extension deemed unresectable via a transsphenoidal approach alone. All patients were in good overall state of health with evidence of significant mass effect on critical neural, vascular or neuroendocrine structures. During the initial experience of the senior author with this subset of patients, a planned staged removal in two different steps was employed for patients that met the above criteria. Each of these two patients underwent a subtotal transsphenoidal surgery first, followed approximately 1 week later with a craniotomy to achieve a gross total resection.
Surgical technique—simultaneous combined above and below approach
Variations of the simultaneous combined above and below approach have been described previously as the “combined supra-infrasellar approach” and the “combined transsphenoidal and pterional craniotomy approach” [4–6]. The procedure as performed at our institution is outlined below.
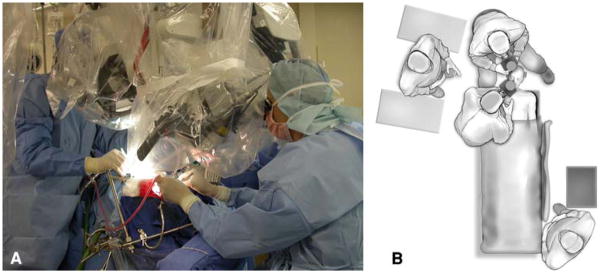
The simultaneous combined “above and below” approach involves an endonasal transsphenoidal approach and fronto-temporal craniotomy and requires two operative fields, two operative microscopes, and two surgical teams. After induction of general anesthesia by endotracheal intubation, a lumbar spinal catheter is placed to provide intraoperative brain relaxation and facilitate potential postoperative cerebrospinal fluid (CSF) leak repair. The patient is positioned supine and secured in a radiolucent, Mayfield three-point head holder. The head is slightly extended and tilted laterally (approximately 15°) towards the left shoulder (for a right-sided craniotomy). If the tumor extends more to the left, a left sided craniotomy can be performed instead. The table is positioned at an angle to facilitate use of portable fluoroscopy to provide localization of the appropriate transsphenoidal trajectory. Alternatively, a frameless stereotactic setup can be used to avoid the need for intraoperative fluoroscopy (Fig. 1).
Fig. 1.
a Photograph of the two operative team set-up utilized in the simultaneous above and below approach. b Aerial sketch demonstrating the separate intracranial and transsphenoidal surgical team locations for a right-sided simultaneous approach
The nose, mouth and scalp are prepped with a povidone–iodine solution and draped in a standard fashion. The abdomen is prepared for fat graft harvest to be performed at the end of the procedure to pack the sella and sphenoid sinus. The transsphenoidal approach is performed first, followed by a standard frontotemporal craniotomy.
Endonasal transsphenoidal approach
Under the operative microscope, an incision is made through the mucosa along the right side of the nasal cartilage. A submucoperichondral plane is developed and submucosal dissection performed along the vomer and nasal cartilage. After developing a submucosal tunnel, the nasal cartilage is disarticulated from the vomer and pushed to the left. A hand-held retractor is advanced through the submucosal tunnel to expose the anterior surface of the sphenoid bone over the sphenoid sinus. The location and trajectory to optimally visualize the anterior face of the sella can be verified using intraoperative fluoroscopy or frameless stereotaxy. The hand-held speculum is replaced with a Hardy retractor to expose the face of the sphenoid sinus which is entered after removing the remaining vomer and anterior wall of the sinus with Kerrison and pituitary rongeurs. The mucosa of the sphenoid sinus is exenterated to prevent postoperative mucocele formation and control mucosal bleeding [16]. This approach allows for optimum exposure of the floor of the sella turcica. At this point, the sellar floor is removed and the dura opened in a U-shaped fashion and reflected inferiorly. Attention is then turned to the completing the transcranial approach.
Transcranial approach
A curvilinear scalp incision is made extending from in front of the tragus over the convexity and ending in the midline behind the hairline. The temporalis muscle and fascia are divided posteriorly and reflected anteriorly with the scalp flap. A standard frontotemporal craniotomy is performed flush with frontal fossa. The sphenoid wing is flattened with a pneumatic drill for better exposure and visualization. Although skull base variations to include orbital and zygomatic osteotomies can be performed, they are not necessary when a craniotomy is performed simultaneous with the transsphenoidal approach. The dura is opened and reflected anteriorly along the floor of the anterior and middle cranial fossa. For tumors with excessive lateral extension, it may be desirable to sharply open the Sylvian fissure. A retractor blade is placed on the undersurface of the frontal lobe back towards the ipsilateral optic nerve. The second operating microscope is brought in and the tumor is visualized in the suprasellar and parasellar region .
