Abstract
To elucidate the Anopheles nuneztovari s.l. taxonomic status at a microgeographic level in four malaria endemic localities from Antioquia and Córdoba, Colombia, fragments of the Cytochrome oxidase subunit I (COI) and the white gene were used. The COI analysis showed low genetic differentiation with FST levels between −0.02 and 0.137 and Nm values between 3 and infinity, indicating the presence of high gene flow among An. nuneztovari s.l. populations from the four localities. The COI network showed a single most common haplotype, 1 (n=55), present in all localities, as the likely ancestral haplotype. Analysis of the white gene showed that An. nuneztovari s.l. populations from both departments grouped with haplotypes 19 and 20, which are part of lineage 3 previously reported. The results of the present study suggest that An. nuneztovari s.l. is a single taxon in the area of the present study.
Keywords: Malaria, Anopheles nuneztovari, white gene, Cytochrome oxidase subunit I, Colombia
Introduction
Malaria is an important public health problem in Colombia, as demonstrated by the fact that, after Brazil, Colombia has the second highest number of malaria cases in Latin America (OPS 2009). In the past ten years, the departments of Antioquia and Córdoba, where this investigation was carried out, have reported nearly 60% of the total cases of the country (Padilla et al. 2011). There are three main vectors, all in subgenus Nyssorhynchus, reported for Colombia present in endemic areas of these departments, including Anopheles albimanus Wiedemann, Anopheles darlingi -Root-, and Anopheles nuneztovari Gabaldón (Olano et al. 2001).
Anopheles nuneztovari s.l. is distributed from eastern Panamá to northern South America and Amazonia (Mirabello & Conn 2008) where it presents differences in behaviour (Montoya-Lerma et al. 2011). Brazilian collections of this species have detected natural infection with Plasmodium (Arruda et al. 1986; Tadei et al. 1998), but the role of An. nuneztovari s.l. in Brazil seems to be limited to local malaria transmission (Galardo et al. 2007). Indeed, populations from Brazil are mainly zoophilic and exophagic with a biting peak near sunset (Tadei & Thatcher 2000). In contrast, in Colombia and Venezuela, An. nuneztovari s.l. is a primary malaria vector (Lounibos & Conn 2000). Recent studies in northeastern Colombia showed that An. nuneztovari s.l. exhibits anthropophilic and endophagic behavior, with a biting peak between 20:00 and 23:00 hours, and can be naturally infected with Plasmodium vivax VK247 (Gutiérrez et al. 2009a). In Venezuela, the highest biting activity is close to midnight indoors and outdoors (Rubio-Palis & Curtis 1992).
Based on morphology, cytology, ecology, behavioral and molecular differences, several authors have suggested that An. nuneztovari s.l is a species complex (summarized in Calado et a l. 2008, Montoya-Lerma et al. 2011). Recent white gene analyses described five lineages across its range, with one (lineage 3) present in Colombia (Mirabello & Conn 2008). An. nuneztovari s.l. is characterized by high intraspecific morphological variability and is thus easily misidentified or confounded with other members of the Oswaldoi Group (Calle et al. 2008, Cienfuegos et al. 2008, Gómez et al. 2010). A deeper dissection of this challenging variability resulted in the description of a new morphological form in the Brazilian Amazon (Bergo et al. 2007), later confirmed as Anopheles goeldii Rozeboom and Gabaldón by sequence analysis of ITS2, COI and white genes (Calado et al. 2008). Scarpassa & Conn (2011, in press) recently determined that, based on a phylogenetic analysis of COI gene sequences, samples from the Amazon Basin could be assigned to two well-supported clades. They hypothesize that one of these clades represents An. goeldii, and the second clade represents a previously undescribed lineage or taxon in An. nuneztovari s.l.
Few studies have attempted to document the taxonomic status and population structure of An. nuneztovari s.l. in Colombia. One study evaluated phenotypic variation in specimens from western and eastern Colombia and detected high levels of wing spot variation, a key character that is often difficult to assess for proper identification (Fajardo et al. 2008). Similarly, morphometric analysis of female specimens of An. nuneztovari s.l. from Montelíbano, Córdoba, found high intraspecific variability (Gómez et al. 2010).
Studies using molecular markers have also attempted to clarify the taxonomic status and population structure of An. nuneztovari s.l. from northern and western Colombia. Using random amplified polymorphic DNA (RAPD), Posso et al. (2003) found low levels of genetic differentiation among three populations. Analysis of ITS2 sequences concluded that in Colombia, An. nuneztovari s.l. from both sides of the Andes mountains comprised a single genetic species (Sierra et al. 2004). Given the wide distribution of An. nuneztovari s.l. in Antioquia and Córdoba, this study was conducted to test the hypothesis that a single taxon is present in this region, to assess the genetic variability at a microgeographic level, and to identify the white gene lineage(s) present.
