Abstract
Hyperhomocysteinemia confers a high risk for thrombotic vascular events, but homocysteine-lowering therapies have been ineffective in reducing the incidence of secondary vascular outcomes, raising questions regarding the role of homocysteine as a mediator of cardiovascular disease. Therefore, to determine the contribution of elevated homocysteine to thrombosis susceptibility, we studied Cbs−/− mice conditionally expressing a zinc-inducible mutated human CBS (I278T) transgene. Tg-I278T Cbs−/− mice exhibited severe hyperhomocysteinemia and endothelial dysfunction in cerebral arterioles. Surprisingly, however, these mice did not display increased susceptibility to arterial or venous thrombosis as measured by photochemical injury in the carotid artery, chemical injury in the carotid artery or mesenteric arterioles, or ligation of the inferior vena cava. A survey of hemostatic and hemodynamic parameters revealed no detectible differences between control and Tg-I278T Cbs−/− mice. Our data demonstrate that severe elevation in homocysteine leads to the development of vascular endothelial dysfunction but is not sufficient to promote thrombosis. These findings may provide insights into the failure of homocysteine-lowering trials in secondary prevention from thrombotic vascular events.
Introduction
Hyperhomocysteinemia is defined as an elevation of plasma total homocysteine (tHcy)1 and is an independent risk factor for arterial and venous vascular diseases.2–4 The classic form of severe hyperhomocysteinemia arises from a rare homozygous deficiency in cystathionine β-synthase (CBS) which metabolizes homocysteine to cystathionine. Patients with severe hyperhomocysteinemia have plasma tHcy levels > 200μM and, if untreated, ∼ 50% suffer from life-threatening thromboembolic events before the age of 30.5 Treatment with B vitamins and betaine in conjunction with restriction of methionine improves vascular outcomes,6 though in most patients this intervention only partially lowers tHcy levels. Therefore, the causal relationship between elevated tHcy and vascular events remains poorly defined.
Mild to moderate hyperhomocysteinemia, with plasma tHcy levels of 15-50μM, occurs more commonly than severe hyperhomocysteinemia and is also associated with elevated risk for myocardial infarction, stroke, and thrombosis.2–4 Despite this established clinical association, treatments to lower tHcy with B vitamins paradoxically fail to decrease secondary vascular events in these patients.7–12 This lack of benefit of homocysteine-lowering therapy is in contrast to patients with severe hyperhomocysteinemia and raises further questions regarding whether homocysteine is a mediator or biomarker of vascular disease.
Both genetic and dietary animal models of hyperhomocysteinemia have been used to examine the vascular effects of elevated tHcy levels. Mouse models with genetic deficiency in CBS have been demonstrated to have endothelial dysfunction.13–15 With dietary induction of hyperhomocysteinemia, mice develop endothelial dysfunction16–22 as well as enhanced responses to vascular injury, such as thrombosis23,24 and neointima formation,25 though in these models it is not clear whether the vascular effects are due to elevated tHcy or other dietary factors. To more directly address the relationship between elevated homocysteine and vascular abnormalities, herein we used a novel genetic mouse model of severe hyperhomocysteinemia.26 This model of CBS deficiency is homozygous for a targeted deletion in the murine Cbs gene and conditionally expresses a mutant human CBS transgene I278T26 which is the most common mutation found in patients with severe hyperhomocysteinemia.5 Using this model, we analyzed the effects of severe hyperhomocysteinemia due to elevations in tHcy on endothelial function and thrombosis. We demonstrate that, as expected, genetic deficiency of murine Cbs produced severe hyperhomocysteinemia and endothelial dysfunction. Paradoxically, however, Cbs deficiency failed to increase susceptibility to arterial or venous thrombosis. Our data suggest that loss of CBS alone is not sufficient to produce the advanced thrombotic vascular events observed in patients with severe hyperhomocysteinemia.
Methods
Mice
Mice homozygous deficient for murine Cbs but expressing a zinc-inducible mutant human CBS transgene (Tg-I278T Cbs−/−)26 were initially bred with mice heterozygous deficient in murine Cbs (Cbs+/−) to obtain Tg-I278T Cbs+/− mice. We then crossed Cbs+/− mice with Tg-I278T Cbs+/− mice to obtain wild-type Cbs+/+, Tg-I278T Cbs+/+, and Tg-I278T Cbs−/− littermates for study. Both Tg-I278T Cbs−/− and Cbs+/− mice were bred for more than 7 generations with C57BL6/J mice before crossbreeding with each other. As previously described,26 all breeders received 25mM zinc sulfate-supplemented drinking water to allow the expression of the human I278T CBS transgene, which rescues Cbs−/− pups from growth retardation and early lethality. After weaning, mice were fed standard mouse chow (LM485; Harlan Teklad) and drinking water unsupplemented with zinc. Genotyping for the targeted murine Cbs23 and for human I278T CBS27 transgenes was performed as previously described. Animal protocols were approved by the University of Iowa Animal Care and Use Committee. Both male and female mice were included in the study.
Plasma tHcy and methionine
Blood was collected by cardiac puncture into EDTA (final concentration 5mM), and plasma was flash frozen. Plasma tHcy and methionine were measured using a Biochrom 30 amino-acid analyzer as previously described.28 In brief, 50 μL of plasma was treated with 5 μL of 12% DTT for 5 minutes at room temperature followed by the addition of 55 μL of 10% sulfosalicylic acid. After incubation for 1 hour at 4°C, samples were centrifuged and the supernatant was analyzed.