Tumor removal
One of the key features of the above and below approach is to internally decompress the tumor initially from the transsphenoidal approach using ring curettes and tumor forceps. Once the tumor has been sufficiently decompressed transsphenoidally, the surgeon from the transcranial approach opens the arachnoid over the tumor and carefully dissects the mass from the optic chiasm and nerves, the carotid vasculature, and the surrounding brain parenchyma while attempting to preserve arachnoid planes and perforating vessels [4, 17]. It is important to free up the tumor capsule, consisting mostly of an extended but thinned-out diaphragm, from surrounding structures and push the remaining tumor bulk through the sellar opening. The bulk of tumor removal is through the transsphenoidal approach as the tumor is pushed from above by the intracranial surgeon. It is critical for the intracranial surgeon to avoid opening the tumor capsule in order to control bleeding and avoid blood in the subarachnoid space. Since the tumor capsule can be fragile and its integrity easily violated, the surgeon should work the tumor interface with care.
The methodical and stepwise dissection of the tumor from surrounding intracranial structures with the simultaneous debulking of tumor transsphenoidally is rewarded by direct visualization and preservation of the optic nerves and chiasm. As the dissection proceeds, the pituitary stalk can be identified and the residual portion of the gland can be identified and preserved in most instances. The ability to dissect the margin of the tumor without violating the integrity of the capsule allows the intracranial surgeon to preserve arachnoid planes and avoid damage to delicate vessels branching off from the carotid and anterior cerebral arteries supplying critical structures including the visual apparatus and hypothalamus. In the final stages of tumor removal, the slackened, patulous capsule is inverted through the suprasellar space and into the sphenoid sinus where the transsphenoidal surgeon can inspect the sac to ensure complete tumor removal.
With firm or fibrous tumors, internal debulking and manipulation of the tumor capsule can be more difficult. Under such conditions where the debulking arrives at a surgical impasse, it may be necessary for the intracranial surgeon to make a small opening in the capsule and proceed with further surgical debulking. This capsular opening should be kept as small as possible to avoid bleeding that might obscure the tumor plane. This variation of the standard above and below technique may also apply to tumors with a large cavernous sinus component. This component of the tumor is often more easily removed under direct vision by the transcranial surgeon who must open the capsule to follow the tumor into the cavernous sinus.
Closure
As with most pituitary tumors, bleeding is minimal once the tumor has been completely removed. Any residual bleeding is usually of a low pressure and easily controlled with the surgeons choice of hemostatic agents (FLOSEAL®(Baxter International, Inc., Deerfield, IL) or Avitene®(Davol, Inc., Cranston, RI) with a temporary compression by a large bulky hemostatic agent such as Gelfoam® (Pharmacia & UpJohn Company, Kalamazoo, MI). Once the bleeding is stopped, the Gelfoam® is removed and any loose hemostatic agent is irrigated away.
Removal of a tumor of this size results in a direct communication between the CSF and sphenoid sinus so a watertight closure is essential. An abdominal fat graft is harvested and a small piece is placed in the resection cavity within the sella making sure to avoid any mass effect. A dural substitute (Duragen®, Integra Life Sciences Corp., Plainsboro, NJ) is placed intradurally and extradurally followed by a larger piece of fat filling the sphenoid sinus and sealing the Duragen® “sandwich”. The spinal drain is left in place for 2–3 days draining 5–8 cc of CSF every hour.
While the transsphenoidal surgeon is closing, the transcranial team reapproximates the dura primarily. The bone flap is plated in place and the subcutaneous tissue and scalp are reapproximated in the standard fashion. Nasal breathing tubes are placed and subsequently removed between 12 and 24 h postoperatively. Foley catheters are left in place to accurately assess urine output and specific gravity for 24 h.
Surgical technique—intentionally staged approach
The intentionally staged transsphenoidal approach followed by craniotomy utilizes the standard transsphenoidal resection described above at the first operation followed by an appropriately designed transcranial approach for tumor residual approximately 1 week later. Depending on individual circumstance, the patient may be discharged home after the transsphenoidal resection and return several days later for the craniotomy or the patient may remain in house if closer surveillance is deemed necessary.
All patients are monitored in the Intensive Care Unit per routine craniotomy protocol. At our institution, the care of patients who have undergone pituitary surgery involves the management of neurosurgical, endocrinological, and nursing issues by a coordinated multi-disciplinary team comprised of members of each of these specialties [18].
