Abstract
To clarify the potential for respiratory transmission of Saffold cardiovirus (SAFV) and characterize the pathogen, we analyzed respiratory specimens from 1,558 pediatric patients in Beijing. We detected SAFV in 7 (0.5%) patients and identified lineages 1–3. However, because 3 patients had co-infections, we could not definitively say SAFV caused disease.
Keywords: Saffold cardiovirus, viruses, pediatric patients, respiratory tract infections, dispatch
Saffold cardiovirus (SAFV) is a new piconavirus, originally identified from fecal samples of a female infant with fever of unknown origin (1). SAFV has since been reported worldwide, and 8 lineages have been identified (1–6). Although serologic surveys have shown that SAFV-3 infection occurs early in life (7), the pathogenicity of SAFV is still unclear.
Because SAFVs are mainly detected in fecal samples, virus transmission is thought to occur by the fecal–oral route (1,3–7). However, 2 research groups also found SAFV-2 lineage in respiratory secretions (2,4). Thus, we investigated whether the respiratory tract route could be an additional transmission route and whether SAFV lineages other than SAFV-2 may also infect the respiratory tract. We identified and characterized 7 SAFV strains, which belonged to 3 distinct lineages, from respiratory samples of children with lower and upper respiratory tract infections (LRTIs and URTIs, respectively).
The Study
We assessed 2 cohorts. Cohort 1 comprised 1,032 children (617 boys and 415 girls) with acute LRTIs, hospitalized in Beijing Children’s Hospital (BCH), from whom nasopharyngeal aspirates were collected from May 2007 through March 2009. The patients ranged in age from 2 weeks to 16 years (mean age 31.3 months, median 9 months). Cohort 2 comprised 506 BCH outpatient children (277 boys and 229 girls) with acute URTIs, from whom throat swabs were collected from May through August 2009. These patients ranged in age from 4 months to 16 years (mean age 46.7 months, median 37 months).
Virus nucleic acids in clinical samples were extracted by using the NucliSens easyMAG system (bioMérieux, Marcy-l’Etoile, France) according to the manufacturer’s instructions. SAFV RNA was detected by nested reverse transcription–PCR (RT-PCR) by using primers selective for the 5′ untranslated region (UTR) (3). The viral protein (VP) 1 gene was amplified by using 3 pairs of primers as previously described (3,5,6). The full genomic sequences were obtained by a genome walking method (7). The 5′ and 3′ UTR sequences were determined by using the RACE System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After being cloned into the pGEM-T Easy vector (Promega, Madison, WI, USA), all PCR products were verified by sequencing. In addition, all screened specimens were tested for known respiratory viruses as previously described (8,9). Mycoplasma pneumoniae was detected by using the gelatin particle agglutination test kit (SERODIA-MYCO II, Fujirebio, Japan).
For phylogenetic analysis, we constructed neighbor-joining trees based on the distances of SAFV nucleotide or amino acid sequences by using MEGA 4.0 (10). We used SimPlot (version 3.5.1) to analyze possible recombination between viral genome sequences (11).
LLC-MK2 cells were used to isolate SAFV as previously described (12). Cells were collected either when cytopathic effects were observed or after 12 days postinoculation, and then they were tested for SAFV by RT-PCR.
We detected SAFV RNA in 4 (0.4%) of the 1,032 nasopharyngeal aspirates from patients with LRTIs and in 3 (0.6%) of the 506 throat swab specimens from outpatients with URTIs. The SAFV-positive patients (4 girls and 3 boys) were 5 months to 9 years of age (Table). SAFV infection did not appear to have a predominant time for occurrence: cases were detected in a range of months for the periods covered (August and December 2007, October and November 2008, and June 2009). All SAFV-positive patients exhibited symptoms of respiratory tract infection, such as coughing, gasping, sneezing, or fever. Of the 4 SAFV-positive patients with LRTIs, 2 had underlying illnesses, i.e., tuberculosis, hepatic dysfunction, or respiratory failure (Table). All SAFV-positive patients recovered within 7–11 days. No major differences were found in disease duration between patients who had underlying diseases and those who did not.