Dilatation responses in cerebral arterioles
Dilatation of cerebral arterioles was measured as described previously.16 Briefly, mice were anesthetized with sodium pentobarbital (70-90 mg/kg IP) and ventilated mechanically with room air and supplemental oxygen. A cranial window was made over the left parietal cortex, and a segment of a randomly selected pial arteriole (∼ 30 μm in diameter) was exposed. The diameter of the cerebral arteriole was measured using a video microscope coupled to an image-shearing device, under control conditions and during superfusion with acetylcholine (an endothelium-dependent vasodilator, 10−6 and 10−5 M), and nitroprusside (an endothelium-independent vasodilator, 10−7 and 10−6 M).
Blood pressure and vessel size
Measurement of systemic arterial blood pressure has been described previously29; briefly, a catheter was inserted into the femoral artery of mice anesthetized with sodium pentobarbital and ventilated mechanically. To measure the size of the carotid artery, the heart and carotid artery were perfusion fixed with 4% paraformaldehyde in anesthetized mice after withdrawing blood from the heart. The left common carotid artery was harvested in formalin and embedded in paraffin for quantitative histologic analysis. Up to 5 serial sections (5 μm in thickness, every 100 μm) were collected below the bifurcation and stained with Van Giessen. Sections were imaged and the cross-sectional area of vessel wall and luminal area were measured using National Institutes of Health ImageJ software.
In vivo thrombosis assays
Carotid artery thrombosis.
Carotid artery thrombosis was induced by photochemical injury30 or chemical injury31 as described previously. Mice were anesthetized with sodium pentobarbital and ventilated mechanically. The right common carotid artery was dissected free and carotid artery blood flow was measured with a 0.5 PSB Doppler flow probe (Transonic Systems Inc) and digital recording system (Gould Ponemah Physiology Platform Version 3.33). To induce photochemical injury to the endothelial layer, the right common carotid artery was transilluminated continuously with a 1.5-mV, 540-nm green laser (Melles Griot) from a distance of 6 cm, and rose bengal (35 mg/kg) was injected via a femoral vein catheter. Blood flow was monitored continuously for 90 minutes or until stable occlusion occurred, at which time the experiment was terminated. First occlusion was defined as the time at which blood flow first decreased to 0 for ≥ 10 seconds, and stable occlusion was defined as the time at which blood flow remained absent for ≥ 10 minutes. To induce chemical injury, a 1 mm × 1 mm Whatman no. 1 filter paper soaked in 10% FeCl3 was applied to the right common carotid artery for 3 minutes. Blood flow was monitored continuously for 30 minutes or until stable occlusion occurred, at which time the experiment was terminated.
Mesenteric arteriole thrombosis.
Susceptibility to thrombosis in mesenteric arterioles was measured in 3- to 4-week-old mice using intravital microscopy as described previously.32 Briefly, blood was collected from the retro-orbital venous plexus of 4- to 6-month-old C57Bl6J donor mice into a polypropylene tube containing enoxaparin. Platelets were isolated, washed, and fluorescently labeled as previously described.32 The study mice were anesthetized with avertin, and labeled platelets were infused (2.5 × 109 platelets/kg) through retro orbital plexus. Mesenteric arterioles between 80-100μM in diameter were studied. A 300 × 300μM Whatman no. 1 filter paper saturated with 5% FeCl3 solution was applied topically for 2 minutes. Subsequently, vessels were monitored for 40 minutes after injury or until stable occlusion.
IVC thrombosis.
Susceptibility to thrombosis in the venous system was measured by a blood stasis model as described previously33 with minor modifications. Briefly, mice were anesthetized using ketamine/xylazine (87.5 mg/kg ketamine and 12.5 mg/kg xylazine, IP). A midline laparotomy was made, and the inferior vena cava (IVC) was exposed directly via blunt dissection. The IVC was ligated inferior to the left renal vein using a 6.0-silk suture. The incision was closed in a 2-layer fashion. Two days later, blood was collected directly from the heart into sodium citrate (9:1, v/v) for measurement of plasma tHcy, and the IVC was harvested for measurement of length and weight of thrombus.
Bleeding time, complete blood cell counting, and clotting factor assays
Bleeding time.
Ten-week-old mice were briefly anesthetized with 75% CO2/ 25% O2. A 4-mm long tail tip was immediately sliced off with a scalpel blade and the wound immersed in a 45-mL conical tube containing saline at 37°C. Throughout the entire procedure, the mice were restrained in a mouse holder positioned just above the waterbath. Blood flow from the wound was monitored for 15 minutes. All cessations and resumptions of bleeding were recorded. Any animal still bleeding after 15 minutes was treated with silver nitrate to stop further bleeding. Bleeding time was defined as time from start of the bleeding until the complete cessation of bleeding.
Complete blood cell counting.
Blood was collected by cardiac puncture into EDTA and analyzed on a Hemavet Multispecies Hematology System (Drew Scientific).
Clotting factor assays.
Blood was collected in ACD buffer (9:1, v/v). Plasma was flash frozen and stored at −80°C. A modified dilute activated plasma prothrombin time (APTT) was performed using a BBL fibrometer (BD Diagnostics). Bovine fibrinogen was added to Tris/BSA buffer to a final concentration of 2 mg/mL. Citrated mouse plasma (15 μL) was incubated at 37°C with 60 μL of the fibrinogen mix, plus 50 μL of Dade Actin FSL APTT Reagent. After 3 minutes, clotting was initiated with the addition of 50 μL of 60mM CaCl2. All samples were tested in triplicate. An in-house pool of normal mouse plasma was used for comparison. Fibrinogen levels were determined by AssayMax Mouse Fibrinogen ELISA (Assaypro), which uses a polyclonal Ab specific for mouse fibrinogen.