Follow-up
All patients’ perioperative and postoperative medical records and imaging studies were reviewed and all postoperative complications recorded. On an outpatient basis, patients were seen and evaluated at regular intervals by the primary surgeon. Endocrine function, visual field deficits, and neurological status were followed and recorded. Serial MRI studies were routinely obtained for evaluation of tumor growth or recurrance, however, these imaging studies were obtained on a patient-by-patient basis and not necessarily at standardized intervals.
Results
Preoperative evaluation
Between 1993 and 1996, 2 patients harboring giant pituitary adenomas underwent an intentionally staged resection. Between 1997 and 2008, 9 patients with similar tumor characteristics underwent surgery via the combined supra-infrasellar approach. The average patient age at presentation was 48 years (range 36–69). Eight patients were male (73%) and 3 were female (27%). Presenting signs and symptoms included visual loss (100%), endocrine dysfunction (64%), hydrocephalus (27%), headache (27%), seizures (9%) and syncope (9%). Nine patients (82%) presented with giant non-secreting adenomas. Two patients (18%) presented with giant prolactinomas refractory to medical management. Based on preoperative MRI evaluation, 1 patient (9%) had a tumor classified as Hardy configuration C, 7 patients (64%) as configuration D, and 3 patients (27%) as configuration E. Patient demographics, presenting signs and symptoms, endocrine abnormalities, tumor characteristics, and previous interventions are listed in Table 1.
Table 1.
Preoperative patient characteristics
| Patient | Age | Gender | Presenting singns/symptoms | Tumor type | Tumor configuration | Previous invention |
|---|---|---|---|---|---|---|
| Combined approach | ||||||
| 1 | 36 | M | VL, SZ, hypopituitarism | NSA | E | No |
| 2 | 69 | F | VL, glaucoma | NSA | D | No |
| 3 | 37 | M | VL, HCP, HA, galacturia | PRO | D | Pergolide |
| 4 | 51 | F | VL, DI | NSA | D | No |
| 5 | 39 | M | VL | PRO | D | Bromocriptine, pergolide, cabergoline |
| 6 | 66 | M | VL | NSA | D | No |
| 7 | 54 | M | VL, DI, hypogonadism | NSA | E | Previous transsphenoidal restriction |
| 8 | 40 | M | VL, HCP, HA, syncope | NSA | D | No |
| 9 | 53 | F | VL, hypopituitarism | NSA | C | Previous transsphenoidal restriction and craniotomy |
| Staged approach | ||||||
| 1 | 40 | M | VL, HCP, HA, hypopituitarism, memory and behavioral changes | NSA | D | No |
| 2 | 41 | M | VL, HA, hypopituitarism | NSA | E | No |
Tumor configuration A, small, superior tumoral extension protruding into the suprasellar cistern; B, medium-size extension indenting the floor of the third ventricle; C, large symmetrical, superior extension filling the anterior portion of the third ventricle; D, asymmetrical superior extension sprouting anteriorly, laterally, or posteriorly (intracranial, intradural); and E, lateral extension into the adjacent cavernous sinus (extracranial, extradural)
VL visual loss, SZ seizures, HCP hydrocephalus, HA headache, DI diabetes insipidus, INC incontinence, NSA non-secreting adenoma, PRO prolactinoma refractory to medical management
Postoperative results, adjuvant treatment and long-term follow-up
Gross total resection (100% tumor volume) was achieved in 6 patients (55%); near total removal (>90% tumor volume) in 1 patient (9%); and subtotal removal (80–90% tumor volume) in 4 patients (36%). Immediate postoperative complications included CSF rhinorrhea in 2 patients (18%), syndrome of inappropriate antidiuretic hormone secretion (SIADH) in 1 patient (9%), transient diabetes insipidus (DI) in 3 patients (27%), transient third nerve palsy in 1 patient (9%), and a urinary tract infection (UTI) in 1 patient (9%). There were no major operative complications, postoperative wound infections or intracranial hemorrhages. All post-operative complications were transient and resolved prior to hospital discharge.
Seven patients (64%) experienced visual improvement postoperatively, while the remaining 4 patients (36%) had no change in visual function. With respect to long-term pituitary function, panhypopituitarism developed in 4 patients, partial hypopituitarism in 4 patients, persistent DI in 2 patients and persistent SIADH in 1 patient. Long-term follow-up ranged from 6 to 182 months, averaging 51.6 months. Postoperative results and long-term follow-up are presented in Table 2.
Table 2.