Materials and methods
Mosquito collection and processing
Adult female specimens of An. nuneztovari s.l. were collected between November 2007 and February 2010 in El Bagre (BAG), Zaragoza (ZAR), and San Pedro de Urabá (SPU), in the department of Antioquia, and in Montelíbano (MTL) and Puerto Libertador (PTL), in Córdoba (Figure 1, Table I). The collection protocols and informed consent were reviewed and approved by a Bioethics Committee for Human Research at the University of Antioquia (SIU-UdeA). Mosquitoes were identified by morphological keys (González & Carrejo 2009, Faran 1980, Faran & Linthicum 1981). A subset of specimens collected from each field trip, is maintained in the University collection. For morphological reference, we retain the wings and legs fixed on slides of a percentage of the specimens. We also maintain photographic records of most specimens that have been processed for molecular analyses.
Figure 1.
Distribution of collection sites for An. nuneztovari s.l. in Antioquia and Córdoba, Colombia. San Pedro de Urabá (SPU), El Bagre (BAG), Zaragoza (ZAR), Montelíbano (MTL), Puerto Libertador (PTL).
Table I. Study sites, dates of collection, coordinates and sample sizes (n) of An. nuneztovari s.l. collected from Antioquia and Córdoba, Colombia.
| Municipality (Abbreviation) | Year | Month (number of days) | Coordinates | n |
|---|---|---|---|---|
| Montelíbano (MTL) | 2007 | July (5) August (2) |
7°59′ N, 75°25′ W | 29 |
| Puerto Libertador (PLB) | 2007 | January (6) | 07°43′N, 75°51′W | 32 |
| El Bagre (BAG) | 2009 | March (3) May (3) |
07° 35′N, 74°49′W | 41 |
| Zaragoza (ZAR) | 2008 | January (1) March (3) Jun (1) |
07°29′N, 74°51′W | 8 |
| San Pedro de Urabá (SPU) | 2010 | February (5) | 06°28′N, 75°33′W | 37 |
| Total n = | 147 |
Molecular analyses
Total DNA was extracted from individual mosquito abdomens by a standard protocol (Rosero et al. 2010), used to confirm species identification following a PCR-RFLP-ITS2 assay developed by our group (Zapata et al. 2007, Cienfuegos et al. 2008, Cienfuegos et al. 2011), and then amplified for the COI and white gene fragments as outlined below. A 1,300 bp fragment of the mtDNA COI gene was amplified by PCR using 10 μM primers UEA3/UEA10 (Lunt et al. 1996), in a 25 μl final reaction volume as previously described (Gutierrez et al. 2010). PCR products were purified using Wizard SV Gel and PCR Cleanup System (Promega, Madison, WI) following the protocol recommended by the manufacturer and sequenced in both directions.
An 800 bp region of the nuclear white gene was amplified using primers WF and W2R in a 25 μl PCR reaction with conditions previously described by Mirabello & Conn (2008). Because of allelic variation (Mirabello & Conn 2008), white gene PCR products were cloned as described in Onyabe & Conn (1999). Five clones corresponding to five specimens from each locality were sequenced. Sequences were edited, assembled by pairwise alignment and then multiple alignments with Clustal W were performed using the Geneious software 5.3.4 (Drummond et al. 2011).
Descriptive statistics and population differentiation test
Haplotype and nucleotide diversities of mtDNA COI sequences were obtained using DnaSP version 5.00.07 (Rozas et al. 2003) and Arlequin version 3.11 software (Excoffier et al. 2005). Analysis of molecular variance (AMOVA) was used to evaluate within and among population variation using Arlequin software. The COI haplotype distribution was evaluated by a statistical parsimony network (TCS) with 95% support (Clement et al. 2000).
Phylogenetic relatedness of An. nuneztovari s.l. populations from Antioquia and Córdoba with populations from South America using white gene sequences
Split networks are successful tools for visualizing incompatible and ambiguous signals and have been used previously for phylogenetics studies (Gutiérrez et al. 2009b, Gutiérrez et al. 2010). Therefore, a neighbor-net network was estimated using SplitsTree4 version 4.10 (Huson & Bryant 2006, Huson & Steel 2004), for An.nuneztovari s.l. white gene sequences obtained in this study and for those in GenBank corresponding to specimens from Brazil, Bolivia, Surinam, Venezuela and Colombia (Accession numbers EU500727-EU500758). Bayesian analysis was carried out using Mr Bayes v3.1 (Huelsenbeck & Ronquist 2001), available in Geneious software 5.3.4 (Drummond et al. 2011), using the default HKY85 model and gamma distributions. The settings for the Markov Chain Monte Carlo (MCMC) were four chains running every 100,000 generations.
