Abstract
Background
The propionic acid derivative Benoxaprofen was introduced for the treatment of rheumatic disorders in 1980. Its product license was then withdrawn 2 years later due to concerns over serious dermatologic, hepatic and renal side effects. Photosensitivity was the most common side effect with reported incidence of up to 50%.
Main observations
We present the first case report of a patient who presented with a melanoma diagnosed 27 years after a benoxaprofen-induced photosensitivity reaction. With an estimated 1.5 million patients previously on benoxaprofen, a large number of patients may potentially face increased risk of developing malignant melanoma. This case report can only suggest an association between solar injury secondary to benoxaprofen-related photosensitivity and subsequent melanoma. However the primary factor that improves survival from melanoma is early diagnosis, and so clinicians treating this group of patients should be aware of this risk.
Conclusion
Although benoxaprofen is no longer in clinical use, the long-term sequelae to its photosensitizing effects may still be clinically important. Clinicians treating this group of patients should be vigilant, and consider a low threshold for diagnostic biopsy of suspicious skin lesions.
Keywords: benoxaprofen, malignant melanoma, photosensitivity, Opren®
Introduction
The propionic acid derivative Benoxaprofen was introduced for the treatment of rheumatic disorders in 1980 under the trade name Opren®,[1] and marketed as a non-steroidal anti-inflammatory drug.[2] Its product license was then withdrawn 2 years later due to concerns over serious dermatologic, hepatic and renal side effects that included rare but severe complications.[1,2,3] Among these, photosensitivity was the most common side effect with reported incidence of up to 50%.[2] A case of melanoma, arising at a site of previous benoxaprofen-related photosensitivity is presented, since this association may suggest these patients to be at increased risk.
Case Report
A 64-year-old woman presented with a 5 mm wide ulcerated lesion over her left trapezius. Initial excision biopsy diagnosed a 2 mm Breslow thickness, Clark level IV nodular malignant melanoma [Fig. 1], with no regression, lymphovascular or perineural involvement. She proceeded to 2 cm wide local excision, and direct closure. No further tumour was present, and locoregional recurrence has not been found after 2 years of follow up. This patient has also had non-melanoma skin cancer of the scalp (basal cell carcinoma). Neither tumour has been unduly aggressive in behaviour, or pathological features. A number of dysplatic naevi have been excised also.
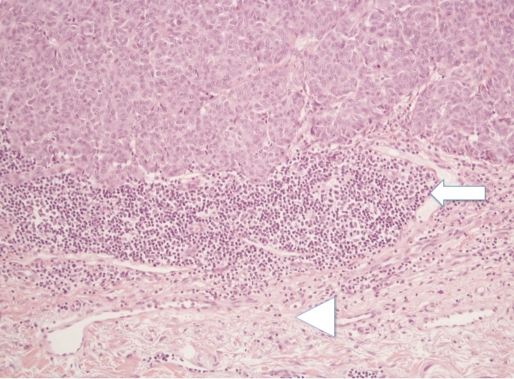
Figure 1.
Haematoxylin and eosin staining of melanoma with 200x magnification showing the deep edge of the tumour (white arrow) with an underlying chronic inflammatory cell infiltrate. Deep to this is a band of elastic tissue (arrow head), which would be consistent with sun damage.
She has no family history of malignancy, and sun exposure history was moderate. However she clearly recalled having suffered sunburn at the site of the subsequent melanoma formation 27 years previously. This solar injury was due to a photosensitivity reaction secondary to osteoarthritis management with benoxapren (Opren®) for a 5-month period before its withdrawal from the market.