Postoperative results, adjuvant treatment and length of follow-up
| Patient | Extent of resection | Complications | Adjuvant treatment | Length of follow-up (months) |
|---|---|---|---|---|
| Combined approach | ||||
| 1 | ST | None | Craniotomy, GKRS | 136 |
| 2 | GT | CSF leak, SIADH, UTI | None | 98.5 |
| 3 | NT | None | None | 34.5 |
| 4 | GT | DI | None | 35.8 |
| 5 | GT | DI | None | 14.2 |
| 6 | ST | CN III palsy | LINAC | 79 |
| 7 | GT | None | None | 6.5 |
| 8 | ST | None | GKRS | 52.2 |
| 9 | ST | DI, briefly unresponsive postop | IMRT | 7.6 |
| Staged approach | ||||
| 1 | GT | CSF leak | None | Pt expired |
| 2 | GT | None | TSA, IMRT | 182 |
ST subtotal, GT gross total, NT near total, CFS cerebrospinal fluid, SIADH syndrome of inappropriate antidiuretic hormone release, UTI urinary tract infection, DI diabetes insipidus, CN cranial nerve, GKRS gamma knife radiosurgery, IMRT intensity modulated radiation therapy, LINAC linear acceleration external beam radiation therapy
Follow-up MRI scans were obtained within 1 year of operation and annually thereafter when able. Four patients, in which subtotal tumor resection was achieved at first surgery, demonstrated evidence of radiographic recurrence on follow-up imaging. Patient 1 required a second trans-cranial operation 7 months postoperatively for residual tumor. Five years later, this patient developed progressive visual loss and seizures. Imaging demonstrated tumor recurrence in the right cavernous sinus that was subsequently treated with gamma knife radiosurgery (GKRS). Patient 6 required a course of fractionated external beam radiation 8 months postoperatively for residual tumor surrounding and compressing the optic chiasm. Patient 8 received gamma knife radiosurgery. Patient 9 received intensity modulated radiation therapy (IMRT) 7 months postoperatively to treat residual tumor around her optic nerve. At latest follow-up, all patients who underwent the combined approach had stable MRI studies with no evidence of recurrence.
In the intentionally staged group, a subtotal resection was achieved via the transsphenoidal approach. Six days later, each patient returned to the operating room where a gross total resection was achieved with a pterional craniotomy. The first patient had a transient CSF leak which resolved prior to discharge. This patient did well immediately postoperatively at the time of discharge, however, suddenly expired 5 days after discharge due to seizure and cardiac arrest. The precise cause of death was uncertain. The second patient had progressive tumor recurrence 12 years after the staged surgery, and underwent a subtotal transsphenoidal resection followed by post-operative IMRT. His latest MRI has remained stable, and his vision has improved. Figures 2, 3 and 4 illustrate preoperative tumor characteristics and postoperative results for several representative cases.
Fig. 2.
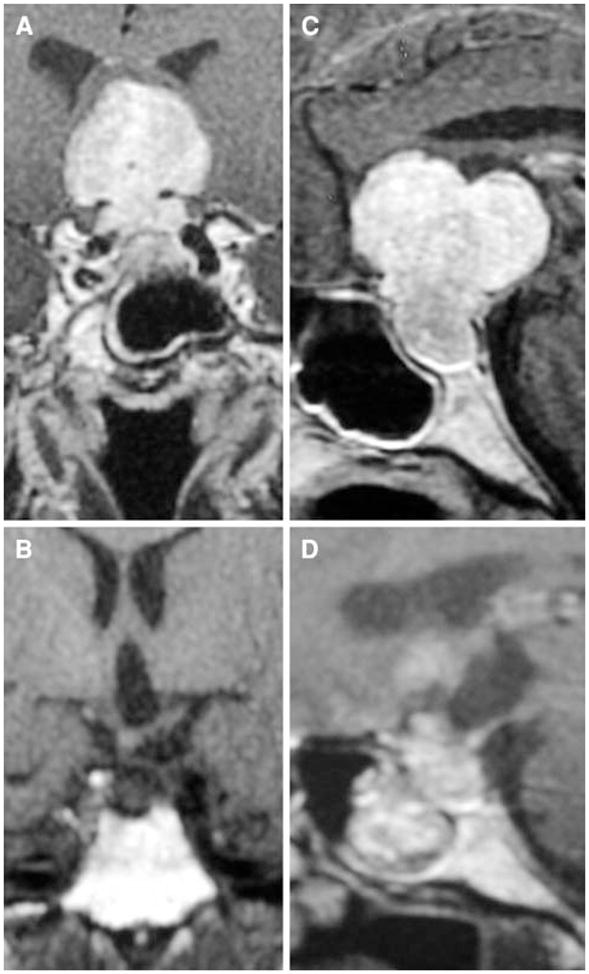
Preoperative coronal (a) and sagittal (c) and postoperative coronal (b) and sagittal (d) contrast-enhanced T1 MRI scans from patient 2 who had a gross total resection of her pituitary tumor. She had a transient CSF leak which resolved post-operatively
Fig. 3.