Results
An. nuneztovari s.l. genetic variation at the intra-population level
Out of 2,490 Anopheles mosquitoes collected in Antioquia and Córdoba, Colombia, 147 An. nuneztovari s.l. specimens were chosen (Table I, Figure 1). A 1,180 bp sequence of the COI gene was analyzed. The aligned sequences showed 23 variable sites and 11 that were parsimony informative. The highest nucleotide diversity was detected in San Pedro de Urabá (Pi= 0.00249+/−0.001315) and moderate to lowest diversity was detected in Puerto Libertador (0.00172+/−0.001120). The haplotype diversity was highest for San Pedro de Urabá (0.8134/−0.0418) and lowest for Puerto Libertador (0.709+/−0.0578; Table II). To compare the population pairs using the COI gene, samples from BAG and ZAR were combined. These localities are 11 km apart and Nm detected infinite migrants between them. There were 20 unique haplotypes (GenBank accession numbers JN255752 to JN255771), all of which connected parsimoniously. Four haplotypes were shared among all localities. The most widespread haplotype (type 1), showed a frequency of 38% (55/147), and was found in all localities. Three other haplotypes were distributed among all localities: type 2 (21%), type 3 (12%) and type 4 (10%). Haplotype 3 was not found in SPU (Table II). There were few private haplotypes (Figure 2).
Table II. Shared COI haplotypes and genetic polymorphism statistics for An. nuneztovari s.l. collected in localities of Antioquia and Córdoba, Colombia.
| Locality | n | COI Haplotype | S | h | Hd (DE) | Pi (DE) |
|---|---|---|---|---|---|---|
| MTL | 29 | 1(11), 2(5), 3(2), 4(5), 6(2), 8(2) | 9 | 8 | 0.807+/−0.5240 | 0.00189+/−0.001199 |
| PTL | 32 | 1(15), 2(8), 3(1), 4(5), 6(2), 8(2) | 7 | 7 | 0.709+/−0.0578 | 0.00172+/−0.001120 |
| Total Córdoba |
61 | 1(26), 2(13), 3(13), 4(10), 6(4) | 10 | 10 | 0.758+/−0.0397 | 0.00180+/−0.001128 |
|
| ||||||
| SPU | 37 | 1(10), 2(13), 4(2), 5(6), 6(2), 9(2) | 15 | 10 | 0.8134/−0.0418 | 0.00249+/−0.001315 |
| BAG+ZAR | 49 | 1(21), 2(5), 3(15), 4(3), 7(2) | 9 | 8 | 0.720+/−0.0441 | 0.00237+/−0.001417 |
| Total Antioquia |
86 | 1(29), 2(17), 3(15), 4(2), 5(6) | 19 | 13 | 0.773+/−0.0280 | 0.00271+/−0.001445 |
|
| ||||||
| Overall Total |
147 | 1(55), 2(230), 3(28), 4(12), 5(6), 6(4) | 23 | 20 | 0.781+/−0.0302 | 0.00234+/−0.001288 |
S: number of segregating sites, h: number of haplotypes, Hd: haplotypic diversity, Pi: nuclear diversity, SD: standard deviation. Numbers inside parenthesis are the frequency of haplotypes by locality. Underline haplotypes are share among populations of Antioquia and Córdoba. Montelíbano (MTL), Puerto Libertador (PTL), San Pedro de Urabá (SPU), El Bagre (BAG), Zaragoza (ZAR).
Figure 2.
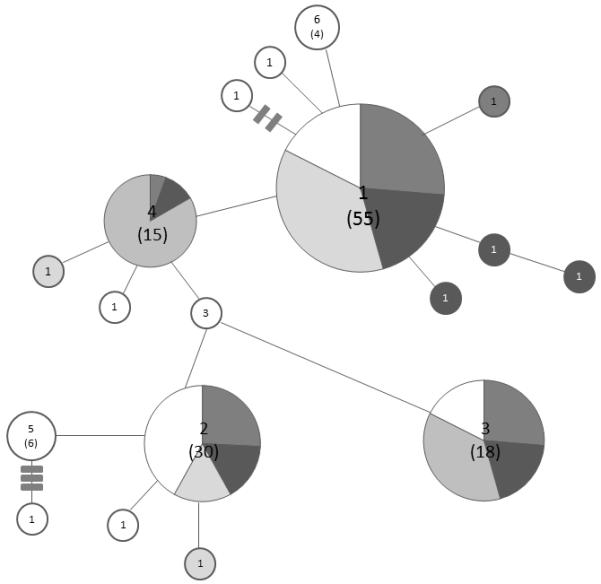
Parsimony based haplotype network of 20 COI haplotypes of 147 An. nuneztovari s.l. specimens. Each locality is represented by a distinct shade. Numbers out of the parentheses represent the haplotype designation and those inside the parentheses indicate the number of sequences included in each haplotype. The size of the circle is proportional to the frequency of detected haplotype. San Pedro de Urabá (SPU), El Bagre (BAG), Zaragoza (ZAR), Montelíbano (MTL), Puerto Libertador (PTL).