Co-infections with additional respiratory pathogens were detected for 3 of 7 SAFV-positive patients, all in the first cohort. These pathogens were respiratory syncytial virus (1 patient), enterovirus (1 patient), and M. pneumoniae (1 patient) (Table). A SAFV-3 strain was isolated from sample BCH1031. Starting at 5 days postinoculation, cytopathic effects were observed. Virus was not isolated from other SAFV-positive samples (data not shown).
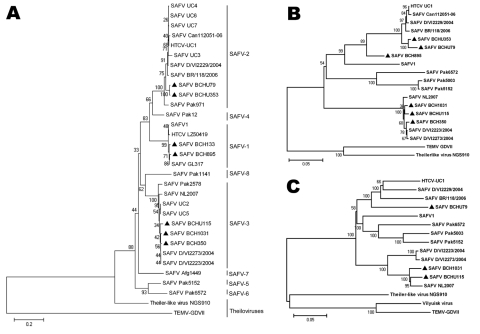
We identified 3 genetic lineages, SAFV-1, -2 and -3, on the basis of phylogenetic analysis of VP1, the most diverse protein of picornaviruses (6) (Figure 1). Multiple-alignment analysis, based on reference sequences of each lineage available in GenBank, showed that the identity of the VP1 amino acid sequences among the strains in the same lineage was 92.6%–100% and among strains belonging to different lineages, 63.3%–100% (Table A1). We found no obvious differences in amino acid sequences, nor any new motifs in these sequences, between strains detected in respiratory samples and those in fecal samples within the same lineage.
Figure 1.
Phylogenetic analysis of Saffold cardiovirus (SAFV) strains obtained in Beijing, China, 2007–2009, based on viral protein (VP) 1 (A), P1 capsid proteins (B), and full-length genomes (C). The trees, with 500 bootstrap replicates, were generated by using the neighbor-joining algorithm in MEGA 4.0 (10). Strains identified in this study are indicated by a specific identification code (BCH or BCHU), followed by the patient number and labeled with dark triangles (BCH133, BCH350, BCH895, BCH1031, BCHU79, BCHU115, and BCHU353). GenBank accession nos. of the complete genome sequences are BCH1031 GU943513, BCHU115 GU943514, and BCHU79 GU943518; of the P1 gene, BCH350 GU943515, BCH895 GU943516, and BCHU353 GU943517; and of the VP1 gene of BCH133, GU126461. SAFV-1 (prototype), UC1-UC7, Can112051-06, D/VI2229/2004, BR/118/2006, D/VI2273/2004, D/VI2223/2004, LZ50419, GL317, Pak12, Pak971, Afg1449, Pak1141, Pak2578, Pak5003, Pak5152, Pak6572, and NL2007, TEMV-GDVII, Theiler-like virus NGS910, and Vilyuisk virus were used as reference sequences (GenBank accession nos. NC009448, NC010810, EU604745-EU604750, AM922293, EU681176-EU681179, FJ586240, FJ464767, FJ463600-FJ463602, FJ463604, FJ463605, FJ463615-FJ463617, FM207487, X56019, AB090161, and EU723237). Scale bars indicate nucleotide substitutions per site.
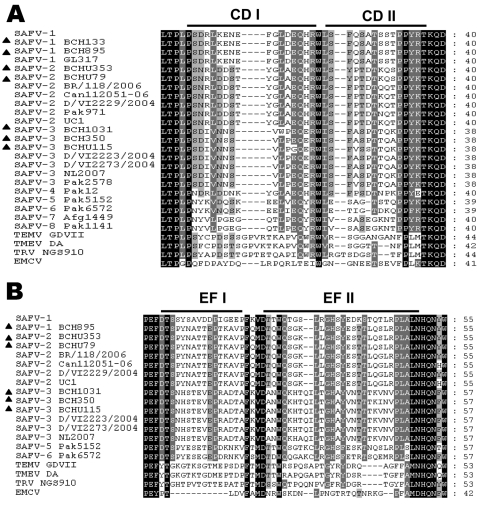
To further characterize the variation of SAFVs, we amplified the P1 region sequences of 6 strains identified in this study. The identity of all available nucleotide sequences of the P1 region among SAFVs was 68.5%–97.2%, whereas that of amino acid sequences was 74.9%–99.4%. The VP1 CD and VP2 EF loop structures, which display the greatest amino acid divergence among different SAFVs and are associated with tropism and virulence (6,7), were analyzed by amino acid alignment. Similar to previously reported findings (6,7), we found that the sequences in both loops among SAFVs were highly diverse among SAFVs (Figure 2). The amino acid identities among all known SAFVs were 53.1%–100% in CD loops and 40.3%–100% in EF loops; amino acid identities of SAFVs versus animal cardioviruses were 29.1%–41.1% in CD loops and 19.2%–32.6% in EF loops. We did not find any major differences among the available amino acid sequences of CD loops (91.1%–100.0%) or EF loops (97.9%–100.0%) of SAFVs within the same lineage for samples collected from either the gastrointestinal or respiratory tracts.