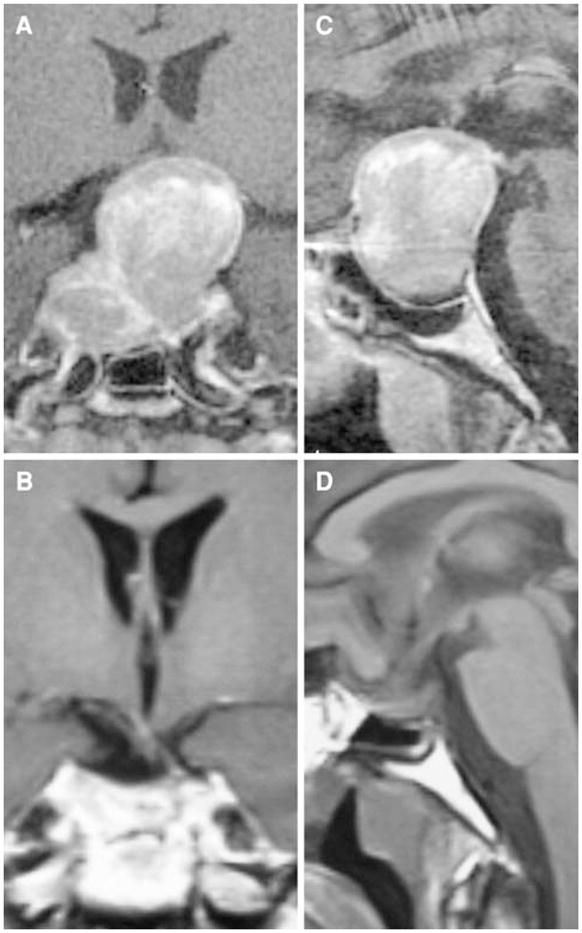
Preoperative coronal (a) and sagittal (c) and postoperative coronal (b) and sagittal (d) contrast-enhanced T1 MRI scans on patient 7 who underwent gross total resection of his pituitary tumor after a failed transsphenoidal approach
Fig. 4.
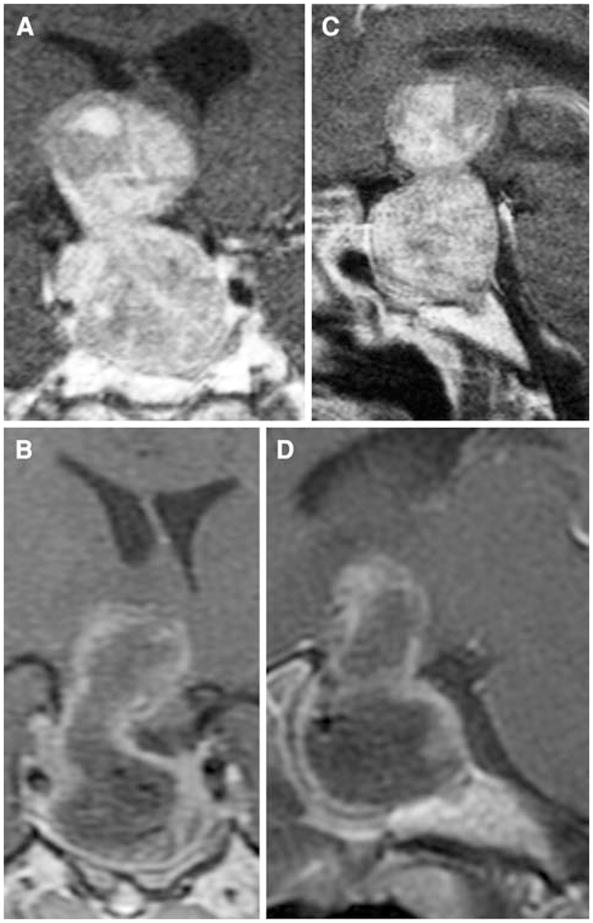
Preoperative coronal (a) and sagittal (c) and postoperative coronal (b) and sagittal (d) contrast-enhanced T1 MRI scans on patient 8 who presented acutely with apoplexy from a dumbbell-shaped hemorrhagic giant pituitary adenoma. This patient received a full course of fractionated radiation therapy post-operatively
Discussion
When confronted with large, non-secreting pituitary adenomas, the primary goal of surgical resection is to decompress critical neurovascular structures without causing further injury. The transsphenoidal route is often the preferred approach due to the lower morbidity rates and a more direct trajectory when compared to transcranial approaches [19, 20]. Dexterity with standard transsphenoidal approaches enables many of these tumors to be resected by experienced pituitary surgeons. However, there exists a small subset of pituitary adenomas that cannot be adequately treated via a traditional transsphenoidal resection alone. These lesions may be shaped in such a way that significant portions of the tumor lie outside the surgical corridor created by the transsphenoidal approach [7–9]. Such pituitary lesions may be dense, fibrous, or nodular in nature which may also limit resection transsphenoidally where critical structures are not directly visualized [1].