Statistical analysis
One-way ANOVA with the Tukey test for multiple comparisons was used to analyze cerebral arteriole dilatation response and occlusion times in different groups. The Student t test was used to analyze plasma tHcy, methionine, bleeding time, fibrinogen, APTT, and complete blood cell counting (CBC). Statistical significance was defined as a P value < .05. Values are reported as mean ± SE.
Results
Tg-I278T Cbs−/− mice develop severe hyperhomocysteinemia
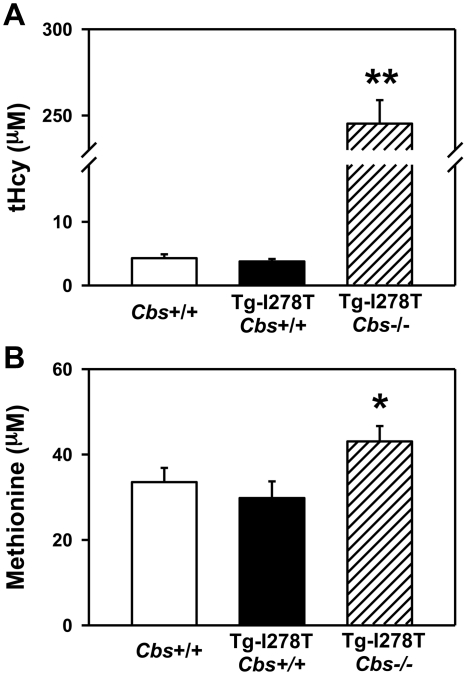
Consistent with the previous study,26 the body weight of Tg-I278T Cbs−/− mice was ∼ 20% less than age-matched Tg-I278T Cbs+/+ or Cbs+/+ mice (P < .05, Table 1). At 20 weeks of age, plasma tHcy levels were similar in Cbs+/+ and Tg-I278T Cbs+/+ mice (4.3 ± 0.6μM and 3.8 ± 0.4μM, respectively, P = .5, Figure 1A). Tg-I278T Cbs−/− mice had severe hyperhomocysteinemia (plasma tHcy: 245 ± 14μM), and tHcy levels were significantly higher than in Cbs+/+ or Tg-I278T Cbs+/+ mice (P < .001, Figure 1A). The tHcy levels in Tg-I278T Cbs−/− mice were similar to those in patients with homozygous CBS deficiency5 and thus validate these mice as a pathophysiologically relevant genetic model of the human disease. Plasma methionine levels were modestly elevated in Tg-I278T Cbs−/− mice (43 ± 3.6μM) compared with Tg-I278T Cbs+/+ mice (30 ± 3.9μM, P < .05, Figure 1B).
Table 1.
Body weight and hemodynamic parameters
| Genotype | Cbs+/+ | Tg-I278T Cbs+/+ | Tg-I278T Cbs−/− |
|---|---|---|---|
| Body weight, g | 28.5 ± 1.8 | 28.2 ± 1.5 | 23.07 ± 0.8* |
| Mean systemic arterial blood pressure | 71.8 ± 2.8 | 71.9 ± 2.5 | 66.1 ± 2.3 |
| Carotid artery | |||
| Baseline blood flow, mL/min | 0.35 ± .05 | 0.40 ± .05 | 0.45 ± .05 |
| Total vessel area, μm2 | 105 382 ± 6149 | 104 821 ± 22 057 | 128 390 ± 1212 |
| Luminal area, μm2 | 40 300 ± 12 180 | 35 396 ± 11 468 | 48 570 ± 3523 |
P < .05 compared with Cbs+/+ and Tg-I278T Cbs+/+ mice (n = 6-14 in each group).
Figure 1.
Plasma tHcy levels are elevated with CBS deficiency. Plasma levels of (A) tHcy and (B) methionine were measured in 20-week-old mice (n = 20-30 mice per group). Values are mean ± SEM; **P < .001 compared with Cbs+/+ and Tg-I278T Cbs+/+ mice and *P < .05 compared with Tg-I278T Cbs+/+ mice.
Tg-I278T Cbs−/− mice exhibit endothelial dysfunction
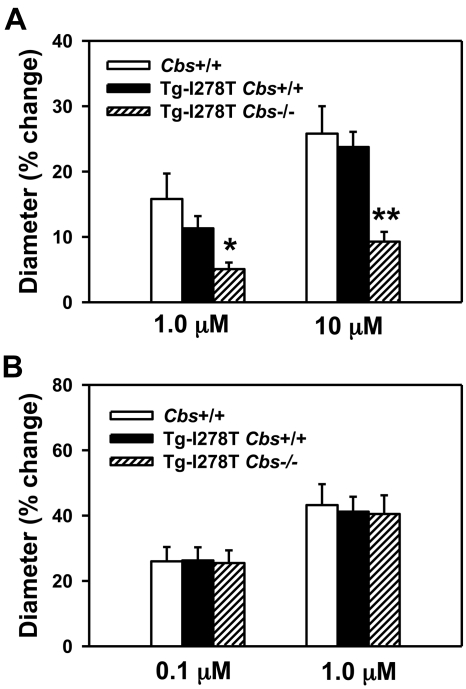
To confirm that endothelial function is impaired in Tg-I278T Cbs−/− mice,14 we measured dilator responses to acetylcholine in cerebral arterioles, which have been consistently found to be sensitive to hyperhomocysteinemia in other murine models.34 Acetylcholine produced dose-dependent dilatation of cerebral arterioles in all 3 groups of mice (Figure 2A). The dilatation responses to both doses of acetylcholine were similar in Cbs+/+ and Tg-I278T Cbs+/+ mice, but were significantly decreased in Tg-I278T Cbs−/− mice (P < .05 with 1μM acetylcholine, and P < .001 with 10μM acetylcholine). Similar to acetylcholine, nitroprusside also produced dose-dependent dilatation of cerebral arterioles in all 3 groups (Figure 2B). However, unlike acetylcholine, no differences in dilatation response were observed between the 3 groups, which confirms that the decreased acetylcholine dilatation response in Tg-I278T Cbs−/− mice is endothelium-dependent.