Figure 2.
Alignment of Saffold cardiovirus (SAFV) viral protein (VP) 1 CD (A) and VP2 EF (B) loop sequences from strains isolated in Beijing, China, 2007–2009. Columns highlighted in black show absolute amino acid conservation; those highlighted in gray show amino acids with highly similar properties. Strains identified in this study are labeled with dark triangles (BCH133, BCH1031, BCHU115, BCH350, BCH895, BCHU353 and BCHU79) (GenBank accession nos. GU126461, GU943513–GU943518), SAFV-1 (prototype), SAFV-1 GL317, SAFV-2 Can112051-06, SAFV-2 BR/118/2006, SAFV-2 D/VI/2229/2004, SAFV-2 Pak971, SAFV-2 UC1, SAFV-3 Pak2578, SAFV-3 D/VI/2223/2004, SAFV-3 D/VI/2273/2004, SAFV-3 NL2007, SAFV-4 Pak12, SAFV-5 Pak5152, SAFV-6 Pak 6572, SAFV-7 Afg1449, SAFV-8 Pak1141, Theiler-like virus NGS910, TMEV GDVII, and EMCV were used as reference sequences (GenBank accession nos. NC009448, FJ464767, AM922293, EU681177, EU681176, FJ463601, NC010810, FJ463605, EU681179, EU681178, FM207487, FJ463600, FJ463616, FJ463617, FJ463602, FJ463604, AB090161, X56019, X87335).
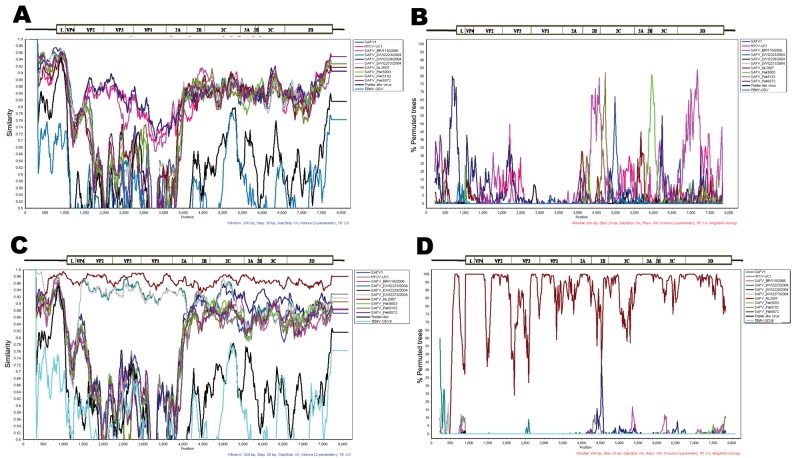
The complete genome sequence of BCH1031 (SAFV-3) was obtained, as were nearly complete genome sequences of BCHU115 (SAFV-3) and BCHU79 (SAFV-2), which covered full-length coding sequences as well as the 3′ UTR and a partial 5′ UTR (Figure 1). SimPlot analysis showed relatively high (>86%) identity between strains within the same lineage. No clear evidence of genetic recombination between the SAFV strains was found (Figure A1).
Conclusions
Although 8 lineages of SAFV have been detected in fecal samples worldwide, only SAFV-2 has been detected in respiratory samples (2,4). In addition, only SAFV-1 had been reported in China (5,13). In this study, we found that SAFV lineages 1, 2, and 3 co-circulated in patients with respiratory infections in Beijing.