Genetic structure and demographic inference
The FST values estimated for COI sequences from localities in Antioquia and Córdoba indicated low to moderate genetic differentiation, with values ranging between −0.02 and 0.137. The number of migrants per generation, Nm, was between 3 and infinity, indicating gene flow among all An. nuneztovari s.l. tested (Table III). Analysis of molecular variance, AMOVA, based on COI haplotype frequencies and conducted with all localities (populations) as one group showed that 12.49% (p=0.18) of the variance could be attributed to among localities (populations) and 87.51% within (Table IV).
Table III. Pairwise genetic differentiation - FST and gene flow - Nm among An. nuneztovari s.l. collected from Antioquia and Córdoba, Colombia.
| COI | ||||
|---|---|---|---|---|
| Localities | PTL | MTL | BAG+ZAR | SPU |
| PTL | — | α | 6.02 | 4.09 |
| MTL | −0.02020 | — | 6.88 | 3.13 |
| BAG+ZAR | 0.07657 | 0.06767 | — | 3.12 |
| SPU | 0.10872 | 0.13743 | 0.13778 | — |
Above diagonal: Nm value, below diagonal: FST values, * p<0.05, α: infinity. San Pedro de Urabá (SPU), El Bagre (BAG), Zaragoza (ZAR), Montelíbano (MTL), Puerto Libertador (PTL).
Table IV. Analysis of molecular variance (AMOVA) of An. nuneztovari s.l. based on COI haplotypes.
| Source of variation |
Variance components |
Percentage of variation |
Fixation Index |
|---|---|---|---|
| Among populations | 0.1896 | 12.49 | FST = 0.12* |
| Within populations | 1.3234 | 87.51 |
not significant (p > 0.05)
The COI network showed a single most common haplotype (type 1, n=55), which was present in all the localities. There were only 16 private haplotypes, which demonstrates high demographic stability and the existence of gene flow among populations (Figure 2). After removing the intron sequence, a 630 bp fragment of the nuclear white gene was analyzed. From 20 sequences obtained, the consensus sequence was submitted to GenBank with accession number JN255772. Phylogenetic neighbor-net network based on white gene sequences showed that An. nuneztovari s.l. specimens from Antioquia and Córdoba grouped with haplotypes 19 and 20, which are part of lineage 3 (Figure 3)as reported by Mirabello & Conn (2008). However, the Bayesian analysis was completely unresolved and uninformative; whereas the neighbor-net network had an area of boxes, which indicated the need for larger sample sizes from these regions.
Figure 3.
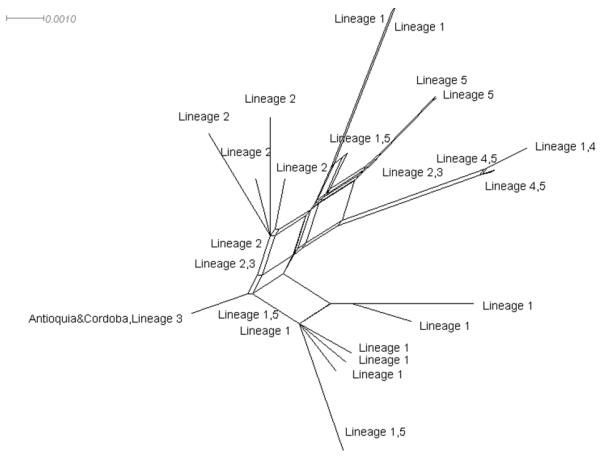
Neighbor-Net network of Anopheles nuneztovari s.l. white gene lineages from Antioquia and Córdoba compared with other Anopheles nuneztovari s.l from South America.
Discussion
The results obtained with COI sequences of specimens An. nuneztovari s.l. collected in localities of high malaria transmission of Antioquia and Córdoba showed low genetic differentiation and gene flow among these localities. In general, levels of COI nucleotide diversity were higher for the populations of Antioquia than for populations of Córdoba. Further, most haplotypes were shared between both regions, suggesting that these populations have a common demographic history (Castelloe & Templeton 1994) (Figure 2). Avise (2000) proposed that populations with high nucleotide and haplotype diversity are more stable, constant in size and have higher effective population sizes. In this study, An. nuneztovari s.l. from San Pedro de Urabá had the highest nucleotide and haplotype diversity, indicating that this population is the most stable among the populations studied. FST and Nm values obtained suggest that An. nuneztovari s.l. from Antioquia and Córdoba may actually comprise a single population. Nm values in malaria vectors are of relevance because they can be used to predict gene spread, e.g. of those genes important for insecticide resistance or parasite refractoriness. An understanding of gene flow is essential for the introduction of transgenic mosquitoes carrying genes for Plasmodium resistance.