Figure 2.
CBS deficiency promotes endothelial dysfunction. Dilatation of cerebral arterioles to (A) acetylcholine or (B) nitroprusside was measured in 20-week-old mice (n = 10-16 mice per group). Values are mean ± SEM; *P < .05, and **P < .001 compared with Cbs+/+ and Tg-I278T Cbs+/+ mice at the same dose of acetylcholine.
CBS deficiency does not produce a prothrombotic phenotype
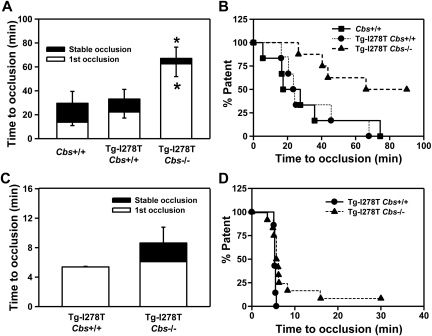
Next, we examined whether CBS deficiency influences the susceptibility to thrombosis. Experimental thrombosis of the carotid artery was first induced by photochemical injury. As expected, the times to stable occlusion were similar between Cbs+/+ and Tg-I278T Cbs+/+ mice (30 ± 9.9 minutes and 33 ± 8.1 minutes, respectively, P = .9, Figure 3A-B). Surprisingly, the time to form stable occlusions was not shortened, but was instead significantly longer, in the Tg-I278T Cbs−/− mice (67 ± 9.5 minutes) compared with Cbs+/+ or Tg-I278T Cbs+/+ mice (P < .05). To confirm this paradoxical finding, we used an alternative method to induce thrombosis in the common carotid artery using 10% FeCl3. After exposure to FeCl3, the first occlusion formed in 5.4 ± 0.07 minutes in Tg-I278T Cbs+/+ mice and remained stable in every mouse in that group (Figure 3C-D). Similar to the photochemical injury studies, the times to occlusion were not shortened in Tg-I278T Cbs−/− mice, and a trend toward prolonged stable occlusion time was detected in mice compared with Tg-I278T Cbs+/+ mice (8.6 ± 2.1 minutes, P = .2).
Figure 3.
CBS deficiency fails to increase susceptibility to carotid artery thrombosis. Time to first occlusion or stable occlusion in the carotid artery after (A) photochemical injury or (C) chemical injury was measured in 20-week-old mice. (B,D) The percentage of mice with a patent carotid artery (ie, free of stable occlusion) as a function of time after photochemical or chemical injury, respectively. Seven to 12 mice were studied in each group. Values are mean ± SE; *P < .05 compared with Cbs+/+ or Tg-I278T Cbs+/+ mice.
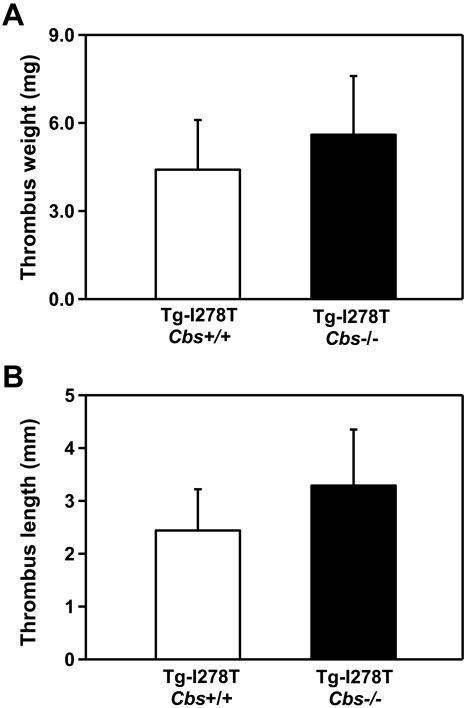
Given the unexpected finding that CBS deficiency did not shorten the time to arterial occlusion, we next studied susceptibility to venous thrombosis to determine whether elevated tHcy has vascular site-specific effects. Using an IVC stasis method, we measured the length and weight of the thrombi that developed 48 hours after IVC ligation. No significant differences in either the length (P = .7) or weight of the thrombi (P = .6) were detected between the Tg-I278T Cbs+/+ and Tg-I278T Cbs−/− mice (Figure 4), which implies that CBS deficiency alone is not sufficient to increase susceptibility to arterial or venous thrombosis in mice.
Figure 4.
Susceptibility to venous thrombosis is not increased in Tg-I278T Cbs−/− mice. The (A) weight and (B) length of thrombus developed in the IVC after 48 hours of ligation was measured in 20-week-old mice (n = 10-11 mice per group). Values are mean ± SE.