Different surgical techniques have been reported attempting to address this complex surgical issue. Some surgeons have injected small amounts of saline or air into the lumbar cistern during transsphenoidal resection in attempts to push the suprasellar portions of the tumor into the operative field [21–24]. Zhang et al. [9] found this technique helpful for soft pituitary lesions with symmetric suprasellar extension, however, for dumbbell-shaped, fibrous, or nodular-type adenomas, this technique was ineffective. Occasionally these tumors reach an excessively large size or extend laterally or superiorly to such a degree that a craniotomy is indicated for tumor removal. A transcranial approach can be used as a primary treatment modality [12], a secondarily staged procedure [3, 8, 10, 11, 13, 25], or can be performed simultaneously with a transsphenoidal resection [4–6].
The combined above and below approach should be considered in any patient undergoing a transcranial approach to take advantage of bloodless tumor dissection and access to the infrasellar portion of the tumor. The transsphenoidal component is “simple” for experienced transsphenoidal surgeons. The ability to decompress and remove the tumor with minimal violation of the tumor capsule is a major advantage to this combined approach. More importantly, the first operation is the best opportunity to achieve a complete resection. Not only does this avoid the difficulties of reoperation but it also avoids the bleeding complications that can occur in the immediate postoperative period when residual tumor is left behind.
Although it is always desirable to obtain a gross total resection, this is not always essential because of tumor radiosensitivity. In our series, a gross or near total resection was achieved in a majority of patients, temporarily postponing the initiation of radiation therapy when necessary. In some instances, given the tumor size and the potential for microscopic residual, radiation may be inevitable. In these cases, the combination of minimal tumor burden with decompression of critical adjacent structures will optimize the radiation response. These giant pituitary adenomas are not in the same prognostic category as smaller tumors, however, with aggressive initial management, excellent long-term outcomes can be appreciated.
Over the past 12 years, a total of 9 procedures using the combined above and below approach have been performed at our institution. In all cases, the decision to implement the combined approach was due to complex tumor characteristics demonstrated on preoperative imaging. In 8 of these cases, the tumor was preoperatively classified as Hardy configuration D or E. In 1 case classified as Hardy configuration C, an intact diaphragm sellae would have inhibited adequate resection of the compressive suprasellar component of this dumbbell-shaped lesion via a traditional transsphenoidal approach alone.
The complication rate in our series is comparable to those of other series describing transsphenoidal resection of large pituitary adenomas [4, 6, 8, 9, 13, 26–29]. Postoperative complications tend to be transient and included DI, SIADH, and CSF rhinorrhea. More importantly, despite open communication between the nasal flora and intradural space, no intracranial infections were encountered in this series. Furthermore, there were no postoperative hemorrhages or complications related to brain retraction such as venous occlusion or infarction. The only complication attributable to the transcranial portion of this combined approach included one transient third nerve palsy.
Conclusions
Based on this relatively small number of patients with these rare tumors, the simultaneous above and below approach is both safe and effective. The simultaneous addition of the transcranial route to the transsphenoidal approach offers improved visualization of critical neurovascular structures during tumor decompression. This improved exposure allows early visualization and preservation of the pituitary stalk, preventing hypothalamic injury from excessive retraction. When dealing with more firm or adherent tumors, this approach allows the intracranial surgeon to safely dissect the suprasellar portions of the tumor away from the optic apparatus and internal carotid artery preventing excessive retraction on these structures as well. This approach may provide for a more complete surgical resection, potentially decreasing postoperative recurrence rates and the need for adjuvant radiation therapy. Furthermore, by performing the transcranial and transsphenoidal approaches simultaneously, the inherent risks of a second general anesthesia are eliminated.