White gene phylogenetic analyses of samples from Antioquia and Córdoba together with other South American populations produced mixed results. Our sequences were identical to haplotypes 19 and 20 reported by Mirabello & Conn (2008), which corresponded to their lineage 3. The area of boxes in the neighbor-net network combined with the unresolved Bayesian analysis, suggest that a broader sampling of this species complex with analyses of white gene and other nuclear genes should be undertaken to more formally test the five lineages proposed in Mirabello & Conn (2008). Nevertheless, our results are consistent with previous studies with various markers that detected differentiation between An. nuneztovari s.l. populations from Colombia and Venezuela west of the Andes with those in other regions of South America (Elliott 1972, Kitzmiller et al. 1973, Conn et al. 1993, Scarpassa et al. 1996, 1999, 2000, Fritz et al. 1994, Mirabello & Conn 2008, Scarpassa & Conn in press 2011).
In comparison with the results presented here, a study using the ribosomal DNA internal transcribed spacer (ITS2) suggested low differentiation between An. nuneztovari s.l. populations located on opposite sides of the Andes mountains (NW and NE Colombia), indicating that An. nuneztovari s.l. is conspecific in Colombia (Sierra et al. 2004). Further, a study using RAPD markers showed low levels of genetic differentiation for An. nuneztovari s.l. populations from NW and NE Colombia, with higher levels of gene flow among western populations (Posso et al. 2003). Although Posso et al. (2003) used RAPD for discrimination of populations in western Colombia, their results are in agreement with the ones in the present study with COI, also in western Colombia. Collectively, results of these studies with An. nuneztovari s.l. populations from NW or NE Colombia are consistent in the detection of low genetic differentiation among the populations evaluated.
Localities in the present study are placed in the Magdalena biogeographic province (Morrone 2006), which encompasses western Venezuela and NW Colombia. The Magdalena province is characterized by temperatures above 24°C, plains with altitudes no greater than 500 meters, fragmented forest, the presence of livestock and agriculture, and active mining (IGAC 2002). The concurrence of similar ecologic and geographic conditions in this biogeographic region may influence An. nuneztovari s.l. populations, favoring the low genetic differentiation observed. The location of An. nuneztovari s.l. Cytotypes B and C coincide with the Magdalena biogeographic province, while Cytotype A was reported in the Brazilian Amazon, south of the country. Given that An. nuneztovari s.l. is broadly distributed in Colombia, including regions in the south in close proximity to the Brazilian Amazon, genetic analyses of An. nuneztovari s.l. should be expanded to include specimens from the southern regions such as the Orinoquía and the Amazonia and other markers such as microsatellites to detect recent mutational events.
Previous work by our group evaluated microgeographic genetic variation of An. darlingi, another important Colombian malaria vector, in Antioquia and Córdoba departments. Those results were based on microsatellites and COI analysis and also showed that An. darlingi populations were highly homogeneous and presented high levels of gene flow (Gutierrez et al. 2010). In the report presented here, microsatellite analysis was not performed because at the time of our collections and analyses, these markers had not been available for An. nuneztovari (Cardoza et al. http://tomato.bio.trinity.edu/manuscripts/11-4/mer-11-0032.pdf). It is possible that microsatellite analysis of An. nuneztovari, as with An. darlingi, would have revealed low genetic differentiation and high gene flow. In particular, analyses of An. darlingi in Brazil at the microgeographic level (152 km apart) showed low levels of genetic differentiation (FST=0.001-0.095) and Nm values between 4.7 and 363.8, indicating that at this distance and within similar ecological and geographic conditions, anopheline differentiation may be low (Scarpassa & Conn 2007). However, differentiation at the microgeographic level has been documented for other important malaria vectors such as Anopheles gambiae (McLain et al. 1989). Specifically, in seven Kenyan localities less than 10 km apart, the authors found significant differences in the genetic distribution of this important African vector indicating the absence of gene flow, probably due to poor dispersal abilities of these populations (McLain et al. 1989).