CBS deficiency does not alter susceptibility to thrombosis in young mice with pronounced hypermethionenemia
One difference between human patients with CBS deficiency and the Tg-I278T Cbs−/− mouse model is that CBS-deficient patients have marked hypermethioninemia in addition to severe hyperhomocysteinemia, whereas adult Tg-I278T Cbs−/− mice have only a very modest elevation of plasma methionine at 20 weeks of age (Figure 1B). We therefore hypothesized that methionine may contribute to the increased risk of thrombosis in the setting of hyperhomocysteinemia. To attempt to examine the combined effect of high tHcy and high methionine on thrombotic susceptibility, we fed Tg-I278T Cbs−/− mice a range of methionine-rich diets containing 1.2%-2.0% methionine. We found, however, that all of the Tg-I278T Cbs−/− mice fed these diets died within 24-48 hours (data not shown), precluding studies of thrombosis.
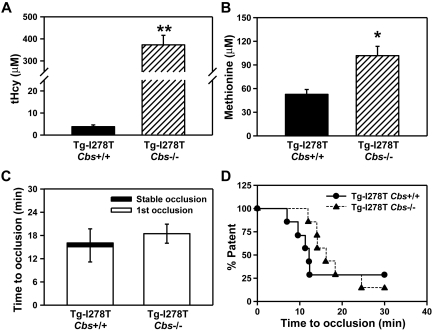
As an alternative approach, we measured plasma levels of tHcy and methionine in young Tg-I278T Cbs−/− mice fed a normal chow diet. At 3-4 weeks of age, Tg-I278T Cbs−/− mice not only had severely elevated plasma tHcy (373 ± 43μM vs 3.8 ± 0.8μM in age-matched Tg-I278T Cbs+/+ mice, P < .0001, Figure 5A) but also a pronounced, 2-fold elevation of plasma methionine (102 ± 12 μM vs 53 ± 6.2 μM, P < .01, Figure 5B). Because of the young age of the mice, we were not able to perform carotid artery injury experiments but instead used intravital microscopy to measure thrombus formation in mesenteric arterioles after injury with 5% FeCl3. The results show that Tg-I278T Cbs−/− mice failed to demonstrate a shortening of the time to thrombotic occlusion of mesenteric arterioles compared with Tg-I278T Cbs+/+ mice (18.5 ± 2.5 minutes vs 16.1 ± 3.7 minutes, respectively, P = .85, Figure 5C-D). These data demonstrate that CBS deficiency in mice fails to increase thrombotic susceptibility in microvessels, even in the presence of pronounced hypermethioninemia.
Figure 5.
Elevated methionine in young Tg-I278T Cbs−/− mice does not alter risk for microvessel thrombosis. Plasma levels of (A) tHcy and (B) methionine were measured in 3-week-old mice. (C) The time to first occlusion or stable occlusion was examined in mesenteric arterioles after chemical injury. (D) The percentage of mice with a patent mesenteric arteriole as a function of time after chemical injury. Seven mice were studied in each group. Values are mean ± SE; **P < .001 and *P < .01 compared with Tg-I278T Cbs+/+ mice.
Baseline hemodynamic parameters, vessel size, and hematologic profile remain unchanged with CBS deficiency
We also examined several other parameters that might contribute to the failure of CBS deficiency to promote a prothrombotic phenotype. First, the arterial blood pressure in anesthetized mice was monitored through the cannulated femoral artery, and we observed similar mean arterial pressures in Cbs+/+, Tg-I278T Cbs+/+, and Tg-I278T Cbs−/− mice (P = .19, Table 1). In addition, there were no significant differences between groups in baseline blood flow through the common carotid artery (P = .5). Next, in perfusion fixed mice, the noninjured left common carotid artery was harvested and the cross-sectional area of the whole vessel and lumen measured. The luminal area (P = .4) and total vessel area (P = .3) were similar in all groups (Table 1).
Finally, we evaluated the effects of CBS deficiency on the hematologic profile (Table 2). Hemoglobin concentration and hematocrit were similar in all 3 groups of mice. A small but significant increase in platelet count was observed in Tg-I278T Cbs−/− mice compared with Cbs+/+ mice (P < .05) but not compared with Tg-I278T Cbs+/+ mice (P = .13). APTT and plasma fibrinogen levels were similar in all groups of mice (P = .42, and P = .28, respectively). Tail bleeding time was also similar in all groups (P = .7).
Table 2.
Hematological profile
| Genotype | Cbs+/+ | Tg-I278T Cbs+/+ | Tg-I278T Cbs−/− |
|---|---|---|---|
| Hemoglobin, g/L | 12.2 ± 0.4 | 10.4 ± 0.5 | 11.6 ± 0.5 |
| Hematocrit, % | 41.3 ± 2.2 | 40.7 ± 1.6 | 44.2 ± 1.1 |
| Platelet count, ×1000/μL | 720.5 ± 40 | 786 ± 46 | 923.1 ± 67* |
| Bleeding time, min | 9.3 ± 1.1 | 9.1 ± 0.4 | 9.1 ± 0.5 |
| APTT, min | ND | 65.6 ± 6.7 | 79.6 ± 17.2 |
| Fibrinogen, mg/dL | ND | 171 ± 14 | 148 ± 15 |
P < .05 compared with Cbs+/+ mice (n = 6-12 mice in each group).
Discussion
Hyperhomocysteinemia is an established risk factor for myocardial infarction, stroke, and thrombosis.2–4 However, it is unclear whether elevated tHcy is a mediator or a biomarker of vascular disease.35 In the present study, we used a recently established murine model of CBS deficiency to address the significance of elevated homocysteine in vascular thrombosis. A key feature of the model was the absence of dietary interventions to influence plasma tHcy because dietary factors may have confounded prior studies of the association between hyperhomocysteinemia and thrombosis in mouse models and human subjects. The novel finding in this study is that, unlike patients with CBS deficiency, Tg-I278T Cbs−/− mice did not exhibit increased susceptibility to arterial or venous thrombosis, though as expected they did develop endothelial dysfunction. The failure of elevated tHcy to accelerate thrombosis in this model may provide insights into the failure of homocysteine-lowering therapy to prevent secondary thrombotic events in most patients with hyperhomocysteinemia.