Although SAFV is known to be transmitted by the fecal–oral route (1,3–7), as are other picornaviruses (14), our detection of SAFV in respiratory samples suggests that various SAFV lineages may also be transmitted through the respiratory tract and may be associated with disease. However, other respiratory viruses and M. pneumoniae were co-detected in 3 of the 7 SAFV-positive patients. Given that we did not conduct assays for common respiratory bacteria (and the number of SAFV-positive cases was limited), whether SAFV actually caused the observed symptoms in the patients cannot be definitively determined.
The genetic diversity of SAFVs in respiratory samples can complicate the relationship between SAFVs and disease because different genotypes of the same picornavirus species may cause different clinical signs and symptoms (6,14). Further investigations are needed to clarify any possible link between the pathogenicity and genetic diversity of SAFV.
Acknowledgments
We thank Yaowu Yang and Li Guo for their assistance with sequence analysis.
This study was supported in part by grants from the International Scientific and Technological Cooperative Project of Chinese Ministry of Science and Technology (S2010KR0788); Fondation Mérieux; and Institute of Pathogen Biology, Chinese Academy of Medical Sciences.
Biography
Dr Ren works as a scientist at the Dr Christophe Mérieux Laboratory, Institute of Pathogen Biology, Chinese Academy of Medical Sciences–Fondation Mérieux and State Key Laboratory of Molecular Virology and Genetic Engineering. Her research is focused on the etiology of infection and pathogenesis of respiratory viruses.
Table A1. Viral protein 1 amino acid sequence identities between Saffold cardiovirus strains.
| Genotype and sample code | BCH133 | BCH895 | SAFV-1 | GL317 | BCHU79 | BCHU353 | UC4 | D/VI2229 | Can112051-06 | Pak971 | BCHU115 | BCH350 | BCH1031 | D/VI2223 | UC2 | Pak2678 | Pak12 | Pak5152 | Pak6572 | Afg1449 | Pak1141 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAFV-1 | |||||||||||||||||||||
| BCH133 | 100 | ||||||||||||||||||||
| BCH895 | 97.8 | 100 | |||||||||||||||||||
| SAFV-1 | 97.4 | 98.1 | 100 | ||||||||||||||||||
| GL317 |
98.5 |
99.2 |
98.9 |
100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SAFV-2 | |||||||||||||||||||||
| BCHU79 | 73.7 | 74.0 | 75.5 | 74.8 | 100 | ||||||||||||||||
| BCHU353 | 73.7 | 74.0 | 75.5 | 74.8 | 99.2 | 100 | |||||||||||||||
| UC4 | 74.8 | 74.8 | 76.2 | 75.5 | 95.2 | 95.2 | 100 | ||||||||||||||
| D/VI2229 | 74.4 | 74.4 | 75.9 | 75.1 | 93.7 | 93.7 | 97.4 | 100 | |||||||||||||
| Can112051-06 | 75.1 | 75.1 | 76.6 | 75.9 | 94.4 | 94.4 | 98.5 | 96.6 | 100 | ||||||||||||
| Pak971 |
75.1 |
75.1 |
76.6 |
75.9 |
93.3 |
93.3 |
94.1 |
92.6 |
93.7 |
100 |
|
|
|
|
|
|
|
|
|
|
|
| SAFV-3 | |||||||||||||||||||||
| BCHU115 | 65.3 | 65.3 | 65.6 | 66.0 | 70.9 | 70.9 | 70.9 | 70.5 | 71.6 | 69.1 | 100 | ||||||||||
| BCH350 | 66.0 | 66.0 | 66.4 | 66.7 | 71.3 | 71.3 | 71.3 | 70.9 | 72.0 | 69.1 | 96.6 | 100 | |||||||||