In summary, the results obtained in the present study provide critical data for understanding the primary malaria vector An. nuneztovari s.l. in localities with the highest annual reports of malaria cases in Colombia (Padilla et al. 2011). In particular, the presence of low genetic differentiation and high gene flow for important malaria vector species such as An. darlingi and An. nuneztovari s.l., indicate that malaria control programs could apply the same strategies, for example insecticide spraying, in these localities of Antioquia and Córdoba, as has been previously suggested (Gutiérrez et al. 2010).
ACKNOWLEDGMENTS
We thank G. Gómez, M. Altamiranda, N. Naranjo, and A. Yates who cooperated in specimen collection and identification. We also are grateful to Sara A. Bickersmith (Wadsworth Center, New York State Department of Health, Albany, NY) for preliminary manuscript evaluation and critical editorial comments. LMJ received support for her MSc training from “Estrategia para la Sostenibilidad-Universidad de Antioquia 2007-2008”, code BUPP E01335 to MMC.
Financial support: This study received support from Comité para el Desarrollo de la Investigación, CODI-Universidad de Antioquia, Grant number 8700-074 to MMC, the United States National Institutes of Health grants R03AI076710-02 to MMC and AI R01 54139-02 to JEC.
REFERENCES
- Arruda M, Carvalho MB, Nussenzweig RS, Maracic M, Ferreira AW, Cochrane AH. Potential vectors of malaria and their different susceptibility to Plasmodium falciparum and Plasmodium vivax in northern Brazil identified by immunoassay. Am J Trop Med Hyg. 1986;35:873–881. doi: 10.4269/ajtmh.1986.35.873. [DOI] [PubMed] [Google Scholar]
- Avise J. Phylogeography: The history and formation of species. Harvard University Press; London: 2000. p. 447. [Google Scholar]
- Bergo ES, Souto RN, Galardo AK, Nagaki SS, Calado DC, Sallum MA. Systematic notes on Anopheles meigen (Diptera: Culicidae) species in the state of Amapa, Brazil. Mem Inst Oswaldo Cruz. 2007;102:373–376. doi: 10.1590/s0074-02762007005000053. [DOI] [PubMed] [Google Scholar]
- Calado D, Foster P, Bergo E, Santos C, Galardo A, Sallum M. Resurrection of Anopheles goeldii from synonymy with Anopheles nuneztovari (Diptera, Culicidae) and a new record for Anopheles dunhami in the Brazilian Amazon. Mem Inst Oswaldo Cruz. 2008;103:791–799. doi: 10.1590/s0074-02762008000800009. [DOI] [PubMed] [Google Scholar]
- Calle DA, Quiñones ML, Erazo HF, Jaramillo N. Discriminación por morfometría geométrica de once especies de Anopheles (Nyssorhynchus) presentes en Colombia. Biomedica. 2008;28:371–385. [PubMed] [Google Scholar]
- Cardoza TB, Barbosa De, Sousa AC, Prado Lima M, Pereira De Souza A, Scarpassa VM. Microsatellite markers for the neotropical malaria vector Anopheles nuneztovari sensu lato: isolation, development and characterization of eighteen loci. Mol Ecol Resources. Available at: http://tomato.bio.trinity.edu/manuscripts/11-4/mer-11-0032.pdf. [Google Scholar]
- Castelloe J, Templeton A. Root probabilities for intraspecific gene trees under neutral coalescent theory. Mol Phylogenet Evol. 1994;3:102–113. doi: 10.1006/mpev.1994.1013. [DOI] [PubMed] [Google Scholar]
- Cienfuegos A, Gómez G, Córdoba L, Luckhart S, Conn J, Correa M. Diseño y evaluación de metodologías basadas en PCR-RFLP de ITS2 para la identificación molecular de mosquitos Anopheles spp. (Diptera: Culicidae) de la Costa Pacífica de Colombia. Rev Biomed. 2008;19:35–44. [Google Scholar]
- Cienfuegos AV, Rosero DA, Naranjo N, Luckhart S, Conn JE, Correa MM. Evaluation of a PCR-RFLP-ITS2 assay for discrimination of Anopheles species in northern and western Colombia. Acta Trop. 2011;118:128–135. doi: 10.1016/j.actatropica.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Conn J, Puertas Y, Seawright J. A new cytotype of Anopheles nuneztovari from western Venezuela and Colombia. J Am Mosq Control Assoc. 1993;9:294–301. [PubMed] [Google Scholar]
- Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A. Research software for biologists, not computer scientists. 2011 Available from: http://www.geneious.com. [Google Scholar]