Several observations suggest that there is a disconnect between elevated tHcy levels and thrombotic events. First, in homocystinuric patients with severe hyperhomocysteinemia, treatments to lower tHcy reduce the risk for secondary thrombotic events despite failing to completely normalize tHcy levels.6 The residual levels of plasma tHcy in “successfully” treated homocystinuric patients often remain moderately elevated to a level (30-80μM) that still might be expected to be associated with a high risk of thrombosis based on data in nonhomocystinuric patients. It is quite striking that homocystinuric patients have such a marked improvement in thrombotic complications despite having this degree of residual hyperhomocysteinemia. This observation raises a question of whether the beneficial effects of these treatments in lowering thrombotic risk can be attributed to the partial reduction in tHcy or to other factors that may be independent of plasma tHcy levels. Second, in subjects with mild to moderate hyperhomocysteinemia, treatment with B vitamins does not appear to reduce secondary risks for myocardial infarction, stroke, or venous thromboembolism despite lowering tHcy to normal levels,7–12 which raises further questions regarding whether elevated tHcy is a direct mediator of vascular events. Finally, although diet-induced hyperhomocysteinemia in mouse models is associated with an increased susceptibility to experimental thrombosis, it is unclear whether this prothrombotic phenotype is caused by elevation of homocysteine or other dietary metabolites.23,24 The genetic murine model used in the present study is advantageous because it excludes any secondary effects associated with diet and therefore allowed us to directly examine the contributions of elevated tHcy to thrombosis susceptibility.
Surprisingly, and in contrast to a dietary model of hyperhomocysteinemia,23 Tg-I278T Cbs−/− mice did not exhibit accelerated thrombosis after photochemical injury of the carotid artery; rather, these mice paradoxically had prolonged time to occlusion compared with control mice. Given the unexpected lack of accelerated thrombosis in Tg-I278T Cbs−/− mice, we expanded our study to an alternative model of injury. Using chemical injury with FeCl3 in the carotid artery, we confirmed that Tg-I278T Cbs−/− mice do not have increased susceptibility to arterial thrombosis, which suggests that elevated tHcy alone is not sufficient to induce a prothrombotic phenotype. Because epidemiologic data demonstrate a strong clinical association between deep vein thrombosis (DVT) and hyperhomocysteinemia,4 we also examined susceptibility to DVT using an IVC stasis method. Again, we observed no prothrombotic effect; Tg-I278T Cbs−/− mice developed similar sized thrombi as control mice after IVC ligation. Others have attempted to assess thrombotic phenotypes in murine models of hyperhomocysteinemia using indirect assays of hemostasis. For example, some studies have reported that CBS deficiency in mice alters tail bleeding time,36 whereas other studies did not observe this phenotype.37 CBS deficiency has been reported to not alter in vitro hemostatic parameters such as prothrombin time or APTT.28,37 In our study, we did not detect any significant differences in tail bleeding time, APTT, or plasma fibrinogen levels between Tg-I278T Cbs−/− and control mice. Moreover, using more physiologic methods to assess susceptibility to thrombosis, our data strongly suggest that elevation in tHcy is not sufficient to accelerate arterial or venous thrombosis.
One potentially interesting finding from our study is that Tg-I278T Cbs−/− mice not only failed to exhibit accelerated thrombosis, but also had prolonged times to occlusion in the photochemical injury model. In an attempt to understand this paradoxical finding, we examined several baseline hemostatic parameters. In Tg-I278T Cbs−/− mice, we detected no differences in CBC except for a small increase in platelet counts compared with control mice, a difference that would not be expected to protect from thrombosis. Because Tg-I278T Cbs−/− mice can develop hepatic steatosis,26 we considered the possibility that these mice may have defects in the synthesis of clotting factors by the liver that may mask a prothrombotic phenotype. Consistent with previous studies,28,37 however, we observed no changes in APTT with CBS deficiency, nor did we detect altered plasma levels of fibrinogen. Next, because Tg-I278T Cbs−/− mice are 20% smaller than control mice, it is possible that they have smaller (in diameter) carotid arteries with increased blood flow velocity, potentially causing more frequent embolization and/or delayed thrombosis.30 However, the carotid artery luminal area and blood flow were similar in Tg-I278T Cbs−/− mice and control mice (Table 1). Collectively, these results demonstrate that alterations in hemostatic or hemodynamic parameters are unlikely to account for the failure of CBS deficiency to produce a prothrombotic phenotype.