The small number of patients treated in this series reflects the low incidence of large, asymmetric, pituitary adenomas encountered in a large clinical pituitary tumor practice. Specifically, this is a small subset of patients with giant pituitary tumors that cannot be adequately decompressed with one surgery alone as determined by an experienced pituitary surgeon (J.N·B.) Due to this small study size, the potential advantages this approach might provide over a traditional transsphenoidal resection are speculative. The combined approach does require two separate operative teams and increases the total operative time by about 1 h compared to a single transcranial operation. Although this approach may be seemingly “radical”, the complications encountered in the current series are not unlike those reported in other series in which large, complex pituitary adenomas are resected by other means whereas the excellent immediate and long-term advantages justify the approach.
Contributor Information
Anthony L. D’Ambrosio, Department of Neurological Surgery, New York, Presbyterian Hospital, Columbia University Medical Center, New York, NY, USA
Omar N. Syed, Email: ons2101@columbia.edu, Department of Neurological Surgery, New York, Presbyterian Hospital, Columbia University Medical Center, New York, NY, USA
Bartosz T. Grobelny, Department of Neurological Surgery, New York, Presbyterian Hospital, Columbia University Medical Center, New York, NY, USA
Pamela U. Freda, Department of Medicine – Endocrinology, New York, Presbyterian Hospital, Columbia University Medical Center, New York, NY, USA
Sharon Wardlaw, Department of Medicine – Endocrinology, New York, Presbyterian Hospital, Columbia University Medical Center, New York, NY, USA.
Jeffrey N. Bruce, Department of Neurological Surgery, New York, Presbyterian Hospital, Columbia University Medical Center, New York, NY, USA
References
- 1.Hardy J. Transsphenoidal hypophysectomy. J Neurosurg. 1971;34:582–594. doi: 10.3171/jns.1971.34.4.0582. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Vezina JL. Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol. 1976;15:261–273. [PubMed] [Google Scholar]
- 3.Hardy J, Wigser SM. Transsphenoidal surgery of pituitary fossa tumors with televised radiofluoroscopic control. J Neurosurg. 1965;23:612–619. doi: 10.3171/jns.1965.23.6.0612. [DOI] [PubMed] [Google Scholar]
- 4.Alleyne CH, Jr, Barrow DL, Oyesiku NM. Combined transsphenoidal and pterional craniotomy approach to giant pituitary tumors. Surg Neurol. 2002;57:380–390. doi: 10.1016/S0090-3019(02) 00705-X (discussion 390). [DOI] [PubMed] [Google Scholar]
- 5.Barrow DL, Tindall GT. Combined simultaneous transsphenoidal transcranial operative approach to selected sellar tumors. Perspect Neurol Surg. 1992;3:49. [Google Scholar]
- 6.Loyo M, Kleriga E, Mateos H, de Leo R, Delgado A. Combined supra-infrasellar approach for large pituitary tumors. Neurosurgery. 1984;14:485–488. doi: 10.1097/00006123-198404000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Kato T, Sawamura Y, Abe H, Nagashima M. Transsphenoidal-transtuberculum sellae approach for supradiaphragmatic tumours: technical note. Acta Neurochir (Wien) 1998;140:715–718. doi: 10.1007/s007010050167 (discussion 719). [DOI] [PubMed] [Google Scholar]
- 8.Saito K, Kuwayama A, Yamamoto N, Sugita K. The transsphenoidal removal of nonfunctioning pituitary adenomas with suprasellar extensions: the open sella method and intentionally staged operation. Neurosurgery. 1995;36:668–675. doi: 10.1097/000061 23-199504000-00005 (discussion 675–666). [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Fei Z, Zhang J, et al. Management of nonfunctioning pituitary adenomas with suprasellar extensions by transsphenoidal microsurgery. Surg Neurol. 1999;52:380–385. doi: 10.1016/S0090-3019 (99)00120-2. [DOI] [PubMed] [Google Scholar]
- 10.Burian KG, Pendl G, Salah S. The recurrence of pituitary adenoma after transfrontal, transsphenoidal or two stage combined operations. Wien Med Wochenschr. 1970;120:833–838. [PubMed] [Google Scholar]