- Elliott R. The Influence of vector behavior on malaria transmission. Am J Trop Med Hyg. 1972;21:755–763. doi: 10.4269/ajtmh.1972.21.755. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol Bioinform Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Fajardo Ramos M, González Obando R, Suárez M, López David, Wilkerson R, Sallum M. Morphological analysis of three populations of Anopheles (Nyssorhynchus) nuneztovari Gabaldón (Diptera: Culicidae) from Colombia. Mem Inst Oswaldo Cruz. 2008;103:85–92. doi: 10.1590/s0074-02762008000100013. [DOI] [PubMed] [Google Scholar]
- Faran ME. Mosquito studies (Diptera: Culicidae) XXXIV. A revision of the Albimanus section of the subgenus Anopheles Nyssorhynchus (Diptera: Culicidae) Am Ent Inst. 1980;15:1–215. [Google Scholar]
- Faran M, Linthicum K. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae) Mosq Syst. 1981;13:1–81. [Google Scholar]
- Fritz G, Conn J, Cockburn A, Seawright J. Sequence analysis of the ribosomal DNA internal transcribed spacer 2 from populations of Anopheles nuneztovari (Diptera: Culicidae) Mol Biol Evol. 1994;11:406–416. doi: 10.1093/oxfordjournals.molbev.a040122. [DOI] [PubMed] [Google Scholar]
- Galardo AK, Arruda M, D’Almeida Couto AA, Wirtz R, Lounibos LP, Zimmerman RH. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am J Trop Med Hyg. 2007;76:461–469. [PubMed] [Google Scholar]
- Gómez G, Cienfuegos A, Gutiérrez L, Conn J, Correa M. Análisis morfológico y molecular evidencia problemas al identificar Anopheles nuneztovari (Diptera: Culicidae) por claves dicotómicas. Rev Col Entomol. 2010;36:68–75. [Google Scholar]
- Gonzalez R, Carrejo N. Introducción al estudio taxonómico de Anopheles de Colombia: claves y notas de distribución. 2nd ed Programa Editorial Universidad de Valle; Cali: 2009. p. 260. [Google Scholar]
- Gutiérrez LA, Gomez GF, Gonzalez JJ, Castro MI, Luckhart S, Conn JE, Correa MM. Microgeographic genetic variation of the malaria vector Anopheles darlingi Root (Diptera: Culicidae) from Córdoba and Antioquia, Colombia. Am J Trop Med Hyg. 2010;83:38–47. doi: 10.4269/ajtmh.2010.09-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez LA, Gonzalez JJ, Gomez GF, Castro MI, Rosero DA, Luckhart S, Conn JE, Correa MM. Species composition and natural infectivity of anthropophilic Anopheles (Diptera: Culicidae) in the states of Córdoba and Antioquia, Northwestern Colombia. Mem Inst Oswaldo Cruz. 2009a;104:1117–11124. doi: 10.1590/s0074-02762009000800008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez LA, Naranjo NJ, Cienfuegos AV, Muskus CE, Luckhart S, Conn JE, Correa MM. Population structure analyses and demographic history of the malaria vector Anopheles albimanus from the Caribbean and the Pacific regions of Colombia. Malar J. 2009b;8:259. doi: 10.1186/1475-2875-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Huson DH, Steel M. Phylogenetic trees based on gene content. Bioinformatics. 2004;20:2044–2049. doi: 10.1093/bioinformatics/bth198. [DOI] [PubMed] [Google Scholar]
- IGAC, Instituto Geográfico Agustín Codazzi . Atlas de Colombia. 5a ed Imprenta Nacional de Colombia; Bogotá DC: 2002. p. 342. [Google Scholar]
- Kitzmiller J, Kreutzer R, Tallaferro E. Chromosomal differences in populations of Anopheles nuneztovari. Bull World Health Org. 1973;48:435–445. [PMC free article] [PubMed] [Google Scholar]
- Lounibos L, Conn J. Malaria vector heterogeneity in South America. Am Entomol. 2000;46:238–249. [Google Scholar]
- Lunt DH, Zhang DX, Szymura JM, Hewitt GM. The insect cytochrome oxidase I gene: evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol Biol. 1996;5:153–165. doi: 10.1111/j.1365-2583.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- McLain DK, Collins FH, Brandling-Bennett AD, Were JB. Microgeographic variation in rDNA intergenic spacers of Anopheles gambiae in western Kenya. Heredity. 1989;62:257–264. doi: 10.1038/hdy.1989.36. [DOI] [PubMed] [Google Scholar]