The fact that our data in mice with severe hyperhomocysteinemia do not parallel the prothrombotic phenotype observed in patients with similarly elevated levels of tHcy5 implies that other factors besides plasma tHcy contribute to the prothrombotic phenotype of hyperhomocysteinemia in humans. It also is possible that interactions between hyperhomocysteinemia and certain prothrombotic factors may differ between mice and humans. One such candidate prothrombotic factor is methionine because CBS-deficient patients develop more profound hypermethioninemia than we observed in Tg-I278T Cbs−/− mice.5 In addition, most of the dietary models of hyperhomocysteinemia in which a prothrombotic or proatherosclerotic phenotype was observed in mice have used a methionine-enriched diet.23,24,38–40 Collectively, these observations imply that a combination of elevated methionine and elevated tHcy might be necessary to increase susceptibility to thrombosis. To address this hypothesis, we examined very young (3-4 weeks old) Tg-I278T Cbs−/− mice which have a pronounced (2-fold) increase in plasma methionine levels along with severe elevations in plasma tHcy compared with age-matched control mice. Using a microvascular thrombosis model, we found that these young mice with combined hypermethioninemia and severe hyperhomocysteinemia still did not exhibit accelerated thrombosis. This finding does not completely exclude the possibility that methionine may be a contributing factor, however, because a higher level of methionine, or a longer duration of exposure to hypermethioninemia, may be necessary to increase susceptibility to thrombosis. When we performed weekly blood draws to measure plasma methionine levels from 3 to 7 weeks of age in Tg-I278T Cbs−/− mice, we found that marked elevation of methionine levels was maintained only until ∼ 4 weeks of age, after which plasma methionine fell to levels similar to those seen in Tg-I278T Cbs−/− at 20 weeks of age (data not shown). These observations indicate that a different mouse model may be needed to understand the interplay between methionine and homocysteine in the pathophysiology of thrombosis.
Despite the absence of a prothrombotic phenotype, Tg-I278T Cbs−/− mice did exhibit endothelial vasomotor dysfunction, an early event in the development of cardiovascular diseases.41 This finding is consistent with other studies using genetic as well as dietary models of hyperhomocysteinemia, in which endothelial dysfunction has been observed in multiple vascular systems, including cerebral and mesenteric arterioles.13–22 Interestingly, the degree of endothelial dysfunction observed in the cerebral arterioles of Tg-I278T Cbs−/− mice with extremely high tHcy levels (> 200μM) in this study was very similar to that seen in other models of mild to moderate16,19,21 hyperhomocysteinemia. Collectively, these findings imply that elevation in tHcy produces a threshold rather than a graded impairment on endothelial function.
A possible limitation of this study is that the examination of endothelial dysfunction and thrombosis was performed in different vascular beds. Impairment of acetylcholine-induced endothelial-dependent dilation is a well-established phenotype in several mouse models of hyperhomocysteinemia, including the Tg-I278TCbs−/− model used in this study.13–15,34 Because the focus of the current manuscript is on thrombosis, we did not repeat measurements of endothelial function in multiple vascular beds. We did, however, assess endothelial function in at least one vascular bed as a positive control experiment to confirm that the model was behaving in the expected manner with respect to the endothelial dysfunction phenotype. For this purpose, we chose to study endothelial function in cerebral arterioles, which we have found to have an extremely reliable and consistent endothelial phenotype in several models of hyperhomocysteinemia.34
In summary, we found that a genetic model of severe hyperhomocysteinemia produces endothelial dysfunction, yet is not sufficient to elicit a prothrombotic phenotype in mice. This unanticipated result implies that elevated levels of homocysteine alone are unlikely to account for the increased thrombotic risk associated with hyperhomocysteinemia. In light of these new findings, the bulk of evidence from murine models now suggests that the prothrombotic effects associated with hyperhomocysteinemia are probably mediated not by elevation of homocysteine itself but instead by other factors. These findings may provide insights into the failure of homocysteine-lowering trials to demonstrate a reduction in secondary cardiovascular events.7–12 Further studies are warranted to define the factors that contribute to development of advanced cardiovascular events in humans with hyperhomocysteinemia, and to determine whether these factors act alone or in combination with elevated tHcy to increase susceptibility to thrombosis.
Acknowledgments
The authors thank Kristina W. Thiel for assistance in manuscript editing, and Dr Sapna Gupta for running plasma samples for tHcy and methionine levels.
This work was supported in part by an American Heart Association Beginning Grant-in-aid (S.D.), and by National Institutes of Health grants HL062984 (F.M.F.), and HL063943 and NS024621 (S.R.L.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.D. conceived of the study, designed and performed a majority of the research, collected, analyzed, and interpreted data, and wrote the manuscript; A.K.C. performed the mesenteric arteriole experiments; M.J. performed the carotid artery thrombosis experiments; L.L. performed the hemostasis assays; C.M.L. performed studies of vasomotor function in cerebral arterioles; F.M.F. provided expertise and reagents for endothelial function measurements; W.D.K. provided mice for the study and assisted with biochemical analyses; and S.R.L. conceived of the study, advised in study design, assisted in data interpretation, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sanjana Dayal, PhD, Department of Internal Medicine, The University of Iowa Carver College of Medicine, 200 Hawkins Dr, 3160 ML, Iowa City, IA 52242; e-mail: sanjana-dayal@uiowa.edu.