| BCH1031 | 66.0 | 66.0 | 66.4 | 66.7 | 71.3 | 71.3 | 71.3 | 70.9 | 72.0 | 69.1 | 97.4 | 98.5 | 100 | ||||||||
| D/VI2223 | 66.4 | 66.4 | 66.7 | 67.1 | 71.6 | 71.6 | 71.6 | 71.3 | 72.4 | 69.4 | 97.4 | 99.2 | 99.2 | 100 | |||||||
| UC2 | 66.4 | 66.4 | 66.7 | 67.1 | 72.0 | 72.0 | 72.0 | 71.6 | 72.4 | 69.4 | 96.2 | 98.1 | 98.1 | 98.8 | 100 | ||||||
| Pak2578 |
65.6 |
65.6 |
66.0 |
66.4 |
71.6 |
71.6 |
70.9 |
70.9 |
71.6 |
70.2 |
94.8 |
95.9 |
95.9 |
96.6 |
96.2 |
100 |
|
|
|
|
|
| SAFV-4 | |||||||||||||||||||||
| Pak12 |
79.5 |
79.9 |
81.7 |
80.6 |
81.6 |
81.9 |
81.9 |
81.2 |
81.6 |
83.0 |
67.6 |
67.6 |
68.0 |
68.0 |
68.3 |
68.0 |
100 |
|
|
|
|
| SAFV-5 | |||||||||||||||||||||
| Pak5152 |
67.2 |
67.6 |
68.3 |
67.6 |
67.0 |
67.0 |
65.2 |
65.5 |
65.5 |
67.7 |
67.3 |
68.1 |
68.1 |
68.4 |
68.4 |
67.3 |
66.6 |
100 |
|
|
|
| SAFV-6 | |||||||||||||||||||||
| Pak6572 |
64.7 |
64.3 |
65.0 |
64.7 |
63.3 |
63.3 |
63.0 |
63.3 |
63.3 |
63.7 |
64.4 |
65.2 |
65.2 |
65.5 |
65.9 |
64.8 |
64.8 |
80.8 |
100 |
|
|
| SAFV-7 | |||||||||||||||||||||
| Afg1449 |
67.8 |
67.8 |
69.3 |
68.6 |
68.0 |
68.0 |
68.3 |
68.7 |
68.3 |
68.3 |
69.8 |
70.9 |
70.9 |
71.3 |
71.3 |
70.5 |
69.8 |
69.9 |
67.0 |
100 |
69.1 |
| SAFV-8 | |||||||||||||||||||||
| Pak1141 | 64.5 | 64.5 | 64.9 | 65.3 | 64.3 | 64.3 | 65.4 | 65.4 | 65.8 | 67.6 | 75.5 | 75.1 | 75.1 | 75.5 | 75.5 | 74.8 | 66.1 | 69.9 | 67.0 | 69.1 | 100 |
Figure A1.
Recombination analyses of Saffold cardioviuses (SAFVs), isolated in Beijing, China, 2007–2009. The relationships among the aligned cardiovirus genome sequences were analyzed by SimPlot software version 3.5.1 (11). A) Sliding-window SimPlot graph generated by using the sequence of the BCHU79 (SAFV-2) as a query against SAFVs, Theiler-like virus, and Theiler murine encephalomyelitis virus–GDVII. B) Bootscan analysis of the sequence of SAFV BCHU79 in comparison to SAFVs, Theiler-like virus, and Theiler murine encephalomyelitis virus–GDVII. C) Sliding-window SimPlot graph generated by using the sequence of the BCH1031 (SAFV-3) as a query against SAFVs, Theiler-like virus, and Theiler murine encephalomyelitis virus–GDVII. D) Bootscan analysis of the sequence of SAFV BCH1031 in comparison to SAFVs, Theiler-like virus, and Theiler murine encephalomyelitis virus–GDVII. Because the genome sequence identity between BCH1031 and BCH115 is as high as 94%, BCH1031 was used to conduct the SimPlot analysis. The nucleotide position is given on the x-axis, and the percent nucleotide sequence similarity is given on the y-axis. The positions relative to viral genes are shown by genome diagrams above the plots and scans. SAFV-1 (prototype), SAFV-2 UC1, SAFV-2 D/VI/2229/2004, SAFV-2 BR118/2006, SAFV-3 D/VI/2273/2004, SAFV-3 D/VI/2223/2004, SAFV-3 NL2007, SAFV-5 Pak5003, SAFV-5 Pak5152, SAFV-6 Pak 6572, Theiler-like virus NGS910, and Theiler murine encephalomyelitis virus–GDVII were used as reference sequences (GenBank accession nos. NC009448, NC010810, EU681176–EU681179, FM207487, FJ463615–FJ463617, AB090161, and X56019).