- Mirabello L, Conn JE. Population analysis using the nuclear white gene detects Pliocene/Pleistocene lineage divergence within Anopheles nuneztovari in South America. Med Vet Entomol. 2008;22:109–119. doi: 10.1111/j.1365-2915.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- Montoya-Lerma J, Solarte Y, Giraldo-Calderón G, Quiñones M, Ruiz-López F, Wilkerson R, González R. Malaria vector species in Colombia - A review. Mem Inst Oswaldo Cruz. 2011;106(Suppl.1):114–122. doi: 10.1590/s0074-02762011000900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone JJ. Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu Rev Entomol. 2006;51:467–494. doi: 10.1146/annurev.ento.50.071803.130447. [DOI] [PubMed] [Google Scholar]
- Olano VA, Brochero HL, Sáenz R, Quiñones ML, A MJ. Mapas preliminares de la distribución de especies de Anopheles vectores de malaria en Colombia. Biomédica. 2001;21:402–408. [Google Scholar]
- Onyabe D, Conn J. Intragenomic heterogeneity of a ribosomal DNA spacer (ITS2) varies regionally in the neotropical malaria vector Anopheles nuneztovari (Diptera: Culicidae) Insect Mol Biol. 1999;8:435–442. doi: 10.1046/j.1365-2583.1999.00134.x. [DOI] [PubMed] [Google Scholar]
- Padilla JC, Álvarez G, Montoya R, Chaparro P, Herrera S. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011;106(Suppl.1):114–122. doi: 10.1590/s0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPS . Informe de la situación del paludismo en las Américas. Organización Panamericana de la Salud; Washington DC: 2009. p. 9. [Google Scholar]
- Posso CE, González R, Cárdenas H, Gallego G, Duque M, Suarez M. Random Amplified Polymorphic DNA analysis of Anopheles nuneztovari (Diptera: Culicidae) from western and northeastern Colombia. Mem Inst Oswaldo Cruz. 2003;98:469–476. doi: 10.1590/s0074-02762003000400007. [DOI] [PubMed] [Google Scholar]
- Rosero D, Gutiérrez L, Cienfuegos A, Jaramillo L, Correa M. Optimización de un procedimiento de extracción de ADN para mosquitos anofelinos. Rev Col Entomol. 2010;36:260–263. [Google Scholar]
- Rozas J, Sanchez-DelBarrio J, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- Rubio-Palis Y, Curtis C. Biting and resting behaviour of anophelines in western Venezuela and implications for control of malaria transmission. Med Vet Entomol. 1992;4:325–334. doi: 10.1111/j.1365-2915.1992.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Scarpassa VM, Conn JE. Tracking of Complex Evolutionary History and Pleistocene Epoch Divergence for Neotropical Malaria Vector Anopheles nuneztovari Sensu Lato with Mitochondrial DNA. Am J Trop Med Hyg. 2011 doi: 10.4269/ajtmh.2011.11-0150. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpassa V, Conn J. Population genetic structure of the major malaria vector Anopheles darlingi (Diptera: Culicidae) from the Brazilian Amazon, using microsatellite markers. Mem Inst Oswaldo Cruz. 2007;102:319–327. doi: 10.1590/s0074-02762007005000045. [DOI] [PubMed] [Google Scholar]
- Scarpassa VM, Geurgas S, Azeredo-Espin AM, Tadei WP. Genetic divergence in mitochondrial DNA of Anopheles nuneztovari (Diptera: Culicidae) from Brazil and Colombia. Genet Mol Biol. 2000;23:71–78. [Google Scholar]
- Scarpassa VM, Tadei WP, Suarez MF. Population structure and genetic divergence in Anopheles nuneztovari (Diptera: Culicidae) from Brazil and Colombia. Am J Trop Med Hyg. 1999;60:1010–1018. doi: 10.4269/ajtmh.1999.60.1010. [DOI] [PubMed] [Google Scholar]
- Scarpassa V, Tadei W, Suarez M. Alloenzyme differentiation among allopatric populations of Anopheles nuneztovari (Diptera: Culicidae) Braz J Genet. 1996;19:265–269. [Google Scholar]
- Sierra DM, Velez ID, Linton YM. Malaria vector Anopheles (Nyssorhynchus) nuneztovari comprises one genetic species in Colombia based on homogeneity of nuclear ITS2 rDNA. J Med Entomol. 2004;41:302–307. doi: 10.1603/0022-2585-41.3.302. [DOI] [PubMed] [Google Scholar]
- Tadei WP, Thatcher B, Santos JM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- Tadei WP, Thatcher DB. Malaria vectors in the Brazilian amazon: Anopheles of the subgenus Nyssorhynchus. Rev Inst Med Trop Sao Paulo. 2000;42:87–94. doi: 10.1590/s0036-46652000000200005. [DOI] [PubMed] [Google Scholar]
- Zapata MA, Cienfuegos AV, Quiros OI, Quinones ML, Luckhart S, Correa MM. Discrimination of seven Anopheles species from San Pedro de Uraba, Antioquia, Colombia, by polymerase chain reaction-restriction fragment length polymorphism analysis of its sequences. Am J Trop Med Hyg. 2007;77:67, 72. [PubMed] [Google Scholar]