References
- 1.Mudd SH, Finkelstein JD, Refsum H, et al. Homocysteine and its disulfide derivatives: a suggested consensus terminology. Arterioscler Thromb Vasc Biol. 2000;20(7):1704–1706. doi: 10.1161/01.atv.20.7.1704. [DOI] [PubMed] [Google Scholar]
- 2.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. J Am Med Assoc. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 3.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. J Am Med Assoc. 1995;274(13):1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 4.den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3(2):292–299. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 5.Mudd SH, Skovby F, Levy HL, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985;37(1):1–31. [PMC free article] [PubMed] [Google Scholar]
- 6.Yap S, Boers GH, Wilcken B, et al. Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol. 2001;21(12):2080–2085. doi: 10.1161/hq1201.100225. [DOI] [PubMed] [Google Scholar]
- 7.Armitage JM, Bowman L, Clarke RJ, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. J Am Med Assoc. 2010;303(24):2486–2494. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 8.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 9.Clarke R, Halsey J, Lewington S, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170(18):1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 10.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 11.Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke. 2010;41(6):1205–1212. doi: 10.1161/STROKEAHA.109.573410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Heijer M, Willems HP, Blom HJ, et al. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: a randomized, placebo-controlled, double-blind trial. Blood. 2007;109(1):139–144. doi: 10.1182/blood-2006-04-014654. [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt RT, Forgione MA, Cap A, et al. Endothelial dysfunction in a murine model of mild hyperhomocyst (e) inemia. J Clin Invest. 2000;106(4):483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Z, Jiang X, Kruger WD, et al. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118(7):1998–2006. doi: 10.1182/blood-2011-01-333310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Yang F, Tan H, et al. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 2005;25(12):2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayal S, Arning E, Bottiglieri T, et al. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke. 2004;35(8):1957–1962. doi: 10.1161/01.STR.0000131749.81508.18. [DOI] [PubMed] [Google Scholar]
- 17.Dayal S, Bottiglieri T, Arning E, et al. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res. 2001;88(11):1203–1209. doi: 10.1161/hh1101.092180. [DOI] [PubMed] [Google Scholar]
- 18.Dayal S, Brown KL, Weydert CJ, et al. Deficiency of glutathione peroxidase-1 sensitizes hyperhomocysteinemic mice to endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2002;22(12):1996–2002. doi: 10.1161/01.atv.0000041629.92741.dc. [DOI] [PubMed] [Google Scholar]
- 19.Dayal S, Devlin AM, McCaw RB, et al. Cerebral vascular dysfunction in methionine synthase-deficient mice. Circulation. 2005;112(5):737–744. doi: 10.1161/CIRCULATIONAHA.104.529248. [DOI] [PubMed] [Google Scholar]
- 20.Dayal S, Rodionov RN, Arning E, et al. Tissue-specific downregulation of dimethylarginine dimethylaminohydrolase in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008;295(2):H816–H825. doi: 10.1152/ajpheart.01348.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devlin AM, Arning E, Bottiglieri T, Faraci FM, Rozen R, Lentz SR. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood. 2004;103(7):2624–2629. doi: 10.1182/blood-2003-09-3078. [DOI] [PubMed] [Google Scholar]
- 22.Lentz SR, Erger RA, Dayal S, et al. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine beta-synthase-deficient mice. Am J Physiol Heart Circ Physiol. 2000;279(3):H970–H975. doi: 10.1152/ajpheart.2000.279.3.H970. [DOI] [PubMed] [Google Scholar]
- 23.Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood. 2006;108(7):2237–2243. doi: 10.1182/blood-2006-02-005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacovina AT, Deora AB, Ling Q, et al. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J Clin Invest. 2009;119(11):3384–3394. doi: 10.1172/JCI39591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan H, Jiang X, Yang F, et al. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovasc Res. 2006;69(1):253–262. doi: 10.1016/j.cardiores.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Chen X, Tang B, Hua X, Klein-Szanto A, Kruger WD. Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet. 2005;14(15):2201–2208. doi: 10.1093/hmg/ddi224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Jhee KH, Hua X, DiBello PM, Jacobsen DW, Kruger WD. Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ Res. 2004;94(10):1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Kuhnisch J, Mustafa A, et al. Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 2009;23(3):883–893. doi: 10.1096/fj.08-120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobey CG, Faraci FM. Effects of a novel inhibitor of guanylyl cyclase on dilator responses of mouse cerebral arterioles. Stroke. 1997;28(4):837–842. doi: 10.1161/01.str.28.4.837. [DOI] [PubMed] [Google Scholar]
- 30.Wilson KM, Lynch CM, Faraci FM, Lentz SR. Effect of mechanical ventilation on carotid artery thrombosis induced by photochemical injury in mice. J Thromb Haemost. 2003;1(12):2669–2674. doi: 10.1111/j.1538-7836.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 31.Fay WP, Parker AC, Ansari MN, Zheng X, Ginsburg D. Vitronectin inhibits the thrombotic response to arterial injury in mice. Blood. 1999;93(6):1825–1830. [PubMed] [Google Scholar]
- 32.Chauhan AK, Motto DG, Lamb CB, et al. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203(3):767–776. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald AP, Meier TR, Hawley AE, et al. Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thromb Res. 2010;125(1):72–78. doi: 10.1016/j.thromres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Dayal S, Lentz SR. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler Thromb Vasc Biol. 2008;28(9):1596–1605. doi: 10.1161/ATVBAHA.108.166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loscalzo J. Homocysteine trials–clear outcomes for complex reasons. N Engl J Med. 2006;354(15):1629–1632. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- 36.Maclean KN, Sikora J, Kozich V, et al. A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment. Mol Genet Metab. 2010;101(2-3):153–162. doi: 10.1016/j.ymgme.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maclean KN, Sikora J, Kozich V, et al. Cystathionine beta-synthase null homocystinuric mice fail to exhibit altered hemostasis or lowering of plasma homocysteine in response to betaine treatment. Mol Genet Metab. 2010;101(2-3):163–171. doi: 10.1016/j.ymgme.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofmann MA, Lalla E, Lu Y, et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107(6):675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci U S A. 2003;100(25):15089–15094. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson KM, McCaw RB, Leo L, et al. Prothrombotic effects of hyperhomocysteinemia and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27(1):233–240. doi: 10.1161/01.ATV.0000251607.96118.af. [DOI] [PubMed] [Google Scholar]
- 41.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110(14):1926–1932. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]