Table. Characteristics of patients with Saffold cardiovirus, Beijing, China, 2007–2009*.
| Patient no. | Age/sex | Sampling date | Diagnosis | Clinical signs | Other underlying diseases | Co-infecting organisms | SAFV lineage |
|---|---|---|---|---|---|---|---|
| BCH133 | 9 y/F | 2007 Aug 7 | Pneumonia | Cough | Tuberculosis, hepatic dysfunction | Mycoplasma pneumoniae | 1 |
| BCH350 | 5 mo/M | 2007 Dec 7 | Broncho- pneumonia | Cough, gasping | Respiratory failure | RSV | 3 |
| BCH895 | 2 y, 8 mo/M | 2008 Oct 6 | Pneumonia | Fever, cough, spitting, gasping, chills | No | EV | 1 |
| BCH1031 | 4 y/M | 2008 Nov 16 | Peribronchitis | Fever, cough, spitting, gasping, chills, sneezing | No | None | 3 |
| BCHU79 | 1 y, 6 mo/F | 2009 Jun 5 | URTI | Fever, cough | Unknown | None | 2 |
| BCHU115 | 1 y, 10 mo/F | 2009 Jun 6 | URTI | Fever, cough | Unknown | None | 3 |
| BCHU353 | 4 y, 6 mo/F | 2009 Jun 17 | URTI | Fever, cough | Unknown | None | 2 |
*SAFV, Saffold cardiovirus; RSV, respiratory syncytial virus; EV, enterovirus; URTI, upper respiratory tract infection.
Footnotes
Suggested citation for this article: Ren L, Gonzalez R, Xie Z, Xiao Y, Li Y, Liu C, et al. Saffold cardioviruses of 3 lineages in children with respiratory tract infections, Beijing, China. Emerg Infect Dis [serial on the Internet]. 2010 Jul [date cited]. http://dx.doi.org/10.3201/eid1607.091682
References
- 1.Jones MS, Lukashov VV, Ganac RD, Schnurr DP. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J Clin Microbiol. 2007;45:2144–50. 10.1128/JCM.00174-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abed Y, Boivin G. New Saffold cardioviruses in 3 children, Canada. Emerg Infect Dis. 2008;14:834–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drexler JF, Luna LK, Stöcker A, Almeida PS, Ribeiro TC, Petersen N, et al. Circulation of 3 lineages of a novel Saffold cardiovirus in humans. Emerg Infect Dis. 2008;14:1398–405. 10.3201/eid1409.080570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu CY, Greninger AL, Kanada K, Kwok T, Fischer KF, Runckel C, et al. Identification of cardioviruses related to Theiler’s murine encephalomyelitis virus in human infections. Proc Natl Acad Sci U S A. 2008;105:14124–9. 10.1073/pnas.0805968105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren L, Gonzalez R, Xiao Y, Xu X, Chen L, Vernet G, et al. Saffold cardiovirus in children with acute gastroenteritis, Beijing, China. Emerg Infect Dis. 2009;15:1509–11. 10.3201/eid1509.081531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blinkova O, Kapoor A, Victoria J, Jones M, Wolfe N, Naeem A, et al. J Cardioviruses are genetically diverse and cause common enteric infections in South Asian children. J Virol. 2009;83:4631–41. 10.1128/JVI.02085-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoll J, Erkens Hulshof S, Lanke K, Verduyn Lunel F, Melchers WJ, Schoondermark–van de Ven E, et al. Saffold virus, a human Theiler’s-like cardiovirus, is ubiquitous and causes infection early in life. PLoS Pathog. 2009;5:e1000416. 10.1371/journal.ppat.1000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren L, Gonzalez R, Wang Z, Xiang Z, Wang Y, Zhou H, et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin Microbiol Infect. 2009;15:1146–53. 10.1111/j.1469-0691.2009.02746.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung JY, Han TH, Kim CK, Kim SW. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis. 2006;12:1254–6. 10.3201/eid1708.060261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- 11.Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C–infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg A, Brewster L, Clark J, Simoes E; ARIVAC Consortium. Evaluation of R-Mix shell vials for the diagnosis of viral respiratory tract infections. J Clin Virol. 2004;30:100–5. 10.1016/j.jcv.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 13.Xu ZQ, Cheng WX, Qi HM, Cui SX, Jin Y, Duan ZJ. New Saffold cardiovirus in children, China. Emerg Infect Dis. 2009;15:993–4. 10.3201/eid1506.090109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pallansch MA, Roos RP. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In: Knipe DM, Howley PM, editors. Fields virology, vol. 1, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 840–84. [Google Scholar]