- 11.Bynke O, Hillman J. Role of transsphenoidal operation in the management of pituitary adenomas with suprasellar extension. Acta Neurochir (Wien) 1989;100:50–55. doi: 10.1007/BF01405274. [DOI] [PubMed] [Google Scholar]
- 12.Patterson RH. The role of transcranial surgery in the management of pituitary adenoma. Acta Neurochir Suppl (Wien) 1996;65:16–17. doi: 10.1007/978-3-7091-9450-8_6. [DOI] [PubMed] [Google Scholar]
- 13.Takakura K, Teramoto A. Management of huge pituitary adenomas. Acta Neurochir Suppl (Wien) 1996;65:13–15. doi: 10.1007/978-3-7091-9450-8_5. [DOI] [PubMed] [Google Scholar]
- 14.Mortini P, Barzaghi R, Losa M, Boari N, Giovanelli M. Surgical treatment of giant pituitary adenomas: strategies and results in a series of 95 consecutive patients. Neurosurgery. 2007;60:993–1002. doi: 10.1227/01.NEU.0000255459.14764.BA (discussion 1003–1004). [DOI] [PubMed] [Google Scholar]
- 15.Wilson CB. A decade of pituitary microsurgery. The Herbert Olivecrona lecture. J Neurosurg. 1984;61:814–833. doi: 10.3171/jns.1984.61.5.0814. [DOI] [PubMed] [Google Scholar]
- 16.Liu JK, Weiss MH, Couldwell WT. Surgical approaches to pituitary tumors. Neurosurg Clin N Am. 2003;14:93–107. doi: 10.1016/ S1042-3680(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 17.Day JD. Surgical approaches to suprasellar and parasellar tumors. Neurosurg Clin N Am. 2003;14:109–122. doi: 10.1016/S1042-3680(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 18.Ausiello JC, Bruce JN, Freda PU. Postoperative assessment of the patient after transsphenoidal pituitary surgery. Pituitary. 2008;11(4):391–401. doi: 10.1007/s11102-008-0086-6. [DOI] [PubMed] [Google Scholar]
- 19.Lanzino G, Laws ER., Jr Key personalities in the development and popularization of the transsphenoidal approach to pituitary tumors: an historical overview. Neurosurg Clin N Am. 2003;14:1–10. doi: 10.1016/S1042-3680(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 20.Liu JK, Das K, Weiss MH, Laws ER, Jr, Couldwell WT. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083–1096. doi: 10.3171/jns.2001.95.6.1083. [DOI] [PubMed] [Google Scholar]
- 21.Barrow DL, Tindall GT. Loss of vision after transsphenoidal surgery. Neurosurgery. 1990;27:60–68. doi: 10.1097/00006123-19900 7000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Ebersold MJ, Quast LM, Laws ER, Jr, Scheithauer B, Randall RV. Long-term results in transsphenoidal removal of nonfunctioning pituitary adenomas. J Neurosurg. 1986;64:713–719. doi: 10.3171/jns.1986.64.5.0713. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto N, Handa H, Yamagami T. Transsphenoidal extracapsular approach to pituitary tumors. J Neurosurg. 1986;64:16–20. doi: 10.3171/jns.1986.64.1.0016. [DOI] [PubMed] [Google Scholar]
- 24.Spaziante R, de Divitiis E. Forced subarachnoid air in transsphenoidal excision of pituitary tumors (pumping technique) J Neurosurg. 1989;71:864–867. doi: 10.3171/jns.1989.71.6.0864. [DOI] [PubMed] [Google Scholar]
- 25.Kouri JG, Chen MY, Watson JC, Oldfield EH. Resection of suprasellar tumors by using a modified transsphenoidal approach Report of four cases. J Neurosurg. 2000;92:1028–1035. doi: 10.3171/jns.2000.92.6.1028. [DOI] [PubMed] [Google Scholar]
- 26.Black PM, Zervas NT, Candia G. Management of large pituitary adenomas by transsphenoidal surgery. Surg Neurol. 1988;29:443–447. doi: 10.1016/0090-3019(88)90138-3. [DOI] [PubMed] [Google Scholar]
- 27.Guidetti B, Fraioli B, Cantore GP. Results of surgical management of 319 pituitary adenomas. Acta Neurochir (Wien) 1987;85:117–124. doi: 10.1007/BF01456107. [DOI] [PubMed] [Google Scholar]
- 28.Kaptain GJ, Vincent DA, Sheehan JP, Laws ER., Jr Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2001;49:94–100. doi: 10.1097/00006123-200107000-00014 (discussion 100–101). [DOI] [PubMed] [Google Scholar]
- 29.Symon L, Jakubowski J, Kendall B. Surgical treatment of giant pituitary adenomas. J Neurol Neurosurg Psychiatry. 1979;42:973–982. doi: 10.1136/jnnp.42.11.973. [DOI] [PMC free article] [PubMed] [Google Scholar]