Discussion
Benoxaprofen is a proprionic acid derivative, which has a marked inhibitory effect on lipoxygenase activity, leading to depressed leukotriene formation and leukocyte chemokinesis. It also acts directly on mononuclear cells, inhibiting mononuclear cell chemotaxis.[2,4] Worldwide, an estimated 1.5 million people took benoxaprofen over the 2-year period (available from May 1980 - August 1982),[2] when benoxaprofen was on the market. 3500 cases of adverse reactions had been reported to the Committee on Safety of Medicines, with 65 deaths, most of which were elderly patient renal or hepatic impairment, or both.[2]
In a study by Halsey and Cardoe[1] of 300 patients using benoxaprofen, about 70% of patients suffered cutaneous side effects, with 29% of the total suffering from photosensitivity (described as redness, itching and burning sensation felt on the skin within a few minutes of sun exposure). In the summer months, this incidence increased to 50%, confirming the drug as a potent photosensitiser. Hindson et al[4] used irradiation monochromator on the patients' skin before and during benoxaprofen treatment and found all patients demonstrating photosensitivity while on the drug, with reddening of skin accompanied by sensation of severe burning, itching with formation of weals in some patients. Wavelengths between 310 and 330 nm were found to be most the most effective in producing this response.[4] Patients were advised to undertake measures like using sunscreens which are effective in preventing the symptoms.[2]
Although photosensitivity from benoxaprofen does not persist after the treatment has been stopped, there are potential long-term effects from the damage caused by previous photosensitivity episodes. Malignant melanoma arises from malignant transformation of melanocytes, and represents 4% of all cancers. A major risk factor for development of malignant melanoma is UV exposure, with substantial evidence of contribution from UVB (280-320 nm) and UVA (320-400 nm).[5] The peak action spectrum of benoxaprofen as stated above, falls within the UVB spectrum and overlaps UVA suggesting a possible mechanism contributing to melanoma development. UV exposure risk primarily involves intermittent damaging sun exposure,[6] where a history of severe sunburn (even in youth) conferring approximately 2-fold increase in risk.[7] Therefore patients who have suffered from episodes of photosensitivity with benoxaprofen use are at higher risk of developing malignant melanoma. This risk also increases with adequate lag time (>10 years) after commencement of exposure[6] as seen with this patient. Although a direct relationship between benoxaprofen-induced photosensitivity reaction and development of melanoma has not been proven, it seems highly probable.
The use of antifungal drug voriconazole has been associated with development of melanoma in the setting of photosensitivity,[8] with recommendations of skin surveillance in these patients. Similarly, clinicians seeing patients previously on benoxaprofen who suffered from photosensitivity reactions should be aware of this risk and be vigilant in skin surveillance.
Conclusion
Although benoxaprofen is no longer in clinical use, the long-term sequelae to its photosensitizing effects may still be clinically important. With an estimated 1.5 million patients previously on benoxaprofen and up to 50% incidence of photosensitivity, a large number of patients may potentially face increased risk of developing malignant melanoma. This first case report can only suggest an association between solar injury secondary to benoxaprofen-related photosensitivity and subsequent melanoma. However the primary factor that improves survival from melanoma is early diagnosis,[9] and so clinicians treating this group of patients should be vigilant, and consider a low threshold for diagnostic biopsy of suspicious skin lesions.
References
- Halsey JP, Cardoe N. Benoxaprofen: side-effect profile in 300 patients. Br Med J (Clin Res Ed) 1982;284:1365–1368. doi: 10.1136/bmj.284.6326.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BR. Benoxaprofen and the skin. Br J Dermatol. 1983;109:361–364. doi: 10.1111/j.1365-2133.1983.tb03554.x. [DOI] [PubMed] [Google Scholar]
- Hindson C, Daymond T, Diffey B, Lawlor F. Side effects of benoxaprofen. Br Med J (Clin Res Ed) 1982;284:1368–1369. doi: 10.1136/bmj.284.6326.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous author. Benoxaprofen. Br Med J (Clin Res Ed) 1982;285:459–460. [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS. Ultraviolet A and melanoma: a review. J Am Acad Dermatol. 2001;44:837–846. doi: 10.1067/mjd.2001.114594. [DOI] [PubMed] [Google Scholar]
- Gallagher RP, Spinelli JJ, Lee TK. Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14:562–566. doi: 10.1158/1055-9965.EPI-04-0564. [DOI] [PubMed] [Google Scholar]
- Hussussain CJ. Malignant Melanoma in Thorne CH, Beasley RW, Aston SJ, Bartlett SP, Gurtner GC, Spear SL (ed) Grabb and Smith's Plastic Surgery 6th Edition. pp. 124–131. [Google Scholar]
- Miller DD, Cowen EW, Nguyen JC, McCalmont TH, Fox LP. Melanoma associated with long-term voriconazole therapy: a new manifestation of chronic photosensitivity. Arch Dermatol. 2010;146:300–304. doi: 10.1001/archdermatol.2009.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]